Abstract
Type 1 iodothyronine deiodinase (Dio1), a selenoenzyme catalyzing the bioactivation of thyroid hormone, is highly expressed in the liver. Dio1 mRNA and enzyme activity levels are markedly reduced in the livers of hepatocyte nuclear factor 4α (HNF4α)-null mice, thus accounting for its liver-specific expression. Consistent with this deficiency, serum T4 and rT3 concentrations are elevated in these mice compared with those in HNF4α-floxed control littermates; however, serum T3 levels are unchanged. Promoter analysis of the mouse Dio1 gene demonstrated that HNF4α plays a key role in the transactivation of the mouse Dio1 gene. Deletion and substitution mutation analyses demonstrated that a proximal HNF4α site (direct repeat 1 [TGGACAAAGGTGC]; HNF4α-RE) is crucial for transactivation of the mouse Dio1 gene by HNF4α. Mouse Dio1 is also stimulated by thyroid hormone signaling, but a direct role for thyroid hormone receptor action has not been reported. We also showed that thyroid hormone-inducible Krüppel-like factor 9 (KLF9) stimulates the mouse Dio1 promoter very efficiently through two CACCC sequences that are located on either side of HNF4α-RE. Furthermore, KLF9 functions together with HNF4α and GATA4 to synergistically activate the mouse Dio1 promoter, suggesting that Dio1 is regulated by thyroid hormone in the mouse through an indirect mechanism requiring prior KLF9 induction. In addition, we showed that physical interactions between the C-terminal zinc finger domain (Cf) of GATA4 and activation function 2 of HNF4α and between the basic domain adjacent to Cf of GATA4 and a C-terminal domain of KLF9 are both required for this synergistic response. Taken together, these results suggest that HNF4α regulates thyroid hormone homeostasis through transcriptional regulation of the mouse Dio1 gene with GATA4 and KLF9.
Thyroid hormone plays important roles in growth, development, differentiation, and the basal metabolic rate in vertebrates. Synthesis of thyroid hormone occurs exclusively in the thyroid gland, whose predominant secretory product is the prohormone thyroxin (T4) and which produces only a small amount of the biologically active hormone 3,5,3′-triiodothyronine (T3) (29). The majority of plasma T3 derives from extrathyroid tissues via outer-ring deiodination of T4 (9). This activation is catalyzed by two different deiodinases, type 1 iodothyronine deiodinase (Dio1) and type 2 iodothyronine deiodinase (Dio2). Dio1 is one of a family of selenoenzymes extensively expressed in the liver, kidney, thyroid, and pituitary in mammals (5, 6). Unlike Dio2, Dio1 can catalyze both activation of T4 by outer-ring deiodination (5′D) to generate T3 and inactivation of T4 by inner-ring deiodination to produce 3,3′,5′-triiodothyronine (rT3) (5D) (9). The expression and activity of Dio1 are modulated by a variety of hormonal, nutritional, and developmental factors, the most potent being thyroid hormone. Thyroid hormone-induced Dio1 expression contributes to the T3 excess commonly found in hyperthyroidism. Propylthiouracil and amiodarone are the two commonly used drugs that inhibit Dio1, which can have substantial effects on circulating thyroid hormone levels. Previous studies reported that T3 and retinoids induce the expression of the Dio1 gene via two complex thyroid hormone response-retinoic acid response elements located in the promoter region of the human Dio1 gene (66). Although both the rat and mouse liver Dio1 mRNAs are markedly increased by T3, canonical thyroid hormone response elements (TREs) have not yet been identified in the available 5′-flanking regions (5′-FR) of these genes (9, 35, 36).
Hepatocyte nuclear factor 4α (HNF4α; NR2A1) is a highly conserved member of the nuclear receptor superfamily. It is highly expressed in the liver, kidney, intestine, and pancreas in mammals (54). The active form of HNF4α is a homodimer which recognizes a direct repeat (DR) of the AGGTCA motif separated by 1 nucleotide (DR1) as a binding site. Binding sites for HNF4α have been found in the regulatory regions of many genes encoding proteins preferentially expressed in the liver, such as apolipoproteins, coagulation factors, serum proteins, and cytochromes P450, and those genes involved in the metabolism of fatty acids, amino acids, and glucose. Mice lacking hepatic expression of HNF4α have revealed that HNF4α regulates the expression of these target genes (17, 21-24, 49). HNF4α, as an orphan nuclear receptor, activates gene transcription in the absence of exogenous ligand (28, 55, 56); therefore, unlike classic nuclear receptors, the transcriptional activity of HNF4α is largely dependent on the selective interaction of tissue-specific or independently regulated coregulators with its activation function 2 (AF-2) domain to stimulate target genes in a tissue-specific and metabolically regulated gene-specific manner (16). For example, GATA factors function together with HNF4α to synergistically activate transcription through a direct protein-protein interaction (58).
The six GATA factors show homology in two zinc finger domains that mediate DNA binding and cofactor interactions. GATA1, -2, and -3 are expressed in developing bone marrow cells and are critical for hematopoiesis (14, 43), whereas GATA4, -5, and -6 have a more diverse pattern of expression that includes the liver, small intestine, heart, lungs, and gonads (3, 25, 31, 42, 60). Although the wide-ranging expression patterns of GATA4, -5, and -6 argue against the notion that these proteins are master regulators of tissue- or cell type-specific gene expression, there is increasing evidence that this subfamily might be critical in regulating cell-specific gene expression through unique interactions with other semirestricted transcription factors and cofactors (34, 41, 61, 64, 67). A recent study showed that GATA4 also forms a complex with Krüppel-like factor 13 (KLF13), a member of the KLF family of zinc finger proteins, and this interaction is important for activating genes involved in cardiac growth and differentiation (30).
To uncover possible additional roles for HNF4α in gene expression in an intact-animal model, we compared the gene expression profiles of liver-specific HNF4α-null mice and littermate HNF4α-floxed control mice. Through the evaluation of transcriptional changes by microarray and quantitative real-time PCR, we revealed that expression of the Dio1 gene is dependent on HNF4α expression and further demonstrated that the mouse Dio1 promoter is directly regulated by HNF4α. In this study, we also describe a likely mechanism by which T3 induces the expression of Dio1 in mouse liver and show that T3-inducible Krüppel-like transcription factor 9 (KLF9) functions together with HNF4α and GATA4 to synergistically activate the mouse Dio1 promoter through direct interaction between these transcriptional factors. These data suggest that HNF4α plays a key role in thyroid hormone homeostasis by cooperatively regulating the 5′ deiodination of T4 with GATA4 and KLF9 and also reveal that regulation of mouse Dio1 by T3 is likely due to an indirect mechanism responding to the T3-dependent stimulation of KLF9.
MATERIALS AND METHODS
Reagents.
T3, rT3, and 6-propyl-2-thiouracil (PTU) were obtained from Sigma; 125I-labeled rT3 was from Perkin-Elmer Life Sciences (Boston, MA). Other reagents were obtained from sources described previously (18, 19, 58, 68).
Antibodies.
Mouse monoclonal antibodies immunoglobulin G (IgG)-H1415 against human HNF4α2 (amino acids [aa] 394 to 461) (62) and IgG-H2429 against human GATA4 (aa 332 to 442) (58) were described in the indicated references. Other antibodies were obtained from the following sources. Goat polyclonal anti-HNF4α (sc-6556), goat polyclonal anti-KLF9 (sc-12996), rabbit polyclonal anti-GATA4 (directed against aa 328 to 439 of human GATA4) (sc-9053), and control goat IgG (sc-2028) were from Santa Cruz Biotechnology. Goat polyclonal anti-human GATA4 antibody (directed against aa 27 to 211 of human GATA) (AF2606) was from R&D Systems. Monoclonal anti-FLAG M2 (F3165), peroxidase-conjugated anti-FLAG M2 (A8592), mouse monoclonal anti-β-actin (A1978), and affinity-purified goat anti-mouse IgG (A4416) were from Sigma. Monoclonal antinucleoporin antibody (610497) was from BD- Biosciences. Control mouse IgG (132-13723) was from Wako (Osaka, Japan). Affinity-purified donkey anti-goat IgG was from Jackson ImmunoResearch Laboratories.
Animals.
Liver-specific HNF4α-null mice were generated as described previously (17). All experiments were performed with 2-month-old male mice (HNF4αflox/flox × albumin-Cre+/− [H4LivKO] and HNF4αflox/flox × albumin-Cre−/− [FLOX]). Mice were housed under a standard 12-h light/12-h dark cycle with ad libitum water and chow.
Affymetrix microarray analysis and quantitative real-time PCR (QRT-PCR).
Total RNA for microarray analyses was extracted from three independent H4LivKO and FLOX livers with ISOGEN (Wako Pure Chemical Industries, Japan). Equivalent amounts from each animal were pooled before the preparation of biotin-labeled cRNA according to the Affymetrix GeneChip Expression Analysis Technical Manual. Hybridizations were performed with 10 μg of fragmented cRNA in triplicate samples, on GeneChip Mouse Genome 430 2.0 arrays (Affymetrix), three for H4LivKO samples and three for FLOX samples. After washing, arrays were stained with streptavidin-phycoerythrin and image data were collected and analyzed with an Affymetrix GeneChip Scanner 3000 (Affymetrix). The GeneChip Analysis Suite software version 5.0 was used to calculate the average difference for each gene probe on the array. The average differences were normalized for each array so as to have a mean value of 100. The average change (2n-fold) is expressed relative to FLOX mice. The methods for QRT-PCR have been described previously (33, 63, 68). The sequences of all of the primer used in this study are available on request.
Dio1 assay.
Dio1 activity was determined by the release of radioiodide from 125I-labeled rT3 according to the method of Leonard and Rosenberg (32). Mouse liver or kidney was homogenized in ice-cold homogenization buffer (0.1 M potassium phosphate at pH 7.2, 1 mM dithiothreitol, 2 mM EDTA) with a Polytron (Kinematica AG, Lucerne, Switzerland) and clarified by centrifugation at 1,000 × g for 10 min at 4°C, and the supernatants were used for the Dio1 assay. Prior to the assay, 125I-labeled rT3 was purified by column chromatography with Sephadex LH-20 (GE Healthcare) on the day of the experiment. Aliquots of the supernatants (20 μg protein for liver and 75 μg for kidney) were incubated with rT3 (1.0 μM for liver and 0.5 μM for kidney) as a substrate and 125I-labeled rT3 (100,000 cpm/tube) as a tracer in deiodinase buffer (0.1 M potassium phosphate at pH 7.2, 10 mM dithiothreitol, 2 mM EDTA) at 37°C for 30 min. The reaction was stopped by the addition of 100 μl of 5% (wt/vol) bovine serum albumin, and the protein-bound iodothyronines were precipitated by adding 800 μl of ice-cold 10% trichloroacetic acid. After centrifugation, the supernatants were applied to minicolumns packed with AG 50W-X2 resin (Bio-Rad Laboratories) and eluted in 1 ml of 10% acetic acid. The released 125I− was counted in a gamma counter (1480 WIZARD Automatic Gamma Counter; Perkin-Elmer). Background levels of deiodination were assessed under the same conditions with the addition of 1 mM PTU. The difference between incubations with and without PTU represented Dio1 activity. Reaction rates were linear with time from 5 to 60 min and with protein content in the range used. The enzymatic activity was expressed as picomoles of I− released per milligram of protein per minute.
Assays for serum T4, T3, rT3, and TSH concentrations.
Total serum T4 and T3 levels were determined with the competitive enzyme-linked immunosorbent assay kits from Alpha Diagnostic International (San Antonio, TX) and Diagnostic Systems Laboratories (Webster, TX), respectively. The reference ranges for T4 and T3 were 1.5 to 24 μg/dl and 50 to 750 ng/dl, respectively. Total serum rT3 was measured by radioimmunoassay with a kit from Adaltis Italia (Rino, Italy). The reference range was 2.5 to 200 ng/dl. Serum thyroid-stimulating hormone (TSH) was measured by radioimmunoassay with the rat TSH assay kit obtained from GE Healthcare. The reference range for TSH was 1.0 to 64 ng/ml. All assays were performed according to the manufacturers' instructions.
Construction of the promoter reporter gene for the luciferase assay.
pDio1(1998) is the mouse Dio1 promoter-luciferase reporter gene that spans positions −1998 to −1 relative to the translation initiation site. pDio1(978), pDio1(500), pDio1(380), pDio1(190), pDio1(110), pDio1(75), and pDio1(65) each contain deletion-containing constructs of the 5′-FR with the 5′ end of each noted in parentheses and the same 3′ endpoint at −1. pDio1(1998) was constructed by the application of PCR to mouse genomic DNA with a forward primer starting at −1998 (5′-GCTGTTAGAAGGACTGGTGGTCGTGTCGTTCTTG-3′) and reverse primer Oli-1R (5′-CCCAAGCTTCTCAGCACGGGGCAGAAGTGGC-3′) flanked by a HindIII site. The digested PCR product was cloned into the SmaI-HindIII sites of pGL3 basic. pDio1(978), pDio1(500), pDio1(380), pDio1(190), pDio1(110), pDio1(75), and pDio1(65) were constructed in a manner identical to that used for pDio1(1998), with forward primers starting at positions −978, −500, −380, −190, −110, −75, and −65, respectively, and coupled with a common reverse primer, Oli-1R. Base substitution mutants were generated in pDio1(500) with the QuikChange II site-directed mutagenesis kit according to the manufacturer's protocol. Oligonucleotides were designated to mutate each element as follows: DR1 motif at −400, from 5′-GGATCTTCTGACT-3′ to 5′-GGATCC-3′; DR1 motif at −88, from 5′-GCACCTTTGTCCA-3′ to 5′-GGATCC-3′; KLF motif at −165, from 5′-CCACCC-3′ to 5′-GGATCC-3′; KLF motif at −71, from 5′-CCACCC-3′ to 5′-GGATCC-3′.
Expression plasmids.
Cytomegalovirus promoter-driven mammalian expression vectors for full-length and deletion mutant HNF4α1 that encode aa 1 to 455 and an internal deletion, Δ359-368 [pCMV-HNF4α1 and pCMV-HNF4α1(Δ359-368)], were constructed in pcDNA3 (Invitrogen). Cytomegalovirus promoter-driven expression vectors for full-length and deletion mutant GATA4 that encode aa 1 to 442, 1 to 303, 1 to 332, and 202 to 442 and internal deletions Δ202-303, Δ304-332, and Δ202-332 constructed in pcDNA3 were described previously (58). Cytomegalovirus promoter-driven expression vectors for mutant GATA4 that encode internal deletions Δ202-244, Δ265-303, and C274G were constructed in pcDNA3 in an identical manner. pCMV-KLF9, a cytomegalovirus promoter-driven expression vector encoding full-length mouse KLF9, pCMV-KLF15 encoding rat KLF15, and pCMV-KLF6 encoding mouse KLF6 were described previously (68). pCMV-FLAG-KLF13 encoding mouse KLF13 containing a FLAG epitope at the N terminus was constructed by reverse transcription-PCR with total RNA from mouse liver as a template and by insertion of the full-length coding sequence into pCMV-Tag2 (Stratagene). The mammalian expression plasmid for KLF2 (pBK-LKLF) (2) was generously provided by Jerry Lingrel (University of Cincinnati College of Medicine), and the mammalian expression plasmid for KLF4 (pcDNA3-GKLF) (69) was a kind gift from Shaw-Fang Yet (Harvard Medical School). The mammalian expression plasmid for KLF5 (45) was kindly provided by Ryozo Nagai (University of Tokyo Graduate School of Medicine). pCMVβ, a plasmid encoding the Escherichia coli β-galactosidase reference gene, was obtained from Stratagene. All mutants were confirmed by DNA sequencing.
Cell culture.
Monolayers of HepG2 cells, a human hepatoma cell line; HEK293 cells, a human embryonic kidney cell line; and NMuLi (ATCC CRL1638) (46) cells, a murine normal liver epithelial cell line, were maintained in Dulbecco's modified Eagle's medium containing 100 μg/ml streptomycin sulfate and 100 U/ml penicillin supplemented with 10% (vol/vol) fetal bovine serum. Chinese hamster ovary (CHO) cells were maintained in Ham F-12 medium containing 5% (vol/vol) fetal bovine serum. All cells were cultured in 5% CO2 at 37°C.
Luciferase reporter assay.
HEK293 and HepG2 cells were plated at a density of 1 × 105/24-well plate on the day prior to transfection. On day 1, cells were transfected with luciferase reporter plasmid (0.1 μg), the indicated amount of expression plasmids, and pCMVβ (0.05 μg) with Lipofectamine 2000 reagent according to the manufacturer's instructions. The total amount of DNA per well was kept constant by adding the corresponding amount of expression vector without a cDNA insert. On day 2, the cells were harvested and assayed for firefly luciferase activity and normalized to β-galactosidase activity as described previously (18, 20, 58). All experiments were performed at least three times in duplicate or triplicate, and the most representative results are shown.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed as previously described (18, 19, 58). Frozen mouse liver (∼0.2 g) was chopped into small pieces with a mortar and pestle and cross-linked for 15 min at room temperature with formaldehyde at a final concentration of 1% (wt/vol) in phosphate-buffered saline. The samples were subsequently washed twice with phosphate-buffered saline containing protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 2.8 μg/ml aprotinin, 10 μg/ml leupeptin, and 5 μg/ml pepstatin A), and then tissue samples were disaggregated with a Dounce homogenizer. After centrifugation, the pellets were resuspended in sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris-HCl at pH 8.1, 1% SDS, 10 mM EDTA) containing protease inhibitors and sonicated to generate a 200- to 1,000-bp DNA fragment. ChIP was performed with anti-HNF4α (IgG-H1415), anti-GATA4 (AF2606) (R&D Systems), anti-KLF9 (sc-12996) (Santa Cruz Biotechnology), or control IgG according to the manufacturer's protocol (Upstate). The primers used to amplify the proximal mouse Dio1 promoter sequences were 5′-AGAGAGAGCTCTGTGCCCTGG-3′ and 5′-GAAGTGGCTCTGAGCCTGCAG-3′ (positions −15 to −199 with respect to the first ATG codon). The primers for the distal mouse Dio1 promoter sequences were 5′-GCGACAATGCCCTGATCAAGA-3′ and 5′-GATTCCACATCTGTTGCTTCA-3′ (positions −1769 to −1948).
Immunoprecipitation and immunoblot analysis.
For protein expression, HEK 293 cells were transfected with 1 μg of the indicated constructs by FuGENE 6 (Roche Applied Science). Cell lysates were prepared with Nonidet P-40 lysis buffer (50 mM HEPES-HCl at pH 7.4, 100 mM NaCl, 1.5 mM MgCl2, 1% [vol/vol] Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 5 μg/ml pepstatin A, 25 μg/ml N-acetyl-leucinal-leucinal-norleucinal, 1 mM dithiothreitol) as previously described (50) and immunoprecipitated with immune monoclonal or polyclonal antibodies. The eluate was separated by denaturing SDS-polyacrylamide gel electrophoresis (PAGE), transferred to Hybond-C extra nitrocellulose filters (GE Healthcare), and immunoblotted with one of the antibodies described above.
Gel mobility shift assay.
The gel mobility shift assay was performed with double-stranded probes as previously described (58). The sequences of the oligonucleotides used are listed in Table 1.
TABLE 1.
Double-stranded oligonucleotides used for gel shift analyses
| Probe | Sequencea |
|---|---|
| Dio1 (−95/−66) wild | 5′-GTAGCCTGCACCTTTGTCCAGCTCCACCCA-3′ |
| 5′-TGGGTGGAGCTGGACAAAGGTGCAGG-3′ | |
| Dio1 (−95/−66) mutant | 5′-GTAGCCTGTGTTCCCACTCAGCTCCACCCA-3′ |
| 5′-TGGGTGGAGCTGAGTGGGAACACAGG-3′ | |
| Dio1 (−407/−379) wild | 5′-GCAGCTGGGATCTTCTGACTCCTTTTTGC-3′ |
| 5′-GCAAAAAGGAGTCAGAAGATCCCAG-3′ | |
| Human CYP8B1 | 5′-GGCCACAGGGCAAGGTCCAGGTGCTCAGAC-3′ |
| 5′-GTCTGAGCACCTGGACCTTGCCCTGTGG-3′ |
Bold and underlined base pairs represent the specific mutations in each oligonucleotide.
Glutathione S-transferase (GST) pull-down and mammalian two-hybrid assay.
GST fusion constructs containing the full length and aa 41 to 245 of KLF9 were created in bacterial expression vector pGEX4T-2 (GE Healthcare), expressed in BL21 bacteria, and purified with the MagneGST protein purification system (Promega) as described previously (58). Purified GST or GST fusion proteins were incubated with 35S-labeled GATA4 synthesized with the TNT Quick Coupled Transcription/Translation system (Promega) for 2 h at 4°C and washed three times before SDS-PAGE was carried out. For the mammalian two-hybrid assay, we used the CheckMate Mammalian Two-Hybrid System (Promega) as described previously (58). The reporter plasmid pGAL4-luc, GAL4-HNF4α(117-465), and the full length and deletion mutant (Δ202-332) of VP16-GATA4 were previously described (58). The various VP16-GATA4 mutants (Δ202-244, Δ268-303, and C274G) were created in the full-length VP16-GATA4 fusion construct by site-directed mutagenesis.
Statistical analysis.
Student's t test was used to compare data between the control and treated groups. A value of P < 0.05 was considered significant.
RESULTS
Expression of the Dio1 gene is reduced in livers from liver-specific HNF4α-null mice.
To reveal an additional role for HNF4α in the liver, the gene expression profile was examined in a liver-specific HNF4α-null mouse. The expression of ∼39,000 transcripts was measured by using Affymetrix Mouse Genome 430 2.0 arrays. To identify genes that are likely to be direct targets of HNF4α, genes were filtered with a stringent cutoff. For a list of the genes whose expression was decreased to less than 0.05 (2−4.4-fold) in the livers of hepatic HNF4α-null mice (designated H4LivKO) compared with their littermate control mice (designated FLOX), see Table S1 in the supplemental material. They include two genes encoding apolipoproteins, four genes encoding cytochromes P450, six genes encoding enzymes involved in lipid and steroid metabolism, one gene encoding an enzyme involved in amino acid metabolism, and one gene encoding an enzyme associated with glucose metabolism. Two genes had been characterized previously as direct HNF4α targets (23, 48) (see Table S1 in the supplemental material; denoted by the symbol *). Eight genes had been reported to be down-regulated in HNF4α-null embryonic livers (4) (see Table S1 in the supplemental material; denoted by the symbol **). With our stringent cutoff, other potential HNF4α target genes were identified (see Table S1 in the supplemental material; for the potential target genes with a low-stringency cutoff, see Table S2 in the supplemental material). One of these new putative HNF4α target genes was Dio1, and it encodes a member of a small family of selenoenzymes and catalyzes the bioactivation of thyroid hormone.
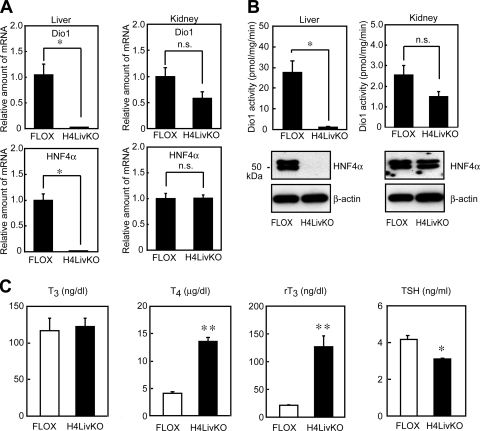
To validate the Dio1 array results, the levels of Dio1 mRNA in the livers were determined by QRT-PCR. As shown in Fig. 1, the expression of Dio1 was profoundly reduced in the livers of H4LivKO mice compared with the control group (FLOX, Fig. 1A). This reduction is due to the loss of HNF4α expression, since the levels of Dio1 mRNA in the kidney, where HNF4α was still present, did not show any significant changes. As expected, HNF4α mRNA was present and absent in the livers of FLOX and H4LivKO mice, respectively (Fig. 1A).
FIG. 1.
Dio1 gene expression is profoundly reduced in the livers of liver-specific HNF4α-null mice. (A) Dio1 mRNA levels in the livers and kidneys of control (FLOX) mice or mice lacking liver HNF4α (H4LivKO). Total RNA from the livers and kidneys of FLOX or H4LivKO mice (17) (2-month-old male mice, n = 4 or 5 per group) was subjected to QRT-PCR quantification for Dio1 and HNF4α as a control. Cyclophilin was used as the invariant control. Values represent the amount of mRNA relative to that in the control group (FLOX), which is arbitrarily defined as 1. Each bar represents the mean ± the standard error. *, P < 0.01 compared with FLOX; n.s., not significant. (B) Dio1 activities in the livers and kidneys from FLOX and H4LivKO mice. Tissue homogenates from FLOX and H4LivKO mice (2-month-old male mice, n = 5 per group) were prepared, and Dio1 activities were determined with rT3 as the substrate as described in Materials and Methods. Each bar represents the mean ± the standard error. *, P < 0.01 (compared with FLOX); n.s., not significant (top). The tissue homogenates from each group were separately pooled, and 10-μg aliquots of whole-cell lysate were subjected to SDS-PAGE and immunoblot analysis with anti-HNF4α (IgG-H1415) and β-actin antibody (bottom). (C) Serum T3, T4, rT3, and TSH levels in FLOX and H4LivKO mice. Serum samples from FLOX and H4LivKO mice (2-month-old male mice, n = 6 or 7 per group) were analyzed. Each bar represents the mean ± the standard error. *, P < 0.05; **, P < 0.01 (compared with FLOX).
Dio1 activity is reduced in the livers of liver-specific HNF4α-null mice.
To determine whether the reduced Dio1 mRNA expression seen results in reduced enzymatic activity, Dio1 activity was assessed in livers from FLOX and H4LivKO mice with 125I-labeled rT3, the preferred substrate for Dio1 in the absence and presence of PTU. As a control, we also examined Dio1 activity in the kidney. As shown in Fig. 1B, high levels of 5′D activity (outer-ring deiodination activity) were found in the livers of FLOX mice. In contrast, consistent with mRNA levels, 5′D activity in the livers of H4LivKO mice was markedly reduced, by more than 90%, while 5′D activities in the kidneys were comparable between FLOX and H4LivKO mice. In these assays, 5′D activity was reduced by more than 99% in the presence of PTU (data not shown), indicating that it was primarily a result of Dio1 (27, 52). These data indicate that hepatic Dio1 gene expression and Dio1 activity are regulated by HNF4α. To further confirm that Dio1 activity was lost in the liver, serum T4, T3, rT3, and TSH concentrations were assessed in FLOX and H4LivKO mice. Both T4 and rT3 concentrations in serum were approximately three- to fourfold higher in H4LivKO mice (Fig. 1C). In contrast, serum T3 concentrations were comparable. The serum TSH concentration was slightly, yet significantly, reduced in H4LivKO mice (Fig. 1C). The above thyroid hormone profile was similar to that of Dio1KO mice (52), with the exception of the serum TSH concentration. In the Dio1KO mice, the serum TSH concentration was comparable to that of wild-type mice. This may be explained in part by the high serum T4 concentration contributing to T3 production in the pituitaries of the H4LivKO mice, where, unlike in the complete Dio1KO mice, expression of Dio1 and Dio2 is normal.
Dio1 is a direct transcriptional target of HNF4α.
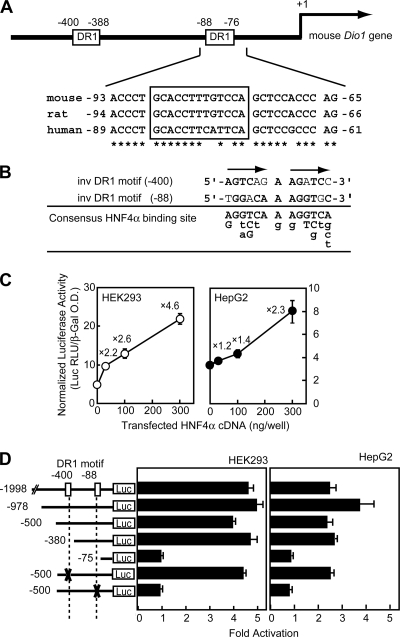
To determine whether HNF4α directly regulates Dio1 expression, the Dio1 gene promoter was examined for the presence of an HNF4α binding site and transactivation studies were carried out. HNF4α preferentially binds DNA as a homodimer to the HNF4α-responsive element (HNF4α-RE), which is composed of two nuclear receptor consensus half sites of AG(G/T)TCA organized as a direct repeat and separated by a single nucleotide (direct repeat 1, DR1). Scanning the 5′-flanking sequences of the mouse, rat, and human genes for the presence of putative DR1 motifs by a computer-assisted homology search (Genomatix) revealed a highly conserved DR1-like element (namely, DR1 motif at −88). This region is located 88 nucleotides upstream of the translation start site for Dio1 in mice (Fig. 2A) and adjacent to a conserved GC/CA-rich sequence, a binding motif for the Sp1/KLF family transcription factor (59). In addition, another potential HNF4α binding site was located at position −400 (DR1 motif at −400) in the mouse promoter (Fig. 2A and B).
FIG. 2.
Dio1 is the direct transcriptional target of HNF4α. (A) Schematic representation of the mouse Dio1 promoter, illustrating two DR1 motifs (top). The DR1 motif at position −88 is conserved in the mouse, rat, and human promoters. Conserved nucleotides are indicated by asterisks (bottom). Nucleotide position +1 is assigned to the A of the ATG initiator codon. (B) Alignment of the two DR1 motifs in the mouse Dio1 promoter. The sequences of DR1 motifs at −400, −88, and the consensus nucleotide(s) found in the HNF4α binding sequences are aligned. Since the DR1 motifs at −400 and −88 are in the inverted orientation, the bottom strand of the DNA is presented. At the consensus site, the consensus nucleotide(s) found in the HNF4α binding sequences is represented by capital letters; the lowercase letters point out divergences from the consensus that are represented as described in reference 54. (C) HNF4α activates the mouse Dio1 promoter. The indicated amounts of HNF4α expression plasmids were transfected into either HEK293 or HepG2 cells together with pDio1(500) and incubated for 24 h, after which the cells were harvested and assayed for luciferase and β-galactosidase activities as described in Materials and Methods. The values above the open and closed circles refer to the induction levels relative to those measured in the absence of the HNF4α expression plasmid. O.D., optical density. (D) Identification of an HNF4α-RE in the mouse Dio1 promoter by progressive deletion and mutation analyses. HEK293 or HepG2 cells were transfected with the indicated Dio1-luciferase reporter, in which each DR1 motif was deleted or mutated, together with 0.3 μg of the HNF4α expression plasmid and assayed for transcriptional activity. Transfection and luciferase assays were performed as described above. The relative activation level (normalized luciferase activity cotransfected with HNF4α expression plasmid versus empty vector) is shown. (C and D) Each value represents the mean ± the standard deviation of triplicate experiments.
To determine whether HNF4α has the potential to activate the mouse Dio1 promoter, a DNA fragment containing the 5′-FR of the mouse Dio1 gene was subcloned into the promoterless luciferase reporter gene, pGL3 basic, and transiently transfected into HEK293 and HepG2 cells along with increasing amounts of HNF4α expression plasmid. As shown in Fig. 2C, the expression levels of luciferase were increased in proportion to the amount of cotransfected HNF4α expression plasmid (maximum, 4.6- and 2.3-fold, respectively), suggesting that Dio1 is an HNF4α target gene.
Identification of the HNF4α binding site in the mouse Dio1 promoter.
To define the location of the control element that mediates the effects of HNF4α, a series of 5′ deletions were introduced into the promoter region. Deletion of the sequence from −1998 to −380 did not change the sensitivity to overexpressed HNF4α (Fig. 2D). A further deletion to −75 resulted in complete loss of induction by HNF4α in both HEK293 and HepG2 cells. Furthermore, in the context of pDio1(500), mutation of the DR1 motif at −88 reduced activity in HepG2 cells, indicating that this proximal HNF4α site is crucial for activation of the Dio1 gene, while mutation of the DR1 motif at −400 did not abolish the responsiveness to HNF4α. It should be noted that the response to transfected HNF4α expression plasmid was significantly higher in HEK293 than in HepG2 cells. This is likely due to the higher cellular levels of endogenous HNF4α in HepG2 cells.
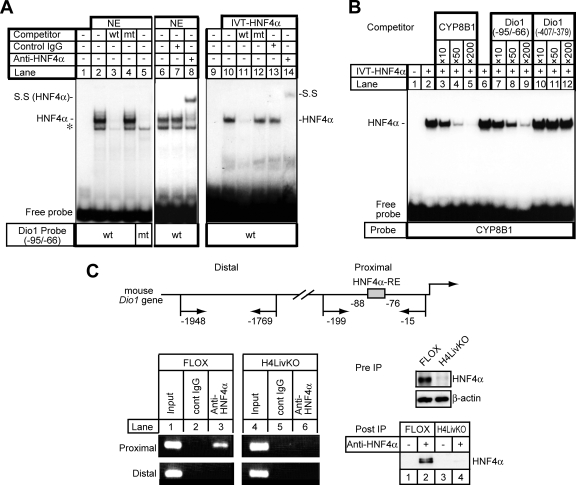
To determine whether HNF4α is capable of binding to the DR1 motif at −88 of the mouse Dio1 promoter, an electrophoretic mobility shift assay (EMSA) was performed with 32P-labeled probes covering the HNF4α binding site (double-stranded 30-mer corresponding to −95 to −66) with either nuclear extracts from HepG2 cells (Fig. 3A, lanes 1 to 8) or in vitro-translated HNF4α (Fig. 3A, lanes 9 to 14). HepG2 nuclear extracts contain a protein that binds to the Dio1 DR1 motif and reacts with the HNF4α antibody (IgG-H1415; Fig. 3A, lanes 2, 6, and 8). Analyses with in vitro-translated HNF4α produced similar results (Fig. 3A, lanes 10 and 14). In both cases, the protein-DNA complex was eliminated by the addition of an excess of unlabeled wild-type probe (Fig. 3A, lanes 3 and 11) but not by the addition of the corresponding mutant oligonucleotides (Fig. 3A, lanes 4 and 12). Also, no specific complex was detected with the labeled mutant probe and the HepG2 nuclear extract (Fig. 3A, lane 5). In contrast, 32P-labeled probes covering the other putative DR1 motif at −400 (corresponding to −407 to −379) did not produce any specific bands under these conditions (data not shown).
FIG. 3.
Specific binding of HNF4α to the mouse Dio1 gene promoter. (A) EMSA. Nuclear extracts (NE) isolated from HepG2 cells (20 μg) or in vitro-translated HNF4α protein (IVT-HNF4α) were incubated with a 32P-labeled wild-type (wt) (lanes 1 to 4 and 6 to 8) or mutant (mt) (lane 5) oligonucleotide probe carrying HNF4α-RE in the absence or presence of a 200-fold excess of the unlabeled wild-type (lanes 3 and 11) or mutant oligonucleotide (lanes 4 and 12). For the supershift assay, an anti-HNF4α antibody, IgG-H1415 (lanes 8 and 14), was added to the binding reaction mixture. Each anti-HNF4α antibody (2 μg) was added to the reaction mixture 30 min after the addition of the probe and incubated for an additional 10 min. The HNF4α-DNA complex and its supershifted complex (S.S) is indicated. Nonspecific bands are denoted by an asterisk. (B) IVT-HNF4α was incubated with a double-stranded, radiolabeled oligonucleotide probe containing the sequence of the high-affinity HNF4α binding site of the human CYP8B1 promoter (70), followed by EMSA. Competition experiments were performed in the presence of a 10- to 200-fold molar excess of the unlabeled oligonucleotides for the HNF4α binding site of the human CYP8B1 promoter (lanes 3 to 5), the DR1 motif at −88 (Dio1 −95/−66) (lanes 7 to 9), or the DR1 motif at −400 (Dio1 −407/−379) (lanes 10 to 12) of the mouse Dio1 promoter. (C) HNF4α-RE of the mouse Dio1 promoter binds to HNF4α in the context of an intact chromatin structure as demonstrated by ChIP. Schematic representation of the 5′-FR of the mouse Dio1 gene. Positions of primer sets (arrow) relative to the translation start site are denoted (top). Cross-linked DNA-protein complexes from either FLOX or H4LivKO mouse liver extracts were immunoprecipitated with anti-HNF4α and control IgG, followed by PCR amplification with specific primers as schematically depicted in the diagram. Genomic DNA in the input cell lysates was used as a positive control. As a negative control, the distal region, which has no HNF4α binding sites, was used for PCR amplification. Amplified PCR products were separated on an ethidium bromide-stained 2% agarose gel (bottom left). The presence of HNF4α in cell lysate before immunoprecipitation (Pre IP) and the samples immunoprecipitated with anti-HNF4α antibody (Post IP), detected by immunoblot analysis with anti-HNF4α antibody, is shown (bottom right).
To further evaluate the ability of the proximal and distal DR1 motifs (at −88 and −400, respectively) to compete for binding to a consensus HNF4α-RE found in the human CYP8B1 promoter (70), an EMSA was carried out. The Dio1 probe carrying the DR1 motif at −88 was able to compete for binding to a radiolabeled band corresponding to the human CYP8B1 promoter HNF4α binding site (70) (Fig. 3B, lanes 7 to 9), while the Dio1 probe carrying the DR1 motif at −400 was not (Fig. 3B, lanes 10 to 12), indicating that only the proximal DR1 motif at −88 is able to displace binding over the well-characterized HNF4α-RE of the CYP8B1 promoter with an efficiency similar to that of the unlabeled self probe. Taken together, these results indicate that the Dio1 promoter has a single HNF4α binding site that is critical for the activation of gene expression. Therefore, hereafter we refer to the DR1 motif at −88 as an HNF4α-RE in the mouse Dio1 promoter.
Finally, we assessed HNF4α binding to the Dio1 promoter in the mouse liver by a ChIP assay. After formaldehyde-based cross-linking of protein and DNA, chromatin fragmented by sonication was immunoprecipitated with anti-HNF4α antibody or with IgG as a control, followed by PCR amplification with specific primers for HNF4α-RE, as schematically depicted in the upper part of Fig. 3C. As a control, a ChIP was carried out with liver extracts from H4LivKO mice. In FLOX mouse liver extracts, endogenous HNF4α bound to the promoter of the Dio1 gene (Fig. 3C, bottom left, compare lanes 2 and 3), while in H4LivKO liver extracts, no band was detected (Fig. 3C, bottom left, compare lanes 5 and 6). In addition, there was no enrichment of DNA corresponding to another unrelated distal region of the Dio1 promoter (positions −1948 to −1769). The immunoblot in the bottom right part of Fig. 3C shows that HNF4α protein levels were decreased in the H4LivKO mice (Fig. 3C, Pre IP) and that HNF4α was efficiently captured by the immunoprecipitation procedure with anti-HNF4α antibody (Fig. 3C, Post IP) in proportion to the overall levels (Fig. 3C, compare the gels labeled “Pre IP” for direct immunoblotting with those labeled “Post IP”).
Consistent with the EMSA results showing that HNF4α binds to HNF4α-RE in vitro, the ChIP results revealed that HNF4α associates with the HNF4α-RE-containing region of the mouse Dio1 promoter in vivo.
Regulation of the Dio1 gene by T3-regulated KLF9.
Although the above data demonstrated that HNF4α activates the mouse Dio1 promoter through a single HNF4α-RE, HNF4α is a very weak activator of this promoter (Fig. 2; a maximum of 2.3-fold in HepG2 cells). Like most eukaryotic genes, Dio1 is likely to be regulated by additional transcriptional mechanisms in addition to HNF4α. In fact, Dio1 is highly regulated by T3 (8, 9). Studies with thyroid hormone receptor (TR) knockout mice indicate that TRβ is primarily responsible for T3-mediated Dio1 stimulation (1). However, unlike the human promoter, canonical TREs or TRE-like elements have not been identified in the available 5′-FR of mouse and rat Dio1 genes (9, 35, 36). We therefore tested whether its transcriptional potency might be enhanced in the presence of a thyroid hormone-regulated transcription factor, which may coactivate HNF4α transcriptional activity of the Dio1 promoter.
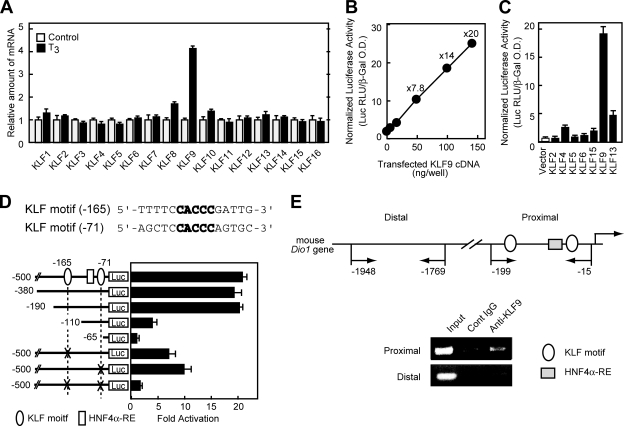
KLF9 (or BTEB1) is a member of the Sp/KLF family of transcription factors and is robustly activated by thyroid hormone T3 treatment (15). Like Dio1, the regulation of this gene is specific to the TRβ1 isoform (15). KLF9 is one of 25 Sp/KLF family members that are characterized by the presence of a highly conserved carboxy (C)-terminal region containing three zinc finger domains that bind GC-rich sequence motifs in DNA and extremely divergent amino (N)-terminal regions that confer specificity and selectivity on their transcriptional activities and protein-protein interactions (26, 59). We evaluated thyroid hormone regulation of KLF9 and other KLF family members in mouse NMuLi hepatocytes. We chose the mouse line because of the differences in thyroid hormone regulation of mouse and human Dio1 genes as mentioned in the introduction. As shown in Fig. 4A, consistent with other reports (12, 15, 39), the expression of the KLF9 gene, but not that of other KLF family members, was increased by the treatment of normal mouse NMuLi hepatocytes with T3. To examine whether KLF9 might act as a transcriptional activator of the Dio1 promoter, transient cotransfection assays were carried out. Several other representative KLF proteins were chosen on the basis of structural similarity to KLF9 (30), and their effects on the Dio1 promoter were tested. As shown in Fig. 4B, KLF9 dose dependently activated the Dio1 promoter up to 20-fold in HepG2 cells. Closely related KLF13 also stimulated the Dio1 promoter, albeit less efficiently than KLF9, whereas other, more distantly related, KLF proteins were ineffective (Fig. 4C). The KLF expression plasmids used in this study were characterized by the original investigators, from whom we obtained the constructs (2, 45, 69). For the expression of KLF6 and KLF13, since they contain a FLAG epitope tag, immunoblot analyses were performed with an anti-FLAG antibody, confirming that the proteins were expressed properly (data not shown).
FIG. 4.
T3-regulated KLF9 activates the mouse Dio1 promoter. (A) Effects of T3 treatment on various KLF mRNA levels in the normal mouse liver epithelial NMuLi cell line. On day 0, cells were set up at 5 × 105/six-well plate in 2 ml of Dulbecco modified Eagle medium supplemented with 10% charcoal-dextran-treated fetal bovine serum (HyClone). On day 2, the cells were switched to the medium with or without 30 nM T3. On day 3, cells were harvested for RNA analysis by QRT-PCR. (B) KLF9 activates the mouse Dio1 promoter. The indicated amounts of KLF9 expression plasmids were transfected into HepG2 cells together with pDio1(500) and incubated for 24 h, after which the cells were harvested and assayed for luciferase and β-galactosidase activities. The values above the closed circles refer to the level of induction relative to that found in the absence of the KLF9 expression plasmid. O.D., optical density. (C) Transactivation of the mouse Dio1 promoter by various KLF proteins in HepG2 cells. HepG2 cells were transfected with pDio1(500) and 0.1 μg of KLF expression plasmids. Luciferase assays were performed as described above. (D) Localization of KLF9-responsive element in the mouse Dio1 promoter by progressive deletion and mutation analyses. HepG2 cells were transfected with the indicated Dio1-luciferase reporter, in which each KLF motif was deleted or mutated, together with 0.1 μg of KLF9 expression plasmid, and assayed for transcriptional activity. The relative activation level (normalized luciferase activity cotransfected with KLF9 expression plasmid versus empty vector) is shown. (B to D) Each value represents the mean of duplicate experiments. Error bars indicate the range of the duplicates. (E) ChIP assays for KLF9 association with the Dio1 gene promoter. Cross-linked DNA-protein complexes from mouse liver extracts were immunoprecipitated with anit-KLF9 or control IgG, followed by PCR amplification with specific primers as schematically depicted in the diagram. Genomic DNA in the input cell lysates was used as a positive control.
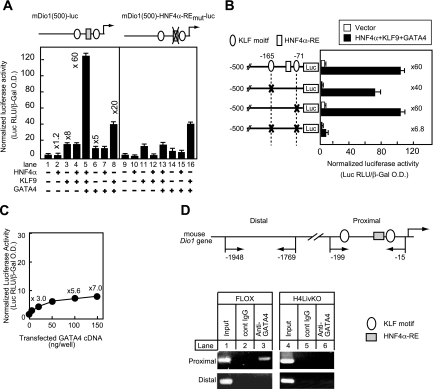
There are two potential KLF9 binding sites within the 0.5-kb region of the mouse Dio1 promoter that was activated by KLF9 in the transfection assay (Fig. 4D). Both of the sequences contain the same CACCC core sequence that is specifically bound by the KLF family of transcription factors (44, 47, 68). To define the location of the control sequence that mediates the effects of KLF9, a series of 5′ deletions was introduced into the promoter region. Deletion of the sequence from −500 to −190 did not change the sensitivity to overexpressed KLF9 (Fig. 4D). A deletion to −110 resulted in profound loss of induction by KLF9, and further deletion to −65 resulted in almost complete loss of induction. Furthermore, in the context of pDio1(500), mutation of either the CACCC site at −165 (KLF motif at −165) or the CACCC site at −71 (KLF motif at −71) decreased the response of the promoter to overexpressed KLF9 by 40 to 60%. Furthermore, when both sites were mutated simultaneously, activation of the Dio1 promoter was almost negligible, indicating that both KLF sites are crucial for the transactivation of the mouse Dio1 gene by KLF9 (see also Fig. 5B below).
FIG. 5.
KLF9 and GATA4 synergistically activate the mouse Dio1 promoter with HNF4α and HNF4α-RE, and KLF motifs are required for the synergy. (A) HepG2 cells were transfected with either pDio1(500) or its mutant in which HNF4α-RE is mutated, together with HNF4α, KLF9, and GATA4 expression plasmids (0.05 μg each), as indicated. The values above the bars refer to the level of induction relative to that found the absence of the expression plasmid. O.D., optical density. (B) HepG2 cells were transfected with the indicated Dio1-luciferase reporter, in which either each KLF motif or both were mutated, together with HNF4α, KLF9, and GATA4 expression plasmids (0.05 μg each). The values on the right of the bars are the levels of induction relative to that found in the absence of the expression plasmid. (C) HepG2 cells were transfected with the indicated amounts of GATA4 expression plasmids together with pDio1(500). (A to C) Luciferase activities were measured and normalized to β-galactosidase activity as described in the legend to Fig. 2. Each value represents the mean of duplicate experiments. Error bars indicate the range of the duplicates. (D) ChIP analysis of the Dio1 promoter in either FLOX or H4LivKO mouse liver with anti-GATA4 (Af2606) or control IgG, followed by PCR amplification with specific primers as schematically depicted in the diagram. Genomic DNA in the input cell lysates was used as a positive control. The results are representative of multiple different ChIP measurements.
To determine whether endogenous KLF9 protein associates with the Dio1 promoter in the liver, ChIP assays were performed with mouse liver extracts and an anti-KLF9 antibody. As shown in Fig. 4E, the mouse Dio1 promoter fragment encompassing the putative KLF9 site was enriched by the anti-KLF9 antibody relative to the signal derived from control IgG. A promoter distal fragment lacking an identifiable KLF site (positions −1948 to −1769) did not show specific enrichment. These data demonstrated that endogenous KLF9 occupies the KLF site in the chromatin-associated Dio1 promoter in vivo and strongly suggest that KLF9 is critical for the activation of hepatic Dio1 gene expression.
Synergistic activation of the mouse Dio1 promoter by HNF4α, GATA4, and KLF9.
Next we examined whether KLF9 can act as a coactivator with HNF4α. We previously reported that GATA4 is capable of binding directly to HNF4α and coexpression of GATA4 with HNF4α resulted in the synergistic activation of the intergenic promoter of the HNF4α target genes for ABCG5 and ABCG8 (58). In addition, previous studies demonstrated that KLF and GATA transcription factors interact to stimulate gene expression. For example, KLF1 (erythroid KLF), the founding member of a KLF family, was initially isolated as a protein that functions in concert with GATA to stimulate genes required for erythropoiesis (11). KLF13, the family member most closely related to KLF9 (59), was identified as a novel GATA4-interacting protein critical for cardiac gene transcription and heart development (30). These reports suggest that KLF9 may also interact with GATA proteins and may contribute to the transcriptional regulation of KLF9 by T3 in the promoter of the Dio1 gene, a target gene for HNF4α. We thus tested this hypothesis by using cotransfections and immunoprecipitation assays.
To examine synergy, we transfected a relatively small amount (50 ng) of each plasmid based on the dose dependency curve (Fig. 2C, 4B, and 5C). When the Dio1-luc promoter reporter construct was cotransfected with this amount of either HNF4α or KLF9 expression plasmid alone into HepG2 cells, luciferase activity was minimally affected or relatively modestly affected (∼1.2- and 8-fold, respectively) (Fig. 5A, lanes 2 and 3), and cotransfection of the HNF4α and KLF9 expression constructs together did not result in any significant further stimulation (Fig. 5A, lane 4). However, when both expression constructs were cotransfected with GATA4 expression constructs, there was a dramatic (60-fold) stimulation of promoter activity (Fig. 5A, lane 5). Interestingly, the coexpression of GATA4 and KLF9 resulted in a lower level of synergy: expression of either the GATA4 or the KLF9 plasmid activated the Dio1 promoter 5-fold and 8-fold, respectively, and coexpression of GATA4 and KLF9 resulted in 20-fold activation (Fig. 5A, lanes 3, 6, and 8). The strong synergy of the three activators was dependent on the proximal HNF4α DNA-binding site, since the mutation of HNF4α-RE significantly reduced the level of stimulation (Fig. 5A, compare lanes 5 and 13). In this transfection assay, endogenous HNF4α may not be sufficient to form an optimal transcription complex with transfected GATA4 and KLF9 to synergize with KLF9 and GATA4, since mutation of HNF4α-RE did not change the activation by KLF9 and GATA4 (Fig. 5A, compare lanes 8 and 16). This strong synergy of three activators was also dependent on the KLF sites. When both motifs were mutated simultaneously, activation of the Dio1 promoter was markedly reduced (Fig. 5B).
Further supporting a role for GATA4 in the activation of Dio1, titration of increasing amounts of the expression plasmid for GATA4 stimulated the Dio1 promoter up to sevenfold in proportion to the amount of DNA transfected (Fig. 5C). ChIP assays demonstrated that endogenous GATA4 bound to the chromatin-associated Dio1 promoter in the wild-type mouse liver, which was profoundly reduced in the livers of H4LivKO mice, indicating that endogenous GATA4 associates with the Dio1 promoter and HNF4α is required for the efficient recruitment of GATA4 to the Dio1 gene (Fig. 5D).
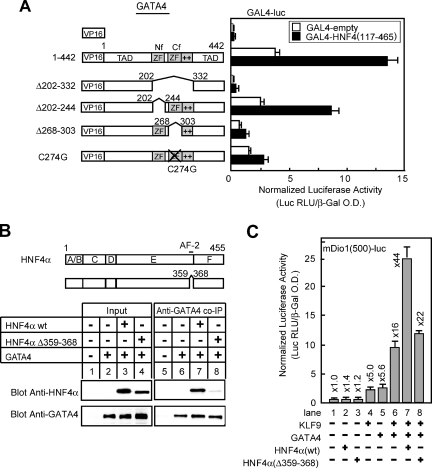
To better understand the mechanisms and protein motifs involved in KLF9-GATA4-HNF4α synergy, we carried out a structure-function analyses of GATA4. A series of GATA4 mutants were cotransfected with KLF9 and HNF4α expression constructs, and Dio1 promoter activity was measured. The GATA proteins contain two transcriptional activation domains, one at the N terminus and one at the C terminus flanking the two zinc finger DNA-binding domains (Fig. 6A). Removal of either the N-terminal activation domain by deletion of the first 201 aa (mutant 1-201) or deletion of the C-terminal activation domain by eliminating aa 333 to 442 at the C terminus (mutant 1-332) reduced but did not abrogate synergy; deletion (Δ202-303) or mutation (C274G) of the C-terminal zinc finger domains (Cf) and deletion of the adjacent basic domain (Δ304-332) profoundly decreased GATA transcriptional activity and abolished KLF9-GATA-HNF4α-mediated transcription over the mouse Dio1 promoter (Fig. 6A). These results are unlikely to be due to inefficient protein expression by the various mutants, as the only one that may be expressed at reduced levels upon transfection is the GATA4 variant with the Δ202-332 mutation (Fig. 6D).
FIG. 6.
KLF9 interacts functionally and physically with the basic domain adjacent to the Cf of GATA4. (A) Schematic diagram of various GATA deletion mutants and the ability to stimulate the mouse Dio1 promoter in association with KLF9 and HNF4α as evaluated by luciferase reporter assay. TAD, transcriptional activation domain. The data shown are the mean of duplicate experiments. Error bars indicate the range of the duplicates. (B) Interaction of GATA4 and KLF9 in transfected HEK293 cells. HEK293 cells were transfected with expression plasmids for GATA4 along with FLAG-tagged KLF9. Cells were harvested, and whole-cell lysate were immunoprecipitated with polyclonal anti-GATA4 (AF2606; R&D Systems) or control IgG (top) or monoclonal anti-FLAG M2 or control IgG (bottom) as described in Materials and Methods. The pellets from immunoprecipitation were subjected to SDS-PAGE and immunoblotted with anti-FLAG (top) or anti-GATA antibody (bottom). The expression of GATA4 or KLF9 was confirmed by immunoblot analysis of 10 μg of whole-cell lysate (Input) with anti-FLAG or anti-GATA4 antibody (AF2606), respectively. Ab, antibody; IP, immunoprecipitation; Cont, control. (C and D) Mapping of the region of GATA4 required for the interaction with KLF9 by coimmunoprecipitation analyses. HEK293 cells expressing FLAG-KLF9 and various GATA4 deletion mutants were immunoprecipitated with polyclonal antibody directed against the C-terminal part of GATA4 (aa 328 to 439) (sc-9053; Santa Cruz Biotechnology) (C) or the N-terminal part of GATA4 (aa 27 to 211) (AF2606) (D) as described for panel B. To detect KLF9 in the protein complex, peroxidase-conjugated anti-FLAG M2 antibody was used, and to detect GATA4, monoclonal anti-GATA4 (IgG-H2429) (for panel C) and polyclonal GATA4 (AF2606) antibodies (for panel D) were used for the immunoblot analyses. (E) Nuclear fractions were prepared from lysates of HEK293 cells expressing full-length (aa 1 to 442) or mutant (Δ304-332) GATA4. The presence of GATA4 in these subcellular fractions was detected by immunoblotting with an anti-GATA4 antibody. The nuclear fraction was confirmed by detection of the nuclear protein nucleoporin. (F) GST pull-down assays were performed with various 35S-labeled GATA4 mutants in the presence of GST or GST fusions containing either the full length (aa 1 to 244) or the deletion mutant form of KLF9 [KLF9(Δ1-41)] immobilized on glutathione beads. After washing, specifically bound proteins were eluted, separated by SDS-PAGE, and detected by autoradiography. The input samples contain 1% of the material added to each GST assay. ZF, zinc finger.
KLF9 interacts functionally and physically with GATA4.
To determine whether the modulation of KLF9 activity by GATA4 requires direct interaction between the proteins, coimmunoprecipitation assays were performed. HEK293 cells were transfected with FLAG-tagged KLF9 and GATA4 expression vectors, immunoprecipitated with either anti-GATA4 or anti-Flag (anti-KLF9) antibody, and subjected to SDS-PAGE and immunoblotting with either anti-GATA4 or anti-Flag antibody. As shown in Fig. 6B, Flag-KLF9 and GATA4 were coprecipitated with anti-GATA4 and anti-Flag antibodies, respectively, indicating that the two proteins are present as a complex in vivo.
To determine the region of GATA4 required for its interaction with KLF9, we tested the abilities of various GATA4 mutants to interact with KLF9. These experiments demonstrated that the N-terminal region of GATA4 (aa 1 to 201) was not required for interaction with KLF9 proteins since even deletion of the entire N-terminal transactivation domain (deletion of aa 1 to 201 [Δ1-201]) did not hinder protein interaction with KLF9 (Fig. 6C). GATA4(Δ1-201) appeared to be recognized by the anti-GATA4 antibody less efficiently than the full-length GATA protein in the immunoprecipitation (Fig. 6C). Nonetheless, KLF9 was associated with both the wild type and GATA4(Δ1-201) at proportional levels (compare lanes 2 and 3). Moreover, as shown in Fig. 6D, deletion of either or both zinc fingers did not compromise the ability to interact with KLF9 (Δ202-303, Δ202-244, and Δ268-303; lanes 4, 7, and 8, respectively). In contrast, deletions of the basic region sequence following the Cf of GATA4(Δ304-332) resulted in a loss of interaction with KLF9 (lane 5). Consistently, deletion mutants that lack this domain, i.e., mutants 1-303 (lane 2) and Δ202-332 (lane 6), hindered the protein-protein interaction. These results are unlikely to be due to a failure to accumulate in the nucleus, as the nuclear fraction showed that the Δ304-332 mutant was present in the nucleus of transfected cells. Here, the integrity of the subcellular fractionation was confirmed by detection of the nucleoporin protein in the nuclear extract fraction (Fig. 6E).
With a GST pull-down assay, we next determined whether KLF9 directly binds to GATA4. Full-length KLF9 and a deletion mutant that lacks 40 aa at the N terminus of KLF9 were expressed in Escherichia coli as fusion proteins with GST, a series of 35S-labeled GATA4 constructs were synthesized in vitro, and GST pull-down assays were performed. The data in Fig. 6F show that deletion of aa 304 to 332 of GATA4 abolished the interaction with full-length KLF9 and the first 40 aa of KLF9 are not required for binding to GATA4.
Functional interaction between the Cf of GATA4 and AF-2 of HNF4α.
By using two-hybrid and GST pull-down assays, we previously demonstrated a physical interaction between GATA4 and HNF4α through two zinc fingers in GATA4 (58). By making smaller deletions in the present study, we further deduced that the Cf is required for the interaction in a mammalian two-hybrid assay (Fig. 7A). The deletion of aa 268 to 303 or the replacement of cysteine 274 with glycine in the Cf with an intact N-terminal zinc finger (Nf) resulted in the loss of stimulation of Gal4 reporter gene expression. The loss of stimulation was not observed with the deletion of aa 202 to 244 in the Nf. We also previously demonstrated that HNF4α interacts with GATA4 through its AF-2 motif by mammalian two-hybrid and GST pull-down assays (58). The crucial role of AF-2 for HNF4α in the physical interaction was further supported by coimmunoprecipitation assays in this study (Fig. 7B). It should be noted that deletion of aa 268 to 303 and the C274-to-G mutation of GATA4 also abrogated synergistic activation with KLF9 and HNF4α (Fig. 6A) and the deletion of the AF-2 domain of HNF4α abolished the synergistic activation (Fig. 7C, compare lanes 6, 7, and 8). Thus, the domains critical for protein-protein interaction were required for synergistic activation of the Dio1 promoter in the transfection assays.
FIG. 7.
The AF-2 domain of HNF4α and the Cf of GATA4 are required for both their association and the synergistic activation of the mouse Dio1 promoter with KLF9. (A) The Cf of GATA4 is required for its association with HNF4α, as demonstrated by mammalian two-hybrid assay. CHO cells were transfected with the reporter vector pGAL4-luc (0.15 μg) and hybrid constructs or empty plasmids (0.015 μg each) indicated. The values represent the mean luciferase activity of duplicate experiments. Error bars indicate the range of the duplicates. β-Galactosidase activity was used as an internal control. ZF, zinc finger; O.D., optical density. (B) Schematic diagram of the AF-2 deletion mutant [HNF4α(Δ359-368)] and the ability to bind to GATA4, as evaluated by coimmunoprecipitation analyses (bottom). Letters A to F in the diagram indicate conventional nuclear receptor domains. AF-2, activation function 2. Anti-HNF4α antibody (IgG-H1415) was used to detect HNF4α in protein complexes immunoprecipitated (IP) with an anti-GATA4 antibody (AF2606) from lysates of HEK293 cells expressing wild-type (wt) or AF-2 deletion mutant HNF4α along with GATA4. (C) Cotransfections were carried out with HepG2 cells with pDio1(500) and 0.05 μg of the wild type (wt) or the AF-2 deletion mutant (Δ359-368) of HNF4α together with either 0.05 μg of KLF9 or GATA4 expression plasmid or both together, as indicated. The values above the bars refer to the level of induction relative that found in the absence of the expression plasmid. The values represent the mean luciferase activity of duplicate experiments. Error bars indicate the range of the duplicates.
DISCUSSION
HNF4α plays a central role in the regulation of the genes preferentially expressed in the liver (54). In the present study, by using liver-specific HNF4α-null mice made by using the Cre-loxP system, we found that HNF4α is essential for thyroid hormone metabolism in the liver. Expression of the Dio1 gene and the 5′-deiodination activity of Dio1 were markedly reduced in the livers of liver-specific HNF4α-null mice. The 5′-FR of the mouse Dio1 gene contains sufficient elements to control Dio1 expression in HepG2 hepatoblastoma cells. Indeed, there are two potential HNF4α-binding sites in the mouse promoter, and results obtained with mutated constructs revealed the proximal HNF4α-binding site be the crucial site. We previously found that Dio1 gene expression is reduced in HepG2 cells when HNF4α expression is knocked down by RNA interference (Table 2 in reference 58). The Dio1 activity in HepG2 cells was also reduced by knockdown of HNF4α, and HNF4α binds to the endogenous human Dio1 promoter, indicating that HNF4α is also crucial for human Dio1 promoter activity (see Fig. S1 in the supplemental material).
Currently, four mouse models have been reported with various deficiencies in the deiodinases: mice with a targeted disruption of the Dio1 gene (Dio1KO) (52); mice with a targeted disruption of the Dio2 gene (Dio2KO mice) (51); C3H mice, which have genetically low levels of Dio1 (7, 53); and Dio2KO mice backcrossed into a C3H background (13). Each of these animals has a normal serum T3 concentration and an increased serum T4 concentration (8). Consistent with these findings, H4LivKO mice also exhibit a normal serum T3 concentration and an increased serum T4 concentration. Clearly, the potent compensatory mechanisms for maintaining optimum levels of serum T3 function efficiently. One difference between H4LivKO and Dio1KO mice was in the serum TSH concentration, i.e., reduced in H4LivKO mice and unchanged in Dio1KO mice. One explanation for this finding is that much (threefold) higher levels of T4 in H4LivKO mice may lead to the suppression of TSH gene expression in the pituitary. Expression of the TSH gene in the pituitary is negatively regulated by local T3 that is generated from T4 by Dio2 in the pituitary, which is critically important in the feedback regulation of TSH secretion (9, 51). Because H4LivKO mice only have reduced Dio1 in the liver, the pituitary expression which is important for TSH regulation is unaffected, resulting in local T3 for suppression of TSH.
Physiological regulation of Dio1 expression likely requires additional regulatory proteins in addition to HNF4α. In the process of searching for proteins that act with HNF4α to coregulate Dio1, we found that KLF9 could activate the Dio1 promoter very efficiently through a CACCC sequence that lies both distal and proximal to the HNF4α-RE identified here. Activation of the mouse Dio1 promoter by overexpressed KLF9 does not absolutely require HNF4α since KLF9 itself could efficiently activate the mouse Dio1 promoter harboring the mutated HNF4α-RE. Thus, it is possible that KLF9 may play a role in the expression of the Dio1 gene in the pituitary and the thyroid gland, where KLF9 is expressed but HNF4α is absent (see the gene expression array database of our laboratory at http://www.lsbm.org/site_e/).
However, interestingly, HNF4α and KLF9 dramatically activate the mouse Dio1 promoter, which is even more active when GATA4 is also expressed. We propose that GATA4 serves as a coactivator of HNF4α and KLF9 and that all three proteins are required for maximal activation of hepatic Dio1 gene expression. This is due to the formation of protein interactions among KLF9, GATA4, and HNF4α. The mutation of their interaction domain or mutation of the DNA-binding site(s) of HNF4α or KLF9 diminished the synergy of the three proteins. KLF9 contacts the basic region of GATA4 (aa residues 304 to 332), which follows the Cf. This domain is critical for KLF9 interaction but not for nuclear localization. With the exception of CEBPα (65), which also contacts the basic region following the Cf of GATA proteins, most protein-protein interactions by the GATA factors involve either N-terminal or C-terminal activation domains (reviewed in reference 40). Moreover, KLF proteins, including KLF9, associate with coactivators/corepressors such as CBP/p300, PCAF, and ctBP2 (10, 57). As GATA factors also interact physically with CBP/p300, corecruitment of these coactivators by the GATA and KLF proteins may be the mechanism underlying transcriptional cooperativity. Together with the reported interaction of KLF1 and GATA1 in erythroid cells (11) and KLF13 and GATA4 in the heart (30), our findings suggest that GATA-KLF interaction may be relevant to transcriptional regulation in a broader array of other cell types and tissues.
We previously found, by using transfection and GST pull-down assays, that GATA4 and GATA6 can directly bind to HNF4α, leading to synergistic activation by HNF4α and GATA4 (58). We have recently succeeded in isolating the endogenous GATA4 and HNF4α protein complex in HepG2 cells by means of targeted proteomic analysis by mass spectrometry (K. Daigo et al., submitted for publication). In addition, ChIP assays revealed that the coexistence of native GATA4 protein and HNF4α on the chromatin-associated Dio1 promoter in the mouse liver.
Recent papers reported that the carboxy zinc finger of GATA2 directly binds to the DNA-binding domain of TRβ. This GATA2-TRβ protein complex is essential for T3-regulated expression of the TSH gene (38). In this case, TRβ does not bind the DNA sequence directly but interacts with GATA2 via its DNA-binding domain. A number of studies have shown that Dio1 mRNA and activity are regulated by thyroid hormone (reviewed in references 8 and 9). Additionally, the human Dio1 promoter contains thyroid hormone response-retinoic acid response elements that mediate TR activation of the human Dio1 gene (66). In rodents, a TRE has not been identified in the 5′-FR of the Dio1 promoter and the precise mechanism of T3-mediated induction of Dio1 gene expression is not clearly understood. Maia et al. reported that the response of the Dio1 gene to T3 in FRTL5 cells and in a rat pituitary cell line is due to transcriptional activation and is not blocked by cycloheximide, indicating that this is a direct effect of T3 not requiring the synthesis of an intermediate protein (37). If this is the case, it is tempting to speculate that TRβ is recruited to the GATA4-HNF4α complex on the Dio1 promoter, and upon T3 binding, it may regulate Dio1 gene transcription through a non-DNA-binding mechanism similar to GATA2-TRβ in TSH gene regulation. On the basis of our results, it is likely that at least part of the T3 regulation may be indirect through that T3-regulated KLF9 expression which would contribute to the KLF9, HNF4α, and GATA4 synergy revealed by the present studies.
Supplementary Material
Acknowledgments
We thank Nagaoki Toyoda (Kansai Medical University) for helpful discussion of the Dio1 assay and Shaw-Fang Yet (Harvard Medical School), Jerry Lingrel (University of Cincinnati College of Medicine), Christina Teng (National Institutes of Health), Ichiro Manabe, and Ryozo Nagai (University of Tokyo Graduate School of Medicine) for plasmids.
This study was supported in part by the grants from the Program of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), by the NFAT project of the New Energy and Industrial Technology Development Organization (NEDO), and by the Special Coordination Fund for Science and Technology of the Ministry of Education, Culture, Sports, Science, and Technology, the Uehara Memorial Foundation, and the Ono Medical Foundation. J.S. is a recipient of funds from the Special Coordination Fund for Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Published ahead of print on 21 April 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Amma, L. L., A. Campos-Barros, Z. Wang, B. Vennstrom, and D. Forrest. 2001. Distinct tissue-specific roles for thyroid hormone receptors β and α1 in regulation of type 1 deiodinase expression. Mol. Endocrinol. 15467-475. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, K. P., C. B. Kern, S. C. Crable, and J. B. Lingrel. 1995. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Krüppel-like factor: identification of a new multigene family. Mol. Cell. Biol. 155957-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arceci, R. J., A. A. King, M. C. Simon, S. H. Orkin, and D. B. Wilson. 1993. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 132235-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battle, M. A., G. Konopka, F. Parviz, A. L. Gaggl, C. Yang, F. M. Sladek, and S. A. Duncan. 2006. Hepatocyte nuclear factor 4α orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc. Natl. Acad. Sci. USA 1038419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, M. J., L. Banu, Y. Y. Chen, S. J. Mandel, J. D. Kieffer, J. W. Harney, and P. R. Larsen. 1991. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 353273-276. [DOI] [PubMed] [Google Scholar]
- 6.Berry, M. J., L. Banu, and P. R. Larsen. 1991. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349438-440. [DOI] [PubMed] [Google Scholar]
- 7.Berry, M. J., D. Grieco, B. A. Taylor, A. L. Maia, J. D. Kieffer, W. Beamer, E. Glover, A. Poland, and P. R. Larsen. 1993. Physiological and genetic analyses of inbred mouse strains with a type I iodothyronine 5′ deiodinase deficiency. J. Clin. Investig. 921517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco, A. C., and B. W. Kim. 2006. Deiodinases: implications of the local control of thyroid hormone action. J. Clin. Investig. 1162571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianco, A. C., D. Salvatore, B. Gereben, M. J. Berry, and P. R. Larsen. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2338-89. [DOI] [PubMed] [Google Scholar]
- 10.Bieker, J. J. 2001. Krüppel-like factors: three fingers in many pies. J. Biol. Chem. 27634355-34358. [DOI] [PubMed] [Google Scholar]
- 11.Cantor, A. B., and S. H. Orkin. 2002. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 213368-3376. [DOI] [PubMed] [Google Scholar]
- 12.Cayrou, C., R. J. Denver, and J. Puymirat. 2002. Suppression of the basic transcription element-binding protein in brain neuronal cultures inhibits thyroid hormone-induced neurite branching. Endocrinology 1432242-2249. [DOI] [PubMed] [Google Scholar]
- 13.Christoffolete, M. A., R. Arrojo e Drigo, F. Gazoni, S. M. Tente, V. Goncalves, B. S. Amorim, P. R. Larsen, A. C. Bianco, and A. M. Zavacki. 2007. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology 148954-960. [DOI] [PubMed] [Google Scholar]
- 14.Dame, C., M. C. Sola, J. Fandrey, L. M. Rimsza, P. Freitag, G. Knopfle, R. D. Christensen, and J. Bungert. 2002. Developmental changes in the expression of transcription factors GATA-1, -2 and -3 during the onset of human medullary haematopoiesis. Br J. Haematol. 119510-515. [DOI] [PubMed] [Google Scholar]
- 15.Denver, R. J., L. Ouellet, D. Furling, A. Kobayashi, Y. Fujii-Kuriyama, and J. Puymirat. 1999. Basic transcription element-binding protein (BTEB) is a thyroid hormone-regulated gene in the developing central nervous system. Evidence for a role in neurite outgrowth. J. Biol. Chem. 27423128-23134. [DOI] [PubMed] [Google Scholar]
- 16.Glass, C. K. R., and G. Michael. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14121-141. [PubMed] [Google Scholar]
- 17.Hayhurst, G. P., Y. H. Lee, G. Lambert, J. M. Ward, and F. J. Gonzalez. 2001. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 211393-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iguchi, H., Y. Ikeda, M. Okamura, T. Tanaka, Y. Urashima, H. Ohguchi, S. Takayasu, N. Kojima, S. Iwasaki, R. Ohashi, S. Jiang, G. Hasegawa, R. X. Ioka, K. Magoori, K. Sumi, T. Maejima, A. Uchida, M. Naito, T. F. Osborne, M. Yanagisawa, T. T. Yamamoto, T. Kodama, and J. Sakai. 2005. SOX6 attenuates glucose-stimulated insulin secretion by repressing PDX1 transcriptional activity and is down-regulated in hyperinsulinemic obese mice. J. Biol. Chem. 28037669-37680. [DOI] [PubMed] [Google Scholar]
- 19.Iguchi, H., Y. Urashima, Y. Inagaki, Y. Ikeda, M. Okamura, T. Tanaka, A. Uchida, T. T. Yamamoto, T. Kodama, and J. Sakai. 2007. SOX6 suppresses cyclin D1 promoter activity by interacting with β-catenin and histone deacetylase 1, and its down-regulation induces pancreatic β-cell proliferation. J. Biol. Chem. 28219052-19061. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda, Y., J. Yamamoto, M. Okamura, T. Fujino, S. Takahashi, K. Takeuchi, T. F. Osborne, T. T. Yamamoto, S. Ito, and J. Sakai. 2001. Transcriptional regulation of the murine acetyl-CoA synthetase 1 gene through multiple clustered binding sites for sterol regulatory element-binding proteins and a single neighboring site for Sp1. J. Biol. Chem. 27634259-34269. [DOI] [PubMed] [Google Scholar]
- 21.Inoue, Y., G. P. Hayhurst, J. Inoue, M. Mori, and F. J. Gonzalez. 2002. Defective ureagenesis in mice carrying a liver-specific disruption of hepatocyte nuclear factor 4α (HNF4α). HNF4α regulates ornithine transcarbamylase in vivo. J. Biol. Chem. 27725257-25265. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, Y., L. L. Peters, S. H. Yim, J. Inoue, and F. J. Gonzalez. 2006. Role of hepatocyte nuclear factor 4α in control of blood coagulation factor gene expression. J. Mol. Med. 84334-344. [DOI] [PubMed] [Google Scholar]
- 23.Inoue, Y., A. M. Yu, J. Inoue, and F. J. Gonzalez. 2004. Hepatocyte nuclear factor 4α is a central regulator of bile acid conjugation. J. Biol. Chem. 2792480-2489. [DOI] [PubMed] [Google Scholar]
- 24.Inoue, Y., A. M. Yu, S. H. Yim, X. Ma, K. W. Krausz, J. Inoue, C. C. Xiang, M. J. Brownstein, G. Eggertsen, I. Bjorkhem, and F. J. Gonzalez. 2006. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4α. J. Lipid Res. 47215-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley, C., H. Blumberg, L. I. Zon, and T. Evans. 1993. GATA-4 is a novel transcription factor expressed in endocardium of the developing heart. Development 118817-827. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, A., K. Sogawa, H. Imataka, and Y. Fujii-Kuriyama. 1995. Analysis of functional domains of a GC box-binding protein, BTEB. J. Biochem. (Tokyo) 11791-95. [DOI] [PubMed] [Google Scholar]
- 27.Köhrle, J., F. Jakob, B. Contempre, and J. E. Dumont. 2005. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 26944-984. [DOI] [PubMed] [Google Scholar]
- 28.Ladias, J. A., M. Hadzopoulou-Cladaras, D. Kardassis, P. Cardot, J. Cheng, V. Zannis, and C. Cladaras. 1992. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J. Biol. Chem. 26715849-15860. [PubMed] [Google Scholar]
- 29.Larsen, P. R., J. E. Silva, and M. M. Kaplan. 1981. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr. Rev. 287-102. [DOI] [PubMed] [Google Scholar]
- 30.Lavallée, G., G. Andelfinger, M. Nadeau, C. Lefebvre, G. Nemer, M. E. Horb, and M. Nemer. 2006. The Krüppel-like transcription factor KLF13 is a novel regulator of heart development. EMBO J. 255201-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laverriere, A. C., C. MacNeill, C. Mueller, R. E. Poelmann, J. B. Burch, and T. Evans. 1994. GATA-4/5/6, a subfamily of three transcription factors transcribed in developing heart and gut. J. Biol. Chem. 26923177-23184. [PubMed] [Google Scholar]
- 32.Leonard, J. L., and I. N. Rosenberg. 1980. Characterization of essential enzyme sulfhydryl groups of thyroxine 5′-deiodinase from rat kidney. Endocrinology 106444-451. [DOI] [PubMed] [Google Scholar]
- 33.Liang, G., J. Yang, J. D. Horton, R. E. Hammer, J. L. Goldstein, and M. S. Brown. 2002. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2779520-9528. [DOI] [PubMed] [Google Scholar]
- 34.Lu, J. R., T. A. McKinsey, H. Xu, D. Z. Wang, J. A. Richardson, and E. N. Olson. 1999. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol. Cell. Biol. 194495-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maia, A., M. Berry, R. Sabbag, J. Harney, and P. Larsen. 1995. Structural and functional differences in the dio1 gene in mice with inherited type 1 deiodinase deficiency. Mol. Endocrinol. 9969-980. [DOI] [PubMed] [Google Scholar]
- 36.Maia, A. L., J. Kieffer, J. Harney, and P. Larsen. 1995. Effect of 3,5,3′-triiodothyronine (T3) administration on dio1 gene expression and T3 metabolism in normal and type 1 deiodinase-deficient mice. Endocrinology 1364842-4849. [DOI] [PubMed] [Google Scholar]
- 37.Maia, A. L., J. W. Harney, and P. R. Larsen. 1995. Pituitary cells respond to thyroid hormone by discrete, gene-specific pathways. Endocrinology 1361488-1494. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita, A., S. Sasaki, Y. Kashiwabara, K. Nagayama, K. Ohba, H. Iwaki, H. Misawa, K. Ishizuka, and H. Nakamura. 2007. Essential role of GATA2 in the negative regulation of thyrotropin β gene by thyroid hormone and its receptors. Mol. Endocrinol. 21865-884. [DOI] [PubMed] [Google Scholar]
- 39.Moeller, L. C., A. M. Dumitrescu, R. L. Walker, P. S. Meltzer, and S. Refetoff. 2005. Thyroid hormone responsive genes in cultured human fibroblasts. J. Clin. Endocrinol. Metab. 90936-943. [DOI] [PubMed] [Google Scholar]
- 40.Molkentin, J. D. 2000. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 27538949-38952. [DOI] [PubMed] [Google Scholar]
- 41.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrisey, E. E., H. S. Ip, M. M. Lu, and M. S. Parmacek. 1996. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev. Biol. 177309-322. [DOI] [PubMed] [Google Scholar]
- 43.Mouthon, M. A., O. Bernard, M. T. Mitjavila, P. H. Romeo, W. Vainchenker, and D. Mathieu-Mahul. 1993. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood 81647-655. [PubMed] [Google Scholar]
- 44.Nuez, B., D. Michalovich, A. Bygrave, R. Ploemacher, and F. Grosveld. 1995. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature 375316-318. [DOI] [PubMed] [Google Scholar]
- 45.Oishi, Y., I. Manabe, K. Tobe, K. Tsushima, T. Shindo, K. Fujiu, G. Nishimura, K. Maemura, T. Yamauchi, N. Kubota, R. Suzuki, T. Kitamura, S. Akira, T. Kadowaki, and R. Nagai. 2005. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 127-39. [DOI] [PubMed] [Google Scholar]
- 46.Owens, R. B., H. S. Smith, and A. J. Hackett. 1974. Epithelial cell cultures from normal glandular tissue of mice. J. Natl. Cancer Inst. 53261-269. [DOI] [PubMed] [Google Scholar]
- 47.Perkins, A. C., A. H. Sharpe, and S. H. Orkin. 1995. Lethal β-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 375318-322. [DOI] [PubMed] [Google Scholar]
- 48.Rhee, J., H. Ge, W. Yang, M. Fan, C. Handschin, M. Cooper, J. Lin, C. Li, and B. M. Spiegelman. 2006. Partnership of PGC-1α and HNF4α in the regulation of lipoprotein metabolism. J. Biol. Chem. 28114683-14690. [DOI] [PubMed] [Google Scholar]
- 49.Rhee, J., Y. Inoue, J. C. Yoon, P. Puigserver, M. Fan, F. J. Gonzalez, and B. M. Spiegelman. 2003. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc. Natl. Acad. Sci. USA 1004012-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai, J., A. Nohturfft, J. L. Goldstein, and M. S. Brown. 1998. Cleavage of sterol regulatory element-binding proteins (SREBPs) at site-1 requires interaction with SREBP cleavage-activating protein. Evidence from in vivo competition studies. J. Biol. Chem. 2735785-5793. [DOI] [PubMed] [Google Scholar]
- 51.Schneider, M. J., S. N. Fiering, S. E. Pallud, A. F. Parlow, D. L. St. Germain, and V. A. Galton. 2001. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 152137-2148. [DOI] [PubMed] [Google Scholar]
- 52.Schneider, M. J., S. N. Fiering, B. Thai, S.-Y. Wu, E. St. Germain, A. F. Parlow, D. L. St. Germain, and V. A. Galton. 2006. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147580-589. [DOI] [PubMed] [Google Scholar]
- 53.Schoenmakers, C. H., I. G. Pigmans, A. Poland, and T. J. Visser. 1993. Impairment of the selenoenzyme type I iodothyronine deiodinase in C3H/He mice. Endocrinology 132357-361. [DOI] [PubMed] [Google Scholar]
- 54.Sladek, F. M., and S. D. Seidel. 2001. Hepatocyte nuclear factor 4α, p. 309-361. In T. P. Burris and E. R. B. McCabe (ed.), Nuclear receptors and genetic disease. Academic Press, San Diego, CA.
- 55.Sladek, F. M., M. D. Ruse, Jr., L. Nepomuceno, S. M. Huang, and M. R. Stallcup. 1999. Modulation of transcriptional activation and coactivator interaction by a splicing variation in the F domain of nuclear receptor hepatocyte nuclear factor 4α1. Mol. Cell. Biol. 196509-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sladek, F. M., W. M. Zhong, E. Lai, and J. E. Darnell, Jr. 1990. Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 42353-2365. [DOI] [PubMed] [Google Scholar]
- 57.Song, A., A. Patel, K. Thamatrakoln, C. Liu, D. Feng, C. Clayberger, and A. M. Krensky. 2002. Functional domains and DNA-binding sequences of RFLAT-1/KLF13, a Krüppel-like transcription factor of activated T lymphocytes. J. Biol. Chem. 27730055-30065. [DOI] [PubMed] [Google Scholar]
- 58.Sumi, K., T. Tanaka, A. Uchida, K. Magoori, Y. Urashima, R. Ohashi, H. Ohguchi, M. Okamura, H. Kudo, K. Daigo, T. Maejima, N. Kojima, I. Sakakibara, S. Jiang, G. Hasegawa, I. Kim, T. F. Osborne, M. Naito, F. J. Gonzalez, T. Hamakubo, T. Kodama, and J. Sakai. 2007. Cooperative interaction between hepatocyte nuclear factor 4α and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol. Cell. Biol. 274248-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suske, G., E. Bruford, and S. Philipsen. 2005. Mammalian SP/KLF transcription factors: bring in the family. Genomics 85551-556. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki, E., T. Evans, J. Lowry, L. Truong, D. W. Bell, J. R. Testa, and K. Walsh. 1996. The human GATA-6 gene: structure, chromosomal location, and regulation of expression by tissue-specific and mitogen-responsive signals. Genomics 38283-290. [DOI] [PubMed] [Google Scholar]
- 61.Svensson, E. C., R. L. Tufts, C. E. Polk, and J. M. Leiden. 1999. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc. Natl. Acad. Sci. USA 96956-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka, T., S. Jiang, H. Hotta, K. Takano, H. Iwanari, K. Sumi, K. Daigo, R. Ohashi, M. Sugai, C. Ikegame, H. Umezu, Y. Hirayama, Y. Midorikawa, Y. Hippo, A. Watanabe, Y. Uchiyama, G. Hasegawa, P. Reid, H. Aburatani, T. Hamakubo, J. Sakai, M. Naito, and T. Kodama. 2006. Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4α in the pathogenesis of human cancer. J. Pathol. 208662-672. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka, T., J. Yamamoto, S. Iwasaki, H. Asaba, H. Hamura, Y. Ikeda, M. Watanabe, K. Magoori, R. X. Ioka, K. Tachibana, Y. Watanabe, Y. Uchiyama, K. Sumi, H. Iguchi, S. Ito, T. Doi, T. Hamakubo, M. Naito, J. Auwerx, M. Yanagisawa, T. Kodama, and J. Sakai. 2003. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 10015924-15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tevosian, S. G., A. E. Deconinck, A. B. Cantor, H. I. Rieff, Y. Fujiwara, G. Corfas, and S. H. Orkin. 1999. FOG-2: a novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc. Natl. Acad. Sci. USA 96950-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong, Q., J. Tsai, G. Tan, G. Dalgin, and G. S. Hotamisligil. 2005. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol. Cell. Biol. 25706-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toyoda, N., A. M. Zavacki, A. L. Maia, J. W. Harney, and P. R. Larsen. 1995. A novel retinoid X receptor-independent thyroid hormone response element is present in the human type 1 deiodinase gene. Mol. Cell. Biol. 155100-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wada, H., K. Hasegawa, T. Morimoto, T. Kakita, T. Yanazume, and S. Sasayama. 2000. A p300 protein as a coactivator of GATA-6 in the transcription of the smooth muscle-myosin heavy chain gene. J. Biol. Chem. 27525330-25335. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto, J., Y. Ikeda, H. Iguchi, T. Fujino, T. Tanaka, H. Asaba, S. Iwasaki, R. X. Ioka, I. W. Kaneko, K. Magoori, S. Takahashi, T. Mori, H. Sakaue, T. Kodama, M. Yanagisawa, T. T. Yamamoto, S. Ito, and J. Sakai. 2004. A Krüppel-like factor KLF15 contributes fasting-induced transcriptional activation of mitochondrial acetyl-CoA synthetase gene AceCS2. J. Biol. Chem. 27916954-16962. [DOI] [PubMed] [Google Scholar]
- 69.Yet, S. F., M. M. McA'Nulty, S. C. Folta, H. W. Yen, M. Yoshizumi, C. M. Hsieh, M. D. Layne, M. T. Chin, H. Wang, M. A. Perrella, M. K. Jain, and M. E. Lee. 1998. Human EZF, a Krüppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J. Biol. Chem. 2731026-1031. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, M., and J. Y. Chiang. 2001. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of hepatocyte nuclear factor 4α in mediating bile acid repression. J. Biol. Chem. 27641690-41699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.