Abstract
Stimulation through the interleukin-1 receptor (IL-1R) and some Toll-like receptors (TLRs) induces ubiquitination of TRAF6 and IRAK-1, signaling components required for NF-κB and mitogen-activated protein kinase activation. Here we show that although TRAF6 and IRAK-1 acquired Lys63 (K63)-linked polyubiquitin chains upon IL-1 stimulation, only ubiquitinated IRAK-1 bound NEMO, the regulatory subunit of IκB kinase (IKK). The sites of IRAK-1 ubiquitination were mapped to Lys134 and Lys180, and arginine substitution of these residues impaired IL-1R/TLR-mediated IRAK-1 ubiquitination, NEMO binding, and NF-κB activation. K63-linked ubiquitination of IRAK-1 required enzymatically active TRAF6, indicating that it is the physiologically relevant E3. Thus, K63-linked polyubiquitination of proximal signaling proteins is a common mechanism used by diverse innate immune receptors for recruiting IKK and activating NF-κB.
Interleukin 1 (IL-1) is a proinflammatory cytokine that promotes innate immune responses by inducing the production of chemotactic factors, acute phase proteins, and other proinflammatory cytokines (for review, see reference 7). These mediators coordinate an array of biological effects that include lymphocyte activation, leukocyte entry into sites of injury or infection, and fever. IL-1 is primarily produced by macrophages and monocytes in response to a variety of stimuli, most notably Toll-like receptor (TLR) ligands, which are components of pathogenic organisms (1). Although classically known for promoting the innate immune response, TLR ligands also promote the adaptive immune response (41).
Despite differences in their extracellular domains, the IL-1 receptor (IL-1R) and TLRs contain a common cytoplasmic motif termed the Toll/IL-1R (TIR) homology domain, which is required for activation of mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways (29). With few exceptions, IL-1R- and TLR-mediated MAPK and NF-κB activation is initiated by the recruitment of the death domain-containing adaptor myeloid differentiation factor 88 (MyD88) to the TIR (3, 26, 31, 49). MyD88 is a scaffold protein that recruits an array of molecules, including the death domain-containing protein IL-1R-associated serine/threonine kinase 1 (IRAK-1), IRAK-2, IRAK-4, and the ubiquitin protein ligase (E3) tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) (for review, see references 1 and 11). IRAK-4 and TRAF6 are essential signaling components of IL-1R- and TLR-mediated MAPK and NF-κB activation, but the means by which they function are not entirely clear (24, 27, 43). IRAK-1 also plays an important role in IL-1R/TLR signaling, although there is a small amount of NF-κB activation in its absence (13, 44, 46). MyD88-independent NF-κB activation is limited to signaling through TLR3 and TLR4, occurs with slower kinetics, and requires the proximal adaptor protein TIR domain-containing adaptor inducing IFN-β/TIR-containing adaptor molecule 1 (50).
IκB kinase (IKK), a key intermediate in cytokine- and TLR ligand-induced NF-κB activation, phosphorylates IκB, the cytosolic inhibitor of NF-κB (36). Phosphorylated IκB is modified with Lys48 (K48)-linked polyubiquitin chains and degraded by proteasomes. IKK contains two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (NEMO). Although possessing no enzymatic activity, NEMO is required for IKK activity and, thus, NF-κB activation in most circumstances (37, 40). We found that NEMO binds to K63-linked polyubiquitin chains through its coiled-coil 2 (CC2) and leucine zipper (LZ) regions. This activity was required for TNF-α-induced recruitment of IKK to the TNF receptor 1 (TNFR1) signaling intermediate RIP and subsequent NF-κB activation (9, 20, 52). The requirement for NEMO to recognize polyubiquitinated signaling intermediates in pathways leading to IKK activation has recently been extended to T-cell receptor signaling (28, 51).
The prevailing model dealing with the linkage of IL-1R/TLR ligation to IKK and NF-κB activation is that following IL-1R/TLR engagement MyD88 binds to the cytoplasmic tail of the receptor, leading to recruitment of IRAK-4 and IRAK-1 (3, 4, 18, 34, 49). IRAK-1 is then phosphorylated (2, 21, 33), resulting in recruitment of TRAF6 (12). Phosphorylated IRAK-1 and TRAF6 are thought to dissociate from the receptor (45), which is followed by TRAF6 autoubiquitination with K63-linked polyubiquitin chains (19, 48). TAK1-binding protein 2 (TAB2) and/or TAB3 binds ubiquitinated TRAF6, which activates transforming growth factor-β-activated kinase (TAK1) (14). Activated TAK1 phosphorylates IKK and MAPK kinase 6 (MKK6) to activate the NF-κB and MAPK signaling pathways (48). Interestingly, the kinase activity of IRAK-1 is dispensable for IL-1-induced NF-κB and MAP kinase activation, unlike that for IRAK-4 (17, 21-23, 25). Thus, IRAK-1 is believed to act primarily as an adaptor for TRAF6.
We have investigated the role of ubiquitination in IL-1R/TLR proximal signaling and have identified a striking analogy with that of TNFR1 and the T-cell antigen receptor. In this case, IL-1 and TLR ligands induce TRAF6-mediated K63-linked polyubiquitination of IRAK-1, which is required for IKK recruitment and activation.
MATERIALS AND METHODS
Cell lines, plasmids and reagents.
293 cells stably expressing IL-1R, TLR4, and MD2 (293/IL-1R/TLR4) were obtained from Xiaoxia Li (Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, OH). NEMO-deficient murine embryonic fibroblasts (MEFs) were provided by M. Schmidt-Supprian (Harvard University, Boston, MA). NEMO-deficient MEFs reconstituted with wild-type NEMO or NEMOL329P have been described previously (52). Immortalized IRAK-1-deficient MEFs have been described elsewhere (46). TRAF6-deficient MEFs were obtained from Jun-ichiro Inoue (Keio University, Yokohama, Japan). Phoenix Eco cells were obtained from Gary Nolan (Stanford University, Stanford, CA). All adherent cell lines were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM β-mercaptoethanol. Human IRAK-1 cDNA was obtained from Liwu Li (Wake Forest University, Winston-Salem, NC) and subcloned into the Gateway system (Invitrogen) by PCR using the primers 5′-CACCATGGCCGGGGGGCCGGGC-3′ and 5′-TCAGCTCTGAAATTCATCACTTTCTTCGGG-3′. IRAK-1K134R, IRAK-1K180R, and IRAK-1K134/180R were generated by substituting the lysine residues at amino acid positions 134 and 180 with arginine (R) using the QuikChange XL site-directed mutagenesis kit (Stratagene). The mutagenic primers were K134R sense (5′-GGAGCCCCCGGAGGTTGCCATCCTC-3′), K134R antisense (5′-GAGGATGGCAACCTCCGGGGGCTCC-3′), K180R sense (5′-CCTTCTTCTACCAGGCCAGGCCCAGAG-3′), and K180R antisense (5′-CTCTGGGCCTGGCCTGGTAGAAGAAGG-3′), and the substitutions were confirmed by direct sequencing. IRAK-1 and the lysine-to-arginine mutants were subcloned into pDEST-515 (Cloning and Optimization Group, NCI-Frederick), pcDNA3.1 nV5 (Invitrogen), and pMSCV-puro (BD Clontech Laboratories Inc.) that had been converted to a Gateway vector (pMSCV-puro ccdB) using the Gateway vector conversion system (Invitrogen). Murine TRAF6 cDNA was subcloned into the Gateway system using the primers 5′-CACCATGAGTCTCTTAAACTGTGAGAACAGCTGC-3′ and 5′CTACACCCCCGCATCAGTACTTCGTG-3′. TRAF6C70A was generated by substituting cysteine (C) at amino acid position 70 with alanine (A) using the QuikChange XL site-directed mutagenesis kit and the primers 5′-CCTCTGGAGAGCAAGTATGAGGCTCCCATCTGCTTGATGGCTTTACG-3′ and 5′-CGTAAAGCCATCAAGCAGATGGGAGCCTCATACTTGCTCTCCAGAGG-3′. The C70A mutation was confirmed by direct sequencing. TRAF6 and TRAF6C70A were subcloned into pMSCV-puro ccdB and pDEST-15 (Cloning and Optimization Group, NCI-Frederick). pGEX-4T2-NEMO, pGEX-4T2-NEMOL329P, pMSCV-HA-NEMO, and pMSVC-HA-NEMOL329P have been described elsewhere (52). pRK5-HA-tagged ubiquitin and pRK5-HA-tagged K63-only ubiquitin (a mutant in which all the lysines except residue 63 have been replaced with arginine) were obtained from Ted Dawson (Johns Hopkins University, Baltimore, MD). pcDNA3.1/His/LacZ was obtained from Invitrogen, and pNF-κB luc was obtained from Clontech. Human and mouse IL-1β were purchased from R&D Systems. pREP4-groESL was obtained from Philip Cole (Johns Hopkins University, Baltimore, MD). Lipopolysaccharide (LPS; 0111:B4) was purchased from Calbiochem. The anti-IκB polyclonal antibody (C-21), anti-IRAK-1 polyclonal antibody (H-273), anti-IRAK-1 monoclonal antibody (MAb F-4), antiubiquitin (anti-Ub) MAb (P4D1), anti-TRAF6 MAb (D-10), anti-TRAF6 polyclonal antibody (H-274), and anti-NEMO polyclonal antibody (FL-419) were purchased from Santa Cruz Biotechnology. The anti-TRAF6 (CT) polyclonal antibody was purchased from Biochain Institute. The anti-NEMO MAb (C73-764) was purchased from BD Pharmingen. The anti-phospho-p38 polyclonal antibody (Thr 180/Tyr 182) and anti-p38 MAb (5F11) were obtained from Cell Signaling Technology. The antihemagglutinin (anti-HA) MAb (clone 12CA5) was purchased from Roche Applied Science. The anti-β-actin MAb (AC-15) was purchased from Sigma. The sheep anti-mouse immunoglobulin (Ig) horseradish peroxidase-linked polyclonal antibody (NXA931) and donkey anti-rabbit IgG horseradish peroxidase-linked polyclonal antibody (NA934V) were purchased from Amersham Biosciences. Alexa Fluor 680 goat anti-mouse IgG was purchased from Molecular Probes. Protein A-conjugated Sepharose 4B (protein A beads) was purchased from Sigma. Glutathione-Sepharose 4B (GSH beads) was purchased from Amersham Pharmacia. The rabbit E1, Ubc13/Uev1A, and K63-only mutant ubiquitin were purchased from Boston Biochem. The mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit was purchased from BD Biosciences.
Transient transfections and NF-κB-driven luciferase reporter assays.
The lysine 63-linked polyubiquitination of IRAK-1 was determined by transiently transfecting 293/IL-1R/TLR4 cells with pRK5-HA-tagged K63-only ubiquitin using Lipofectamine 2000 (Invitrogen). Twenty-four h later the cells were stimulated with human IL-1, washed with phosphate-buffered saline, and lysed. NF-κB transcriptional activity was assessed by transiently transfecting 293/IL-1R/TLR4 cells with pNF-κB luc, pcDNA3.1/His/LacZ, pDEST515, or pDEST515 containing IRAK-1, IRAK-1K134R, IRAK-1K180R, or IRAK-1K134/180R cDNA and using Lipofectamine 2000 for 24 h. The cells were lysed in 100 μl of luciferase lysis buffer (25 mM Tris pH 7.8, 2 mM dithiothreitol, 10% glycerol, 1 mM EDTA, and 1% Triton X-100), and NF-κB luciferase activity was detected in 10 μl of lysate after the addition of 100 μl of luciferase substrate as described elsewhere (32). β-Galactosidase activity was determined using the β-Gal kit (Invitrogen). To assess NF-κB transcriptional activity in IRAK-1-deficient MEFs, the cells were transfected with pNF-κB luc, pDEST515, or pDEST515 containing IRAK-1, IRAK-1K134R, IRAK-1K180R, or IRAK-1K134/180R cDNA using Lipofectamine 2000 for 36 h. The cells were stimulated with IL-1 (10 ng/ml) and LPS (10 μg/ml) for 5 h and lysed, and NF-κB luciferase activity was determined as described above. For experiments involving NEMO-deficient MEFs, the cells were transiently transfected with pNF-κB luc, pcDNA3.1/His/LacZ, and pMSCV, pMSCV-HA-NEMO, or pMSCV-HA-NEMOL329P using the MEF1 kit (Amaxa Inc.) and, 20 h later, treated with IL-1 or LPS and lysed. Luciferase was normalized to β-galactosidase activity as described above.
Electrophoretic mobility shift assay and IL-6 ELISA.
NF-κB DNA binding was assessed by electrophoretic mobility shift using 4 μg of nuclear extracts prepared from MEFs and an [α-32P]dCTP-end-labeled double-stranded oligonucleotide as described elsewhere (35, 42, 47). To determine IL-6 production, equal amounts of wild-type, TRAF6-deficient, and TRAF6-deficient MEFs reconstituted with TRAF6 or TRAF6-C70A were plated and 12 h later stimulated with 1 ng/ml IL-1 or 10 μg/ml LPS for 48 h. IL-6 production in the supernatants was quantified by ELISA.
Retroviral transduction.
TRAF6-deficient and IRAK-1-deficient MEFs were infected for 48 h with viral supernatant from Phoenix Eco cells transfected with pMSCV-puro ccdB containing the indicated cDNAs. TRAF6-deficient cells stably expressing TRAF6 or TRAF6C70A were selected and maintained in medium containing puromycin (1 μg/ml).
Immunoblotting and immunoprecipitation.
Cells treated with or without IL-1 or LPS were lysed in lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 30 mM NaF, 2 mM sodium pyrophosphate supplemented with protease inhibitor cocktail [Roche], and 1 mM N-ethylmaleimide). Lysates were normalized to protein concentration, denatured in sample buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with the appropriate antibodies. Immunoblots were visualized using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce) or fluorescently conjugated secondary antibodies and the Odyssey imaging system (Li-Cor Biosciences). For immunoprecipitations and coimmunoprecipitations, lysates were normalized to protein concentration and incubated with the appropriate polyclonal antibodies and protein A beads at 4°C for 16 h. After extensive washing, the bead-bound complexes were washed extensively with lysis buffer, eluted in sample buffer, resolved by SDS-PAGE, and immunoblotted with the appropriate monoclonal antibodies. In some experiments, noncovalent protein-protein interactions were disrupted by heating the lysates at 95°C in the presence of 1% SDS and diluted with lysis buffer to a final concentration of 0.1% SDS. The proteins were immunoprecipitated with the appropriate polyclonal antibodies at 23°C for 3 to 4 h. The bead-bound complexes were washed extensively, eluted, resolved by SDS-PAGE, and immunoblotted with the appropriate monoclonal antibodies.
In vitro protein binding.
Glutathione S-transferase (GST) proteins were expressed in DH5α Escherichia coli and purified from bacterial lysates using GSH beads. Purified GST proteins were incubated with cell lysates for 16 h at 4°C. The bead-bound complexes were washed extensively with lysis buffer, eluted with sample buffer, resolved by SDS-PAGE, and immunoblotted with the appropriate antibodies.
In vitro ubiquitination.
GST-TRAF6 was coexpressed in BL21(DE3) E. coli with the chaperone groESL to increase its solubility and purified from bacterial lysates using GSH beads. 35S-labeled IRAK-1 was in vitro translated using the TNT Quick Coupled transcription/translation system (Promega), incubated with GST-TRAF6- or GST-TRAF6-C70A-coated beads, rocked for 2 h at 4°C in GST binding buffer (120 mM NaCl, 10% glycerol, 1% Triton X-100, 50 mM Tris pH 7.5), and washed extensively. Translation of IRAK-1 required the addition of its cDNA to the reticulocyte mixture, and no binding was apparent when 35S-labeled IRAK-1 was offered to beads coated with GST alone. To ubiquitinate IRAK-1, the bead-bound complexes were incubated in ubiquitination buffer (50 mM Tris pH 7.5, 2.5 mM MgCl2, 0.5 mM dithiothreitol, 0.05% NP-40) containing rabbit E1 (50 ng), Ubc13/Uev1A (200 ng of each), ubiquitin or K63-only ubiquitin (10 μg), and ATP (2 mM) for 1.5 h at 30°C. The reactions were stopped with the addition of SDS-containing sample buffer followed by heating at 95°C for 5 min. The eluted material was resolved by SDS-PAGE and transferred to nitrocellulose membranes, and 35S-labeled IRAK-1 was visualized with a Storm PhosphorImager (Molecular Dynamics).
RESULTS
NEMO binds ubiquitinated IRAK-1 but not TRAF6.
TRAF6 and IRAK-1 are rapidly ubiquitinated following IL-1R engagement (19, 22, 48, 53). Although TRAF6 can autoubiquitinate to form K63-linked polyubiquitin chains in vitro (48), whether this occurs in vivo has not been demonstrated. Given the importance of ubiquitination in IL-1 signaling (19), we asked if TRAF6 and IRAK-1 polyubiquitin chains are K63-linked and, if so, are bound by NEMO. To determine this, 293 cells stably expressing IL-1R, TLR4, and the TLR-associated protein, MD2 (293/IL-1R/TLR4) were transfected with a HA-tagged ubiquitin mutant in which all the lysines except residue 63 had been replaced with arginine. The cells were stimulated with IL-1 for the indicated periods, and detergent lysates were prepared. To disrupt noncovalent protein-protein interactions, the lysates were heated in the presence of 1% SDS and diluted with lysis buffer, and IRAK-1 or TRAF6 were immunoprecipitated and immunoblotted with anti-HA (Fig. 1A). K63-linked polyubiquitinated IRAK-1 (Fig. 1A, left panel) and TRAF6 (Fig. 1A, right panel) were evident within 2.5 min of stimulation and persisted for up to 20 min. Because NEMO is required for NF-κB activation downstream of IL-1 (37, 40) and preferentially binds K63- compared to K48-linked polyubiquitin (52), we asked if NEMO binds to K63-linked polyubiquitinated IRAK-1 and/or TRAF6 from stimulated cells. IRAK-1 and TRAF6 were either immunoprecipitated or pulled down with GST-NEMO from cell lysates prepared from untreated or IL-1-treated 293/IL-1R/TLR4 cells and detected by immunoblotting (Fig. 1B). Surprisingly, although stimulation with IL-1 resulted in ubiquitination of both IRAK-1 and TRAF6 (Fig. 1B, left panels), GST-NEMO bound only to ubiquitinated IRAK-1 (Fig. 1B, right panels). There was no appreciable binding of GST-NEMO to unubiquitinated TRAF6 (data not shown) or IRAK-1 (Fig. 1B and 2A). The same specificity was found when endogenous NEMO was immunoprecipitated from IL-1-stimulated cells: ubiquitinated IRAK-1 but not TRAF6 was coimmunoprecipitated (Fig. 1C). Therefore, although both TRAF6 and IRAK-1 undergo K63-linked polyubiquitination following IL-1 stimulation, NEMO binds only to ubiquitinated IRAK-1.
FIG. 1.
NEMO binds ubiquitinated IRAK-1 but not ubiquitinated TRAF6. (A) 293/IL-1R/TLR4 cells transfected with HA-tagged K63-only ubiquitin were stimulated or not with IL-1 for the indicated amounts of time, harvested, and lysed with detergent. The lysates were heated at 95°C in the presence of 1% SDS for 5 min and diluted to a final concentration of 0.1% SDS, and IRAK-1 and TRAF6 were immunoprecipitated (IP). The amounts of K63-linked polyubiquitinated IRAK-1 and TRAF6 were determined by immunoblotting (IB) with anti-HA. The amounts of immunoprecipitated IRAK-1 and TRAF6 were determined by immunoblotting with anti-IRAK-1 and anti-TRAF6. (B) 293/IL-1R/TLR4 cells were stimulated with or without IL-1 for 5 min, harvested, and lysed. Lysates were either heated as for panel A and immunoprecipitated with anti- IRAK-1 or anti-TRAF6 or they were incubated with GST- or GST-NEMO-coated beads. The eluted material and lysates were immunoblotted with anti-Ub (left panels) or anti-IRAK-1 and anti-TRAF6 (right panels). (C) NEMO was immunoprecipitated from cell lysates made from 293/IL-1R/TLR4 cells stimulated with or without IL-1 for 5 min and immunoblotted for IRAK-1, TRAF6, and NEMO. IRAK-1 and TRAF6 in the lysates were detected by immunoblotting with anti-IRAK-1 and anti-TRAF6. (D) NEMO was immunoprecipitated from lysates made from 293/IL-1R/TLR4 cells that had been stimulated with LPS for the indicated times and immunoblotted for IRAK-1 and NEMO.
FIG. 2.
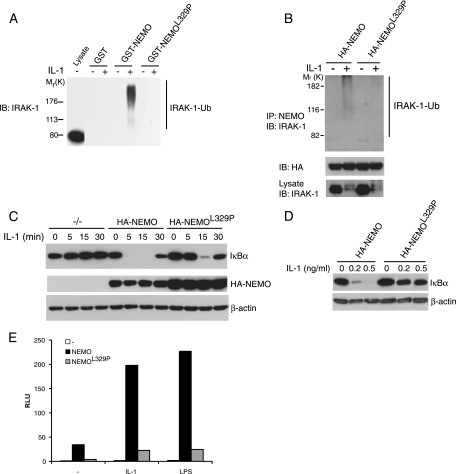
K63-linked polyubiquitin binding of NEMO is required for IRAK-1 binding and IL-1-induced IκB degradation. (A) Cell lysates from 293/IL-1R/TLR4 cells that had been stimulated with or without IL-1 (10 ng/ml) for 5 min were incubated with beads coated with GST, GST-NEMO, or GST-NEMOL329P and immunoblotted (IB) with anti-IRAK-1. (B) NEMO was immunoprecipitated (IP) from cell lysates from NEMO-deficient MEFs stably reconstituted with HA-tagged NEMO (HA-NEMO) or HA-tagged NEMOL329P (HA- NEMOL329P) that had been stimulated with or without IL-1 (5 ng/ml) for 5 min and immunoblotted with anti-IRAK-1 or anti-HA. (C) NEMO-deficient MEFs (-/-) or NEMO-deficient MEFs reconstituted with HA-NEMO or HA-NEMOL329P were stimulated with IL-1 (1 ng/ml) for the indicated periods of time. The cells were harvested and lysed, and the amounts of IκB and NEMO were determined by immunoblotting. (D) HA-NEMO or HA-NEMOL329P cells were stimulated with 0.2 or 0.5 ng/ml IL-1 for 10 min, harvested, and lysed, and the amount of IκB was determined by immunoblotting. The lanes were reordered for clarity. (E) IL-1- and LPS-induced NF-κB luciferase activity as measured by relative light units (RLU) in NEMO-deficient MEFs transiently transfected with vector (-), NEMO, or NEMOL329P.
TLR ligand-induced NF-κB activation requires IRAK-1 (44). To determine if IRAK-1 ubiquitination and binding to NEMO is also a feature of TLR signaling, 293/IL-R/TLR4 cells were stimulated with LPS (TLR4 ligand). Within 10 min of the addition of LPS, ubiquitinated IRAK-1 was coimmunoprecipitated with NEMO, an association that persisted for up to 80 min (Fig. 1D). Thus, NEMO binding to polyubiquitinated IRAK-1 is common to both IL-1R- and TLR-mediated signaling pathways.
The CC2-LZ region of NEMO is required for IL-1-induced interaction with IRAK-1 and NF-κB activation.
Given that after stimulation NEMO bound only ubiquitinated IRAK-1 (Fig. 1), the role of the ubiquitin-binding CC2-LZ region of NEMO was determined. Beads coated with GST-NEMO or GST-NEMOL329P, an LZ mutant with severely impaired K63-linked polyubiquitin-binding activity (52), were used to pull down IRAK-1 from IL-1-stimulated cells (Fig. 2A). GST-NEMO bound to ubiquitinated IRAK-1 in lysates from IL-1-treated 293/IL-1R/TLR4 cells, but GST- NEMOL329P did not. The ubiquitin-binding activity of NEMO was also required for binding to IRAK-1 in cells, because in IL-1-stimulated NEMO-deficient MEFs stably reconstituted with NEMO or NEMOL329P, ubiquitinated IRAK-1 was coimmunoprecipitated with NEMO but not NEMOL329P (Fig. 2B).
To evaluate the relevance of the NEMO-IRAK-1 interaction to IKK activation, IL-1-induced IκB degradation was assessed in NEMO- and NEMOL329P-expressing MEFs. Consistent with previous reports (37, 40), IL-1 stimulation did not cause IκB degradation in the absence of NEMO (Fig. 2C). Cells in which NEMO was introduced responded with complete IκB degradation within 5 min, with the level partially returning to resting levels by 30 min. In contrast, IL-1-induced IκB degradation in cells expressing NEMOL329P was not evident until 15 min and was incomplete. Dose-response analysis showed that NEMOL329P-expressing cells were also much less sensitive to IL-1 than cells expressing NEMO (Fig. 2D). The failure of NEMOL329P to support normal IKK activation was reflected in the lack of IL-1- or LPS-induced NF-κB transcriptional activity in NEMOL329P-expressing cells (Fig. 2E). The ubiquitin-binding activity of NEMO is therefore important for IL-1R/TLR-mediated NF-κB activation.
TRAF6 is required for K63-linked polyubiquitination of IRAK-1.
The RING domain-containing E3 TRAF6 is required for IL-1R/TLR-mediated NF-κB activation (24). Because TRAF6 binds IRAK-1 through an interaction of the TRAF domain of the former and three C-terminal binding sites in the latter (5, 23, 55), we considered the possibility that TRAF6 is the E3 that catalyzes the K63-linked polyubiquitination of IRAK-1. To address this, in vitro-translated IRAK-1 was bound to GST-TRAF6-coated beads and in vitro ubiquitination reactions were performed with the E2 Ubc13/Uev1a, which catalyzes the formation of K63-linked polyubiquitin chains (6). TRAF6 ubiquitinated IRAK-1 in the presence of ATP and Ubc13/Uev1a, but not in the absence of either (Fig. 3A). Ubiquitination of IRAK-1 did not occur when E3-defective TRAF6 containing a point mutation in the RING domain was used (see Fig. S1A in the supplemental material). Because binding to GST-NEMO is a sensitive method for detecting K63-linked polyubiquitin (52), GST-NEMO was used to pull down ubiquitinated IRAK-1 from untreated and IL-1-treated wild-type and TRAF6-deficient cells. Beads coated with GST-NEMO bound ubiquitinated IRAK-1 from stimulated wild-type but not TRAF6-deficient cells (Fig. 3B). Consistent with this, NEMO immunoprecipitated from IL-1-treated wild-type cells brought down ubiquitinated IRAK-1, whereas NEMO immunoprecipitated from TRAF6-deficient cells did not (Fig. 3C). When IRAK-1 ubiquitination was directly assessed, IL-1 was found to induce abundant high-molecular-weight polyubiquitinated material in wild-type cells but only low-molecular-weight material in the absence of TRAF6 (see Fig. S1B in the supplemental material). It is noteworthy that the reduction in unmodified levels of IRAK-1 was similar in wild-type and TRAF6-deficient cells following IL-1 treatment (Fig. 3B and C), indicating that TRAF6 is required for IRAK-1 polyubiquitination and NEMO binding but not recruitment to the cytoplasmic tail of IL-1R.
FIG. 3.
TRAF6 E3 activity is required for K63-linked polyubiquitination of IRAK-1. (A) GST-TRAF6-coated beads were incubated with in vitro-translated and 35S-labeled IRAK-1, washed extensively, and incubated in the presence or absence of ATP, and/or Ubc13/Uev1A, and ubiquitin. IRAK-1 ubiquitination was detected using a phosphorimager. No IRAK-1 binding was detected with beads coated with GST alone. (C) Cell lysates from wild-type (WT) or TRAF6-deficient (T6-/-) MEFs that were stimulated with or without IL-1 for 5 min were incubated with GST-NEMO-coated beads and immunoblotted (IB) with anti-IRAK-1. (C) NEMO was immunoprecipitated (IP) from lysates of WT or TRAF6-deficient MEFs that had been stimulated with or without IL-1 for 5 min and immunoblotted with anti-IRAK-1 and anti-NEMO. (D) Cell lysates from T6−/− MEFs or T6−/− MEFs reconstituted with wild-type TRAF6 (T6-WT) or TRAF6C70A (T6-C70A) were immunoblotted for TRAF6. (E) Lysates made from unstimulated or IL-1-stimulated T6−/−, T6-WT, or T6-C70A MEFs were incubated with GST-NEMO-coated beads and immunoblotted for IRAK-1. (F) IL-1-induced NF-κB DNA binding in T6−/−, T6-WT, and T6-C70A MEFs was assessed in an electrophoretic mobility shift assay. (G) IL-1-induced p38 dual phosphorylation (p-p38) was measured in lysates of T6−/−, T6-WT, and T6-C70A MEFs by immunoblotting.
To determine whether the E3 activity of TRAF6 is required for K63-linked polyubiquitination of IRAK-1 in vivo, GST-NEMO was used to pull down IRAK-1 from cell lysates of IL-1-treated TRAF6-deficient MEFs stably reconstituted with wild-type TRAF6 or an E3-inactive RING mutant, TRAF6C70A (19) (Fig. 3D). IL-1 stimulation induced K63-linked polyubiquitination of IRAK-1 in TRAF6-expressing cells, as judged by NEMO binding (Fig. 3E). In contrast, little if any ubiquitinated IRAK-1 was detected in IL-1-stimulated TRAF6C70A-expressing cells. Consistent with this, whereas TRAF6 restored high-molecular-weight polyubiquitination of IRAK-1 in IL-1-stimulated cells, TRAF6C70A failed to do so (see Fig. S1A in the supplemental material). Furthermore, TRAF6C70A cells had little if any IL-1-induced NF-κB activation (Fig. 3F), p38 phosphorylation (Fig. 3G), or IL-6 production (see Fig. S1C in the supplemental material). These results indicate that TRAF6 is the physiologic E3 for IRAK-1 and that its enzymatic activity is required for downstream signaling.
K63-linked polyubiquitination of human IRAK-1 occurs on lysines 134 and 180.
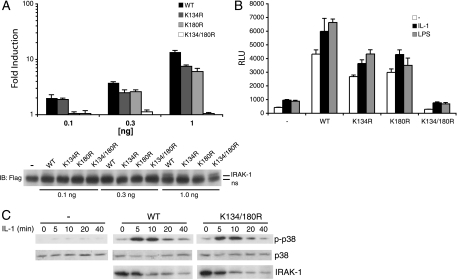
The intermediate domain of IRAK-1 is required for IL-1-induced NF-κB but not MAP kinase activation (23). Given that TRAF6 binds and ubiquitinates IRAK-1, that IRAK-1 shares some organizational similarity with RIP (a death domain, intermediate domain [although it has no significant homology with IRAK-1], and a kinase domain), and that the critical lysine residue for RIP K63-linked polyubiquitination is located within its intermediate domain (9, 20), we assessed the possible contribution of the only conserved lysine residues in the intermediate domain of IRAK-1, K134 and K180, to its K63-linked ubiquitination by TRAF6. IRAK-1 and mutants in which K134 and/or K180 was replaced with arginine (IRAK-1K134R, IRAK-1K180R, and IRAK-1K134/180R) were in vitro translated, bound to GST-TRAF6-coated beads, and ubiquitinated in the presence of Ubc13/Uev1a and a K63-only mutant of ubiquitin (Fig. 4A). Wild-type IRAK-1 was ubiquitinated to a small degree in the absence of K63-only ubiquitin, presumably due to ubiquitin present in the reticulocyte lysate. This level was substantially increased, both in terms of a discrete ubiquitin ladder and the appearance of high-molecular-weight material, in the presence of exogenous K63-only ubiquitin (compare lanes 5 and 6). Mutation of K134 reduced IRAK-1 ubiquitination to levels only slightly higher than when no K63-only ubiquitin was offered, whereas there was little obvious reduction in K180R ubiquitination. Mutation of both K134 and K180 eliminated all but the monoubiquitinated K63-only species, indicating that although K134 is predominant, at least in vitro, both lysines are in fact sites of ubiquitination.
FIG. 4.
IRAK-1 lysines 134 and 180 are required for TRAF6-mediated and IL-1-induced ubiquitination. (A) In vitro ubiquitination of in vitro-translated and 35S-labeled IRAK-1 (WT), IRAK-1K134R (K134R), IRAK-1K180R (K180R), or IRAK-1K134/180R (K134/180R) by GST-TRAF6 in the presence of ATP, Ubc13/Uev1A, and K63-only ubiquitin (K63O). Lanes 1 to 4 are the in vitro-translated products offered to the GST-TRAF6-coated beads. The ubiquitination reactions are shown in lanes 5 to 12. (B) IRAK-1 was either immunoprecipitated (IP) with anti-IRAK-1 or pulled down with GST-NEMO-coated beads from lysates of unstimulated and IL-1-stimulated IRAK-1-deficient MEFs retrovirally transduced with vector (-), WT, K134R, K180R, or K134/180R. IRAK-1 immunoprecipitates were immunoblotted (IB) with anti-Ub, and GST-NEMO-bound material was blotted with anti-IRAK-1. Expression of IRAK-1 and β-actin in the lysates is shown in the bottom two rows.
To determine whether these lysines are required for IL-1-induced IRAK-1 ubiquitination and NEMO binding in vivo, IRAK-1-deficient MEFs were transduced with retroviruses encoding IRAK-1 and the different lysine-to-arginine mutants. After treatment with IL-1, IRAK-1 and the different mutants were immunoprecipitated or pulled down with GST-NEMO-coated beads and immunoblotted with antiubiquitin or anti-IRAK-1, respectively (Fig. 4B). Wild-type IRAK-1 was heavily ubiquitinated, as was IRAK-1K180R. Substitution of arginine for K134 caused a modest but clear reduction in IRAK-1 ubiquitination, whereas K180R was relatively unaffected. Notably, and consistent with the in vitro ubiquitination shown in Fig. 4A, substitution of both lysines resulted in a very large reduction in ubiquitination. Consistent with this, GST-NEMO pulled down considerably less ubiquitinated IRAK-1K134/180R than the wild-type or single point mutants of IRAK-1. Importantly, the lysine-to-arginine substitutions did not affect IRAK-1 recruitment to IL-1R, because the amount of unmodified IRAK-1 and its variants was reduced similarly following IL-1 treatment (Fig. 4B, IRAK-1 immunoblot of lysate). Therefore, both K134 and K180 are required for the majority of IRAK-1 ubiquitination following IL-1 stimulation.
Like the death domain-containing kinase RIP, transient expression of IRAK-1 activates NF-κB. To assess the relative contribution of the different lysines in the intermediate domain to NF-κB activation, IRAK-1 or its variants were cotransfected with an NF-κB-driven reporter into 293/IL-R/TLR4 cells (Fig. 5A). Transient expression of IRAK-1 activated NF-κB in a dose-dependent fashion. The single lysine mutations activated NF-κB but were slightly less potent than the wild-type cDNA. In contrast, IRAK-1K134/180R failed to activate. Similarly, transient expression of IRAK-1 into IRAK-1-deficient MEFs dramatically increased NF-κB reporter activity, which was enhanced by IL-1 or LPS (Fig. 5B). The IRAK-1 single lysine mutants were modestly impaired in this regard, and the IRAK-1 double lysine mutant was inactive. These results are consistent with the reduced ubiquitination and NEMO binding of IRAK-1K134/180R and demonstrate that both lysines contribute to signaling downstream of IRAK-1.
FIG. 5.
IRAK-1 lysines 134 and 180 are required for IL-1-induced NF-κB but not p38 activation. (A) NF-κB activation in 293/IL-1R/TLR4 cells transiently transfected with increasing amounts of IRAK-1 (WT), IRAK-1K134R (K134R), IRAK-1K180R (K180R), or IRAK-1K134/180R (K134/180R). Values were normalized to β-galactosidase activity and are expressed as the induction level compared to luciferase in cells transfected with empty vector (-). Expression of WT, K134R, K180R, and K134/180R was confirmed by immunoblotting (IB) with anti-Flag. A large nonspecific band just below IRAK-1 is denoted by ns. (B) IL-1- and LPS-induced NF-κB luciferase activity was measured in IRAK-1-deficient MEFs transiently transfected with an NF-κB-driven luciferase reporter in combination with vector (-), WT, K134R, K180R, or K134/180R. Each transfection was performed in triplicate, and error bars represent the standard errors of the means. This experiment is representative of five independent experiments. (C) IL-1-induced p38 activation was measured in IRAK-1-deficient MEFs transiently transfected with vector, IRAK-1, or IRAK-1K134/180R. The amount of phosphorylated (p-p38) and total p38 was determined by immunoblotting.
IL-1-induced p38 MAP kinase activation is thought to be the result of upstream TAK1 kinase activation (38) and is impaired in IRAK-1-deficient cells (13). Given that ubiquitinated TRAF6 activates TAK1 by binding TAB2/TAB3 (14, 48), we asked if IRAK-1 ubiquitination had a similar function. IRAK-1-deficient MEFs were transfected with IRAK-1 or IRAK-1K134/180R and stimulated with IL-1, and p38 MAP kinase activation was evaluated by immunoblotting for phosphorylated p38. Whereas IL-1 failed to induce phosphorylated p38 in IRAK-1-deficient cells, cells reconstituted with wild-type IRAK-1 responded rapidly (Fig. 5C). Despite its inability to reconstitute IL-1R/TLR-mediated NF-κB activation, IRAK-1K134/180R restored IL-1-induced p38 activation to wild-type levels (Fig. 5C), indicating that IRAK-1 ubiquitination is not required for TAK1 activation. Thus, K63-linked polyubiquitination of IRAK-1 at lysine residues 134 and 180 is required for binding NEMO and is upstream of NF-κB, but not p38, activation.
DISCUSSION
TRAF6 and IRAK-1 are proximal and essential signaling components in IL-1R/TLR-mediated NF-κB and MAP kinase activation (13, 24, 46). TRAF6 contains a RING domain that confers E3 activity, and its autoubiquitination with K63-linked polyubiquitin chains is necessary for TAK1 activation and ligand-induced NF-κB activation (19, 48). IRAK-1, on the other hand, is a serine/threonine kinase and is thought to be an adaptor important for recruitment of TRAF6 to liganded IL-1R. Because IRAK-1 in many ways resembles RIP (both contain death domains, intermediate domains, and kinase activities that are dispensable for NF-κB activation and are recruited to their respective receptors and ubiquitinated), we considered the possibility that it, too, has the capacity to bind NEMO and activate IKK. Our findings show that although IRAK-1 and TRAF6 are ubiquitinated with K63-linked chains following IL-1 treatment, surprisingly, NEMO binds only to ubiquitinated IRAK-1. The reason for this specificity is not clear, but it may reflect a low but meaningful affinity of NEMO for unubiquitinated IRAK-1. Because the avidity of NEMO for polyubiquitin increases as a function of chain length (52), it is also possible that in stimulated cells the much longer polyubiquitin chain length observed with IRAK-1 compared to TRAF6 makes it a better binding partner for NEMO. The binding of NEMO to ubiquitinated IRAK-1 was confirmed by the observation that an IRAK-1 mutant, which cannot be ubiquitinated, bound poorly to NEMO and failed to promote receptor-mediated NF-κB activation.
The apparent reduction in IRAK-1 levels following IL-1 treatment is due to a shift to higher molecular weights resulting from its recruitment to the cytoplasmic tail of IL-1R and phosphorylation, possibly by IRAK-4 and/or autophosphorylation, and unmodified IRAK-1 can be recovered by phosphatase treatment of IRAK-1 immunoprecipitates from IL-1-stimulated cells (4, 18, 33, 53). Whether there is subsequent degradation of IRAK-1 is unclear, as we were unable to prevent the decrease in the unmodified band with proteasome inhibitors (unpublished observation). Our finding that IRAK-1 and all its lysine mutants were equally reduced after stimulation indicates that the lysine mutants were, in fact, recruited to the IL-1R signaling complex. This is also supported by the finding that IRAK-1K134/180R fully restored IL-1-induced p38 activation.
Substitution of arginine for lysine 134 has been reported to impair IRAK-1 ubiquitination but not its ability to restore IL-1-induced NF-κB activation (54). In contrast, we observed only a small and variable reduction in IRAK-1K134R ubiquitination and binding to NEMO. It is possible that this discrepancy may be due to the differences in cells used. We instead found that mutation of both lysines, 134 and 180, markedly impaired IRAK-1 ubiquitination and function. Our finding that mutation of either lysine alone had a modest (K134) or very little (K180) effect on ubiquitination may reflect the semiquantitative and perhaps nonlinear nature of the assay, or possibly that loss of ubiquitination at one site enhances ubiquitination at the other. The small amount of residual ubiquitination of the K134/180R mutant indicates that there must be other, minor, sites of ubiquitination, but the ubiquitin linkage is unknown. Regardless, this ubiquitination does not appear to be physiologically relevant, because the K134/180R mutant was unable to restore NF-κB activation in IRAK-1-deficient cells.
Pellino is a family of E3s that bind IRAK-1 (39). It was recently proposed that Pellino catalyzes the K63-linked polyubiquitination of IRAK-1, based on in vitro and overexpression studies (30). Our results using TRAF6-deficient cells, however, indicate that TRAF6 is both required for IRAK-1 ubiquitination and that its E3 activity is necessary for generating the high-molecular-weight ubiquitinated IRAK-1, NEMO binding, and activation of NF-κB. Therefore, although it may be possible to enforce ubiquitination of IRAK-1 by Pellino, in cells it appears that TRAF6 is the major functionally relevant E3. It is an interesting speculation that Pellino family members may be responsible for the residual low-molecular-weight ubiquitination of IRAK-1 found in TRAF6-deficient cells.
Our data show that the E3 activity of TRAF6 is not only required for IL-1-induced IRAK-1 ubiquitination and NF-κB activation, but also p38 activation (Fig. 3G). In addition, results obtained with the IRAK-1 double lysine mutant argue that IRAK-1 ubiquitination is not required for the latter (Fig. 5C). Therefore, there must be another target for TRAF6's E3 activity that couples to MAP kinase activation. The kinase TAK1 is required for activation of both IKK and p38 in response to IL-1 and TLR ligands (38). The ability of TAK1 to activate IKK and MKK6, an upstream activator of p38, is thought to result from K63-linked polyubiquitination of TRAF6 and recruitment of TAB2/TAB3/TAK1 complexes (48). It is therefore possible that ubiquitinated TRAF6, or perhaps another substrate, recruits TAK1 through interactions with TAB2 and TAB3, and ubiquitinated IRAK-1 recruits IKK through an interaction with NEMO. Once in proximity, TAK1 could phosphorylate IKKβ and MKK6, leading to the activation of NF-κB and p38, respectively.
The kinase activity of IRAK-4 is required for the recruitment of IRAK-1 to the cytoplasmic tail of IL-1R (18). Patients harboring mutations that disrupt either IRAK-4 expression or the CC2-LZ region of NEMO are susceptible to recurrent bacterial infections (8, 10, 33). Furthermore, knock-in mice containing mutations that disrupt the kinase activity of IRAK-4 are resistant to TLR-induced septic shock (15, 16). Our finding that IRAK-1 ubiquitination is required for normal IL-1/TLR ligand-induced NF-κB activation suggests that patients with IRAK-4 and NEMO CC2-LZ mutations may fail to respond to pathogenic organisms because of an inability to recruit IRAK-1 or NEMO, respectively.
The K63-linked polyubiquitin-binding activity of NEMO has emerged as a mechanism for recruiting IKK to receptor signaling complexes and activating NF-κB (9, 20, 28, 51, 52). Our findings show that this activity is also required for IL-1R/TLR-induced NF-κB activation, demonstrating that NEMO-polyubiquitin binding is a broadly used and highly conserved pathway for the activation of NF-κB by a wide variety of physiologic and pathological extracellular stimuli.
Supplementary Material
Acknowledgments
We thank Bei Dong for subcloning and mutagenesis, Srinivasa Srinivasula for helpful discussions and critical reading of the manuscript, Liwu Li for supplying the human IRAK-1 cDNA, Ted Dawson for pRK5-HA-tagged ubiquitin and pRK5-HA-tagged K63-only ubiquitin, Xiaoxia Li for 293/IL-1R/TLR4 cells, M. Schmidt-Supprian for NEMO-deficient MEFs, and Jun-ichiro Inoue for TRAF6-deficient MEFs.
This work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute.
Footnotes
Published ahead of print on 17 March 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4499-511. [DOI] [PubMed] [Google Scholar]
- 2.Burns, K., S. Janssens, B. Brissoni, N. Olivos, R. Beyaert, and J. Tschopp. 2003. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, K., F. Martinon, C. Esslinger, H. Pahl, P. Schneider, J. L. Bodmer, F. Di Marco, L. French, and J. Tschopp. 1998. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 27312203-12209. [DOI] [PubMed] [Google Scholar]
- 4.Cao, Z., W. J. Henzel, and X. Gao. 1996. IRAK: a kinase associated with the interleukin-1 receptor. Science 2711128-1131. [DOI] [PubMed] [Google Scholar]
- 5.Cao, Z., J. Xiong, M. Takeuchi, T. Kurama, and D. V. Goeddel. 1996. TRAF6 is a signal transducer for interleukin-1. Nature 383443-446. [DOI] [PubMed] [Google Scholar]
- 6.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103351-361. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 872095-2147. [PubMed] [Google Scholar]
- 8.Doffinger, R., A. Smahi, C. Bessia, F. Geissmann, J. Feinberg, A. Durandy, C. Bodemer, S. Kenwrick, S. Dupuis-Girod, S. Blanche, P. Wood, S. H. Rabia, D. J. Headon, P. A. Overbeek, F. Le Deist, S. M. Holland, K. Belani, D. S. Kumararatne, A. Fischer, R. Shapiro, M. E. Conley, E. Reimund, H. Kalhoff, M. Abinun, A. Munnich, A. Israel, G. Courtois, and J. L. Casanova. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat. Genet. 27277-285. [DOI] [PubMed] [Google Scholar]
- 9.Ea, C. K., L. Deng, Z. P. Xia, G. Pineda, and Z. J. Chen. 2006. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22245-257. [DOI] [PubMed] [Google Scholar]
- 10.Filipe-Santos, O., J. Bustamante, M. H. Haverkamp, E. Vinolo, C. L. Ku, A. Puel, D. M. Frucht, K. Christel, H. von Bernuth, E. Jouanguy, J. Feinberg, A. Durandy, B. Senechal, A. Chapgier, G. Vogt, L. de Beaucoudrey, C. Fieschi, C. Picard, M. Garfa, J. Chemli, M. Bejaoui, M. N. Tsolia, N. Kutukculer, A. Plebani, L. Notarangelo, C. Bodemer, F. Geissmann, A. Israel, M. Veron, M. Knackstedt, R. Barbouche, L. Abel, K. Magdorf, D. Gendrel, F. Agou, S. M. Holland, and J. L. Casanova. 2006. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 2031745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssens, S., and R. Beyaert. 2003. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11293-302. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, Z., J. Ninomiya-Tsuji, Y. Qian, K. Matsumoto, and X. Li. 2002. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol. Cell. Biol. 227158-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanakaraj, P., P. H. Schafer, D. E. Cavender, Y. Wu, K. Ngo, P. F. Grealish, S. A. Wadsworth, P. A. Peterson, J. J. Siekierka, C. A. Harris, and W. P. Fung-Leung. 1998. Interleukin (IL)-1 receptor-associated kinase (IRAK) requirement for optimal induction of multiple IL-1 signaling pathways and IL-6 production. J. Exp. Med. 1872073-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanayama, A., R. B. Seth, L. Sun, C. K. Ea, M. Hong, A. Shaito, Y. H. Chiu, L. Deng, and Z. J. Chen. 2004. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15535-548. [DOI] [PubMed] [Google Scholar]
- 15.Kawagoe, T., S. Sato, A. Jung, M. Yamamoto, K. Matsui, H. Kato, S. Uematsu, O. Takeuchi, and S. Akira. 2007. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J. Exp. Med. 2041013-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, T. W., K. Staschke, K. Bulek, J. Yao, K. Peters, K. H. Oh, Y. Vandenburg, H. Xiao, W. Qian, T. Hamilton, B. Min, G. Sen, R. Gilmour, and X. Li. 2007. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 2041025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knop, J., and M. U. Martin. 1999. Effects of IL-1 receptor-associated kinase (IRAK) expression on IL-1 signaling are independent of its kinase activity. FEBS Lett. 44881-85. [DOI] [PubMed] [Google Scholar]
- 18.Koziczak-Holbro, M., C. Joyce, A. Gluck, B. Kinzel, M. Muller, C. Tschopp, J. C. Mathison, C. N. Davis, and H. Gram. 2007. IRAK-4 kinase activity is required for interleukin-1 (IL-1) receptor- and toll-like receptor 7-mediated signaling and gene expression. J. Biol. Chem. 28213552-13560. [DOI] [PubMed] [Google Scholar]
- 19.Lamothe, B., A. Besse, A. D. Campos, W. K. Webster, H. Wu, and B. G. Darnay. 2007. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J. Biol. Chem. 2824102-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, H., M. Kobayashi, M. Blonska, Y. You, and X. Lin. 2006. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-κB activation. J. Biol. Chem. 28113636-13643. [DOI] [PubMed] [Google Scholar]
- 21.Li, S., A. Strelow, E. J. Fontana, and H. Wesche. 2002. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. USA 995567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, X., M. Commane, C. Burns, K. Vithalani, Z. Cao, and G. R. Stark. 1999. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol. 194643-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, X., M. Commane, Z. Jiang, and G. R. Stark. 2001. IL-1-induced NF-κB and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK). Proc. Natl. Acad. Sci. USA 984461-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 131015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maschera, B., K. Ray, K. Burns, and F. Volpe. 1999. Overexpression of an enzymically inactive interleukin-1-receptor-associated kinase activates nuclear factor-κB. Biochem. J. 339227-231. [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. J. Janeway. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2253-258. [DOI] [PubMed] [Google Scholar]
- 27.Naito, A., S. Azuma, S. Tanaka, T. Miyazaki, S. Takaki, K. Takatsu, K. Nakao, K. Nakamura, M. Katsuki, T. Yamamoto, and J. Inoue. 1999. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4353-362. [DOI] [PubMed] [Google Scholar]
- 28.Oeckinghaus, A., E. Wegener, V. Welteke, U. Ferch, S. C. Arslan, J. Ruland, C. Scheidereit, and D. Krappmann. 2007. Malt1 ubiquitination triggers NF-κB signaling upon T-cell activation. EMBO J. 264634-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Neill, L. A. 8 August 2000, posting date. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE. doi: 10.1126/stke.2999.44.re1. [DOI] [PubMed]
- 30.Ordureau, A., H. Smith, M. Windheim, M. Peggie, E. Carrick, N. Morrice, and P. Cohen. 2008. The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem. J. 40943-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey, S., and D. K. Agrawal. 2006. Immunobiology of Toll-like receptors: emerging trends. Immunol. Cell Biol. 84333-341. [DOI] [PubMed] [Google Scholar]
- 32.Petrak, D., S. A. Memon, M. J. Birrer, J. D. Ashwell, and C. M. Zacharchuk. 1994. Dominant negative mutant of c-Jun inhibits NF-AT transcriptional activity and prevents IL-2 gene transcription. J. Immunol. 1532046-2051. [PubMed] [Google Scholar]
- 33.Picard, C., A. Puel, M. Bonnet, C. L. Ku, J. Bustamante, K. Yang, C. Soudais, S. Dupuis, J. Feinberg, C. Fieschi, C. Elbim, R. Hitchcock, D. Lammas, G. Davies, A. Al-Ghonaium, H. Al-Rayes, S. Al-Jumaah, S. Al-Hajjar, I. Z. Al-Mohsen, H. H. Frayha, R. Rucker, T. R. Hawn, A. Aderem, H. Tufenkeji, S. Haraguchi, N. K. Day, R. A. Good, M. A. Gougerot-Pocidalo, A. Ozinsky, and J. L. Casanova. 2003. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 2992076-2079. [DOI] [PubMed] [Google Scholar]
- 34.Qin, J., Z. Jiang, Y. Qian, J. L. Casanova, and X. Li. 2004. IRAK4 kinase activity is redundant for interleukin-1 (IL-1) receptor-associated kinase phosphorylation and IL-1 responsiveness. J. Biol. Chem. 27926748-26753. [DOI] [PubMed] [Google Scholar]
- 35.Rincon, M., B. Derijard, C. W. Chow, R. J. Davis, and R. A. Flavell. 1997. Reprogramming the signalling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct. 151-68. [DOI] [PubMed] [Google Scholar]
- 36.Rothwarf, D. M., and M. Karin. 1999. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci. STKE. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed]
- 37.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14854-862. [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, S., H. Sanjo, K. Takeda, J. Ninomiya-Tsuji, M. Yamamoto, T. Kawai, K. Matsumoto, O. Takeuchi, and S. Akira. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 61087-1095. [DOI] [PubMed] [Google Scholar]
- 39.Schauvliege, R., S. Janssens, and R. Beyaert. 2006. Pellino proteins are more than scaffold proteins in TLR/IL-1R signalling: a role as novel RING E3-ubiquitin-ligases. FEBS Lett. 5804697-4702. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Supprian, M., W. Bloch, G. Courtois, K. Addicks, A. Israel, K. Rajewsky, and M. Pasparakis. 2000. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol. Cell 5981-992. [DOI] [PubMed] [Google Scholar]
- 41.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2947-950. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 176419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki, N., S. Suzuki, G. S. Duncan, D. G. Millar, T. Wada, C. Mirtsos, H. Takada, A. Wakeham, A. Itie, S. Li, J. M. Penninger, H. Wesche, P. S. Ohashi, T. W. Mak, and W. C. Yeh. 2002. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416750-756. [DOI] [PubMed] [Google Scholar]
- 44.Swantek, J. L., M. F. Tsen, M. H. Cobb, and J. A. Thomas. 2000. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 1644301-4306. [DOI] [PubMed] [Google Scholar]
- 45.Takaesu, G., S. Kishida, A. Hiyama, K. Yamaguchi, H. Shibuya, K. Irie, J. Ninomiya-Tsuji, and K. Matsumoto. 2000. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5649-658. [DOI] [PubMed] [Google Scholar]
- 46.Thomas, J. A., J. L. Allen, M. Tsen, T. Dubnicoff, J. Danao, X. C. Liao, Z. Cao, and S. A. Wasserman. 1999. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol. 163978-984. [PubMed] [Google Scholar]
- 47.Tugores, A., M. A. Alonso, F. Sanchez-Madrid, and M. O. de Landazuri. 1992. Human T cell activation through the activation-inducer molecule/CD69 enhances the activity of transcription factor AP-1. J. Immunol. 1482300-2306. [PubMed] [Google Scholar]
- 48.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412346-351. [DOI] [PubMed] [Google Scholar]
- 49.Wesche, H., W. J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7837-847. [DOI] [PubMed] [Google Scholar]
- 50.West, A. P., A. A. Koblansky, and S. Ghosh. 2006. Recognition and signaling by Toll-like receptors. Annu. Rev. Cell Dev. Biol. 22409-437. [DOI] [PubMed] [Google Scholar]
- 51.Wu, C. J., and J. D. Ashwell. 2008. NEMO recognition of polyubiquitinated Bcl10 is required for T cell receptor-mediated NF- 239 B activation. Proc. Natl. Acad. Sci. USA 1053023-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, C. J., D. B. Conze, T. Li, S. M. Srinivasula, and J. D. Ashwell. 2006. NEMO is a sensor of Lys 63-linked polyubiquitination and functions in NF-κB activation. Nat. Cell Biol. 8398-406. [DOI] [PubMed] [Google Scholar]
- 53.Yamin, T. T., and D. K. Miller. 1997. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J. Biol. Chem. 27221540-21547. [DOI] [PubMed] [Google Scholar]
- 54.Yao, J., T. W. Kim, J. Qin, Z. Jiang, Y. Qian, H. Xiao, Y. Lu, W. Qian, M. F. Gulen, N. Sizemore, J. DiDonato, S. Sato, S. Akira, B. Su, and X. Li. 2007. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NF-κB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J. Biol. Chem. 2826075-6089. [DOI] [PubMed] [Google Scholar]
- 55.Ye, H., J. R. Arron, B. Lamothe, M. Cirilli, T. Kobayashi, N. K. Shevde, D. Segal, O. K. Dzivenu, M. Vologodskaia, M. Yim, K. Du, S. Singh, J. W. Pike, B. G. Darnay, Y. Choi, and H. Wu. 2002. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418443-447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.