Abstract
The accumulation of misfolded proteins stresses the endoplasmic reticulum (ER) and triggers cell death through activation of the multidomain proapoptotic BCL-2 proteins BAX and BAK at the outer mitochondrial membrane. The signaling events that connect ER stress with the mitochondrial apoptotic machinery remain unclear, despite evidence that deregulation of this pathway contributes to cell loss in many human degenerative diseases. In order to “trap” and identify the apoptotic signals upstream of mitochondrial permeabilization, we challenged Bax−/− Bak−/− mouse embryonic fibroblasts with pharmacological inducers of ER stress. We found that ER stress induces proteolytic activation of the BH3-only protein BID as a critical apoptotic switch. Moreover, we identified caspase-2 as the premitochondrial protease that cleaves BID in response to ER stress and showed that resistance to ER stress-induced apoptosis can be conferred by inhibiting caspase-2 activity. Our work defines a novel signaling pathway that couples the ER and mitochondria and establishes a principal apoptotic effector downstream of ER stress.
The endoplasmic reticulum (ER) is the major site within the cell for folding, modification, and trafficking of secreted proteins. Various physiological and pathological stresses encountered by the cell (e.g., hypoxia, nutrient deprivation, and disruption of intracellular pH) lead to an increased demand on the ER's protein-folding capacity. When the extent of unfolded proteins in the ER lumen reaches a critical level, the cell engages a set of evolutionarily conserved signal transduction pathways, which are collectively known as the unfolded protein response (UPR) (25). The ER-resident transmembrane sensors IRE1α, PERK, and ATF6 are the major effectors of the UPR in mammalian cells and initially expand the ER network, upregulate chaperones, and arrest global translation in order to restore homeostasis. However, if the ER damage is extensive or prolonged, the cell initiates apoptosis through activation of the multidomain proapoptotic BCL-2 proteins BAX and BAK at the outer mitochondrial membrane (21). Several caspases (caspase-4 and caspase-12), calcium regulatory proteins, and BH3-only proteins (NOXA, PUMA, and BIM) have been implicated in contributing to apoptotic signaling downstream of ER stress (8, 9, 10, 13, 20, 22, 23). Nevertheless, the molecular chain of events leading from ER stress to mitochondrial BAX/BAK activation remains poorly understood.
Mounting evidence suggests that apoptosis triggered by excessive stress on the protein-folding capacity of the ER contributes to pathological cell loss in many common human degenerative diseases (e.g., Alzheimer's, Parkinson's, and type 2 diabetes) (14, 18). Hence, there is great interest among the biomedical community in understanding how this occurs and whether this apoptotic pathway might be a good therapeutic target for these diseases. Until now, the signaling events that connect ER stress with the mitochondrial apoptotic machinery have remained elusive.
Here we report the discovery of two key apoptotic players that signal the mitochondrial death machinery in response to excessive or prolonged stress on the ER. Using rigorous in vitro and in vivo biochemical assays, we show that caspase-2 cleavage of the BH3-only protein BID is a major apoptotic switch downstream of ER stress, which if blocked protects cells against this form of injury.
MATERIALS AND METHODS
Cell culture and biological reagents.
All mouse embryonic fibroblasts (MEFs) were transformed by simian virus 40 (SV40) and maintained in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine, and nonessential amino acids (UCSF cell culture facility). Primary thymocytes from 6-week-old mice were maintained in the same media. z-VAD-fmk, z-VDVAD-fmk, z-IETD-CHO, and annexin V-FITC were purchased from BioVision. Protease inhibitor cocktail (PIC), IPTG (isopropyl-β-D-thiogalactopyranoside), thapsigargin (TG), brefeldin A (BFA), staurosporine (STS), tumor necrosis factor alpha (TNF-α), and cycloheximide were obtained from Sigma. All recombinant caspases were purchased from Calbiochem. Caspase-2 small inhibiting RNA (siRNA; catalog no. L-044184-00-0005) and control siRNA (catalog no. D-001810-10), along with DharmaFECT 3 reagent, were used per the manufacturer's (Dharmacon) protocol. An RNeasy kit (Qiagen) was used for RNA extraction according to the manufacturer's protocol. Lipofectamine and Plus transfection regents were purchased from Invitrogen.
Cellular fractionation and cytochrome c release assay.
MEFs were resuspended in mitochondrial experimental buffer (MEB; 125 mM KCl, 10 mM Tris-MOPS [morpholinepropanesulfonic acid] [pH 7.4], 5 mM glutamate, 1.25 mM malate, 2 μM EGTA) plus 1× PIC and mechanically disrupted, using a 400-μm chamber attached to an M-110S Microfluidizer processor (Microfluidics). Cell lysates were then centrifuged at 100,000 × g for 60 min at 4°C; the resulting supernatant was termed the S100 fraction. To isolate mitochondria, diced mouse liver or pelleted Jurkat cells were resuspended in MEB plus 1× PIC, stroked 10 times through a 27-gauge needle, and centrifuged at 700 × g for 10 min at 4°C to remove nuclei and unbroken cells. The supernatant was centrifuged at 7,000 × g for 10 min at 4°C to pellet the isolated mitochondria. For cytochrome c release assays, 100 μg of S100 extract was incubated with 25 μg of isolated mitochondria for 45 min at room temperature (RT) in MEB. Subsequently, the reactions were centrifuged at 16,100 × g for 5 min at 4°C, and the pellet was lysed in RIPA buffer (20 mM Tris-MOPS [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40) to release the remaining cytochrome c. For experiments using mouse liver mitochondria, the supernatant and pellet fractions were immunoblotted as detailed below. For experiments using Jurkat mitochondria, the supernatant and resuspended pellet fractions were incubated with human cytochrome c enzyme-linked immunosorbent assay (ELISA) plates (R&D Systems) per the manufacturer's instructions. The percentage of cytochrome c release was calculated by dividing the amount of cytochrome c found in the supernatant fraction by the sum of the amount of cytochrome c found in the supernatant and pellet fractions.
Antibodies, immunoprecipitation, in vitro BID cleavage assays, and Western blot analysis.
Antibodies used for Western blot analysis included antiactin from Chemicon, anti-BAK, anti-BAX, anti-α subunit of eukaryotic translation initiation factor 2 (anti-eIF2α), anti-phospho-eIF2α, anti-BAD, anti-BIK, anti-PUMA, and anti-poly(ADP-ribose) polymerase (anti-PARP) from Cell Signaling Technology, anti-BCL-XL from Santa Cruz Biotechnology, anti-BID from R&D Systems, anti-caspase-2 from Chemicon, anti-full-length caspase-8 from Alexis, anti-cleaved caspase-8 from Imgenex, BD Pharmingen anti-cytochrome c, anti-BIM from ProSci Incorporated, and anti-V5 from Sigma. Horseradish peroxidase-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (anti-mouse, anti-rat, and anti-rabbit antibodies) and Bio-Rad (anti-goat antibody). For immunoprecipitation experiments, S100 extracts were incubated with anti-BID or an isotype-matched control antibody and protein A/G beads (Santa Cruz Biotechnology) for 60 min at 4°C and centrifuged at 2,200 × g for 5 min at 4°C. The subsequent supernatant fractions were used in a cytochrome c release assay on isolated Jurkat mitochondria as described above. For in vitro BID cleavage assays, 100 μg of each S100 extract was incubated for 1 h with recombinant BCL-XL, anti-BCL-XL, and protein A/G beads and centrifuged at 2,200 × g. The supernatant fraction was then either left alone or pretreated for 10 min with a caspase inhibitor before recombinant BID was added. Recombinant caspases were incubated with caspase activity buffer (10 mM dithiothreitol, 20 mM Tris-MOPS [pH 7.4], 200 mM KCl) and, if necessary, pretreated with various inhibitors for 10 min before recombinant BID was added. All BID cleavage assay samples were then incubated at 37°C for 1 h. Whole-cell lysates were prepared by disruption of the cells with RIPA buffer plus 1× PIC for 5 min on ice, centrifugation at 16,000 × g to remove insoluble materials, and boiling at 100°C for 5 min. The samples were resolved by 10% NuPAGE gels (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore). Antibodies were detected by using the chemiluminescence method (PerkinElmer), Pierce film, and a Konica SRX-101A developer. Gels were quantified via densitometry by using the open-source ImageJ software (rsb.info.nih.gov/ij/).
Production of recombinant proteins.
To produce recombinant GST-BCL-XL, 1 μg of pGEX-2T-Bcl-XL plasmid was transformed into BL21 bacteria by heat shocking the bacteria in the presence of the plasmid at 42°C for 1 min. The bacteria were subsequently grown on a 100-μg/ml ampicillin-selective LB agar plate. The resulting colonies were expanded into LB plus 100 μg/ml ampicillin and induced to express GST-BCL-XL via treatment with 0.5 mM IPTG. The bacteria were cultured at 37°C until they reached an optical density at 600 nm of 0.6. They were then pelleted via centrifugation at 3,000 × g for 10 min and subsequently resuspended in 1× phosphate-buffered saline (PBS) plus 1% Triton X-100 with PIC and 1 mg/ml lysozyme. The resuspended cells were then frozen overnight at −80°C, thawed, and lysed through a 0.1-μm Microfluidizer chamber (Microfluidics). Cell debris was removed via centrifugation at 10,000 × g for 10 min and by passing the supernatant through a 0.1-μm filter. The lysate was then incubated with glutathione-agarose beads at 4°C for 2 h on a rocker. The beads were then pelleted by centrifugation at 300 × g for 5 min and washed twice in 1× PBS. The GST-Bcl-XL was eluted from the beads by incubation with 50 mM Tris-50 mM reduced glutathione for 30 min at RT on a rocker. To produce recombinant BID, pGEX-2T-BID was expressed and purified from bacteria as described above. After being bound to the agarose beads, BID was cleaved off by the addition of 1 ng of thrombin in 1× thrombin buffer for 60 min at RT. The beads were pelleted, and the supernatant was cleared of thrombin by passing it through a benzamidine-Sepharose matrix at 16,100 × g.
XBP-1 splicing assay.
An XBP-1 splicing assay was performed as described previously (2). After being treated, the total RNA was extracted from cells by using an RNeasy kit (Qiagen) per the manufacturer's instructions. After being treated with DNase I, a 600-bp cDNA product encompassing the IRE1α cleavage site was amplified by a one-step reverse transcription-PCR kit (Invitrogen), using the sense primer mXBP1.3S (5′-AAACAGAGTAGCAGCGCAGACTGC-3′) and the antisense primer mXBP1.2AS (5′-GGATCTCTAAAACTAGAGGCTTGGTG-3′). This fragment was further digested by PstI to reveal a restriction site that is lost upon the splicing of XBP-1 by IRE1α. The products were resolved on a 2% agarose gel.
Annexin V staining and fluorescence-activated cell sorter (FACS) analysis.
Cells were harvested by trypsinization, washed twice in 1 × PBS, and incubated with annexin V binding buffer (2 mM CaCl2, 80 mM NaCl, 1% HEPES plus 1 μg/ml fluorescein isothiocyanate-annexin V for 5 min. The cells were subsequently passed through a FACSCalibur machine (Becton Dickinson) and detected with CellQuest software (BD Biosciences). Statistical analyses were completed using Student's t test.
RESULTS AND DISCUSSION
ER stress can be induced pharmacologically in cells with agents that perturb protein modification or trafficking at the ER, including BFA (an inhibitor of ER-Golgi protein transport), tunicamycin (specific N glycosylation inhibitor), and TG (inhibitor of the ER Ca2+ pump). Our laboratory and others have previously shown that Bax−/− Bak−/− (DKO) fibroblasts are strikingly resistant to apoptosis in response to these ER stress-inducing agents (15, 33). Moreover, targeting BAX stably and exclusively to the outer mitochondrial membrane in DKO cells restores the cell death response to BFA and other ER stress-inducing agents (26), which argues that an accumulation of misfolded proteins in the ER lumen triggers an apoptotic signal that is transmitted to mitochondria, where it induces activation of BAX and/or BAK. Once active, BAX and/or BAK permeabilize the outer mitochondrial membrane, which results in the release of prodeath mitochondrial proteins into the cytosol (e.g., cytochrome c and Smac/DIABLO) and activation of the executioner caspases (e.g., caspase-3) (6, 16, 28, 30). Therefore, we hypothesized that the resistance of the Bax−/− Bak−/− cells to ER stress would allow us to capture and identify the early (premitochondrial) apoptotic signals induced by this form of injury while the cells remained alive and free of prodeath activities resulting from mitochondrial permeabilization.
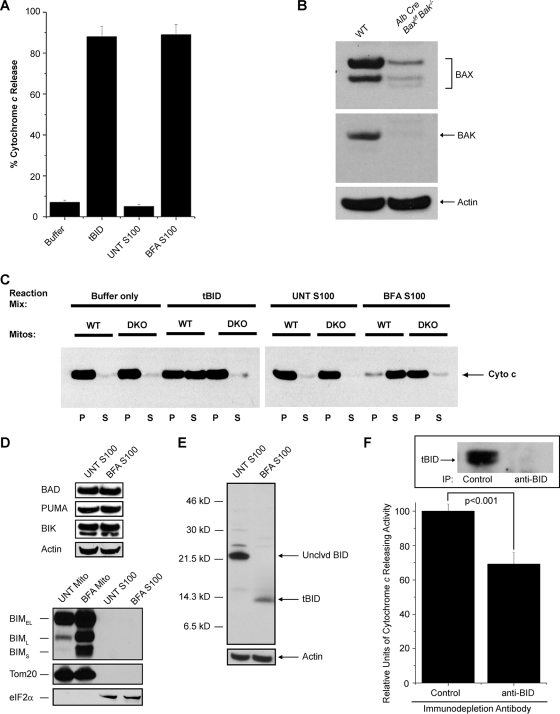
To this end, we induced ER stress in SV40-transformed DKO MEFs via treatment with BFA for 24 h. Following treatment, a cytosolic extract (the S100 fraction) was isolated by mechanical disruption of the cells in an isotonic buffer (to keep organelles intact) followed by high-speed (100,000 × g) centrifugation. To test for the presence of ER stress-induced BAX/BAK activating signals, we incubated the S100 fractions from untreated and BFA-treated DKO MEFs with mitochondria isolated from human Jurkat T cells and then assayed them for cytochrome c release. As shown in Fig. 1A, the S100 fraction from untreated DKO MEFs did not induce significant mitochondrial cytochrome c release above the level seen with buffer alone. In contrast, the S100 of BFA-treated DKO cells released greater than 90% of the mitochondrial cytochrome c into the supernatant (as measured by an enzyme-linked immunosorbent assay against human cytochrome c). The addition of the Ca2+ chelator EGTA or the pancaspase inhibitor zVAD-fmk had no effect on the cytochrome c-releasing activity of the S100 of BFA-treated DKO cells (data not shown). Moreover, to confirm that the S100 fraction from the BFA-treated MEFs was causing cytochrome c release by specifically activating BAX/BAK and not by generally disrupting mitochondrial integrity, we incubated the S100 fractions from BFA-treated and untreated DKO MEFs with isolated liver mitochondria from age-matched wild-type (WT) or conditionally targeted (Baxf/f Bak−/−) mice with a liver-specific Cre deletion (albumin-Cre) (Fig. 1B). The S100 fraction from treated DKO MEFs induced robust cytochrome c release from the WT mitochondria but not from BAX/BAK-deficient mitochondria (Fig. 1C). Together, these data strongly indicate that ER stress induces a cytosolic activity capable of triggering BAX/BAK-dependent cytochrome c release from isolated mitochondria.
FIG. 1.
BID is proteolytically activated following ER stress and induces BAX/BAK-dependent mitochondrial cytochrome c release. (A) Cytosolic (S100) extracts from untreated (UNT) and BFA-treated DKO MEFs, as well as recombinant tBID (positive control) and reaction buffer (negative control), were incubated with isolated Jurkat mitochondria and assayed for the ability to release human cytochrome c. (B) Liver lysates (60 μg) from WT and Alb Cre Baxf/f Bak−/− mice were immunoblotted for BAX and BAK. (C) S100 extracts from untreated DKO MEFs and DKO MEFs treated with 2.5 μg/ml of BFA, as well as recombinant tBID and reaction buffer, were incubated with mitochondria (Mitos) isolated from WT and DKO (Alb Cre Baxf/f Bak−/−) hepatocytes. The mitochondrial pellet (P) and supernatant (S) from each reaction were immunoblotted for murine cytochrome c (Cyto c). BFA-treated S100 extract releases cytochrome c from WT but not DKO mitochondria. (D) S100 extracts from untreated DKO MEFs and DKO MEFs treated with 2.5 μg/ml BFA were immunoblotted for BAD, PUMA, BIK, and actin. Mitochondrial (Mito) and S100 fractions from untreated DKO MEFs and DKO MEFs treated with 2.5 μg/ml BFA were immunoblotted for BIM, Tom20 (a mitochondrial marker), and eIF2α (a cytosolic marker). Note that no BIM was detected in the S100 fractions. BIMEL, BIM extra long; BIML, BIM long; BIMS, BIM short. (E) S100 extracts from untreated DKO MEFs and DKO MEFs treated with 2.5 μg/ml BFA were immunoblotted for BID and actin. Only tBID was detected in the BFA S100 extract. Unclvd, uncleaved. (F) S100 extract from DKO MEFs treated with 2.5 μg/ml BFA was immunodepleted with either an isotype-matched control or an anti-BID antibody and assayed for its ability to induce cytochrome c release from isolated Jurkat mitochondria. The inset shows immunodepleted extracts blotted for tBID. The relative levels of cytochrome c-releasing activity per microgram of extract were significantly reduced when BID was immunodepleted. IP, immunoprecipitation.
In response to various forms of cell injury, mitochondrial BAX and BAK can be activated either directly or indirectly by a subclass of proapoptotic BCL-2 family members known as the BH3-only proteins, so named because of their sequence homology solely in the amphipathic α-helical BH3 domain (4, 27). Therefore, we assayed S100 fractions from untreated and BFA-treated DKO MEFs for differences in expression or posttranslational modifications of known BH3-only proteins. The cytosolic levels of most of the known BH3-only proteins, including BAD, PUMA, and BIK, remain unchanged with ER stress (Fig. 1D). The BH3-protein BIM has recently been implicated in ER stress-induced apoptosis (23). While the levels of mitochondrion-associated BIM clearly increased upon BFA treatment, there was no detectable BIM in our cytosolic extracts either before or after ER stress (Fig. 1D), ruling out the possibility that BIM significantly contributes to the BFA-induced cytosolic cytochrome c-releasing activity. Surprisingly, we found that while the vast majority of the BH3-only protein BID in the untreated S100 is full-length (∼22 kDa), nearly all the BID present in the S100 from the BFA-treated cells is of a smaller size (∼14 kDa) (Fig. 1E). In response to certain death signals, BID is activated by proteolytic cleavage into a smaller 14 -kDa truncated form, termed tBID, which then translocates to mitochondria, where it potently activates BAX and/or BAK (12, 17, 31, 32). The presence of tBID in the BFA S100 fraction from DKO cells suggested the possibility that this activated protein might be an apoptotic signal that links ER stress to the mitochondrial death machinery. To test this hypothesis, we immunodepleted BID/tBID from the S100 fraction of BFA-treated cells and subsequently assayed the ability of this fraction to induce cytochrome c release (Fig. 1F). Interestingly, BFA-treated S100 extract that had been immunodepleted for BID contained approximately a third less cytochrome c releasing-activity than the nonimmunodepleted sample (Fig. 1F). These data indicate that tBID represented one of the major ER stress-induced apoptotic activities in our cytosolic extract.
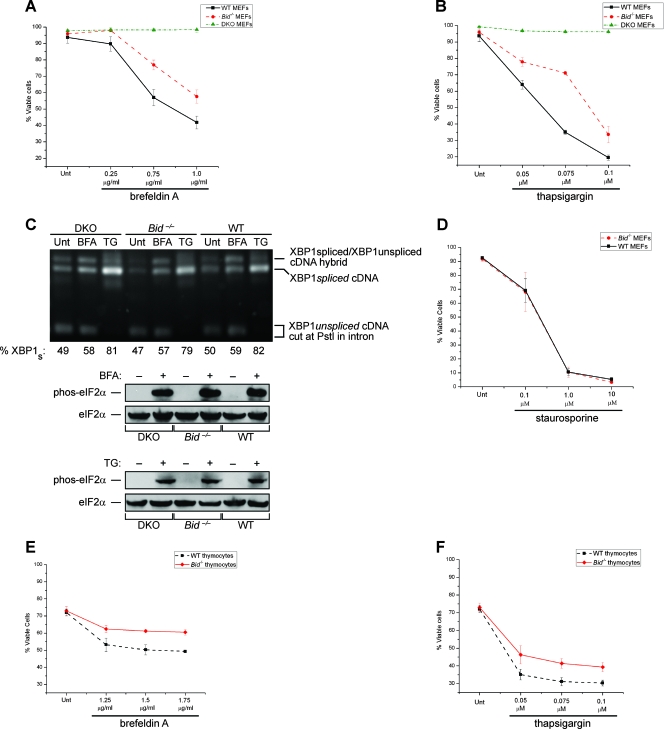
If BID is essential for ER stress-induced death in vivo, then the loss of BID should confer resistance to this form of cell injury. To test this, we challenged SV40-transformed WT, DKO, and Bid−/− MEFs with various concentrations of BFA and TG. The Bid−/− MEFs displayed significant resistance to treatment with both BFA (Fig. 2A) and TG (Fig. 2B), albeit less than did the DKO cells. To rule out the possibility that the apoptotic resistance of Bid−/− and DKO cells was due to changes in UPR signaling, we measured the major downstream targets of both IRE1α and PERK in response to the same doses of BFA and TG used in the apoptotic studies. Unfolded proteins in the ER activate the RNase domain of IRE1α to initiate splicing of the XBP1 mRNA (2, 5). As a consequence of IRE1α-mediated intron removal, XBP1 mRNA is frame shifted and translated to produce XBP1 protein, a transcription factor whose downstream effects increase protein-folding capacity (25). As assessed through an reverse transcription-PCR-based assay, the levels of spliced XBP1 mRNA were essentially identical among the three cell types at baseline and in response to the same doses of BFA and TG used in our apoptosis studies (Fig. 2C). PERK is an ER-localized transmembrane kinase that, when activated by misfolded proteins, phosphorylates eIF2α at Ser51 (7), leading to a global attenuation in cap-dependent protein translation. As shown in Fig. 2C, ER stress-induced phosphorylation of eIF2α at Ser51 occurs equally among the three cell types. These results suggest that the apoptotic resistance of the Bid−/− and DKO cells to ER stress is not attributable to gross differences in UPR signaling at the ER membrane. Moreover, the Bid−/− MEFs did not display resistance to STS (a pankinase inhibitor) treatment, a death stimulus known to induce mitochondrial BAX/BAK-dependent apoptosis (Fig. 2D). These results indicate that Bid−/− cells have a specific resistance to apoptosis induced by ER stress but not to all intrinsic apoptotic stimuli. To ensure that the transformed nature of the MEFs was not affecting our results, we also challenged primary WT and Bid−/− thymocytes with various concentrations of BFA and TG. Again, the Bid−/− thymocytes displayed significantly greater resistance to both BFA (Fig. 2E) and TG (Fig. 2F) than their WT counterparts. Together, these data demonstrate that loss of BID confers cytoprotection against ER stress, confirming that tBID is one of the major apoptotic signals that link the ER and the mitochondrion.
FIG. 2.
Bid−/− cells are resistant to treatment with ER stress agents. Wild-type, Bax−/− Bak−/− (DKO), and Bid−/− simian virus 40-transformed MEFs were treated with the indicated concentrations of either brefeldin A (A) or TG (B) for 24 and 18 h, respectively. Following these treatments, the percentages of viable (annexin V-negative) cells were determined via FACS. (C) Whole-cell lysates from DKO, Bid−/−, and WT MEFs that were left untreated (Unt) or treated with either 0.75 μg/ml BFA (12 h) or 0.075 μM TG (8 h) were immunoblotted for phospho (phos)-eIF2α and total eIF2α. The total RNA from DKO, Bid−/−, and WT MEFs that were untreated or treated with either 0.75 μg/ml BFA (4 h) or 0.075 μM TG (8 h) was isolated and subjected to an XBP1-splicing assay. The XBP1 cDNA products of PstI digestion were revealed on a 2% agarose gel. Unspliced XBP1 mRNA produced the lower doublet (291 bp and 310 bp), whereas spliced XBP1 (XBP1s) mRNA produced one 575-bp band. The highest band shown is a previously described hybrid of spliced and unspliced XBP1 cDNA fragments. Densitometry was used to determine the percent XBP1s [XBP1s/(XBP1s + XBP1us)]. (D) Bid−/− and WT MEFs were treated with the indicated concentrations of STS for 18 h, and the percentages of viable (annexin V-negative) cells were determined via FACS. (E and F) Primary thymocytes from 6-week-old WT and Bid−/− mice were treated with the indicated concentrations of either brefeldin A (E) or TG (F) for 8 h. Following these treatments, the percentages of viable (annexin V-negative) cells were determined via FACS. The results of all the assays were analyzed via Student's t test (n = 3). Together these data demonstrate that cells deficient in BID are resistant to apoptosis induced by ER stress agents.
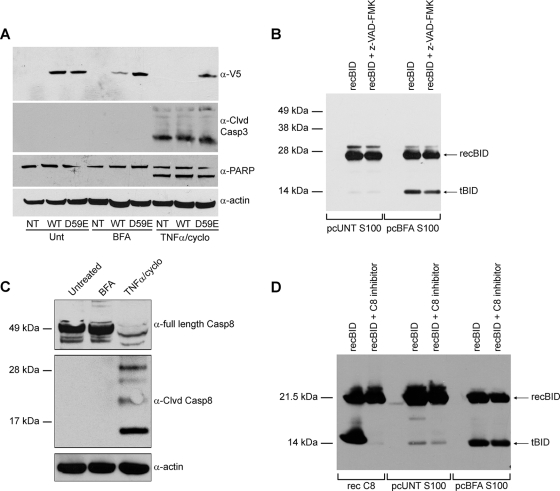
To investigate where BID is cleaved in response to ER stress, we transiently expressed V5-tagged versions of WT BID or a D59E BID mutant in DKO MEFs. The D59E mutation replaces the aspartate residue in BID that is targeted by caspase-8 and other caspases, rendering BID uncleavable by these proteases (34). We monitored changes in the levels of full-length V5-tagged WT or D59E BID as indications of BID cleavage, since the anti-V5 antibody does not recognize V5-tagged tBID. Upon treatment of the transfected cells with TNF-α-cycloheximide (which is known to induce BID cleavage via caspase-8 [12, 17]), the level of full-length V5-tagged WT BID was greatly reduced, indicative of its being cleaved (Fig. 3A). In contrast, the level of full-length D59E BID remained constant in the TNF-α-cycloheximide-treated cells, as it is resistant to caspase-8 activity. Moreover, through activation of initiator caspase-8, TNF-α-cycloheximide led to the processing of caspase-3 and the cleavage of its target, PARP, independently of mitochondrial permeabilization. When the transfected cells were treated with BFA, the level of full-length V5 WT BID was substantially reduced, consistent with our finding that BID is cleaved during ER stress. Yet the D59E mutant of BID remained at a constant level after BFA treatment (Fig. 3A). As expected, BFA was unable to activate caspase-3 in the DKO cells where mitochondrial permeabilization was blocked, which suggests that the ER stress-induced protease is an initiator (premitochondrial) caspase and cleaves BID at this site. As mentioned previously, the classical BID protease is caspase-8, but we found no evidence to suggest that it is cleaved or enzymatically activated in MEFs following BFA treatment (Fig. 3C).
FIG. 3.
ER stress induces BID cleavage at position D59 independently of caspase-8 activation. (A) Nontransfected (NT) DKO MEFS or DKO MEFS transfected with either BID-V5 (WT) or D59E BID-V5 (D59E) were left untreated or treated with 2.5 μg/ml of BFA or 1 ng/ml of TNF-α-2.5 μg/ml of cycloheximide (TNFα/cyclo) for 24 h. Whole-cell lysates immunoblotted for anti-V5 (α-V5) show that full-length WT, but not D59E BID, was reduced upon BFA treatment. Immunoblots of PARP and cleaved (Clvd) caspase-3 demonstrate that caspase-3 activity is present in TNF-α-cycloheximide-treated cells but not in BFA-treated samples. (B) S100 extracts from untreated (UNT) and BFA-treated DKO MEFs were precleared (pc) of endogenous BID/tBID and then incubated with recombinant (rec) BID with or without being pretreated with the broad-range caspase inhibitor z-VAD-fmk (40 μM). Samples were then immunoblotted for BID. Note that the S100 extract from BFA-treated DKO MEFs cleaves recBID and that this activity is not substantially reduced by z-VAD-fmk. (C) DKO MEFs were left untreated or were treated with 2.5 μg/ml BFA or 1 ng/ml of TNF-α-2.5 μg/ml of cycloheximide for 24 h. Whole-cell lysates were then immunoblotted for full-length caspase-8, cleaved caspase-8, and actin. Note that caspase-8 is cleaved and activated in response to TNF-α-cycloheximide but not to BFA. (D) S100 extracts from untreated and BFA-treated DKO MEFs were first precleared of endogenous BID/tBID and then incubated in the presence of recBID with or without pretreatment with the caspase-8 inhibitor z-IETD-fmk (40 μM). As a control, recombinant caspase-8 was incubated with recBID in the absence or presence of z-IETD-fmk (40 μM). The untreated S100 extract did not induce cleavage of recBID. The BFA extract cleaved recBID even in the presence of caspase-8 inhibition with z-IETD-fmk. α, anti.
To investigate the mechanism of BID cleavage following ER stress, we asked whether we could detect its proteolytic cleavage in our cell extracts. Following depletion of endogenous BID/tBID by preclearing (pc) the S100 extracts with agarose beads coated with an antiapoptotic binding partner (BCL-xL), we incubated full-length recombinant BID (recBID) with the pcS100 extracts from untreated and BFA-treated DKO MEFs. While the extract from untreated cells had no effect on the size of recBID, the pcS100 from the BFA-treated cells contained a definite BID cleavage activity (Fig. 3B). Interestingly, this BID proteolytic activity was not substantially reduced by the broad-spectrum caspase inhibitor z-VAD-fmk (Fig. 3B), which is known to block some but not all caspases. Moreover, pretreatment with the specific caspase-8 inhibitor Ac-IETD-CHO did not block this in vitro BID cleavage activity (Fig. 3D), consistent with our previously mentioned results showing that caspase-8 is not activated by ER stress (Fig. 3C). These results suggest that another caspase might be responsible for cleaving BID under conditions of ER stress.
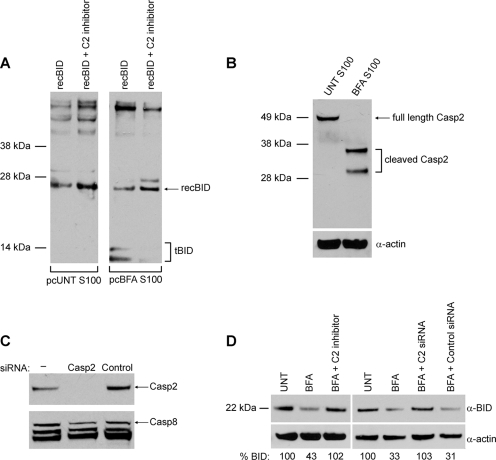
As caspase-2 is resistant to z-VAD-fmk and has been reported to cleave BID in response to heat shock (1), we decided to further investigate whether this protease plays a role in ER stress. The caspase-2-specific inhibitor z-VDVAD-fmk completely blocked the in vitro cleavage of recBID by the pcS100 fraction from BFA-treated DKO MEFs (Fig. 4A). Moreover, we found that caspase-2 is cleaved in the S100 extract from BFA-treated DKO MEFs (Fig. 4B), indicating that it is activated by ER stress upstream of mitochondrial permeabilization. To determine if caspase-2 is responsible for BID activation within intact cells, DKO MEFs were left untreated or were pretreated with z-VDVAD-fmk and then challenged with BFA. In whole-cell extracts, we were not able to consistently detect tBID with our antibody; however, a reduction in the level of full-length BID was obvious. Similar to the in vitro results, caspase-2 inhibition blocked ER stress-induced cleavage of BID (Fig. 4D). To confirm that the observed effects on BID cleavage were a direct result of caspase-2 inhibition and not an off-target effect of the inhibitor, we transfected DKO MEFs with control siRNA or siRNA directed against caspase-2 (Fig. 4C) and then induced ER stress with BFA. In cells with reduced caspase-2 expression, the level of full-length BID did not decrease after BFA treatment (Fig. 4D). Together, these results strongly indicate that caspase-2 is the primary protease that activates BID following ER stress.
FIG. 4.
Caspase-2 cleaves BID in response to ER stress. (A) S100 extracts from untreated (UNT) and BFA-treated DKO MEFs were precleared (pc) of endogenous BID/tBID and then incubated with recBID with or without pretreatment with the specific caspase-2 inhibitor z-VDVAD-fmk (50 μM). Samples were then immunoblotted for BID. Note that pretreatment with z-VDVAD-fmk blocked BID cleavage. (B) S100 extracts from untreated DKO MEFs and DKO MEFs treated with 2.5 μg/ml of BFA were immunoblotted for caspase-2 (Casp2) and actin. Note that BFA treatment led to caspase-2 cleavage. (C) Following a 24-h transfection of siRNA against caspase-2, immunoblotting of DKO whole-cell lysates showed that caspase-2 expression was substantially reduced. (D) DKO MEFs were left untreated or were pretreated with 50 μM z-VDVAD-fmk (C2 inhibitor), control siRNA, or caspase-2 siRNA (C2 siRNA), followed by treatment with 2.5 μg/ml of BFA for 24 h. Immunoblotting for BID and subsequent quantification by densitometry showed that inhibiting or knocking down caspase-2 protected against BFA-induced cleavage of endogenous full-length BID. α, anti.
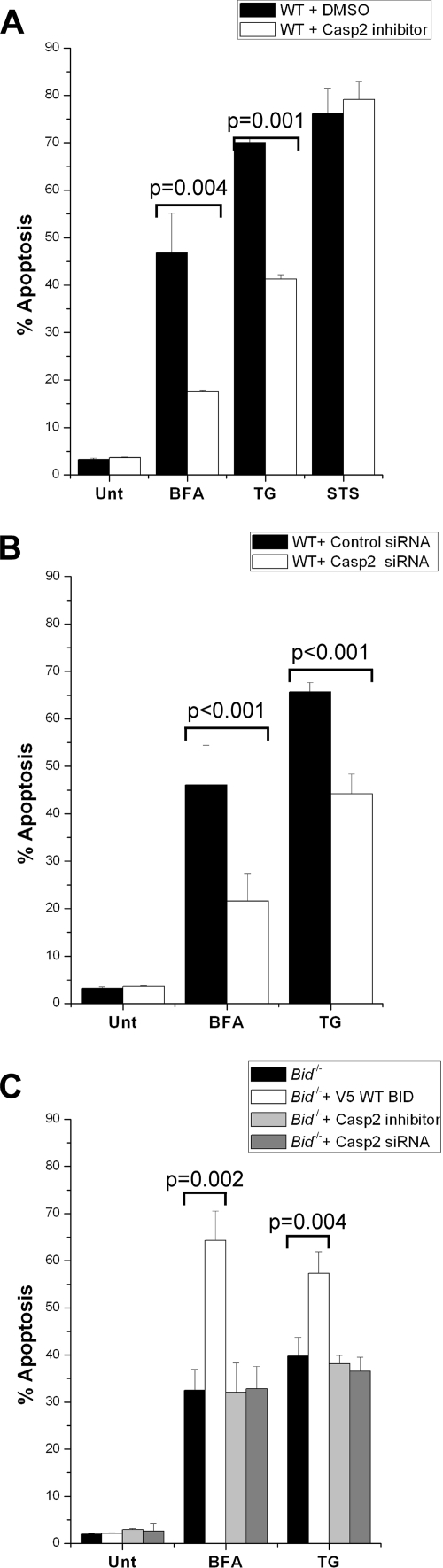
If caspase-2 is critical for linking ER stress to the mitochondrion via BID cleavage, then reducing caspase-2 activity should confer resistance to ER stress-induced apoptosis in a manner similar to genetic loss of BID. To test this, WT MEFs were left untreated or were pretreated with z-VDVAD-fmk and then challenged with BFA, TG, or STS. The cells pretreated with the caspase-2 inhibitor displayed significant resistance to ER stress-induced apoptosis but not to STS, compared to the cells that were not pretreated with z-VDVAD-fmk (Fig. 5A). To rule out nonspecific effects of the inhibitor, we again transiently transfected WT MEFs with either control siRNA or siRNA directed against caspase-2. The transfected cells were then treated with BFA or TG. The cells transfected with control siRNA were significantly more sensitive to ER stress than those treated with caspase-2 siRNA (Fig. 5B). Moreover, expression of V5-tagged WT BID in the Bid−/− MEFs significantly increased their apoptotic response to BFA and TG (Fig. 5C). Importantly, pharmacological inhibition or siRNA knockdown of caspase-2 did not further protect Bid−/− cells against these ER stress agents, suggesting that BID is the major apoptotic target of caspase-2 in response to this form of cell injury (Fig. 5C). Together, these data demonstrate that caspase-2 activity is required for BID processing following ER stress and that activated BID is one of the major signals that link the ER to the mitochondrion.
FIG. 5.
Absence of caspase-2 (Casp2) activity confers resistance to ER stress-induced apoptosis. (A) WT MEFs were pretreated with dimethyl sulfoxide (DMSO) or 50 μM z-VDVAD-fmk (a caspase-2 inhibitor) and challenged with 0.25 μg/ml BFA (24 h), 0.05 μM TG (18 h), or 1.0 μM STS (18 h). Cells were then harvested and analyzed by FACS for annexin V staining. Note that cells pretreated with z- VDVAD-fmk were significantly resistant to the ER stress agents but not to STS. (B) WT MEFs were transfected with either 2 μM control siRNA or caspase-2 siRNA and treated with 0.25 μg/ml of BFA (24 h) or 0.05 μM of TG (18 h). Cells were then harvested and analyzed by FACS for annexin V staining. Note that cells transfected with caspase-2 siRNA were significantly resistant to both of these ER stress agents. (C) Bid−/− MEFs were either pretreated with 50 μM z-VDVAD-fmk (6 h) or transfected with 1 μg V5-tagged WT BID or 2 μM caspase-2 siRNA and then challenged with 0.75 μg/ml of BFA (24 h) or 0.075 μM of TG (18 h). The cells were then harvested and analyzed by FACS for annexin V staining. Note that pharmacological inhibition or siRNA knockdown of caspase-2 did not further protect Bid−/− cells against ER stress agents. The results of all the assays were analyzed via Student's t test (n = 3).
Although BID is known to be important in transducing apoptotic signals for several other death stimuli, we are the first to show that it is a critical mediator of ER stress. Loss of BID increases resistance to ER stress, confirming the importance of BID in apoptotic signaling downstream of this organelle. Nonetheless, Bid−/− cells are not completely resistant to ER stress-induced apoptosis, indicating that BID is not the only signal that can link the ER to the mitochondrial death machinery. Consistent with this finding, depletion of BID from the cytosolic extract of BFA-treated DKO cells only partially reduced the ability of the extract to release cytochrome c from isolated mitochondria in vitro. Given that ER stress conditions are encountered by the most ancient of eukaryotic organisms, it is not surprising that mammalian cells have evolved several pathways to induce apoptosis once the ER is terminally damaged by misfolded proteins. Indeed, it has recently been reported that another BH3-only protein, BIM, can contribute to apoptotic signaling downstream of ER stress (23). However, despite finding increased BIM at mitochondria following BFA treatment, we tested our cytosolic extracts from untreated and BFA-treated DKO cells and found that they contained no detectable BIM. Therefore, it is plausible that other BH3-only proteins (in addition to BID and BIM) can signal from the ER to the mitochondria under certain conditions (10, 13).
Compared to caspase-8, little is known about the activation and function of caspase-2. A role for caspase-2 is best documented in response to genotoxic stress, where it seems to act upstream of mitochondrial permeabilization (11, 24, 29). While there have been hints that caspase-2 may be involved in ER stress (3, 19), we are the first to show that it plays an essential role in this apoptotic pathway and to define its critical target. However, it remains unclear how caspase-2 is activated by ER stress and whether it is downstream of one or more of the stress sensors (IRE1α, ATF6, and PERK) that monitor ER homeostasis. This is a topic we are actively investigating.
Our data offer novel insights into the biochemical links between the ER and the mitochondria during times of ER stress. Given the role of ER stress in the pathogenesis of many prevalent human degenerative disorders, our findings may promote the development of rational antiapoptotic therapeutic strategies to preserve cell viability and function in such diseases.
Acknowledgments
We thank Ronny Drapkin, Barbara Malynn, Averil Ma, and Gerard Evan for scientific advice and encouragement throughout this project. The BAX conditionally deficient and Bid−/− mice were a kind gift from Osamu Takeuchi, Jill Fisher, and the laboratory of the late Stanley J. Korsmeyer of the Dana-Farber Cancer Institute. Andrew Gilmore of the University of Manchester generously provided the wt and mutant Bid constructs. We thank Christine Lin for help in preparing figures for the manuscript.
This work was supported by NIH grants K08 AI054650 (S.A.O.), KO8 DK065671 (F.R.P.), and DP2-OD001925 (F.R.P.), an HHMI Physician-Scientist Early Career Award (S.A.O.), the Steward Trust Foundation (S.A.O.), the Sandler Program in Basic Sciences (S.A.O. and F.R.P.), the Burroughs Wellcome Foundation (F.R.P.), the Juvenile Diabetes Research Foundation (F.R.P.), the Hillblom Foundation (F.R.P.), the Partnership for Cures (F.R.P.), and the UC Cancer Research Coordinating Committee (S.A.O.).
We dedicate this work to the late Stanley J. Korsmeyer (1950-2005), whose mentorship, love of science, and generosity positively influenced so many.
Footnotes
Published ahead of print on 21 April 2008.
REFERENCES
- 1.Bonzon, C., L. Bouchier-Hayes, L. J. Pagliari, D. R. Green, and D. D. Newmeyer. 2006. Caspase-2-induced apoptosis requires Bid cleavage: a physiological role for Bid in heat shock-induced death. Mol. Biol. Cell 172150-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calfon, M., H. Zeng, F. Urano, J. H. Till, S. R. Hubbard, H. P. Harding, S. G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 41592-96. [DOI] [PubMed] [Google Scholar]
- 3.Cheung, H. H., N. Lynn Kelly, P. Liston, and R. G. Korneluk. 2006. Involvement of caspase-2 and caspase-9 in endoplasmic reticulum stress-induced apoptosis: a role for the IAPs. Exp. Cell Res. 3122347-2357. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk, J. E., L. Bouchier-Hayes, and D. R. Green. 2006. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 131396-1402. [DOI] [PubMed] [Google Scholar]
- 5.Credle, J. J., J. S. Finer-Moore, F. R. Papa, R. M. Stroud, and P. Walter. 2005. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 10218773-18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase- activation by eliminating IAP inhibition. Cell 10233-42. [DOI] [PubMed] [Google Scholar]
- 7.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397271-274. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi, T., and T. P. Su. 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131596-610. [DOI] [PubMed] [Google Scholar]
- 9.Hitomi, J., T. Katayama, Y. Eguchi, T. Kudo, M. Taniguchi, Y. Koyama, T. Manabe, S. Yamagishi, Y. Bando, K. Imaizumi, Y. Tsujimoto, and M. Tohyama. 2004. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 165347-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieran, D., I. Woods, A. Villunger, A. Strasser, and J. H. Prehn. 2007. Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc. Natl. Acad. Sci. USA 10420606-20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassus, P., X. Opitz-Araya, and Y. Lazebnik. 2002. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 2971352-1354. [DOI] [PubMed] [Google Scholar]
- 12.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase- 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94491-501. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., B. Lee, and A. S. Lee. 2006. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J. Biol. Chem. 2817260-7270. [DOI] [PubMed] [Google Scholar]
- 14.Lin, J. H., P. Walter, and T. S. Yen. 2008. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 3399-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsten, T., A. J. Ross, A. King, W. X. Zong, J. C. Rathmell, H. A. Shiels, E. Ulrich, K. G. Waymire, P. Mahar, K. Frauwirth, Y. Chen, M. Wei, V. M. Eng, D. M. Adelman, M. C. Simon, A. Ma, J. A. Golden, G. Evan, S. J. Korsmeyer, G. R. MacGregor, and C. B. Thompson. 2000. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 61389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86147-157. [DOI] [PubMed] [Google Scholar]
- 17.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94481-490. [DOI] [PubMed] [Google Scholar]
- 18.Marciniak, S. J., and D. Ron. 2006. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 861133-1149. [DOI] [PubMed] [Google Scholar]
- 19.Murakami, Y., E. Aizu-Yokota, Y. Sonoda, S. Ohta, and T. Kasahara. 2007. Suppression of endoplasmic reticulum stress-induced caspase- activation and cell death by the overexpression of Bcl-xL or Bcl-2. J. Biochem. (Tokyo) 141401-410. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa, T., H. Zhu, N. Morishima, E. Li, J. Xu, B. A. Yankner, and J. Yuan. 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 40398-103. [DOI] [PubMed] [Google Scholar]
- 21.Oakes, S. A., S. S. Lin, and M. C. Bassik. 2006. The control of endoplasmic reticulum-initiated apoptosis by the BCL-2 family of proteins. Curr. Mol. Med. 699-109. [DOI] [PubMed] [Google Scholar]
- 22.Obeng, E. A., and L. H. Boise. 2005. Caspase-12 and caspase-4 are not required for caspase-dependent endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 28029578-29587. [DOI] [PubMed] [Google Scholar]
- 23.Puthalakath, H., L. A. O'Reilly, P. Gunn, L. Lee, P. N. Kelly, N. D. Huntington, P. D. Hughes, E. M. Michalak, J. McKimm-Breschkin, N. Motoyama, T. Gotoh, S. Akira, P. Bouillet, and A. Strasser. 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 1291337-1349. [DOI] [PubMed] [Google Scholar]
- 24.Robertson, J. D., M. Enoksson, M. Suomela, B. Zhivotovsky, and S. Orrenius. 2002. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 27729803-29809. [DOI] [PubMed] [Google Scholar]
- 25.Ron, D., and P. Walter. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8519-529. [DOI] [PubMed] [Google Scholar]
- 26.Scorrano, L., S. A. Oakes, J. T. Opferman, E. H. Cheng, M. D. Sorcinelli, T. Pozzan, and S. J. Korsmeyer. 2003. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300135-139. [DOI] [PubMed] [Google Scholar]
- 27.Strasser, A. 2005. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5189-200. [DOI] [PubMed] [Google Scholar]
- 28.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397441-446. [DOI] [PubMed] [Google Scholar]
- 29.Tinel, A., and J. Tschopp. 2004. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304843-846. [DOI] [PubMed] [Google Scholar]
- 30.Verhagen, A. M., P. G. Ekert, M. Pakusch, J. Silke, L. M. Connolly, G. E. Reid, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 10243-53. [DOI] [PubMed] [Google Scholar]
- 31.Wang, K., X. M. Yin, D. T. Chao, C. L. Milliman, and S. J. Korsmeyer. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 102859-2869. [DOI] [PubMed] [Google Scholar]
- 32.Wei, M. C., T. Lindsten, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 142060-2071. [PMC free article] [PubMed] [Google Scholar]
- 33.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin, X. M. 2006. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene 3697-19. [DOI] [PubMed] [Google Scholar]