Abstract
The homeodomain transcription factor Nkx6.1 plays an important role in pancreatic islet β-cell development, but its effects on adult β-cell function, survival, and proliferation are not well understood. In the present study, we demonstrated that treatment of primary rat pancreatic islets with a cytomegalovirus promoter-driven recombinant adenovirus containing the Nkx6.1 cDNA (AdCMV-Nkx6.1) causes dramatic increases in [methyl-3H] thymidine and 5-bromo-2′-deoxyuridine (BrdU) incorporation and in the number of cells per islet relative to islets treated with a control adenovirus (AdCMV-βGAL), whereas suppression of Nkx6.1 expression reduces thymidine incorporation. Immunocytochemical studies reveal that >80% of BrdU-positive cells in AdCMV-Nkx6.1-treated islets are β cells. Microarray, real-time PCR, and immunoblot analyses reveal that overexpression of Nkx6.1 in rat islets causes concerted upregulation of a cadre of cell cycle control genes, including those encoding cyclins A, B, and E, and several regulatory kinases. Cyclin E is upregulated earlier than the other cyclins, and adenovirus-mediated overexpression of cyclin E is shown to be sufficient to activate islet cell proliferation. Moreover, chromatin immunoprecipitation assays demonstrate direct interaction of Nkx6.1 with the cyclin A2 and B1 genes. Overexpression of Nkx6.1 in rat islets caused a clear enhancement of glucose-stimulated insulin secretion (GSIS), whereas overexpression of Nkx6.1 in human islets caused an increase in the level of [3H]thymidine incorporation that was twice the control level, along with complete retention of GSIS. We conclude that Nkx6.1 is among the very rare factors capable of stimulating β-cell replication with retention or enhancement of function, properties that may be exploitable for expansion of β-cell mass in treatment of both major forms of diabetes.
Type 1 diabetes results from autoimmune destruction of insulin-producing β cells in the islets of Langerhans, whereas type 2 diabetes involves loss of glucose-stimulated insulin secretion (GSIS) and a gradual diminution of β-cell mass (45). Insulin injection therapy has been the standard treatment for type 1 diabetes since the discovery of the hormone more than 80 years ago. Islet transplantation has been investigated as an alternative to insulin injection, but a major obstacle to broad application of this approach has been an inadequate supply of human islets (21). Pharmacotherapy of type 2 diabetes includes administration of agents that enhance insulin secretion, but these drugs often lose efficacy over time and cause complications such as hypoglycemia (30). Moreover, no controlled strategy for restoration of β-cell mass has been identified for the type 2 disease. Thus, a more complete understanding of the mechanisms that control islet β-cell growth and function is required in order to develop more effective therapies for both major forms of diabetes.
Several members of the homeodomain family of transcription factors, including Pdx1, Hb9/Hlxb9, Nkx2.2, Nkx6.1, Isl-1, Pax6, and Pax4, are involved in the development of the various pancreatic islet cell types (α, β, and δ) and maintenance of their differentiated functions (9, 19). Nkx6.1 was initially isolated in a screen for homeodomain-containing transcripts in a hamster insulinoma cell cDNA library (40). Subsequently, functional roles for Nkx6.1 in development of the nervous system (38) and islet β cells (41) were described. Disruption of Nkx6.1 in transgenic mice resulted in a >90% decrease in functional β-cell mass, with no apparent defect in the development of other islet cell types (41). The dearth of β cells in this model was attributed to the absence of the secondary transition, in which insulin-positive cells normally undergo rapid proliferation at approximately embryonic day 15 in the developing mouse embryo.
The paucity of β cells in Nkx6.1-knockout mice limits further phenotypic characterization, leaving potential roles of the transcription factor in maintenance of mature β-cell function and growth unexplored. To address this issue, we have recently begun to manipulate Nkx6.1 expression in mature β cells, resulting in the demonstration of new roles in suppression of glucagon expression and control of GSIS (42). In the present study, we showed that Nkx6.1 overexpression strongly stimulates indices of islet cell replication, such as [methyl-3H]thymidine and 5-bromo-2′-deoxyuridine (BrdU) incorporation, as well as increasing cell numbers in both rat and human islets, whereas small interfering RNA (siRNA)-mediated suppression of Nkx6.1 has the opposite effect. We also demonstrate that the growth-promoting effect of Nkx6.1 is mediated by upregulation of a broad array of cell cycle control genes, some of which are shown by chromatin immunoprecipitation (ChIP) assays to be direct targets for Nkx6.1 binding. Finally, we show that the β-cell-mitogenic effects of Nkx6.1 are accompanied by retention or enhancement of GSIS in human and rat islets, respectively.
MATERIALS AND METHODS
Use of recombinant adenoviruses for overexpression and siRNA-mediated suppression of target genes in primary islets.
For gene overexpression studies, cytomegalovirus promoter-driven recombinant adenoviruses containing the hamster Nkx6.1 cDNA (AdCMV-Nkx6.1), the human cyclin E1 cDNA (AdCMV-CycE), the bacterial β-galactosidase gene (AdCMV-βGAL), green fluorescent protein (GFP) cDNA (AdCMV-GFP), and myc-tagged human cyclin B1 cDNA downstream of the tetracycline (Tet) operator (Ad-t-cyclin B1) and the Tet activator (Ad-tA) were prepared and used as previously described (4, 25, 31, 42). For gene suppression studies, adenoviruses containing siRNAs specific to rat Nkx6.1 (Ad-siNkx6.1) or with no known gene homology (Ad-siRNAcontrol) were used as described previously (2, 42).
Pancreatic islets were harvested from male Sprague-Dawley rats weighing approximately 250 g (33, 35), under a protocol approved by the Duke University Institutional Animal Care and Use Committee. Approximately 200 rat islets per condition were cultured in 2 ml of RPMI medium (10% fetal calf serum, 8 mM glucose) and treated with viruses at a concentration of 5 × 109 particles/ml medium for 18 h. Virus-containing medium was replaced with fresh culture medium, and islets were cultured for various times after treatment, as indicated below and in the figure legends, with fresh medium daily.
Human islet aliquots were procured through the National Center for Research Resources Islet Cell Centers or the Islet Cell Laboratory, Baylor Medical Center, Dallas, TX, and were cultured, treated with recombinant adenoviruses, and used for measurements of gene expression, [3H]thymidine incorporation, and GSIS exactly as described for rat islet cultures.
[3H]thymidine incorporation.
DNA synthesis rates were measured as described previously (12). [Methyl-3H]thymidine was added at a final concentration of 1 μCi/ml to pools of ∼200 islets during the last 18 h of cell culture. Groups of 30 islets were picked in triplicate, washed, and centrifuged twice at 300 × g for 3 min at 4°C. DNA was precipitated with 500 μl of cold 10% trichloroacetic acid and solubilized by the addition of 100 μl of 0.3 N NaOH. The amount of [3H]thymidine incorporated into DNA was measured by liquid scintillation counting and normalized to the amount of total cellular protein (7).
BrdU labeling, immunohistochemistry, and immunofluorescence in rat islets.
For BrdU labeling, a 1:100 dilution of BrdU labeling reagent (Invitrogen) was added to islet culture medium in place of [3H]thymidine for the final 18 h of cell culture. Preparation of islets for immunohistochemistry was performed as described previously (12). Islets were fixed in Bouin's solution for 2 h and maintained in 10% neutral-buffered formalin. Five-micrometer serial sections on glass slides were deparaffinized with xylene and rehydrated in a graded series of ethanol solutions. Antigen retrieval was performed by microwaving the slides for 13.5 min in 10 mM sodium citrate buffer with 0.05% Tween 20, pH 6.0. BrdU was detected with a biotinylated mouse anti-BrdU antibody (Invitrogen) and 3,3′-diaminobenzidine-tetrahydrochloride as the horseradish peroxidase substrate.
For insulin immunostaining, the HistoMouse-MAX kit (Invitrogen) was used with prediluted guinea pig anti-insulin serum (Invitrogen) and an alkaline phosphatase-conjugated secondary antibody by using Fast Red (Invitrogen) as the alkaline phosphatase substrate.
Direct immunofluorescence was performed on sections prepared as described above, using mouse anti-BrdU conjugated to Alexa Fluor 546 (1:25; Invitrogen), guinea pig anti-insulin (1:50; Invitrogen), rabbit antiglucagon (1:50; Invitrogen), and rabbit anti-Nkx6.1 (1:4,000; according to reference 24). Secondary antibodies (1:1,000; Jackson Labs) were fluorescein isothiocyanate-conjugated goat anti-guinea pig or anti-rabbit antibody. All images were acquired using an Eclipse E400 microscope (Nikon) with a Photometrics Coolsnap color charge-coupled-device camera (Roper Scientific). Confocal microscopy was performed using an LSM 410 laser scanning confocal microscope (Zeiss) at the Duke University light microscopy core facility (supported by Heather Galivan).
GSIS.
Pools of ∼200 human or rat islets were treated with various recombinant adenoviruses, and groups of 30 islets per condition were picked in triplicate. Static incubation GSIS assays were performed as described (42). Medium samples were analyzed by radioimmunoassay with the insulin Coat-a-Count kit (Diagnostic Products, Los Angeles, CA) (10, 20).
Islet dispersion and cell counting.
From a starting pool of 150 islets, six groups of 10 islets were placed in 250 μl of Krebs-Ringer bicarbonate buffer (Sigma) with 1 mM EDTA, 0.2% (wt/vol) bovine serum albumin, and 5.6 mM glucose. Trypsin (25 μg/ml; Sigma) and DNase I (2 μg/ml; Sigma) were added, and islets were dispersed into single cells by gentle pipetting every 60 s for 10 min at 37°C (28). Each group was counted twice by two observers using a hemocytometer.
Microarray analysis.
Total RNA was prepared from rat islets and was purified using the RNeasy microkit (Qiagen), which included DNase treatment to eliminate genomic contamination. RNA samples (∼500 ng) from five independent groups of AdCMV-Nkx6.1- and AdCMV-βGAL-treated islets were used for two rounds of amplification and labeled with Cy5. The samples were hybridized with rat reference RNA labeled with Cy3 to a DNA chip containing the version 3 rat microarray (Operon Biotechnologies) printed with 27,649 spots corresponding to 27,095 annotated genes and scanned on a Gene Pix 5000 scanner in the Duke University Microarray Core facility (by Holly Dressman). Analysis of the data was performed using the Genespring GX v7.3.1 software (Agilent). Data were normalized using per-chip and per-spot intensity-dependent LOWESS normalization. Statistical analysis was performed using the software's cross-gene error model. Results of one-way analysis of variance (parametric test, variances not assumed equal) were filtered for changes in expression (≥2-fold upregulation or ≥50% downregulation) and P values of ≤0.05 (Welch t test).
Measurement of RNA levels.
RNA was harvested from 20 to 50 primary rat islets by using the RNeasy microkit (Qiagen). Real-time PCRs were performed using the ABI Prism 7000 sequence detection system and software (Applied Biosystems) and PCR master mix reagents (Bio-Rad) as previously described (42). Sequences for all primers are available upon request. Demonstration of adenovirus-mediated overexpression of the hamster Nkx6.1 (AdCMV-Nkx6.1) and human cyclin B1 (Ad-t-cyclin B1) transgenes was achieved by reverse transcription (RT)-PCR analysis, performed as previously described (42).
Immunoblot analysis.
Islets were lysed in protein extract buffer (MPER; Pierce) and sonicated. Twenty-microgram aliquots of protein were resolved on 10% bis-Tris-HCl-buffered (pH 6.4) polyacrylamide gels (Invitrogen) and transferred to polyvinylidene difluoride membranes. The primary antibodies and dilutions used were anti-Nkx6.1 (1:1,000; Beta Cell Biology Consortium), anti-cyclin D1 (1:500; Neomarkers), anti-cyclin D2 (1:400; Sigma), anti-cyclin E (1:200; Santa Cruz Biotechnologies), anti-cyclin A (1:500; Sigma), and anti-cyclin B1 (1:300; Sigma). Membranes were incubated overnight at 4°C and primary antibodies were detected using appropriate horseradish peroxidase-linked secondary antibodies and visualized using the ECL Advance kit (Amersham) on the Versadoc 5000 system (Bio-Rad). Membranes were stripped (by use of the ReBlot kit; Chemicon) and reprobed with anti-γ-tubulin (1:10,000; Sigma).
ChIP assays.
ChIP assays were performed as detailed previously (8), using anti-Nkx6.1 antiserum or normal rabbit serum. Each ChIP assay was quantified in triplicate by real-time PCR for recovery of rat cyclin B1, A2, and E1 genes. Forward and reverse primers (relative to the transcriptional start site) were as follows: for cyclin B1 (bp −1647 to −1521), 5′-GCTCTGCCATTTATCATCACTGG and 5′-TGACTGCCAAGCAAGGAAGC; for cyclin A2 (bp +759 to +842), 5′-AATAAAAGTTGGTACCCACAGGGC and 5′-GAAGGTCCTTAAGAGGCGCAA; and for cyclin E1 (bp +635 to +734), 5′-CAAGGGAGAGGAAGGAGAGG and 5′-TGCTCCCCCAAAACTCATTC.
Statistical methods.
Statistical significance was determined using a two-tailed Student's t test. P values less than 0.05 were considered significant.
RESULTS
Nkx6.1 simultaneously enhances [methyl-3H]thymidine incorporation and GSIS in rat islets.
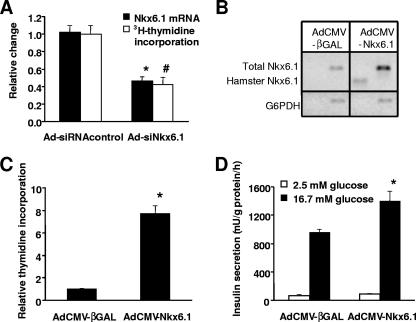
We have recently reported that siRNA-mediated suppression of Nkx6.1 expression in primary rat islets results in impairment of GSIS (42). In the current study, we have expanded our investigation of the biological impact of Nkx6.1 to include studies of islet cell replication. Treatment of rat islets with a recombinant adenovirus containing an Nkx6.1-specific siRNA sequence (Ad-siNkx6.1) caused a 54% ± 5% decrease in Nkx6.1 mRNA levels relative to the level in islets treated with a recombinant virus containing an siRNA sequence with no known sequence homology (Ad-siRNAcontrol) (Fig. 1A). Suppression of Nkx6.1 to this extent caused a 58% ± 8% decrease in [methyl-3H]thymidine incorporation into islet cell genomic DNA (Fig. 1A), suggestive of decreased islet cell proliferation.
FIG. 1.
Manipulation of Nkx6.1 expression in rat islets regulates [3H]thymidine incorporation and GSIS. (A) Rat islets were treated with Ad-siNkx6.1 or Ad-siRNAcontrol for 18 h and then cultured for an additional 80 h. Suppression of Nkx6.1 mRNA levels was confirmed by real-time PCR (black bars). Uptake of [methyl-3H]thymidine into genomic DNA was also measured (white bars). Data represent the means ± standard errors of the means from three independent experiments, each performed in triplicate. Nkx6.1 mRNA levels (*) and [3H]thymidine incorporation (#) in Ad-siNkx6.1-treated islets were lower than those in control islets, with a P value of <0.001. (B to D) Rat islets were treated with AdCMV-Nkx6.1 or AdCMV-βGAL for 18 h and maintained in culture for an additional 80 h. (B) RT-PCR analysis with primers specific for total Nkx6.1 (rat and hamster), hamster Nkx6.1 (AdCMV-Nkx6.1 contains the hamster Nkx6.1 cDNA), and, as a loading control, rat glucose-6-phosphate dehydrogenase (G6PDH). (C) [3H]thymidine incorporation into genomic DNA. Data represent the means ± standard errors of the means for three independent experiments, each involving triplicate pools of 30 islets per condition. *, a P value of <0.0001 in comparison to AdCMV-βGAL-treated islets. (D) GSIS in rat islets. Data are the means ± standard errors of means from three independent experiments, each performed in triplicate. *, a P value of <0.005 in comparison to AdCMV-βGAL-treated islets.
The effect of Nkx6.1 suppression slowing β-cell replication suggests that overexpression of Nkx6.1 might act in the opposite manner to stimulate β-cell mitotic activity. To test this idea, we treated primary rat islets with a recombinant adenovirus containing the hamster Nkx6.1 cDNA sequence (AdCMV-Nkx6.1) or a control adenovirus containing a bacterial β-galactosidase gene (AdCMV-βGAL) and measured [3H]thymidine incorporation. RT-PCR analysis demonstrated a fivefold increase in Nkx6.1 mRNA in AdCMV-Nkx6.1-treated islets compared to that in the AdCMV-βGAL-treated islets (Fig. 1B). Overexpression of Nkx6.1 to this degree resulted in a 7.7-fold ± 0.7-fold increase in thymidine incorporation relative to the AdCMV-βGAL-treated control islets (Fig. 1C).
In almost all cases reported to date, increases in β-cell replication caused by manipulations such as oncogene expression or growth factor and cell matrix additions are accompanied by loss of differentiated function, particularly, decreases in insulin content and GSIS (21). Since it has also been shown that Nkx6.1 can suppress expression of a reporter gene under control of an insulin promoter fragment (34), we sought to determine if the growth-promoting effects of Nkx6.1 overexpression are linked to functional derangements. AdCMV-Nkx6.1-treated islets exhibited no significant change in basal insulin secretion (measured at 2.5 mM glucose) relative to AdCMV-βGAL-treated islets, whereas Nkx6.1 overexpression caused a 46% increase in insulin secretion at a stimulatory glucose concentration (P < 0.005) (Fig. 1D), with no change in insulin content (22.7 ± 4.1 mU insulin/islet for AdCMV-Nkx6.1-treated islets and 24.8 ± 3.2 mU insulin/islet for AdCMV-βGAL-treated islets; P = 0.71). Thus, overexpression of Nkx6.1 in primary rat islets activates β-cell mitosis in concert with enhancement of GSIS and retention of normal insulin content.
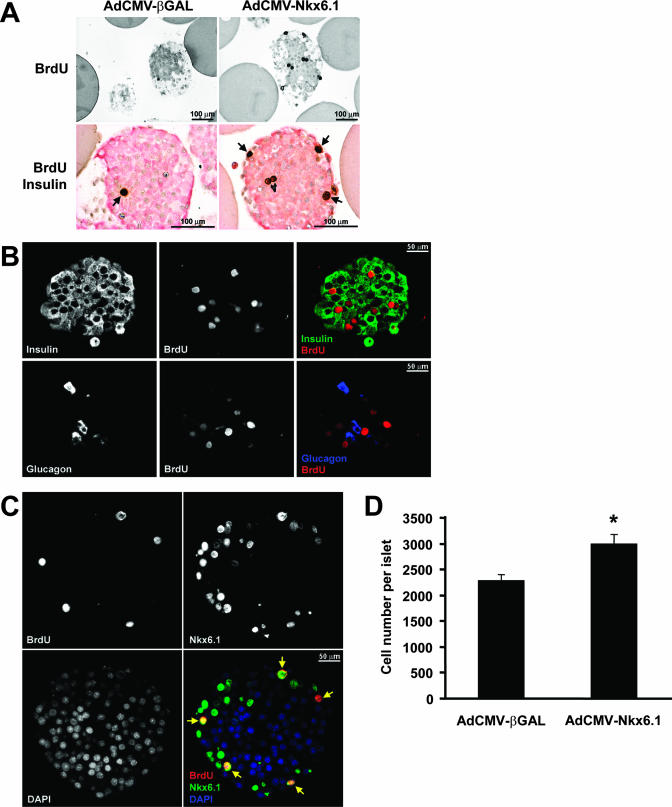
Overexpression of Nkx6.1 increases islet β-cell replication and islet cell numbers.
The increase in thymidine incorporation in AdCMV-Nkx6.1-treated rat islets shown in Fig. 1 could be explained by stimulation of β-cell proliferation but could also be due to replication of other islet cell types or contaminating nonislet cells. To investigate these issues further, we measured uptake of the nucleotide analog BrdU into rat islets by immunohistochemistry. Islets treated with AdCMV-Nkx6.1 had a striking increase in BrdU incorporation (Fig. 2A) such that nearly all sections from islets overexpressing Nkx6.1 had multiple BrdU-positive cells (the range of BrdU-positive cells per section was 0 to 9 for 154 sections, with an average of 3.1 ± 0.2 positive cells/section) with many pairs of adjacent stained cells that appeared to have recently divided. In contrast, sections from untreated or AdCMV-βGAL-treated control islets usually had no BrdU-positive cells (the range of BrdU-positive cells per section was 0 to 4 for 213 sections, with an average of 0.4 ± 0.1 positive cells/section). The 8.1- ± 1.3-fold increase in the number of BrdU-positive cells in AdCMV-Nkx6.1-treated islets in comparison to the number in AdCMV-βGAL-treated islets was well correlated with the increase in thymidine incorporation shown in Fig. 1 and clearly demonstrates a robust increase in the number of islet cells undergoing cell division in response to Nkx6.1 overexpression.
FIG. 2.
Nkx6.1 stimulates rat islet β-cell replication. (A) Histochemical staining of islet sections. Islet sections were stained with antibodies that detect BrdU incorporation (top panels) or BrdU (brown nuclear staining) and insulin (pink cytosolic staining) (bottom panels). (B) Immunofluorescence analysis of BrdU and insulin expression (top panels) or BrdU and glucagon expression (bottom panels) in sections of AdCMV-Nkx6.1-treated rat islets. The far-right panels are the overlay of insulin and BrdU staining (top) or glucagon and BrdU staining (bottom). (C) Serial sections of AdCMV-Nkx6.1-treated islets stained for BrdU (top left), Nkx6.1 (top right), or DAPI (4′,6′-diamidino-2-phenylin-dole) nuclear stain (bottom left). The bottom right panel shows the overlay of all three signals. The yellow arrows identify the BrdU-labeled nuclei, which in all cases costain with Nkx6.1 and DAPI. (D) Effects of Nkx6.1 overexpression on islet cell number. Data represent the means ± standard errors of the means for two independent experiments, each involving six independent groups of 10 islets per condition. *, a P value of <0.005 in comparison to AdCMV-βGAL-treated islets.
Histochemical costaining of 154 separate sections from AdCMV-Nkx6.1-treated islets revealed that 404 of 480 BrdU-positive cells (84%) were also insulin positive (see the representative section in the lower panels of Fig. 2A). In contrast, only 50 of 83 (60%) of the rare BrdU-positive cells in 213 separate sections of AdCMV-βGAL-treated islets were insulin positive. Confocal immunofluorescence studies were carried out to further investigate the relationship between BrdU staining and Nkx6.1 expression. In the representative section shown in Fig. 2B, 8 out of 10 BrdU-positive nuclei are clearly surrounded by green insulin immunofluorescence, and the remaining 2 BrdU-positive nuclei are not immediately surrounded by insulin immunostaining. However, as also shown in Fig. 2B, costaining of islet sections with antibodies for glucagon and BrdU revealed that none of the eight BrdU-positive cells in the section were costained with glucagon. These data are representative of three independent islet aliquots from which ≥40 islet sections were examined per aliquot. Thus, these studies suggest that the vast majority of the BrdU-positive cells in AdCMV-Nkx6.1-treated islets are β cells.
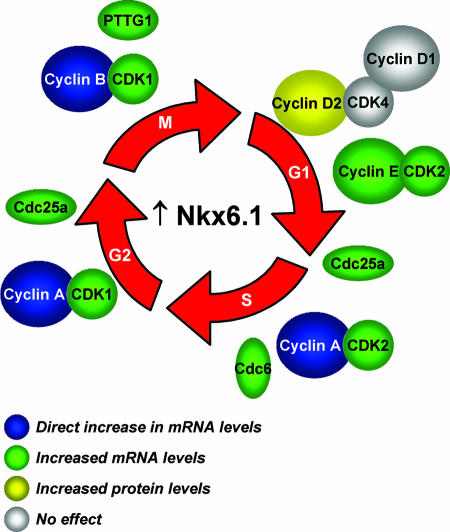
FIG. 8.
Schematic summary of the effects of overexpressed Nkx6.1 on proteins involved in all phases of the cell cycle. Nkx6.1 exerts these effects via an array of direct and indirect actions as indicated in the key at the lower left and as elaborated in the text.
Figure 2C shows the results of costaining for Nkx6.1 and BrdU. In sections of AdCMV-βGAL-treated islets, Nkx6.1 could not be detected via direct immunofluorescence (data not shown). This is consistent with the experience of others with currently available Nkx6.1 antibodies, which generally require an amplification technique called tyramide signal amplification for detection of endogenous Nkx6.1 immunofluorescence (37). Treatment of islets with AdCMV-Nkx6.1 caused the appearance of a clear Nkx6.1 signal in many cells throughout each section with no application of tyramide signal amplification. Importantly, overlay of BrdU and Nkx6.1 nuclear staining (Fig. 2C, lower right panel) revealed that all BrdU-positive cells were also Nkx6.1 positive. Interestingly, the reverse was not true, as not all Nkx6.1-positive cells were also BrdU positive. A likely explanation for this is that BrdU was added only during the last 18 h of the 98-h period following exposure of islets to AdCMV-Nkx6.1; therefore, some Nkx6.1-overexpressing cells may have completed S phase prior to the addition of BrdU. Taken together, these data indicate that treatment of islets with AdCMV-Nkx6.1, which should direct Nkx6.1 expression in all islet cells via its cytomegalovirus promoter, preferentially stimulates β-cell proliferation.
Finally, we sought to determine if the Nkx6.1-induced increases in [3H]thymidine and BrdU actually equate to an increase in islet cell number. Three hundred rat islets of roughly equal sizes were hand picked, split into equal aliquots, and treated with either AdCMV-Nkx6.1 or AdCMV-βGAL for 18 h, followed by an additional 80 h of culture. Thereafter, six aliquots of 10 islets from each group were separated, dispersed into single cells, and subjected to cell counting, with this entire experiment being repeated on two separate occasions. As shown in Fig. 2D, AdCMV-Nkx6.1-treated islets had an average of 31% ± 8% more cells than AdCMV-βGAL-treated islets did (P < 0.005). Therefore, overexpression of Nkx6.1 results in a net increase in islet cell number.
Microarray analysis revealed that Nkx6.1 regulates a broad array of cell cycle-regulatory genes.
To investigate the mechanism by which Nkx6.1 stimulates islet cell replication with retention of function, we performed microarray analysis on rat islets treated with AdCMV-Nkx6.1 or AdCMV-βGAL. RNA samples from five independent islet samples were collected and hybridized to a rat oligonucleotide array containing approximately 27,000 genes. While originally described as a suppressor of gene expression, Nkx6.1 may also serve as an enhancer, based on findings that it can stimulate its own expression (23). Our microarray analysis reveals that overexpression of Nkx6.1 resulted in suppression of 187 genes by ≥50%, consistent with its role as a transcriptional repressor, but also results in ≥2-fold upregulation of 156 genes, suggestive of a broader transcriptional activator role for this factor than previously realized, although it is possible that the upregulation of many of these genes was secondary to a primary suppressor function of Nkx6.1. The list of 187 downregulated and 156 upregulated genes is provided in Table S1 in the supplemental material. The presentation of the data as a heat map for the five independent rat islet microarray experiments demonstrates the consistency with which Nkx6.1 acted to upregulate or downregulate the different groups of genes (Fig. 3A). In examining the list of upregulated genes in Table S1 in the supplemental material, we noticed that Nkx6.1 overexpression resulted consistently in large increases in expression of a number of cell cycle-regulatory genes, including those encoding cyclins A2, B1, B2, E1, and E2 and Cdk1, Cdk2, Cdc6, Cdc25a, and pituitary tumor transforming gene 1 (PTTG1) protein. To confirm the findings of the microarray analysis, we used real-time PCR to measure mRNA levels for the various cell cycle control genes in AdCMV-Nkx6.1-treated and AdCMV-βGAL-treated islets. Importantly, all of the aforementioned cell cycle-regulatory genes that were found to be upregulated in the microarray study were confirmed by real-time PCR analysis to be upregulated (Fig. 3B). The Nkx6.1-upregulated genes are involved in all phases of the cell cycle, from G1 to M. These experiments therefore revealed a heretofore unknown and broad role of Nkx6.1 in the control of islet cell replication.
FIG. 3.
Effects of Nkx6.1 on gene expression in adult rat pancreatic islets. Five independent groups of rat pancreatic islets were treated with AdCMV-Nkx6.1 or AdCMV-βGAL, and duplicate RNA samples were utilized for microarray analysis. (A) Heat map demonstrating consistent upregulation (green) or downregulation (red) of Nkx6.1-regulated genes. (B) Confirmation of microarray results for key cell cycle-regulatory and functional genes by quantitative real-time PCR. Data represent the means ± standard errors of the means for five independent experiments. Rb1, retinoblastoma protein 1; GK, glucokinase. (C) ChIP analysis of interactions of Nkx6.1 with the cyclin A2, B1, and E1 promoters. Data are represented as the relative levels of association of Nkx6.1 with genomic fragments following immunoprecipitation with anti-Nkx6.1 serum, normalized to the amount of DNA recovered with normal serum, and represent the means ± standard errors of the means from three independent experiments, each performed in triplicate. *, a P value of <0.005 for the increase of Nkx6.1 association with the cyclin A2 and B1 genes in AdCMV-Nkx6.1-treated islets compared to that in AdCMV-βGAL-treated islets.
We also investigated the effects of Nkx6.1 overexpression on genes known to confer key functional properties of the mature β cell. Neither mature nor pre-mRNA insulin transcript levels were altered by Nkx6.1 expression; the latter have been shown to reflect acute changes in insulin transcription due to their short half-life (22). In addition, Nkx6.1 overexpression caused no significant changes in levels of Pdx1, GLUT2, or glucokinase mRNAs (Fig. 3B).
Direct interactions of Nkx6.1 with the cyclin A2 and B1 promoters.
To investigate the possibility that Nkx6.1 might exert its effects on cell cycle-regulatory genes via direct interactions, we first analyzed the rat cyclin A2, B1, and E1 promoters for sequences homologous to a recently described Nkx6.1-regulated enhancer sequence (23). All three genes were found to contain multiple copies of the ATTT core element necessary for Nkx6.1-mediated activation, and several of these elements in each gene were found to be flanked by high-GC-content sequences, another characteristic of the Nkx6.1-regulated enhancer. Moreover, the Nkx6.1 enhancer-like elements in the rat cyclin A2, B1, and E1 promoters had significant homology with similar regions in their human cognate genes (data not shown). To determine if Nkx6.1 actually occupies these genes in rat islets, PCR primers were designed to amplify sequences in the cyclin A2, B1, and E1 genes. Using these primers, ChIP analyses were performed on rat islets following treatment with AdCMV-Nkx6.1 or AdCMV-βGAL. Islet chromatin was sheared to an average of 500 to 800 bp for the ChIP assays. Figure 3C shows that treatment of islets with AdCMV-Nkx6.1 caused a 15-fold increase in the levels of a cyclin B1 gene-specific PCR product immunoprecipitated with Nkx6.1 antiserum relative to samples treated with normal rabbit serum. For the cyclin B gene, interaction with an Nkx6.1 enhancer-like element at approximately −1,500 bp of the promoter region was shown, whereas no interaction was detected with an Nkx6.1 enhancer-like sequence at −600 bp. In addition, a ninefold increase in an Nkx6.1-associated product was identified from the cyclin A2 gene in cells treated with AdCMV-Nkx6.1, whereas Nkx6.1 overexpression did not increase Nkx6.1 association with the cyclin E1 gene (Fig. 3C). Consistent with these findings in islets with overexpressed Nkx6.1, AdCMV-βGAL-treated control islets exhibited a clear enrichment of PCR products from the cyclin A2 and cyclin B1 genes in Nkx6.1 antiserum-treated samples compared to control serum-treated samples, whereas no significant enrichment was observed in a product from the cyclin E1 gene (Fig. 3C). We conclude that Nkx6.1 interacts directly with the cyclin A2 and B1 genes. No significant binding of Nkx6.1 to the cyclin E1 gene was detected at the 96-h time point chosen for these studies. It remains possible that Nkx6.1 binds to other regions of the cyclin E1 gene or that its binding occurred at time points earlier than 96 h; these issues await further study.
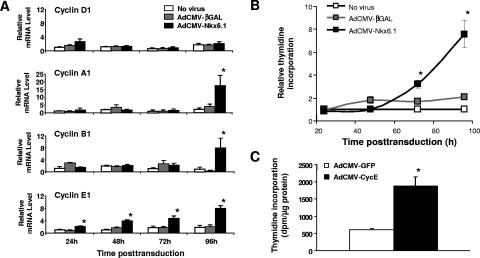
Time course of Nkx6.1-stimulated β-cell proliferation and induction of cell cycle-regulatory genes.
To investigate temporal aspects of Nkx6.1-mediated β-cell proliferation and regulation of cell cycle genes, we treated rat islets with AdCMV-Nkx6.1 or AdCMV-βGAL and performed assays of [3H]thymidine incorporation and regulation of cell cycle genes at 24, 48, 72, and 96 h after viral treatment. With regard to cell cycle genes, cyclin E mRNA was increased at 24 h and continued to rise at all subsequent time points in response to Nkx6.1 expression, whereas cyclins associated with later phases of the cell cycle, such as cyclin B1 and cyclin A2 did not rise until 72 to 96 h after Nkx6.1 expression (Fig. 4A). Consistent with data of Fig. 3, cyclin D2 mRNA did not change at any time in response to Nkx6.1 expression (Fig. 4A). Interestingly, we observed that [3H]thymidine incorporation was not significantly increased until between 72 to 96 h after Nkx6.1 expression (Fig. 4B), despite the fact that Nkx6.1 gene expression was clearly increased at 24 h and remained elevated out to 96 h (Fig. 1 and data not shown).
FIG. 4.
Time course studies and sufficiency of cyclin E for activation of islet cell replication. (A) Time course of induction of cyclins D1, A1, B1, and E1 in response to Nkx6.1 overexpression in rat islets. Relative expression is normalized to the level in untreated islets (no virus) cultured for 24 h. (B) Time course of stimulation of [3H]thymidine incorporation into rat islets by AdCMV-Nkx6.1 treatment relative to AdCMV-βGAL or no treatment, expressed relative to incorporation into no-virus control islets at each time point. For panels A and B, data are means ± standard errors of the means for three independent experiments, and the asterisk indicates significant differences between Nkx6.1-overexpressing cells and controls (P < 0.05). (C) Overexpression of human cyclin E1 is sufficient to increase [3H]thymidine incorporation in rat islets. Rat islets were treated with recombinant adenoviruses containing the human cyclin E cDNA (AdCVM-CycE) or the GFP cDNA (AdCMV-GFP). For these studies, [3H]thymidine was added in the 80- to 96-h time period after viral treatment. Data are means ± standard errors of the means from three independent experiments. *, a P value of <0.05 in comparison to the control.
Cyclin E has been ascribed a role as an initiator of the cell cycle (i.e., sufficient to drive the G1-to-S transition) in other cell types (1) but has never been studied directly in normal islets to our knowledge. Thus, to determine if the rise in cyclin E might be sufficient to initiate islet β-cell proliferation in our studies, we treated rat islets with an adenovirus containing the human cyclin E cDNA (AdCMV-CycE) or a control virus containing GFP (AdCMV-GFP). Treatment with AdCMV-CycE caused an approximately fourfold increase in [3H]thymidine incorporation relative to that in AdCMV-GFP-treated islets (Fig. 4C). Thus, the temporal sequence of Nkx6.1-mediated stimulation of islet β-cell proliferation seems to involve early induction of cyclin E, followed by later upregulation of genes, such as those encoding cyclins A and B, that are involved in later phases of the cell cycle. Also, our studies show that overexpression of cyclin E is sufficient to activate islet cell proliferation.
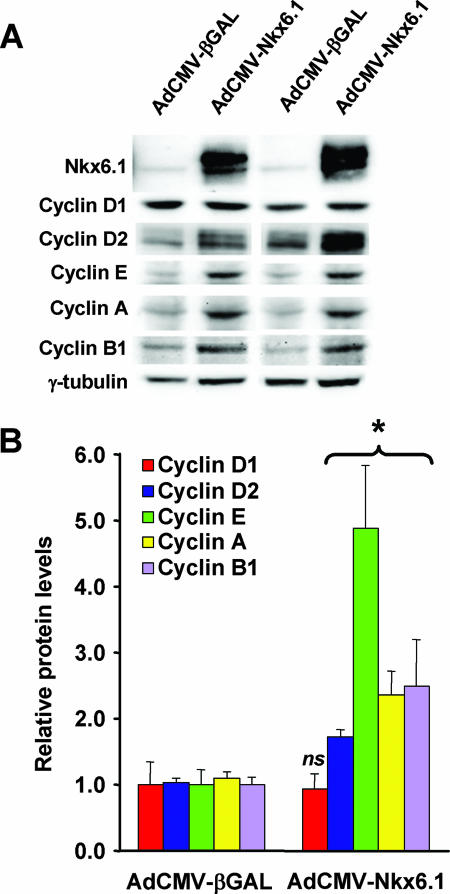
Effects of Nkx6.1 expression on cyclin protein levels.
To determine if the pronounced effects of Nkx6.1 on cyclin mRNA levels are accompanied by increases in protein expression, we performed immunoblot analyses of cyclin A, B1, D1, D2, and E levels in islets treated with AdCMV-Nkx6.1 or AdCMV-βGAL at the 96-h time point. AdCMV-Nkx6.1-treated islets exhibited 2.4- to 4.9-fold increases in cyclin A2, cyclin B1, and cyclin E1 protein levels relative to those of AdCMV-βGAL-treated control islets (Fig. 5). Nkx6.1 overexpression had no effect on cyclin D1 protein levels. However, Nkx6.1 overexpression did cause a modest but significant increase (72%) in cyclin D2 protein levels, despite the lack of effect of AdCMV-Nkx6.1 treatment on cyclin D2 mRNA levels (Fig. 3 and 4). Thus, Nkx6.1 appears to regulate cell cycle-regulatory genes via several distinct mechanisms: (i) direct binding to specific promoter sequences (cyclin A2 and B1 genes), (ii) indirect effects on mRNA levels (cyclin E1 gene), and (iii) changes in protein amount independent of changes in mRNA levels (cyclin D2 gene).
FIG. 5.
Nkx6.1 overexpression in rat islets increases the levels of multiple cyclin proteins. AdCMV-Nkx6.1- or AdCMV-βGAL-treated rat islets were harvested for immunoblot analysis with antibodies specific for Nkx6.1, cyclin A, B1, D1, D2, or E, or γ-tubulin as a loading control. (A) Representative immunoblot. (B) Quantitative gel scan data from three independent experiments. *, a P value of <0.05 in comparison with AdCMV-βGAL-treated islets; ns, not significant.
Effects of Nkx6.1 on selected cell cycle inhibitors.
We also tested the idea that Nkx6.1 could be regulating selected cell cycle inhibitors, such as the Ink family and p27kip1, that have been implicated in control of β-cell proliferation (11, 18, 46). To this end, we measured p16Ink4a, p15Ink4b, p18Ink4c, p19Ink4d, and p27Kip1 mRNA levels at various time points following treatment of rat islets with AdCMV-Nkx6.1 or AdCMV-βGAL. Islet culture per se seemed to have an effect on these cell cycle inhibitors such that p15Ink4b mRNA levels were increased in all three experimental conditions (untreated and AdCMV-βGAL- and AdCMV-Nkx6.1-treated islets) and all time points (days 1, 2, 3, and 4) relative to the levels in freshly isolated islets, whereas p16Ink4a, p18Ink4c, p19Ink4d, and p27kip1 mRNA levels were all decreased at day 1 of culture and decreased individually at various other time points (Table 1). The only significant effect of AdCMV-Nkx6.1, in comparison to the effects of the AdCMV-βGAL control, was a modest increase in p19Ink4d mRNA 3 and 4 days after virus treatment (Table 1). Because p27kip1 is tightly regulated at the level of protein turnover in pancreatic islets (46), we also measured p27kip1 protein levels. No significant changes in p27kip1 protein levels were observed 1, 2, 3, or 4 days after AdCMV-Nkx6.1 treatment relative to the protein levels in time-matched AdCMV-βGAL-treated control islets (data not shown). Overall, our studies of this particular group of cell cycle repressors suggest that they are not highly regulated by Nkx6.1.
TABLE 1.
Time course of Nkx6.1 effects on selected cell cycle inhibitor mRNA levelsa
| Cell cycle inhibitor | Time cultured (h) | Mean relative mRNA level ± SEM in rat islets treated with:
|
||
|---|---|---|---|---|
| No virus | AdCMV-βGAL | AdCMV-Nkx6.1 | ||
| p16Ink4a | 0 | 1.00 ± 0.27 | NA | NA |
| 24 | 0.79 ± 0.10 | 0.65 ± 0.03 | 0.46 ± 0.11 | |
| 48 | 0.72 ± 0.01 | 1.50 ± 0.63 | 1.49 ± 0.05 | |
| 72 | 0.59 ± 0.16 | 1.42 ± 0.13 | 1.21 ± 0.01 | |
| 96 | 0.71 ± 0.13 | 1.33 ± 0.23 | 1.74 ± 0.31 | |
| p15Ink4b | 0 | 1.00 ± 0.56 | NA | NA |
| 24 | 2.91 ± 0.81 | 3.30 ± 0.16 | 1.90 ± 0.74 | |
| 48 | 3.20 ± 0.87 | 6.73 ± 4.12 | 4.35 ± 0.52 | |
| 72 | 2.80 ± 1.19 | 2.71 ± 0.60 | 2.53 ± 0.35 | |
| 96 | 3.47 ± 1.81 | 4.94 ± 2.11 | 4.30 ± 1.73 | |
| p18Ink4c | 0 | 1.00 ± 0.03 | NA | NA |
| 24 | 0.40 ± 0.04 | 0.37 ± 0.05 | 0.27 ± 0.03 | |
| 48 | 0.49 ± 0.01 | 0.85 ± 0.31 | 0.40 ± 0.01 | |
| 72 | 0.47 ± 0.13 | 0.91 ± 0.06 | 0.55 ± 0.01 | |
| 96 | 0.45 ± 0.22 | 1.04 ± 0.25 | 0.87 ± 0.24 | |
| p19Ink4d | 0 | 1.00 ± 0.01 | NA | NA |
| 24 | 0.88 ± 0.09 | 0.75 ± 0.08 | 0.71 ± 0.10 | |
| 48 | 0.65 ± 0.04 | 0.94 ± 0.30 | 1.31 ± 0.06 | |
| 72 | 0.52 ± 0.09 | 0.63 ± 0.05 | 0.87 ± 0.07* | |
| 96 | 0.46 ± 0.19 | 0.70 ± 0.20 | 1.36 ± 0.13* | |
| p27kip1 | 0 | 1.00 ± 0.14 | NA | NA |
| 24 | 0.58 ± 0.01 | 0.70 ± 0.02 | 0.64 ± 0.08 | |
| 48 | 0.89 ± 0.01 | 0.84 ± 0.08 | 0.87 ± 0.09 | |
| 72 | 0.85 ± 0.13 | 1.38 ± 0.14 | 0.97 ± 0.07 | |
| 96 | 0.89 ± 0.31 | 1.53 ± 0.35 | 1.40 ± 0.06 | |
The changes in p16Ink4a, p15Ink4b, p18Ink4c, p19Ink4d, and p27kip1 mRNA levels over time for untreated rat islets or rat islets treated with AdCMV-βGAL or AdCMV-Nkx6.1 are shown. Data for each transcript are normalized relative to the levels of that transcript in untreated freshly isolated islets. Data are means ± standard errors of the means (SEM) for three independent experiments, and asterisks indicate significant differences between AdCMV-Nkx6.1-overexpressing cells and controls (P < 0.05). NA, not applicable.
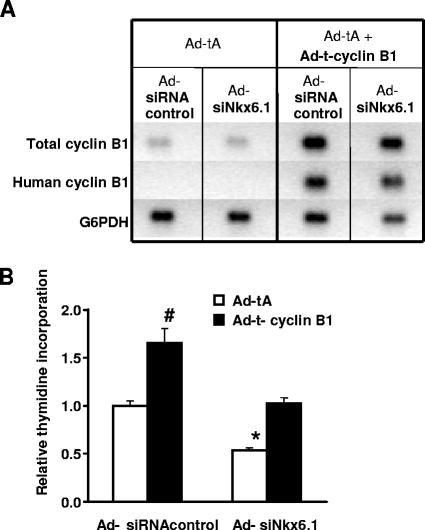
Effects of cyclin B1 silencing and overexpression on β-cell proliferation.
In separate studies, we performed microarray analysis of replicate samples of INS-1-derived 832/13 cells treated with Ad-siNkx6.1 or Ad-siRNAcontrol and found that suppression of Nkx6.1 expression in these cells preferentially suppressed cyclin B1 expression, in concert with suppression of thymidine incorporation (data not shown). For this reason, and because the cyclin B1 gene is one of the two cyclin genes that interact directly with Nkx6.1 (Fig. 3), we sought to determine if Nkx6.1-mediated changes in cyclin B1 expression participate in the control of β-cell proliferation. Rat islets were treated with Ad-siNkx6.1 or Ad-siRNAcontrol in the presence or absence of a Tet-inducible system for cyclin B1 expression (25). As shown in Fig. 6A, human cyclin B1 was detected only in RNA samples from islets transduced with both virus containing the Tet activator and virus containing Tet responder-cyclin B1, resulting in a clear increase in total cyclin B1 levels measured with a primer set that recognizes both rat and human cyclin B1. Treatment of islets with Ad-siNkx6.1 decreased thymidine incorporation by 47% ± 3%, whereas overexpression of cyclin B1 was able to reverse the Ad-siNkx6.1-mediated decrease, bringing thymidine incorporation back to control levels (Fig. 6B). However, while overexpression of Nkx6.1 caused a sevenfold increase in thymidine incorporation (Fig. 1), overexpression of cyclin B1 in rat islets caused only a 66% ± 15% increase in thymidine incorporation (Fig. 6B). Taken together, our data show that regulation of cyclin B1 expression is clearly involved in mediating the effect of Nkx6.1 to suppress islet proliferation but that cell cycle-regulatory factors in addition to cyclin B1 are required to achieve the strong effect of Nkx6.1 overexpression on islet replication.
FIG. 6.
Cyclin B1 overexpression partially rescues the effect of Nkx6.1 suppression on rat islet replication. Primary rat islets were treated with Ad-tA or Ad-tA plus Ad-t-cyclin B1 and cotreated with Ad-siNkx6.1 or Ad-siRNAcontrol. (A) Representative RT-PCR analysis using primer pairs that amplify human cyclin B1, total cyclin B1 (rat and human), or glucose-6-phosphate dehydrogenase (G6PDH) as a loading control. (B) [3H]thymidine incorporation into genomic DNA. Data represent the means ± standard errors of the means from three experiments, each involving triplicate pools of 30 islets per condition. *, significant decrease in thymidine incorporation in Ad-tA-treated/Ad-siNkx6.1-treated islets compared to the Ad-siRNA control-treated islets (P < 0.0005). #, increase in Ad-siRNAcontrol-treated, cyclin B-expressing (Ad-t-cyclin B1-treated) islets compared to control (Ad-tA-treated) islets (P < 0.0001).
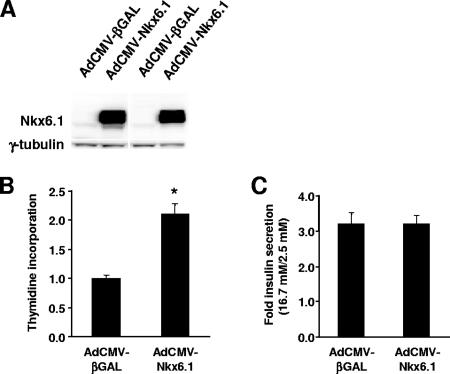
Effect of Nkx6.1 overexpression on cell replication and GSIS in human islets.
Finally, we investigated the effect of Nkx6.1 overexpression on the replication and function of human islets. As shown in Fig. 7A, treatment of human islets with AdCMV-Nkx6.1 caused a clear increase in expression of Nkx6.1 protein. This led to an increase in [3H]thymidine incorporation in the AdCMV-Nkx6.1-treated human islets, making it twice that in the AdCMV-βGAL-treated human islets (Fig. 7B). Importantly, this effect was observed consistently for four separate human islet aliquots from independent donors. In these groups of islets, levels of GSIS were identical in the AdCMV-Nkx6.1- and AdCMV-βGAL-treated groups (Fig. 7C). Thus, Nkx6.1 overexpression did not improve GSIS in human islets as it did in rat islets but, importantly, also did not cause any functional impairment, despite activation of DNA replication.
FIG. 7.
Effects of Nkx6.1 overexpression on human islets. Human islets were treated with AdCMV-Nkx6.1 or AdCMV-βGAL. (A) Immunoblot analysis showing representative data from two of four separate human islet aliquots. (B) [3H]thymidine incorporation. Data are the means from four independent experiments, each performed in triplicate. *, a P value of <0.0005 in comparison to AdCMV-βGAL-treated islets. (C) GSIS in human islets, expressed as the ratio of insulin secreted at a stimulatory glucose concentration (16.7 mM) to insulin secreted at a basal glucose concentration (2.5 mM). Data represent means ± standard errors of the means from four independent experiments performed on the same islet aliquots used in the experiments whose results are shown in panels A and B.
DISCUSSION
Pancreatic islet β-cell mass is controlled by a dynamic balance between cell proliferation and cell death (apoptosis) (6). Diabetes occurs when this balance is disrupted by autoimmune-mediated β-cell destruction (type 1 diabetes) or a failure of β-cell mass to compensate for metabolic demand (type 2 diabetes). Gaining a better understanding of molecular mechanisms that regulate β-cell replication and survival is therefore of great relevance for development of new diabetes therapies. In the present study, we provide evidence that the homeodomain transcription factor Nkx6.1 regulates adult β-cell proliferation via concerted upregulation of a host of cell cycle-regulatory genes. Moreover, unlike many prior studies in which stimulation of β-cell replication was accompanied by deterioration of β-cell function, the effects of Nkx6.1 on islet growth occurred with full retention of GSIS in human islets and enhancement of insulin secretion in rat islets.
Prior studies provide evidence that homeobox transcription factors can regulate proliferation of a variety of cell types (14), although such a role has not been described previously for Nkx6.1. Both direct and indirect modes of regulation appear to be possible. For example, the homeobox protein Oct10 has been shown to regulate the cyclin D1 gene via direct interaction with its promoter (32), whereas the degradation of cyclin A, B1, and E proteins is mediated through protein-protein interactions with the homeobox protein ESXR1 (36). These studies provide a broad precedent for the idea that Nkx6.1, a homeobox protein, might influence β-cell proliferation via direct or indirect regulation of cell cycle factors, but our studies are the first to show that such mechanisms actually exist and are operative in adult β cells. Interestingly, targeted disruption of the Nkx6.1 gene resulted in mice with pancreatic islets that retained only 6% of the normal complement of β cells (41). The decrease in β-cell mass was accompanied by a corresponding decrease in the number of BrdU-positive cells, suggesting that the loss of Nkx6.1 resulted in a decrease in the capacity of β-cell precursors to proliferate and differentiate into insulin-expressing cells. The present study helps to explain these prior results by demonstrating that Nkx6.1 can simultaneously enhance β-cell replication and function.
Despite the critical role of Nkx6.1 in β-cell development, little has been reported about its target genes prior to the present study. The microarray study reported herein reveals that in the adult β cell, Nkx6.1 functions as both an enhancer and a suppressor of gene expression to nearly equal degrees (187 genes suppressed by ≥50% and 156 genes upregulated ≥2-fold). Among the upregulated genes, 37 had ontology classifications related to proliferation, including genes encoding cyclins A2, B1, B2, and E1, Cdk1, Cdk2, Cdc6, Cdc25a, and PTTG1, whereas only seven of the suppressed genes fell into this category. Figure 8 demonstrates schematically that Nkx6.1-upregulated genes have roles in all phases of the cell cycle, from G1 to M, and also summarizes our findings that Nkx6.1 can regulate these target genes by a diverse array of mechanisms, including direct interaction (those encoding cyclins A2 and B1) and alterations in protein levels independent of changes in mRNA (that encoding cyclin D2). The precise mechanisms by which Nkx6.1 regulates expression of other growth-related genes listed above and in Table S1 in the supplemental material remain to be investigated.
The foregoing findings were unanticipated in light of the broadly held view that Nkx6.1 functions primarily as a transcriptional repressor (34, 44) but are made more understandable by other recent structure/function studies. Nkx6.1 is a 364-residue polypeptide with four distinct domains. The homeodomain of Nkx6.1 binds to TAAT- or ATTA-containing promoter elements (27, 34), similar to core DNA-binding elements in other mammalian homeobox proteins (14). Further specificity for Nkx6.1 binding to DNA is derived from flanking nucleotides, which extend the core motif to CATTTAATTACCCT (34). The homeodomain fused to the VP16 activation domain activates reporter constructs containing multiple copies of the TAAT-containing consensus sequence (27). However, in the same assay system, full-length Nkx6.1 is a potent transcriptional repressor (34), demonstrating that the homeodomain is necessary for DNA recognition but that other domains regulate transcriptional activity, including the N-terminal repressor domain, the COOH-terminal binding interference domain region that acts to decrease the binding affinity of Nkx6.1 to its DNA target, and the COOH-terminal activation domain, which is required for Nkx6.1-mediated activation of gene expression (23). The Nkx6.1-extrinsic factors, as opposed to the Nkx6.1-intrinsic factors, that allow repressor or activator functions to predominate in the context of specific target genes remain to be established.
Our studies appear to have defined a pathway for stimulating β-cell proliferation that is distinct from another prominent and recently emergent mechanism in which Akt1/protein kinase B stimulates islet β-cell proliferation via activation of the cyclin-dependent kinase Cdk4, which interacts with the D cyclins (5, 15, 43). Other studies have confirmed that transgenic manipulation of D cyclins or Cdk4 affects islet growth and that cyclins D1 and D2 are essential for postnatal expansion of β-cell mass (17, 29, 39). Moreover, adenovirus-mediated overexpression of hepatocyte growth factor, which causes upregulation of D cyclins, or overexpression of cyclin D1/Cdk4 in rodent islets increases β-cell replication while enhancing or maintaining the secretory function, respectively (12, 16). The growth-promoting effects of Nkx6.1 would appear to be mediated by a pathway that is largely distinct from that activated by hepatocyte growth factor or Akt1, since Nkx6.1 does not increase cyclin D1 or Cdk4 expression. The effect of Nkx6.1 instead seems to involve a broad array of effects on a distinct group of cell cycle-regulatory genes, including those encoding cyclins A, B, D2, and E and the ancillary cell cycle-regulatory genes encoding Cdk1, Cdk2, Cdc6, Cdc25a, and PTTG1. Time course studies revealed that cyclin E1 mRNA is upregulated in the first 24 h of Nkx6.1 overexpression, whereas cyclins A and B were upregulated at later time points (72 to 96 h) and cyclin D was never induced at the mRNA level. In addition, adenovirus-mediated overexpression of cyclin E was sufficient to cause a strong stimulation of [3H]thymidine incorporation. Thus, the findings overall are consistent with a critical initiator role for cyclin E rather than D cyclins in mediating the effects of Nkx6.1. Interestingly, the cyclin E gene is not one of the genes that appear to interact with Nkx6.1 directly, based on ChIP studies performed to date. Explanations for this could include interaction of Nkx6.1 with regions of the cyclin E promoter not contained in genomic fragments studied to date, interactions only at earlier time points (the ChIP studies were performed 96 h after Nkx6.1 expression), or indirect effects of Nkx6.1 on other regulatory genes. The details of this mechanism remain to be defined.
An important finding of the present work was that Nkx6.1 overexpression is sufficient to stimulate cell division in both human and rodent islets. Interestingly, Nkx6.1 overexpression did not enhance GSIS in human islets as it did in rat islets, but neither did it cause impairment of insulin secretion. The lesser stimulation of islet cell replication and the lack of enhancement of GSIS by AdCMV-Nkx6.1 in human islets could be due to the efficiency of β-cell gene transfer in human islets being lower than that in rat islets, to subtle differences in the structure and function of hamster Nkx6.1 (the product of the gene contained in the AdCMV-Nkx6.1 adenovirus) compared to the those of the human protein, or to different levels of expression of cell cycle inhibitory or “pocket” proteins, such as p27kip1 or retinoblastoma protein (11). Also, the genes involved in Nkx6.1-mediated enhancement of GSIS in rat islets remain to be identified. Given the strong suppression of GSIS observed for islets that were induced to grow by oncogene expression or application of certain growth factors or matrix manipulations (21), the suppressor functions of Nkx6.1 may be used to maintain or enhance GSIS via control of genes that are normally induced in response to β-cell proliferation. These ideas remain to be investigated in future studies.
In closing, we have described a novel role for the transcription factor Nkx6.1 in the regulation of β-cell proliferation and function and demonstrated broad-scale gene repressor and activator functions of this transcription factor in the adult β cell. These findings suggest that modulation of Nkx6.1 expression or activity or alteration in Nkx6.1 target gene expression could play a role in the etiologies of both type 1 and type 2 diabetes, as dramatic changes in β-cell mass and function are at the heart of both diseases. Consistent with these ideas, Nkx6.1 expression is markedly decreased in islets of two models of β-cell dysfunction, the partially pancreatectomized rat and the Zucker diabetic fatty rat (26, 42). From the therapeutic perspective, development of methods for expansion of islet β-cell mass has been a long-sought-after but highly elusive goal (21). A wide array of methods have been applied but have resulted almost universally in the loss of differentiated functions in inverse proportion to success in promoting replication (3, 13). Surprisingly, the Nkx6.1 gene seems to be a gene with growth-promoting properties that also contributes to maintenance of the mature β-cell phenotype, an ideal combination for enhancing β-cell function and mass in the context of both major forms of diabetes.
Supplementary Material
Acknowledgments
We thank Tony Means, Sally Kornbluth, and Larry Moss, Duke University Medical Center, for critical reading of the manuscript, and Lisa Poppe and Helena Winfield for expert technical assistance. We are also grateful to Susan Bonner-Weir (Joslin Diabetes Center) and Irene Cozar-Castellano (University of Pittsburgh) for helpful advice on the islet immunohistochemistry studies, Holly Dressman and Zhengzheng Wei (Duke University Medical Center) for their help with the microarray analysis, and Joseph Nevins (Duke University Medical Center) for provision of the AdCMV-CycE adenovirus.
This work was supported by NIH grants U01 DK56047 and P01 DK58398, grant 17-2007-1026 from the Juvenile Diabetes Research Foundation (to C.B.N.), and NIH grant R01 DK60581 (to R.G.M.).
Footnotes
Published ahead of print on 17 March 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aleem, E., H. Kiyokawa, and P. Kaldis. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7831-836. [DOI] [PubMed] [Google Scholar]
- 2.Bain, J. R., J. C. Schisler, K. Takeuchi, C. B. Newgard, and T. C. Becker. 2004. An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes 532190-2194. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, G. M., A. M. Montgomery, A. D. Lopez, E. Hao, B. Perez, M. L. Just, J. R. Lakey, M. E. Hart, and A. Hayek. 2002. A novel approach to increase human islet cell mass while preserving beta-cell function. Diabetes 513435-3439. [DOI] [PubMed] [Google Scholar]
- 4.Becker, T. C., R. J. Noel, W. S. Coats, A. M. Gomez-Foix, T. Alam, R. D. Gerard, and C. B. Newgard. 1994. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 43A161-189. [DOI] [PubMed] [Google Scholar]
- 5.Bernal-Mizrachi, E., W. Wen, S. Stahlhut, C. M. Welling, and M. A. Permutt. 2001. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Investig. 1081631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir, S. 2001. Beta-cell turnover: its assessment and implications. Diabetes 50(Suppl. 1)S20-S24. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti, S. K., J. C. James, and R. G. Mirmira. 2002. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J. Biol. Chem. 27713286-13293. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti, S. K., and R. G. Mirmira. 2003. Transcription factors direct the development and function of pancreatic beta cells. Trends Endocrinol. Metab. 1478-84. [DOI] [PubMed] [Google Scholar]
- 10.Clark, S. A., C. Quaade, H. Constandy, P. Hansen, P. Halban, S. Ferber, C. B. Newgard, and K. Normington. 1997. Novel insulinoma cell lines produced by iterative engineering of GLUT2, glucokinase, and human insulin expression. Diabetes 46958-967. [DOI] [PubMed] [Google Scholar]
- 11.Cozar-Castellano, I., N. Fiaschi-Taesch, T. A. Bigatel, K. K. Takane, A. Garcia-Ocana, R. Vasavada, and A. F. Stewart. 2006. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr. Rev. 27356-370. [DOI] [PubMed] [Google Scholar]
- 12.Cozar-Castellano, I., K. K. Takane, R. Bottino, A. N. Balamurugan, and A. F. Stewart. 2004. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 53149-159. [DOI] [PubMed] [Google Scholar]
- 13.de la Tour, D., T. Halvorsen, C. Demeterco, B. Tyrberg, P. Itkin-Ansari, M. Loy, S. J. Yoo, E. Hao, S. Bossie, and F. Levine. 2001. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol. Endocrinol. 15476-483. [DOI] [PubMed] [Google Scholar]
- 14.Del Bene, F., and J. Wittbrodt. 2005. Cell cycle control by homeobox genes in development and disease. Semin. Cell Dev. Biol. 16449-460. [DOI] [PubMed] [Google Scholar]
- 15.Fatrai, S., L. Elghazi, N. Balcazar, C. Cras-Meneur, I. Krits, H. Kiyokawa, and E. Bernal-Mizrachi. 2006. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 55318-325. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Ocana, A., K. K. Takane, V. T. Reddy, J. C. Lopez-Talavera, R. C. Vasavada, and A. F. Stewart. 2003. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces beta cell death. J. Biol. Chem. 278343-351. [DOI] [PubMed] [Google Scholar]
- 17.Georgia, S., and A. Bhushan. 2004. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Investig. 114963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgia, S., and A. Bhushan. 2006. p27 regulates the transition of beta-cells from quiescence to proliferation. Diabetes 552950-2956. [DOI] [PubMed] [Google Scholar]
- 19.Habener, J. F., D. M. Kemp, and M. K. Thomas. 2005. Transcriptional regulation in pancreatic development. Endocrinology 1461025-1034. [DOI] [PubMed] [Google Scholar]
- 20.Hohmeier, H. E., H. BeltrandelRio, S. A. Clark, R. Henkel-Rieger, K. Normington, and C. B. Newgard. 1997. Regulation of insulin secretion from novel engineered insulinoma cell lines. Diabetes 46968-977. [DOI] [PubMed] [Google Scholar]
- 21.Hohmeier, H. E., and C. B. Newgard. 2005. Islets for all? Nat. Biotechnol. 231231-1232. [DOI] [PubMed] [Google Scholar]
- 22.Iype, T., J. Francis, J. C. Garmey, J. C. Schisler, R. Nesher, G. C. Weir, T. C. Becker, C. B. Newgard, S. C. Griffen, and R. G. Mirmira. 2005. Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J. Biol. Chem. 28016798-16807. [DOI] [PubMed] [Google Scholar]
- 23.Iype, T., D. G. Taylor, S. M. Ziesmann, J. C. Garmey, H. Watada, and R. G. Mirmira. 2004. The transcriptional repressor Nkx6.1 also functions as a deoxyribonucleic acid context-dependent transcriptional activator during pancreatic β-cell differentiation: evidence for feedback activation of the nkx6.1 gene by Nkx6.1. Mol. Endocrinol. 181363-1375. [DOI] [PubMed] [Google Scholar]
- 24.Jensen, J., P. Serup, C. Karlsen, T. F. Nielsen, and O. D. Madsen. 1996. mRNA profiling of rat islet tumors reveals Nkx 6.1 as a beta-cell-specific homeodomain transcription factor. J. Biol. Chem. 27118749-18758. [DOI] [PubMed] [Google Scholar]
- 25.Jin, P., S. Hardy, and D. O. Morgan. 1998. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 141875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonas, J. C., A. Sharma, W. Hasenkamp, H. Ilkova, G. Patane, R. Laybutt, S. Bonner-Weir, and G. C. Weir. 1999. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J. Biol. Chem. 27414112-14121. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen, M. C., H. Vestergard Petersen, J. Ericson, O. D. Madsen, and P. Serup. 1999. Cloning and DNA-binding properties of the rat pancreatic beta-cell-specific factor Nkx6.1. FEBS Lett. 461287-294. [DOI] [PubMed] [Google Scholar]
- 28.Joseph, J. W., V. Koshkin, M. C. Saleh, W. I. Sivitz, C. Y. Zhang, B. B. Lowell, C. B. Chan, and M. B. Wheeler. 2004. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J. Biol. Chem. 27951049-51056. [DOI] [PubMed] [Google Scholar]
- 29.Kushner, J. A., M. A. Ciemerych, E. Sicinska, L. M. Wartschow, M. Teta, S. Y. Long, P. Sicinski, and M. F. White. 2005. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol. Cell. Biol. 253752-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebovitz, H. E. 2004. Chapter 76, Insulin secretagogues: sulfonylureas, meglitinides, and phenylalanine derivatives, p. 1107-1138. In D. LeRoith, S. I. Taylor, and J. M. Olefsky (ed.), Diabetes mellitus, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 31.Leone, G., J. DeGregori, L. Jakoi, J. G. Cook, and J. R. Nevins. 1999. Collaborative role of E2F transcriptional activity and G1 cyclindependent kinase activity in the induction of S phase. Proc. Natl. Acad. Sci. USA 966626-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magne, S., S. Caron, M. Charon, M. C. Rouyez, and I. Dusanter-Fourt. 2003. STAT5 and Oct-1 form a stable complex that modulates cyclin D1 expression. Mol. Cell. Biol. 238934-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milburn, J. L., Jr., H. Hirose, Y. H. Lee, Y. Nagasawa, A. Ogawa, M. Ohneda, H. BeltrandelRio, C. B. Newgard, J. H. Johnson, and R. H. Unger. 1995. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J. Biol. Chem. 2701295-1299. [DOI] [PubMed] [Google Scholar]
- 34.Mirmira, R. G., H. Watada, and M. S. German. 2000. Beta-cell differentiation factor Nkx6.1 contains distinct DNA binding interference and transcriptional repression domains. J. Biol. Chem. 27514743-14751. [DOI] [PubMed] [Google Scholar]
- 35.Naber, S. P., J. M. McDonald, L. Jarett, M. L. McDaniel, C. W. Ludvigsen, and P. E. Lacy. 1980. Preliminary characterization of calcium binding in islet-cell plasma membranes. Diabetologia 19439-444. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa, H., S. Ashizawa, M. Naito, M. Yanagihara, N. Ohnishi, T. Maeda, Y. Matsuda, Y. Jo, H. Higashi, A. Kakita, and M. Hatakeyama. 2004. Paired-like homeodomain protein ESXR1 possesses a cleavable C-terminal region that inhibits cyclin degradation. Oncogene 236590-6602. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen, I. L., R. Klinck, J. Hecksher-Sorensen, S. Zahn, O. D. Madsen, P. Serup, and M. C. Jorgensen. 2006. Generation and characterization of monoclonal antibodies against the transcription factor Nkx6.1. J. Histochem. Cytochem. 54567-574. [DOI] [PubMed] [Google Scholar]
- 38.Qiu, M., K. Shimamura, L. Sussel, S. Chen, and J. L. Rubenstein. 1998. Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech. Dev. 7277-88. [DOI] [PubMed] [Google Scholar]
- 39.Rane, S. G., P. Dubus, R. V. Mettus, E. J. Galbreath, G. Boden, E. P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 2244-52. [DOI] [PubMed] [Google Scholar]
- 40.Rudnick, A., T. Y. Ling, H. Odagiri, W. J. Rutter, and M. S. German. 1994. Pancreatic beta cells express a diverse set of homeobox genes. Proc. Natl. Acad. Sci. USA 9112203-12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sander, M., L. Sussel, J. Conners, D. Scheel, J. Kalamaras, F. Dela Cruz, V. Schwitzgebel, A. Hayes-Jordan, and M. German. 2000. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 1275533-5540. [DOI] [PubMed] [Google Scholar]
- 42.Schisler, J. C., P. B. Jensen, D. G. Taylor, T. C. Becker, F. K. Knop, S. Takekawa, M. German, G. C. Weir, D. Lu, R. G. Mirmira, and C. B. Newgard. 2005. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc. Natl. Acad. Sci. USA 1027297-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuttle, R. L., N. S. Gill, W. Pugh, J. P. Lee, B. Koeberlein, E. E. Furth, K. S. Polonsky, A. Naji, and M. J. Birnbaum. 2001. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat. Med. 71133-1137. [DOI] [PubMed] [Google Scholar]
- 44.Vallstedt, A., J. Muhr, A. Pattyn, A. Pierani, M. Mendelsohn, M. Sander, T. M. Jessell, and J. Ericson. 2001. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron 31743-755. [DOI] [PubMed] [Google Scholar]
- 45.Weir, G. C., and S. Bonner-Weir. 2004. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53S16-S21. [DOI] [PubMed] [Google Scholar]
- 46.Zhong, L., S. Georgia, S. I. Tschen, K. Nakayama, and A. Bhushan. 2007. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J. Clin. Investig. 1172869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.