Abstract
The dynamics of the Aurora B protein kinase during Xenopus oocyte meiotic maturation were examined. Resting G2 oocytes express inactive Aurora B that is not associated with other subunits of the chromosome passenger complex (CPC). Activity increases near the time of germinal vesicle breakdown in progesterone-treated oocytes, and this increase is correlated with the synthesis of inner centromere protein (INCENP) and survivin, components of the CPC. Ablation of INCENP synthesis led to the failure of progesterone treatment to activate Aurora B, but biochemical progression through the meiosis I-to-II transition and arrest at metaphase II were not affected. At fertilization, Aurora B was deactivated in concert with the degradation of INCENP, and the levels of Aurora B kinase activity and INCENP oscillated in subsequent embryonic cell cycles. Prevention of the decrease in Aurora B activity at fertilization by expression of ectopic wild-type INCENP, but not kinase-dead Aurora B INCENP, blocked calcium-induced exit from metaphase arrest in egg extracts.
Aurora B is a key mitotic kinase that plays essential roles in chromosome alignment, segregation, and cytokinesis and is also a critical regulator of the spindle checkpoint (2, 6, 7, 24, 45). Aurora B is a member of the chromosome passenger complex (CPC), which consists of Aurora B, inner centromere protein (INCENP), borealin/Dasra B/Dasra A, TD-60, and survivin (2, 6). Upon binding to INCENP, Aurora B assumes a partially active conformation and phosphorylates two serines at the C terminus of INCENP, designated the IN-Box (37). This phosphorylation facilitates conversion to the fully activated state (37, 46). Deactivation of Aurora B after the metaphase/anaphase transition is poorly understood, but the anaphase-promoting complex/cyclosome (APC/C), activated by Cdh1, can degrade Aurora B in some systems (27, 38). Besides degradation, dephosphorylation of Aurora B is blocked by the protein phosphatase 2A (PP2A) and PP1 inhibitor okadaic acid (40). Chromatin-associated PP1 has also been reported to negatively regulate Aurora B in interphase in vivo (2, 26).
The role of Aurora B in chromosome dynamics has been investigated using Xenopus egg extracts as a model system. Depletion of INCENP/Aurora B/Dasra B from Xenopus egg extracts results in failure of bipolar spindle formation and microtubule nucleation and stabilization (33). Upon inhibition of Aurora B by the inhibitor ZM447439, chromosomes undergo premature decondensation and fail to form microtubules that are nucleated from chromatin (11). These results suggest that Aurora B is required for the formation of condensed metaphase chromosomes, spindle assembly, and chromosome segregation in Xenopus early-embryonic cell cycles.
Recently, several studies have shown that the CPC plays an important role not only in mitosis but also in meiosis. Treatment of pig oocytes with ZM447439 inhibits meiotic progression (17), and depletion by small interfering RNA of the Caenorhabditis elegans Aurora B homolog, AIR-2, causes failure of chiasma resolution during homologous chromosome segregation (18). In budding yeast, loss of function of the Aurora B homolog, Ipl1, results in premature separation of sister chromatids and failed biorientation of homologous chromosomes and sister chromatids during meiosis I and meiosis II, respectively (25, 47). Similar effects are observed after depletion of Aurora B from Drosophila oocytes (31).
Full-grown Xenopus oocytes are arrested in prophase of meiosis I and resume meiosis upon stimulation by progesterone. After resumption of meiosis, the oocyte progresses through the consecutive M phases of meiosis I and meiosis II without an intervening interphase and then arrests again at metaphase of meiosis II (meta-II) until fertilization. This period, encompassing the resumption of meiosis I to the arrest at meta-II, is called oocyte maturation. Upon fertilization, calcium levels increase, and the mature oocyte exits meiosis II by transiting from meta-II to anaphase II with extrusion of a second polar body. The stable meta-II arrest of the mature oocyte/egg is a consequence of cytostatic factor (CSF) activity, which inhibits the APC/C (43). Upon elevation of calcium levels at fertilization, CSF activity declines and the APC/C is activated. Although the regulation of Xenopus Aurora A during oocyte maturation has been studied extensively (22, 23), the role of Aurora B in oocyte maturation and early-embryonic cell cycles is not well understood. Here we report on an analysis of the CPC and the regulation of Aurora B kinase activity in vivo during oocyte maturation and after fertilization.
MATERIALS AND METHODS
Xenopus oocytes, embryos, and CSF extracts.
Oocyte maturation was induced in vitro by progesterone as described previously (44). Progression through maturation was assessed by germinal vesicle breakdown (GVBD) and polar body emission by using a dissecting microscope. Eggs were fertilized in vitro as described previously (14). CSF extracts were prepared from unfertilized eggs as described previously (44), and CSF release was initiated by addition of CaCl2 to 0.8 mM.
Immunoprecipitation of the CPC.
One hundred microliters of protein G-conjugated Dynabeads (Dynal) was coupled to 10 μl of Aurora B serum and washed five times with extraction buffer (80 mM β-glycerophosphate, 20 mM EGTA, 5 mM MgCl2, 20 mM HEPES [pH 7.5]). Twenty oocytes or eggs were suspended with 180 μl of extraction buffer, crushed by up and down pipetting, and centrifuged at 15,000 × g for 1 min. The supernatant between the yolk and lipid was collected, and 20 μl was resuspended in 6 μl of 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The rest of the supernatant was incubated with anti-Aurora B antibody-coupled beads; after incubation for 1 h at room temperature, the flowthrough was collected and either suspended in SDS-PAGE sample buffer for Western blot analysis or used for immune complex kinase assays as described below. The equivalent of one oocyte or egg was loaded onto the gel and analyzed by Western blotting.
Antibodies.
A rabbit polyclonal antibody to a conserved region of human INCENP was purchased from Abcam (ab12183). A rabbit polyclonal antibody to human TD-60 antibody was a gift from William Earnshaw (Wellcome Trust Center, Edinburgh, Scotland); a rabbit polyclonal antibody to Xenopus Dasra A (xDasra A) was a gift from Hironori Funabiki (Rockefeller University); a rabbit polyclonal antibody to xAurora B was a gift from Johne' Liu (Ottawa Health Research Institute, Ottawa, Canada); and a rabbit polyclonal antibody to survivin was a gift from Yixian Zheng (Carnegie). Sheep polyclonal antibodies to cyclin B1 and cyclin B2 have been described previously (44), and a rabbit polyclonal antibody against phospho-T232 of human Aurora B was a gift from Patrick Eyers (University of Manchester) or was purchased from Cell Signaling. The analogous sequence in Aurora B is highly conserved at T248, and both T-loop antibodies failed to react with kinase-dead Aurora B, even in M phase. A mouse monoclonal antitubulin antibody was purchased from Sigma (T9026). A rabbit antibody to Xenopus PP1γ1 was raised against a peptide spanning the 14 C-terminal residues (VTPPRGMITKQAKK) of the PP1γ1 catalytic subunit and was purified on a column of recombinant full-length PP1γ1. Mouse anti-Myc or anti-Flag monoclonal antibodies conjugated to horseradish peroxidase were purchased from Santa Cruz or Sigma-Aldrich, respectively.
Cloning and purification of protein.
A cDNA encoding Xenopus Aurora B was cloned as described previously (9). A cDNA encoding Xenopus INCENP (39) was cloned by standard PCR techniques from a Xenopus oocyte library. A kinase-negative (KN) mutant also was constructed (D234A Aurora B). For bacterial expression of the Aurora B-INCENP complex, a bicistronic cDNA encoding Aurora B and the IN-Box domain of INCENP (amino acids 677 to 874) was cloned into a pET30 LIC vector with the LIC duet minimal adaptor as a connector (Novagen). His-tagged Aurora B or the Aurora B-INCENP complex was purified on Talon beads as described elsewhere (9). For mRNA production, Flag-tagged Aurora B cDNA or Myc-tagged INCENP cDNA was cloned into a pCS2 LIC vector, and mRNA was produced using the mMessage mMachine kit (Ambion). [35S]methionine-labeled securin was synthesized in reticulocyte lysate with the TNT Quick Coupled Transcription/Translation system kit (Promega).
Antisense oligonucleotides.
A phosphorothioate-modified 2′-O-methyl RNA/DNA chimeric antisense oligonucleotide against INCENP was custom made by Integrated DNA Technologies (Coralville, IA). The sequence of the INCENP antisense oligonucleotide was 5′-GCAUCGTTCATGGTGGAAGG-3′, and that of the control (scrambled) oligonucleotide was 5′-GTGTCACACGCCTCAAGGTC-3′. Both antisense oligonucleotides were suspended in nuclease-free water to a concentration of 0.1 mM, and 40 nl was injected into each individual oocyte. Oocytes were incubated overnight after oligonucleotide injection and before induction of maturation or other analysis.
Kinase assays.
Histone H1 kinase assays were performed as described previously (44). To determine specific Aurora B activity, Aurora B immunoprecipitates from 20 oocytes or embryos prepared as described above were incubated in 20 μl of kinase buffer (50 mM Tris-HCl [pH 7.4], 100 mM NaCl, 10 mM MgCl2, 0.1 mM EGTA, 0.1% β-mercaptoethanol, 0.01% Brij 35) containing 1 μg histone H3 and [γ-32P]ATP (500 cpm/pmol) for 30 min at 30°C. The reaction was stopped by addition of 7 μl of 5× SDS-PAGE sample buffer. An amount of the reaction corresponding to seven oocytes was electrophoresed through a 15% SDS gel, and the radiolabeled histone H3 band was detected by autoradiography. The band was excised and radiolabel incorporation quantified by Cerenkov counting in a liquid scintillation counter.
RESULTS
INCENP and survivin accumulate after progesterone treatment.
To explore the meiotic regulation of Aurora B during Xenopus oocyte maturation, we first examined components of the CPC in immature and mature oocytes. The Western blot analysis in Fig. 1A shows that Aurora B, TD-60, and Dasra A protein are already present in immature G2 oocytes and that their abundance does not change upon arrest in meta-II. In contrast, little or no INCENP and no survivin are evident in immature oocytes. However, both INCENP and survivin accumulate to significant levels after progesterone treatment. To examine the formation of the CPC during maturation, the amount of each CPC component that associates with Aurora B was assessed before and after maturation by codepletion with Aurora B from the extract. All detectable Aurora B could be depleted by immunoprecipitation. However, all of the Dasra A and TD-60 present in immature oocytes remained in the supernatants of Aurora B-depleted extracts (Fig. 1A). In contrast, in mature meta-II-arrested oocytes, nearly all of the INCENP and most of the Dasra A and survivin were codepleted with Aurora B, indicating formation of the CPC. However, the level of TD-60 was not significantly reduced by Aurora B depletion.
FIG. 1.
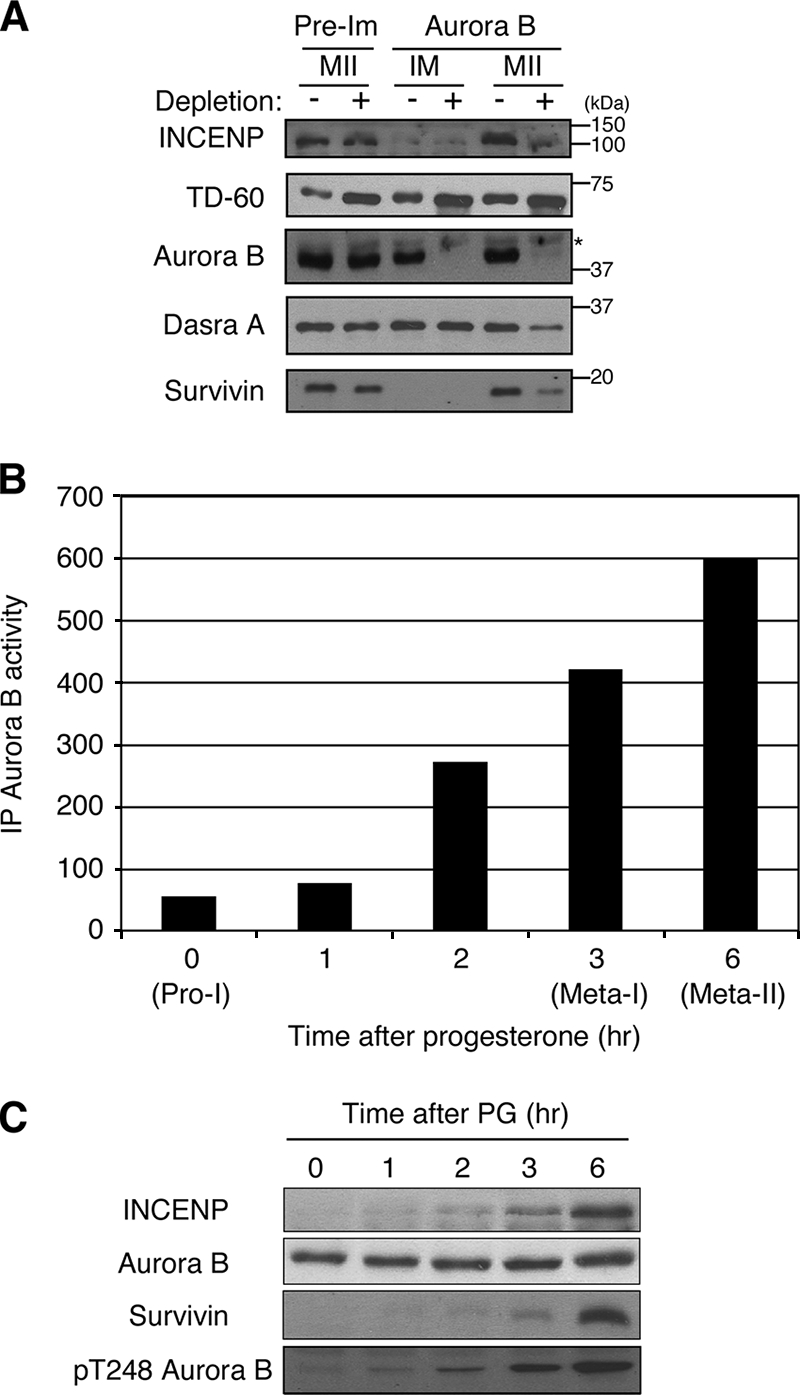
CPC formation during maturation. (A) CPC subunit association. Extracts from immature (IM) and meta-II-arrested (MII) oocytes taken before (−) and after (+) immunodepletion with preimmune serum (Pre-Im) or anti-Aurora B antibodies were immunoblotted with antibodies against either INCENP, TD-60, Aurora B, Dasra A, or survivin, as indicated. The positions of molecular size markers are given on the right. (B) The Aurora B kinase activity of resting (Pro-I) oocytes or of oocytes treated with progesterone was measured at the indicated times with immunoprecipitated (IP) Aurora B by using histone H3 as a substrate as described in Materials and Methods. (C) Kinetics of CPC subunit accumulation. Progesterone (PG)-treated oocytes were homogenized at the indicated times, and extracts were immunoblotted with antibodies against INCENP, Aurora B, survivin, or the phospho-T-loop of Aurora B.
Aurora B kinase activity and INCENP levels increase during oocyte maturation.
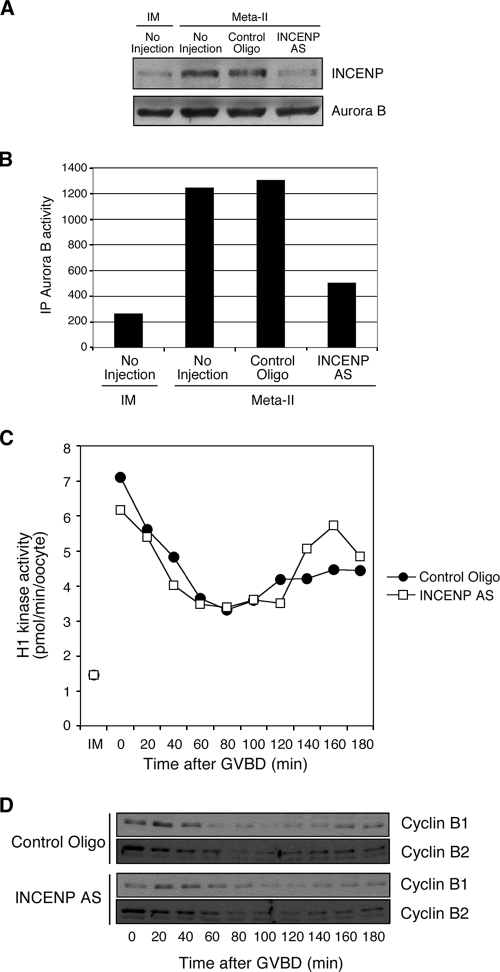
We next measured Aurora B kinase activity during oocyte maturation by using histone H3 as a substrate. At each time point, Aurora B was immunoprecipitated from oocytes and incubated with histone H3 and [γ-32P]ATP. As shown in Fig. 1B, Aurora B activity is low in immature oocytes and gradually increases as oocytes mature. This Aurora B activation was also confirmed by Western blotting using an anti-Aurora B phospho-T-loop antibody that specifically recognizes the active form of Aurora B (46) (Fig. 1C). The increase in Aurora B kinase activity is well correlated with the increased levels of INCENP and survivin (Fig. 1C). INCENP has been reported to be an activator of Aurora B in other cell types (4, 15, 19). To examine whether the newly synthesized INCENP is required to activate Aurora B during oocyte maturation, we ablated the synthesis of INCENP by microinjection of antisense oligonucleotides into oocytes prior to treatment with progesterone. As shown in Fig. 2A, when oocytes reached meta-II, no increased accumulation of INCENP was evident in antisense-oligonucleotide-injected oocytes. Moreover, at meta-II, the kinase activity of Aurora B from antisense-oligonucleotide-injected oocytes was much lower than that of control-oligonucleotide-injected oocytes, which was similar to that of uninjected meta-II-arrested oocytes (Fig. 2B). This result indicates that the synthesis of INCENP is required to activate Aurora B during oocyte maturation.
FIG. 2.
Effect of INCENP accumulation on oocyte maturation. (A) Antisense (AS) oligonucleotide ablation of INCENP. Oocytes were first injected with control oligonucleotides or INCENP AS oligonucleotides and then treated with progesterone. Extracts from immature (IM) and meta-II oocytes were then immunoblotted with antibodies against INCENP or Aurora B, as indicated. (B) Aurora B kinase activity was measured with Aurora B immunoprecipitates (IP) in uninjected oocytes (IM) or progesterone-treated meta-II oocytes that had previously been injected with control oligonucleotides or INCENP AS oligonucleotides, as indicated. Similar results were obtained in several independent experiments. (C) The level of histone H1 kinase activity was determined at the indicated times after GVBD in extracts from progesterone-treated oocytes injected with control oligonucleotides or AS oligonucleotides against INCENP. (D) Cyclin B2 levels after ablation of INCENP during maturation. Oocytes were injected with control oligonucleotides or INCENP AS oligonucleotides, incubated overnight, and then treated with progesterone. Maturing oocytes were crushed at the indicated time points after GVBD (meta-I). Extracts were then immunoblotted with anti-cyclin B1 or anti-cyclin B2 antibodies.
To determine the effect of INCENP ablation and reduced Aurora B kinase activity on progression through oocyte maturation, the levels of histone H1 kinase activity and cyclins B1 and B2 were assessed after GVBD in progesterone-treated oocytes injected with control oligonucleotides or INCENP antisense oligonucleotides. The kinetics of histone H1 kinase activity after GVBD were not significantly different, and activity in both control- and antisense-oligonucleotide-injected oocytes increased after GVBD to the level expected for oocytes arrested at meta-II by CSF (Fig. 2C). At 80 to 100 min in both control- and antisense-oligonucleotide-injected oocytes, the levels of cyclin B1 and B2 declined, reflecting anaphase I and the decline in histone H1 kinase activity. At 140 to 180 min, both cyclins reaccumulated to similar levels, reflecting CSF arrest at meta-II (Fig. 2D). Upon elevation of calcium levels by the ionophore A23187, both cyclins B1 and B2 were degraded as CSF arrest was terminated (data not shown). Thus, no significant difference in the dynamics of cyclin B levels and histone H1 kinase activity during meiotic progression is evident in oocytes lacking increased INCENP and Aurora B kinase activity (Fig. 2C and D).
INCENP is not sufficient to activate Aurora B in immature oocytes.
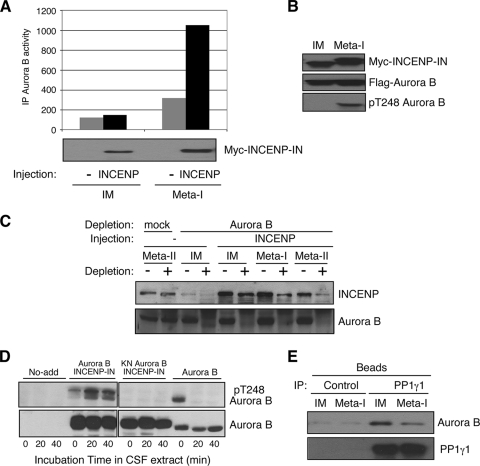
The correlation of Aurora B activation with INCENP synthesis suggests that activation of the kinase occurs as a consequence of the production of INCENP and its association with Aurora B. To evaluate this hypothesis, we injected mRNA encoding Myc-INCENP into immature, G2-arrested oocytes. Western blotting of immature oocytes showed that exogenous Myc-INCENP was successfully translated in injected oocytes. However, kinase assays with Aurora B immunoprecipitated from such oocytes revealed that the exogenously expressed INCENP did not activate Aurora B (Fig. 3A). We also measured Aurora B activity when oocytes expressing Myc-INCENP reached meta-I after progesterone treatment. The results show that the exogenous INCENP does activate Aurora B in meta-I oocytes. Thus, exogenous INCENP is capable of activating Aurora B but does not do so in immature oocytes. The activation of Aurora B was also indirectly monitored on Western blots by phosphorylation of Thr 248 in the Aurora B T-loop, detected by use of a phosphospecific antibody (46). When Aurora B and INCENP were expressed at the same level in both immature and meta-I oocytes, T-loop phosphorylation of Aurora B was detected only in meta-I oocytes, not in immature oocytes (Fig. 3B). These results confirm that Aurora B is not activated by expression of INCENP protein in immature oocytes.
FIG. 3.
Progesterone regulates the association of INCENP with Aurora B. (A) Ectopic INCENP activates Aurora B only after progesterone treatment. Oocytes were injected with mRNA encoding Myc-INCENP-IN and were incubated overnight; then some were treated with progesterone to induce maturation. Extracts from immature (IM) or progesterone-treated (meta-I) oocytes were immunoblotted with anti-Myc antibodies. The same extracts were analyzed for total Aurora B kinase activity in immunoprecipitates (IP), as indicated. Similar results were obtained in several independent experiments. (B) INCENP promotes T-loop phosphorylation of Aurora B. Oocytes were injected with mRNAs encoding both Myc-INCENP-IN and Flag-Aurora B, and some were then treated with progesterone. The expression of INCENP and Aurora B was monitored with antibodies to Myc or Flag, as indicated, and the phosphorylation of T248 (pT248) was measured with a phosphospecific antibody as described in Materials and Methods. (C) Association of INCENP and Aurora B during maturation. Oocytes were injected with mRNAs encoding Myc-INCENP and were incubated overnight; then some were treated with progesterone. Extracts from IM, meta-I (GVBD), and meta-II oocytes before (−) and after (+) Aurora B immunodepletion were immunoblotted with anti-INCENP and anti-Aurora B antibodies, as indicated. Mock depletion was performed with Dyna beads coupled to preimmune serum. (D) Aurora B T-loop dephosphorylation is blocked by INCENP. Xenopus CSF-arrested meta-II egg extracts were immunoblotted with anti-Aurora B or anti-phospho-T248 Aurora B antibodies at the indicated times after the addition of recombinant Aurora B alone or of wild-type or KN Aurora B bound to INCENP. (E) The association of Aurora B with PP1 is reduced during oocyte maturation. Extracts from IM and progesterone-treated meta-I (GVBD) oocytes were incubated with control rabbit immunoglobulin G or anti-PP1 antibodies bound to Sepharose beads; the beads were then washed and eluted with SDS sample buffer, and the eluates were analyzed by SDS-PAGE and immunoblotting with antibodies to PP1γ1 and Aurora B, as indicated.
One possible explanation for the failure of INCENP to activate Aurora B in immature oocytes is the lack of association with endogenous Aurora B. To check complex formation between Aurora B and INCENP in immature oocytes, we injected Myc-INCENP mRNA into oocytes and compared the amounts of Myc-INCENP before and after quantitative immunodepletion of Aurora B from extracts of both control oocytes and oocytes treated with progesterone. As shown in Fig. 3C, in immature oocyte extracts depleted of Aurora B, the level of Myc-INCENP remained high, whereas most INCENP was codepleted with Aurora B in extracts from both meta-I and meta-II oocytes. These results indicate that ectopically expressed INCENP is not able to bind to Aurora B in immature oocytes but does so after progesterone treatment of the oocyte.
Purified bacterially expressed monomeric Aurora B already has T-loop phosphorylation as a result of autophosphorylation (Fig. 3D). However, when this protein is added to metaphase-arrested CSF extracts, phospho-T248 is rapidly dephosphorylated (Fig. 3D). This dephosphorylation is prevented if kinase-active, but not kinase-dead, Aurora B is bound to INCENP, indicating that INCENP prevents dephosphorylation of the Aurora B T-loop. PP1 has been reported to be a negative regulator of Aurora B (26, 40). This might occur by preventing the accumulation of phosphate in the T-loop, as has been reported for Aurora A (3, 10, 34). Given that INCENP blocks Aurora B T-loop dephosphorylation and that PP1 can dephosphorylate the T-loop, we investigated whether Aurora B is bound to PP1 in immature oocytes. mRNA encoding Flag-Aurora B was injected into immature oocytes, and the recombinant protein was allowed to accumulate overnight. PP1γ1 was then immunoprecipitated from extracts of these oocytes, and its association with Aurora B was analyzed by Western blotting with anti-Flag antibodies. Figure 3E shows that Aurora B is bound to PP1γ1 at significant levels in immature oocytes, while the association is markedly reduced in meta-I oocytes.
Aurora B is deactivated at fertilization.
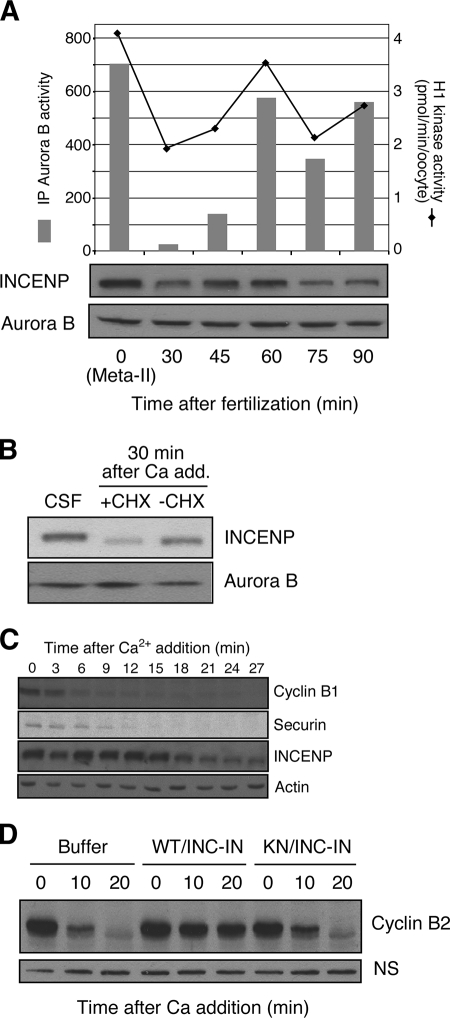
We next investigated the regulation of Aurora B at fertilization and during early-embryonic cell cycles. The kinase activity of Aurora B decreased abruptly at fertilization, consistent with a previous report on activity in metaphase and interphase egg extracts (5), and Aurora B activity then oscillated during early-embryonic cell cycles, being high in M phase and low in interphase (Fig. 4A). Western blotting of Aurora B showed that the amount of Aurora B does not change after fertilization (Fig. 4A), but the phosphorylation of T248 in the T loop is rapidly reversed (data not shown). This result is consistent with evidence that Aurora B degradation is mediated by the APC/Cdh1, and Cdh1 is absent in Xenopus laevis until the midblastula transition (27, 38). The decline in Aurora B activity at fertilization was associated with the degradation of INCENP (Fig. 4A, lower panel). This degradation could be seen most clearly in CSF extracts treated with both calcium, to mimic fertilization/egg activation and CSF release, and cycloheximide, to inhibit resynthesis of INCENP (Fig. 4B). It is evident that INCENP turns over very quickly, since without cycloheximide treatment, INCENP reaccumulated to M-phase levels within 60 min after fertilization (Fig. 4A and B). This rapid resynthesis may explain why earlier studies that examined only interphase and mitotic extracts did not observe changes in INCENP levels or association in the cell cycle (5). In subsequent embryonic cycles, the level of Aurora B kinase activity oscillated, being high in M phase and low in S phase, as judged by histone H3 kinase activity. This oscillation also correlated with periodic changes in the abundance of INCENP (Fig. 4A). The kinetics of INCENP degradation after CSF release in extracts shows that it occurs later than is evident with securin (Fig. 4C), consistent with the expected roles of active Aurora B in late mitotic events and cytokinesis (15, 36).
FIG. 4.
Aurora B inactivation at fertilization. (A) Oscillation of Aurora B kinase activity. Newly fertilized eggs were homogenized at the indicated time points and immunoblotted with anti-INCENP and anti-Aurora B antibodies (lower panel). Aurora B kinase activity was measured with immunoprecipitated (IP) Aurora B by using histone H3 as a substrate, and total histone H1 kinase activity in the extract was assayed as described in Materials and Methods. (B) INCENP is degraded at fertilization. Xenopus CSF extracts, arrested at meta-II, were first supplemented with cycloheximide (+CHX) or water (−CHX), released from CSF arrest by the addition (add.) of CaCl to 0.8 mM, and finally incubated for 30 min. The equivalent of 0.5 μl of extract was immunoblotted with anti-INCENP or anti-Aurora B antibodies. (C) Kinetics of INCENP degradation. Extracts were prepared from meta-II, CSF-arrested eggs. Five microliters of radiolabeled securin in reticulocyte lysate was added to 100 μl of CSF extract. Calcium was added to initiate CSF release, and cycloheximide was added to block new protein synthesis. Western blotting was performed at the indicated times by using antibodies to cyclin B1, INCENP, or actin (loading control) as indicated. The degradation of securin was monitored by autoradiography. (D) Active Aurora B-INCENP blocks CSF release. Xenopus CSF-arrested egg extracts were supplemented with bacterially expressed recombinant wild-type Aurora B-INCENP-IN (WT/INC-IN) complex or the kinase-dead (KN) Aurora B-INCENP-IN complex; after 10 min at room temperature, anaphase and cyclin B proteolysis were initiated by further supplementation with 0.8 mM CaCl2. At the indicated times, 0.5 μl of each extract was assessed for the level of cyclin B2 by Western blotting. A nonspecific band (NS) recognized by the anti-cyclin B1 antibody served as a loading control.
The arrest of the mature oocyte at metaphase II is mediated by a cytoplasmic activity known as CSF (43). Current evidence indicates that CSF arrest occurs in part due to inhibition of the APC/C. Several different pathways of APC/C inhibition during oocyte maturation have been identified; these include the Mos/mitogen-activated protein kinase/p90Rsk pathway, the cyclin E/Cdk2-Mps1 pathway, the Bub1/Mad1/Mad2 pathway, and the xErp1/Emi2 pathway (13, 20, 35, 42, 44). The most important of these pathways appears to be the Mos pathway, since phosphorylation of the APC/C inhibitor Emi2 by p90Rsk is necessary for both the establishment and the maintenance of CSF arrest (16, 28). There are several criteria for identifying a CSF component. Data presented in this paper (Fig. 1B and 4A) show that Aurora B is activated in oocyte maturation by the production of INCENP and is deactivated at fertilization by the degradation of INCENP, changes predicted for a CSF component. Aurora B is well documented as a spindle checkpoint kinase that inhibits the APC/C when spindle defects are present (6, 45). To evaluate whether Aurora B is capable of regulating CSF arrest, meta-II-arrested CSF egg extracts were incubated with wild-type or KN Aurora B-INCENP complexes prior to the addition of calcium. As shown in Fig. 4D, wild-type but not KN Aurora B blocked calcium-induced CSF release into anaphase II.
DISCUSSION
In this study, we analyzed the regulation and function of the CPC, especially Aurora B and INCENP, during Xenopus oocyte maturation and embryonic cell cycles. Our results show that INCENP is the main regulator of Aurora B in vivo.
Among CPC proteins, survivin is completely absent in resting G2 oocytes, and only a slight amount of INCENP exists; members of the CPC such as Dasra A and TD-60 are already expressed in immature oocytes, but only Dasra A was found to be codepleted with Aurora B in meta-II oocytes (Fig. 1). Although the localization of TD-60 suggests that it is a chromosome passenger protein (1), some studies have questioned whether TD-60 is really a CPC component (12, 32). Alternatively, we cannot exclude the possibility that a minor fraction of TD-60 binds Aurora B in oocytes. Our results do not exclude the possibility that other reported CPC components such as Dasra B are also present in Xenopus oocytes, but reagents to evaluate this possibility are currently unavailable. A remarkable finding in this work is that although INCENP production after progesterone treatment is required for Aurora B activation, even overexpression of INCENP is not sufficient to activate Aurora B in immature G2 oocytes. This failure of INCENP to activate Aurora B appears to be a consequence of a failure to bind Aurora B (Fig. 3C). These results indicate that add regulation of Aurora B besides INCENP production is required to activate Aurora B in immature oocytes. One possibility is that an additional CPC or other component is required for the binding of INCENP to Aurora B. We investigated whether a CPC component missing in immature G2 oocytes, survivin, facilitated association; however, it did not, even when coexpressed with INCENP (data not shown). We also considered the existence of an inhibitor that could prevent the binding of Aurora B and INCENP in immature oocytes. An attractive possible candidate is PP1, based on previous reports (40) and the precedent with Aurora A, in which PP1 is thought to constitutively dephosphorylate the T-loop in the absence of the activator TPX2 (9). As shown in Fig. 3E, more Aurora B is bound to PP1 in immature oocytes than in meta-I oocytes. Aurora B possesses a canonical motif, (R/K)(V/I)XL, that is similar to motifs reported to promote the targeting and binding of the PP1 catalytic subunit (8). However, it has been reported that Aurora B interaction with PP1 does not depend on the KVLF motif (40). We attempted to generate an Aurora B mutant in which the KVLF motif was absent, but the mutant was inactive, possibly because the PP1 binding motif is within the kinase domain (data not shown). Further work is necessary to determine whether the binding of INCENP to Aurora B affects the binding of PP1 or its access to phosphorylated T248 in the T-loop.
Recently, several studies have shown the importance of INCENP and Aurora B during meiosis. Our inhibition of Aurora B kinase by microinjection of INCENP antisense oligonucleotides showed that INCENP is necessary for Aurora B activation during oocyte maturation, but we did not observe a significant effect of prevention of Aurora B activation on meiotic progression as judged by first polar body emission, cyclin B degradation and accumulation, and total histone H1 kinase activity. However, injection of an antisense oligonucleotide only prevents the synthesis of new protein; we could not ablate the small amount of INCENP protein already present in immature oocytes (Fig. 2A). If the main function of INCENP protein in meiosis is to support chromosome segregation, the small amount of Aurora B-INCENP prelocalized specifically in the centromeres of the G2 oocyte might be sufficient to carry out a functional role in homologous chromosome segregation, even if progesterone-induced activation of INCENP-Aurora B is blocked. In this connection, it has been reported that in mouse sperm in prophase I, INCENP is already localized on centromeres (29). We observed that the small amount of preexisting INCENP in immature oocytes is localized in the germinal vesicle, but we have been unable to directly visualize centromeres (unpublished data). We also cannot exclude the possibility that instead of Aurora B, a germ cell-specific Aurora kinase, like Aurora C in mouse or human, which is also activated by INCENP, might be specifically involved in oocyte maturation (41).
Recently, Ramadan et al. (30) have shown that addition of Aurora B to CSF extracts causes a delay in histone H3 dephosphorylation, chromosome decondensation, and nuclear formation. Our data in Fig. 4 confirm that Aurora B can function as a regulator of the APC/C during CSF arrest. In this regard, the effect of Aurora B is similar to the effects of the Mos/mitogen-activated protein kinase/p90Rsk/Emi2, cyclin E/Cdk2/Mps1, and Bub1/Mad1/Mad2 pathways. These pathways can inhibit calcium-induced CSF release when components are constitutively active or are overexpressed (13, 21, 35, 42). Immunodepletion of Emi2 from CSF-arrested extracts has been found to cause release of CSF arrest in the absence of calcium (20, 35), but depletion of Mad2, Bub1, Mps1, Aurora B, or cyclin E has no effect on the maintenance of CSF arrest, indicating that Emi2 provides sufficient APC/C inhibition for metaphase arrest even in the absence of the other pathways (13, 20, 43, 44; also data not shown). In oocyte maturation during the meiosis I-to-II transition after GVBD, M phase is maintained after anaphase I for at least 2 h before the appearance of Emi2 (20). Although several spindle checkpoint kinases that regulate APC/C activity are present and active after anaphase I, including Aurora B (Fig. 1), Mps1 (13), and Bub 1 (44), loss-of-function experiments with each of them indicate they are not required to maintain M phase during the meiosis I-to-II transition.
Acknowledgments
We are very grateful to Johne' Liu (Ottawa Health Research Institute) for providing antiserum to xAurora B. We thank Hironori Funabiki (Rockefeller University) for providing antibody to xDasra A, Bill Earnshaw (University of Edinburgh) for antibody to TD-60, Yixian Zheng (Carnegie Institution) for antibody to xSurvivin, Patrick Eyers (University of Manchester) for antibody to the human Aurora B T-loop, and Thierry Lorca (CRBM, Montpellier, France) for a cDNA encoding Xenopus securin. T.M.M. is a Research Associate and J.L.M. an Investigator of the Howard Hughes Medical Institute.
This work was supported by the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Andreassen, P. R., D. K. Palmer, M. H. Wener, and R. L. Margolis. 1991. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J. Cell Sci. 99523-534. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, P. D., E. Knatko, W. J. Moore, and J. R. Swedlow. 2003. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15672-683. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss, R., T. Sardon, J. Ebert, D. Lindner, I. Vernos, and E. Conti. 2004. Determinants for Aurora-A activation and Aurora-B discrimination by TPX2. Cell Cycle 3404-407. [PubMed] [Google Scholar]
- 4.Bishop, J. D., and J. M. Schumacher. 2002. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B kinase stimulates Aurora B kinase activity. J. Biol. Chem. 27727577-27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton, M. A., W. Lan, S. E. Powers, M. L. McCleland, J. Kuang, and P. T. Stukenberg. 2002. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell 133064-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmena, M., and W. C. Earnshaw. 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4842-854. [DOI] [PubMed] [Google Scholar]
- 7.Ducat, D., and Y. Zheng. 2004. Aurora kinases in spindle assembly and chromosome segregation. Exp. Cell Res. 30160-67. [DOI] [PubMed] [Google Scholar]
- 8.Egloff, M. P., D. F. Johnson, G. Moorhead, P. T. Cohen, P. Cohen, and D. Barford. 1997. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 161876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyers, P. A., M. E. Churchill, and J. L. Maller. 2005. The Aurora A and Aurora B protein kinases: a single amino acid difference controls intrinsic activity and activation by TPX2. Cell Cycle 4784-789. [DOI] [PubMed] [Google Scholar]
- 10.Eyers, P. A., E. Erikson, L. G. Chen, and J. L. Maller. 2003. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13691-697. [DOI] [PubMed] [Google Scholar]
- 11.Gadea, B. B., and J. V. Ruderman. 2005. Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol. Biol. Cell 161305-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gassmann, R., A. Carvalho, A. J. Henzing, S. Ruchaud, D. F. Hudson, R. Honda, E. A. Nigg, D. L. Gerloff, and W. C. Earnshaw. 2004. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimison, B., J. Liu, A. L. Lewellyn, and J. L. Maller. 2006. Metaphase arrest by cyclin E-Cdk2 requires the spindle-checkpoint kinase Mps1. Curr. Biol. 161968-1973. [DOI] [PubMed] [Google Scholar]
- 14.Haccard, O., B. Sarcevic, A. Lewellyn, R. Hartley, L. Roy, T. Izumi, E. Erikson, and J. L. Maller. 1993. Induction of metaphase arrest in cleaving Xenopus embryos by MAP kinase. Science 2621262-1265. [DOI] [PubMed] [Google Scholar]
- 15.Honda, R., R. Korner, and E. A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 143325-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue, D., M. Ohe, Y. Kanemori, T. Nobui, and N. Sagata. 2007. A direct link of the Mos-MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 4461100-1104. [DOI] [PubMed] [Google Scholar]
- 17.Jelinkova, L., and M. Kubelka. 2006. Neither Aurora B activity nor histone H3 phosphorylation is essential for chromosome condensation during meiotic maturation of porcine oocytes. Biol. Reprod. 74905-912. [DOI] [PubMed] [Google Scholar]
- 18.Kaitna, S., P. Pasierbek, M. Jantsch, J. Loidl, and M. Glotzer. 2002. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 12798-812. [DOI] [PubMed] [Google Scholar]
- 19.Kang, J., I. M. Cheeseman, G. Kallstrom, S. Velmurugan, G. Barnes, and C. S. Chan. 2001. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 155763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., and J. L. Maller. 2005. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 151458-1468. [DOI] [PubMed] [Google Scholar]
- 21.Lorca, T., A. Castro, A. M. Martinez, S. Vigneron, N. Morin, S. Sigrist, C. Lehner, M. Doree, and J. C. Labbe. 1998. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 173565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma, C., C. Cummings, and X. J. Liu. 2003. Biphasic activation of Aurora-A kinase during the meiosis I-meiosis II transition in Xenopus oocytes. Mol. Cell. Biol. 231703-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maton, G., C. Thibier, A. Castro, T. Lorca, C. Prigent, and C. Jessus. 2003. Cdc2-cyclin B triggers H3 kinase activation of Aurora-A in Xenopus oocytes. J. Biol. Chem. 27821439-21449. [DOI] [PubMed] [Google Scholar]
- 24.Meraldi, P., R. Honda, and E. A. Nigg. 2004. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr. Opin. Genet. Dev. 1429-36. [DOI] [PubMed] [Google Scholar]
- 25.Monje-Casas, F., V. R. Prabhu, B. H. Lee, M. Boselli, and A. Amon. 2007. Kinetochore orientation during meiosis is controlled by Aurora B and the monopolin complex. Cell 128477-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murnion, M. E., R. R. Adams, D. M. Callister, C. D. Allis, W. C. Earnshaw, and J. R. Swedlow. 2001. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J. Biol. Chem. 27626656-26665. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, H. G., D. Chinnappan, T. Urano, and K. Ravid. 2005. Mechanism of Aurora-B degradation and its dependency on intact KEN and A-boxes: identification of an aneuploidy-promoting property. Mol. Cell. Biol. 254977-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiyama, T., K. Ohsumi, and T. Kishimoto. 2007. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 4461096-1099. [DOI] [PubMed] [Google Scholar]
- 29.Parra, M. T., A. Viera, R. Gomez, J. Page, M. Carmena, W. C. Earnshaw, J. S. Rufas, and J. A. Suja. 2003. Dynamic relocalization of the chromosomal passenger complex proteins inner centromere protein (INCENP) and aurora-B kinase during male mouse meiosis. J. Cell Sci. 116961-974. [DOI] [PubMed] [Google Scholar]
- 30.Ramadan, K., R. Bruderer, F. M. Spiga, O. Popp, T. Baur, M. Gotta, and H. H. Meyer. 2007. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature 4501258-1262. [DOI] [PubMed] [Google Scholar]
- 31.Resnick, T. D., D. L. Satinover, F. MacIsaac, P. T. Stukenberg, W. C. Earnshaw, T. L. Orr-Weaver, and M. Carmena. 2006. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev. Cell 1157-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruchaud, S., M. Carmena, and W. C. Earnshaw. 2007. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 8798-812. [DOI] [PubMed] [Google Scholar]
- 33.Sampath, S. C., R. Ohi, O. Leismann, A. Salic, A. Pozniakovski, and H. Funabiki. 2004. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118187-202. [DOI] [PubMed] [Google Scholar]
- 34.Satinover, D. L., C. A. Leach, P. T. Stukenberg, and D. L. Brautigan. 2004. Activation of Aurora-A kinase by protein phosphatase inhibitor-2, a bifunctional signaling protein. Proc. Natl. Acad. Sci. USA 1018625-8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt, A., P. I. Duncan, N. R. Rauh, G. Sauer, A. M. Fry, E. A. Nigg, and T. U. Mayer. 2005. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 19502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schumacher, J. M., A. Golden, and P. J. Donovan. 1998. AIR-2: an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1431635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sessa, F., M. Mapelli, C. Ciferri, C. Tarricone, L. B. Areces, T. R. Schneider, P. T. Stukenberg, and A. Musacchio. 2005. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol. Cell 18379-391. [DOI] [PubMed] [Google Scholar]
- 38.Stewart, S., and G. Fang. 2005. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 658730-8735. [DOI] [PubMed] [Google Scholar]
- 39.Stukenberg, P. T., K. D. Lustig, T. J. McGarry, R. W. King, J. Kuang, and M. W. Kirschner. 1997. Systematic identification of mitotic phosphoproteins. Curr. Biol. 7338-348. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama, K., K. Sugiura, T. Hara, K. Sugimoto, H. Shima, K. Honda, K. Furukawa, S. Yamashita, and T. Urano. 2002. Aurora-B associated protein phosphatases as negative regulators of kinase activation. Oncogene 213103-3111. [DOI] [PubMed] [Google Scholar]
- 41.Tang, C. J., C. Y. Lin, and T. K. Tang. 2006. Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev. Biol. 290398-410. [DOI] [PubMed] [Google Scholar]
- 42.Tung, J. J., D. V. Hansen, K. H. Ban, A. V. Loktev, M. K. Summers, J. R. Adler III, and P. K. Jackson. 2005. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA 1024318-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tunquist, B. J., and J. L. Maller. 2003. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 17683-710. [DOI] [PubMed] [Google Scholar]
- 44.Tunquist, B. J., M. S. Schwab, L. G. Chen, and J. L. Maller. 2002. The spindle checkpoint kinase Bub1 and cyclin E/Cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr. Biol. 121027-1033. [DOI] [PubMed] [Google Scholar]
- 45.Vigneron, S., S. Prieto, C. Bernis, J. C. Labbe, A. Castro, and T. Lorca. 2004. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 154584-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasui, Y., T. Urano, A. Kawajiri, K. Nagata, M. Tatsuka, H. Saya, K. Furukawa, T. Takahashi, I. Izawa, and M. Inagaki. 2004. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J. Biol. Chem. 27912997-13003. [DOI] [PubMed] [Google Scholar]
- 47.Yu, H. G., and D. Koshland. 2007. The Aurora kinase Ipl1 maintains the centromeric localization of PP2A to protect cohesin during meiosis. J. Cell Biol. 176911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]