Abstract
We have identified a highly conserved phenylalanine in motif IV of the DEAD-box helicases that is important for their enzymatic activities. In vivo analyses of essential proteins in yeast showed that mutants of this residue had severe growth phenotypes. Most of the mutants also were temperature sensitive, which suggested that the mutations altered the conformational stability. Intragenic suppressors of the F405L mutation in yeast Ded1 mapped close to regions of the protein involved in ATP or RNA binding in DEAD-box crystal structures, which implicated a defect at this level. In vitro experiments showed that these mutations affected ATP binding and hydrolysis as well as strand displacement activity. However, the most pronounced effect was the loss of the ATP-dependent cooperative binding of the RNA substrates. Sequence analyses and an examination of the Protein Data Bank showed that the motif IV phenylalanine is conserved among superfamily 2 helicases. The phenylalanine appears to be an anchor that maintains the rigidity of the RecA-like domain. For DEAD-box proteins, the phenylalanine also aligns a highly conserved arginine of motif VI through van der Waals and cation-π interactions, thereby helping to maintain the network of interactions that exist between the different motifs involved in ATP and RNA binding.
The putative RNA helicases constitute a ubiquitous group of enzymes that are associated with all of the processes involving RNA, including transcription, ribosome biogenesis, mRNA splicing, RNA export, translation, and RNA degradation (reviewed in references 10, 30, 37, 42, and 46). They are characterized by two linked RecA-like domains (domains 1 and 2) that contain seven to eight conserved motifs (I, Ia, Ib, II, III, IV, V, and VI), and they are closely related to many of the DNA helicases (43). These RNA and DNA helicases are classified into either superfamily 1 (SF1) or superfamily 2 (SF2) according to the characteristics of the conserved motifs (17). Although some DNA and RNA helicases are known to have processive unwinding activity, most putative helicases show no or only weak activity in in vitro tests (46). Because contiguous double-stranded RNA is rare in vivo, RNA helicases may play a role primarily in RNA-RNA or RNA-protein remodeling (20, 25, 40, 48, 50) or as place setters to ensure the unidirectionality of complex reactions such as splicing or ribosomal biogenesis (46). Nevertheless, all of these family members are collectively known as helicases in the literature (17). Hence, we will use the term helicase in this paper as a generic term rather than as a functional description.
The largest family of RNA helicases is the DEAD-box proteins of SF2 that typically have the sequence Asp-Glu-Ala-Asp (DEAD) in motif II (31). In addition to the eight conserved motifs, they contain three other features that are characteristic of this family: the upstream Q motif, which is involved in adenine recognition (11, 45), a conserved GG between motifs Ia and Ib (31), and a conserved QxxR, where x is any amino acid, between motifs IV and V (6). Recently, solved crystal structures have become available with both a bound RNA substrate and a bound ATP analog (1, 4, 41). These structures show remarkably similar interactions with the ligands, and they help clarify the roles of the different motifs. Thus, the Q motif and motifs I, II, V, and VI bind the nucleotide, and the GG and QxxR motifs and motifs Ia, Ib, IV, and V bind the RNA. Motif III forms intramolecular interactions. Many of the motifs also are part of a network of interdomain interactions. The interactions between the protein and the RNA are nonspecific, and most involve the phosphodiester backbone or the 2′ hydroxyl of the ribose group. In all cases, the 3′ end of the RNA shows significant bending around the phosphodiester backbone, and the bases are unstacked. This has been interpreted as a possible mechanism for a helicase activity (41). However, it is unclear whether DEAD-box proteins physically displace duplexes, and the kinking of the phosphodiester backbone could provide a mechanism for preventing the protein from slipping or sliding on the single-stranded RNA (46). It is worth noting that the bending is associated with nucleotide binding; it is not clear what role ATP hydrolysis plays in the reaction.
Although the cocrystallization of the proteins with their ligands represents a major advancement in the field, many questions remain. The solved crystal structures represent a single, thermodynamically stable conformation that does not tell us how the protein uses the energy of ATP binding or hydrolysis to carry out the conformational changes needed for its enzymatic activity. Moreover, the roles of many of the highly conserved residues within and outside the motifs have not been elucidated. Some of them may be involved in protein-protein interactions that occur within large complexes. This can be seen in the eIF4AIII (DDX48) crystal structures with bound cofactors (1, 4). However, the vast majority of these interacting residues are not conserved within the DEAD-box family (N. K. Tanner, unpublished data). This is not surprising, because helicases have diverse cellular locations and roles. Thus, the conserved residues must play roles in common with most, if not all, DEAD-box helicases and potentially with other helicase families as well.
One such residue with no known role is a highly conserved phenylalanine in motif IV that inevitably follows two aliphatic residues. A similar pattern is seen in many other families of SF2 helicases (17, 46). A previous genetic screen in our laboratory of the yeast Saccharomyces cerevisiae DED1 gene revealed that these encoded residues were important for cell viability (R. Bock and P. Gurtner, unpublished data). In the solved crystal structures of various SF2 helicases, the aliphatic residues and the phenylalanine are part of the β-sheet core of the RecA-like domain 2. The solved crystal structures of DEAD-box helicases with RNA substrates show the same structural context; moreover, two residues following the phenylalanine, which are found in a loop-helix region, interact with the RNA substrate (1, 4, 41). These observations suggested that the mutations in this region were affecting RNA binding. However, the effects in vivo were more profound than expected from the limited interactions seen in the structures. Therefore, we decided to undertake biochemical analyses of mutants of the conserved phenylalanine.
Ded1 is an essential protein in the yeast S. cerevisiae; it was first identified as an extragenic suppressor of a Prp8 mutant, which implied a role in mRNA splicing (24), but it also was implicated in the transcription of polymerase III RNAs (47). It was later shown to have a general role in translation initiation (9, 13) and in the 40S ribosome scanning step (3). As determined by sequence alignments, Ded1 belongs to a subfamily of DEAD-box proteins that are probable orthologs (2, 27, 28); these consist of mouse PL10, mammalian DDX3X and DDX3Y, Xenopus An3, Drosophila melanogaster Belle, and Schizosaccharomyces pombe Ded1. It is slightly more distantly related to Drosophila Vasa, for which there is a solved crystal structure with bound substrates (41). Vasa weakly complements a DED1 deletion when overexpressed (J. Banroques, unpublished data). Finally, the S. cerevisiae DED1 deletion is partially complemented by the overexpression of the S. cerevisiae Dbp1 protein, which is an apparent paralog of Ded1 (23).
The yeast Ded1 can be overexpressed in Escherichia coli and purified to high yield. It contains a strong, RNA-dependent ATPase activity and has a high affinity for RNA substrates (11, 22), which makes it a good subject for these studies. We randomly mutated the conserved phenylalanine and analyzed the mutants in vivo and in vitro. In vitro analyses included RNA-dependent ATPase activity, strand displacement, and electrophoretic mobility shift assays (EMSA). Moreover, we obtained intragenic suppressors of the partially viable leucine mutant, which are located in various positions in the protein, and analyzed them in vitro as well. Data were analyzed further in silico with the solved crystal structure of the related Vasa protein (41). We found that mutations of the conserved phenylalanine affected ATP hydrolysis, strand displacement, and the ATP-dependent cooperative binding of RNA substrates. We confirmed the importance of the phenylalanine by making similar mutations in two other essential DEAD-box proteins in yeast, eIF4A and Dbp2. These data indicated that the phenylalanine occupies a critical position in the helicase core that probably maintains the intramolecular interactions between motifs and thereby helps link ATP binding and hydrolysis with the conformational changes needed for activity.
MATERIALS AND METHODS
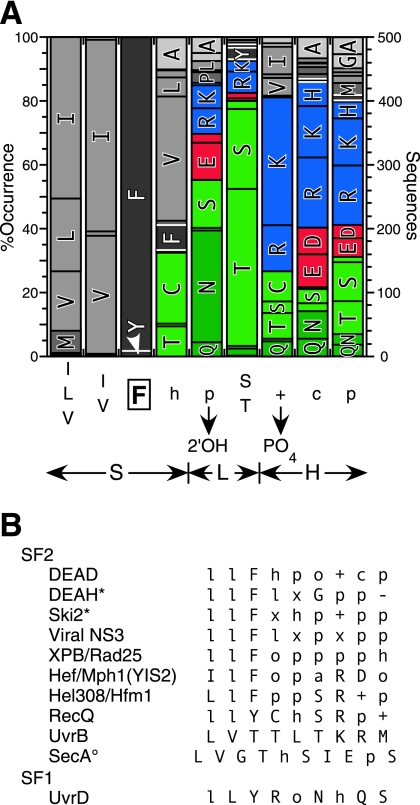
Sequence alignments of motif IV.
A BLAST search was done on the NCBI database (www.ncbi.nlm.nih.gov/BLAST/) using the helicase core sequence of Ded1 (amino acids 139 to 531) and the nonredundant GenBank database to obtain 500 unique sequences (BLAST-500; expectation value, ≤4e−63). These sequences were aligned according to the previously defined motifs (10). Alignments generally were unambiguous because the motifs occurred within fixed distances of each other and because of the presence of conserved residues outside the conserved motifs. However, some sequences had significant insertions between motifs, which were particularly common around motif IV. Because motif IV more often is defined simply as two aliphatic groups (I, L, or V) followed by a phenylalanine (l·l·F, where l is an aliphatic group) and because such sequences sometimes were found within the insertions, we were not always able to clearly identify the motif. Moreover, we were interested in revealing variants that did not contain a conserved phenylalanine. Fortunately, a number of solved crystal structures of DEAD-box proteins are available in the RCSB Protein Data Bank database (www.rcsb.org/pdb/home/home.do) (reviewed in references 10, 21, and 26). We examined them to determine the structural context of motif IV with SwissPdbViewer (www.expasy.org/spdbv/) (18); in all cases it occurs in a β-sheet-loop-helix structure. Therefore, we used PredictProtein (www.predictprotein.org/) (38) to determine the predicted secondary structures of the ambiguous sequences and to identify the residues that had the correct context for motif IV. We then determined the frequency of each residue using StatAln (www.expasy.ch/tools/stataln.html; Alexandre Gattiker). We did an additional Blast2 search on the ExPASy site against the full database with yeast Spb4 (because it had an unusual motif IV) to obtain 76 new sequences, for a total of 576.
We examined the crystal structures of other families of SF2 helicases in the Protein Data Bank, and we conducted other Blast2 searches. Many of these sequences were retrieved and then aligned by Clustal XXL on the chEMBnet site (www.ch.embnet.org/software/ClustalW-XXL.html). Motif IV was identified based on the sequence, on the structural context in the solved structures, and on previous publications. The consensus sequences were determined using the Bork website (http://coot.embl.de/Alignment/consensus.html).
Cloning, vectors, and strains.
All yeast manipulations were done using standard techniques (19). The DED1 coding region (1,850 bp) plus 470 bp upstream and 420 bp downstream of the flanking yeast genomic DNA and also containing the DED1 promoter and terminator was cloned into the HindIII and SalI sites of YCplac111 (CEN-LEU) to yield pRB34-DED1 (a gift of Ronald Bock). This plasmid was used as a template for all subsequent PCR amplifications. Mutations were introduced by a two-step, overlapping fusion PCR using Pfu polymerase as previously described (11, 45). Mutations were introduced with oligonucleotides completely randomized at the three positions of the codon corresponding to the amino acid at position 405. Additional oligonucleotides were used to insert specifically tryptophan and histidine. The amplified DNAs were digested with AgeI and then cloned into the same site of the pRB34-DED1 plasmid. Similarly, we made mutations at equivalent positions of two other essential yeast proteins, eIF4A (F266L) and Dbp2 (F366L), in the previously described p415-PL-ADH yeast plasmid (45). All clones were verified by sequencing. The Ded1-R486A mutation in p415-PL-ADH was a kind gift of Pierre-Alain Braillard.
Ded1 plasmids were transformed into the ΔDED1/ΔDBP1 strain (ded1::HIS3 dbp1::KANMX6) (11) containing the DBP1 open reading frame (ORF) cloned into the multicopy p416-TEF plasmid (34); using the DBP1 plasmid instead of DED1 minimized recombination between plasmids and the appearance of pseudorevertants. The eIF4A and Dbp2 plasmids were transformed, respectively, into the ΔTIF1/ΔTIF2 strain SS13-3A (tif1::HIS3 tif2::ADE2) containing the YCplac33-TIF1 plasmid (45) and the ΔDBP2 strain (DBP2::KANMX6) containing the DBP2 ORF, including the intron 1,100 bp residues upstream and 880 bp downstream in the YCplac33 plasmid. The transformants that could grow on synthetic minimal medium plates lacking leucine (SD-Leu) were selected and streaked on 5-fluoroorotic acid (5-FOA) medium and tested for their ability to grow at 18, 30, and 36°C. Selected constructs were verified by sequencing the recovered plasmids.
Isolation of intragenic suppressors.
Intragenic suppressors of the conditional ded1-F405L mutation were obtained by two methods. In the first, mutagenic PCR was performed using Taq DNA polymerase and two oligonucleotides hybridized at either end of the ded1-F405L ORF. Four cycles of mutagenic PCR were done using 6.5 mM MgCl2, 0.5 mM MnCl2, 1 mM dCTP and dTTP, and 0.2 mM dATP and dGTP. Thirty more cycles then were done, using 2% of the mutagenized PCR as the template, under normal PCR conditions. This library of DNA fragments was digested and subcloned into the HindIII and SalI sites of the pRB34-LEU CEN vector. The DNA was amplified in E. coli and then used to transform the ΔDED1/ΔDBP1 yeast strain containing the DBP1 plasmid. Transformants were selected and screened as described above.
In the second method, several independent yeast cultures of the ΔDED1/ΔDBP1 strain containing the ded1-F405L plasmid, which were derived from single colonies, were irradiated with UV light for 10 s to obtain approximately 50% survival. About 500 to 1,000 cells were spread on SD-Leu plates and incubated at the permissive temperature (30°C) for 3 to 6 days. The colonies obtained then were restreaked on SD-Leu plates and incubated at 18, 30, and 36°C. Plasmids were recovered from cultures that grew better than those of the mutant; these were reintroduced into the ΔDED1/ΔDBP1 strain containing the DED1 plasmid and selected on 5-FOA-containing plates at the three temperatures. For both methods, plasmids supporting enhanced growth were recovered and sequenced.
Protein expression and purification.
The AgeI fragments of DED1, which contained the mutations, were cloned into the equivalent sites of the pET-22b expression vector (Novagen) containing wild-type DED1. The TIF1 constructs were subcloned into the NdeI and XhoI sites of pET22b. The Origami (DE3) and Rosetta (DE3) E. coli strains (Novagen) were used for expressing the proteins. Expression and purification were performed as previously described (11, 45). Protein concentrations were determined by the Bio-Rad protein assay, using bovine serum albumin (BSA) as a standard. The proteins were judged to be 90 to 95% pure on Laemmli sodium dodecyl sulfate (SDS) polyacrylamide gels.
RNA-dependent ATPase assays.
For RNA-dependent ATPase assays, we used a colorimetric assay based on molybdate-malachite green and used total yeast RNA (Type III; Sigma) and ATP (GE-Pharmacia) as substrates as previously described for Ded1 (11) and for eIF4A (45). Reaction mixtures were incubated at 30°C for various times, and reactions were stopped by making the reaction mix 4.5 mM in EDTA and placing it on ice. Data were analyzed using Kaleidagraph 4.02 (Synergy). Time courses at each ATP concentration were repeated three or more independent times. Time courses also were carried out in the absence of RNA to determine the background hydrolysis of the ATP and in the absence of ATP to monitor the contribution due to RNA degradation. In both cases, the signals were only a small percentage of the reactions including ATP and RNA; these background signals were subtracted from the subsequent calculations of the reaction rates.
Strand displacement assay.
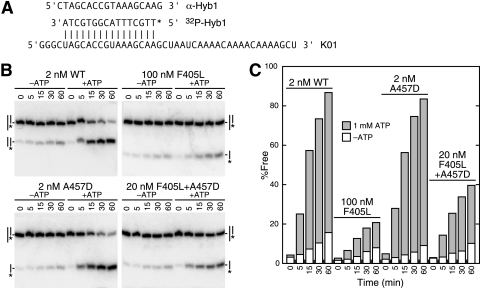
A 44-nucleotide-long RNA (R1; 5′ GGG CGA AUU CAA AAC AAA ACA AAA CUA GCA CCG UAA AGC AAG CU 3′) was transcribed off a HindIII-cut plasmid, polyacrylamide gel electrophoresis (PAGE) purified, and hybridized to a 32P-end-labeled, 16-nucleotide-long DNA oligonucleotide (Hyb1; 5′-TTG CTT TAC GGT GCT A-3′) as previously described to form a 5′ single-stranded RNA with a 3′ RNA-DNA duplex (11). In addition, we transcribed another 44-nucleotide-long RNA (see Fig. 5) that formed a 5′ DNA-RNA duplex with the same oligonucleotide. Reaction conditions were as previously described, with 50 nM of RNA-DNA duplexes (11); because the duplex efficiently annealed during the course of the reaction, we added a 25-fold excess of cold Hyb1 or another oligonucleotide that was complementary to Hyb1 (see Fig. 5A) as a trap to block the reformation of the labeled duplex. Reactions were carried out at 30 or 37°C for various times, the products were separated by PAGE at 4°C, and the corresponding bands were quantified with a Cyclone phosphorimager (Packard) and Optiquant software (Packard). Because the proteins had a low intrinsic affinity for the RNA substrates, we often saw ATP-independent strand displacement that was dependent on the protein concentration. This was low for the Ded1 wild-type protein, which was used at a low protein/duplex ratio, but gave significant background activity for many of the poorly active mutants that were used at higher protein concentrations. Moreover, there was some strand exchange with the oligonucleotide trap, which resulted in an apparent protein-independent displacement of up to 10% (depending on the duplex and temperature) during the time course. This background activity was subtracted from the results.
FIG. 5.
Strand displacement assay. (A) The 5′ duplex consisted of K01 RNA and the 32P-labeled DNA oligonucleotide Hyb1. An excess of either unlabeled Hyb1 or α-Hyb1 oligonucleotide, which is complementary to Hyb1, was used as a trap to prevent the rehybridization of the labeled oligonucleotide with the RNA. (B) Strand displacement activity of the 5′ duplex with the wild-type (WT) and mutant proteins. The protein concentrations were varied to optimize the sensitivity. Reactions were done with and without 1 mM ATP, the duplex concentration was 50 nM, and the protein concentrations are as shown. Reactions were done at 30°C for the times indicated in minutes. Products were separated as described in the text. Note that an excess of α-Hyb1 was used with the wild-type protein, which formed a DNA-DNA duplex as shown (‖), and an excess of Hyb1 was used with the other proteins (|). The asterisk indicates the labeled oligonucleotide. Both traps gave similar results, but the α-Hyb1 trap gave a higher background displacement. (C) Quantification of the results shown in panel B.
Measuring RNA binding by EMSA.
Approximately 5 nmol of PAGE-purified R1 RNA was dephosphorylated with 30 U of calf intestinal phosphatase (Promega) for 1.5 h at 37°C, extracted with buffer-saturated phenol-chloroform-isoamyl alcohol (Fluka), precipitated with ethanol, centrifuged, washed with 70% ethanol, dried, and resuspended in 20 mM Tris base, pH 8.0, with 0.1 mM EDTA. We kinased 125 pmol of this material with 20 μCi of [γ-32P]ATP and 20 U of T4 polynucleotide kinase (Biolabs) for 1 h at 37°C in the presence of 1 U/μl RNasin (Promega). Similarly, we directly end labeled two 25-nucleotide-long RNAs that were chemically synthesized (RNA01 and RNA02; PAGE purified; Dharmacon). Reaction mixtures were stored at −20°C until needed.
Because the specific activity of the labeled RNA limited the usable range of concentrations, we typically used a constant RNA concentration (5 nM) and varied the protein concentration. The protein and RNA were incubated together in 20-μl volumes containing 20 mM Tris base, pH 7.5, 70 mM potassium acetate, 2 mM MgCl2, 1 mM dithiothreitol, and 0.1 μg/μl BSA for 20 min on ice with no nucleotide, with 5 mM ADP (Sigma), or with 5 mM adenosine 5′-β,γ-imino-triphosphate (AMP-PNP; Roche). In some cases, 1 U/μl of RNasin was added to reaction mixtures containing AMP-PNP because of RNase contamination in the product. After this, 5 μl of 50% glycerol containing 0.02% bromophenol blue dye was added, and 20 μl of the sample was loaded onto a 1.5-mm-thick 6% polyacrylamide (29:1) Mini-Protean gel (Bio-Rad) containing 25 mM Tris base, pH 8.8, and 250 mM glycine. The gel was prerun for 1 h at 70 V and 4°C and was run for 100 min with the samples. The gel was dried, subjected to autoradiography, and quantified with a phosphorimager.
Data were analyzed with Kaleidagraph. To facilitate the analyses, all of the radioactivity above the level of the free RNA was counted as retarded on the gel. This was because of variability between gels and because of the variable extents of smearing. A lane with no protein was used as a control for background radiation; it was subtracted from the other values. However, discrete bands sometimes were seen above the free RNA that probably reflected RNA secondary structures, which were still substrates for protein binding. Thus, our values slightly underestimated the RNA-protein affinities. Data were fit to the Hill equation (see Table 2). Binding assays were conducted multiple times for each protein.
TABLE 2.
RNA binding affinities of the proteinsa
| Protein | RNAb | K1/2 (without NTP) | K1/2c (with ADP) | K1/2c (with AMP-PNP) |
|---|---|---|---|---|
| Wild type | R1 | 180 ± 50 | 210 ± 30 | NDd |
| Wild type | RNA01 | 230 ± 20 | 270 ± 30 | 35 ± 12 |
| Wild type | RNA02 | 200 ± 50 | 290 ± 60 | 40 ± 14 |
| F405A | R1 | 890 ± 180 | 1000 ± 200 | 860 ± 200 |
| F405A | RNA01 | ND | ND | 700 ± 70 |
| F405L | R1 | 250 ± 30 | 220 ± 40 | 120 ± 20 |
| F405M | R1 | ND | ND | 53 ± 5 |
The K1/2s (half saturation values) were derived from the best fit of the data to the equation θ = nK[L]m/(1 + K[L]m), where θ is the fraction of RNA bound, n is the number of binding sites, [L] is the ligand concentration, K is the binding constant, and m is the Hill coefficient. Best fits were obtained with either n = 1 or n as a variable (always approximately 1). A minimum of 10% error was assumed in the protein concentrations; larger errors are the standard deviations from the means of three or more independent experiments.
RNA substrates were 44-mer R1 (5′ GGGCGAAUUCAAAACAAAACAAAACUAGCACCGUAAAGCAAGCU 3′), 25-mer RNA01 (5′ UCAUACUUUUCUUUUCUUUUCCAUC 3′), or 25-mer RNA02 (5′ GAUGGAAAAGAAAAGAAAAGUAUGA 3′).
ADP and AMP-PNP concentrations were 5 mM.
ND, not determined.
RESULTS
Sequence alignments of motif IV.
Sequence alignments were made as described in Materials and Methods, and the results are shown in Fig. 1A. The consensus sequence was two aliphatic groups, a phenylalanine, a hydrophobic group, and then five polar and charged groups (l·l·F·h·p·S/T·+·c·p) (Fig. 1A). The residues immediately following motif IV are less well conserved, but hydrophobic residues typically occur every three to four residues; these amino acids are located in regions of a helix that interact with the rest of the RecA-like core. We found a phenylalanine in 98% of the aligned sequences, a tyrosine in about 2%, and a leucine once. However, we also found sequences containing two adjacent aromatic groups in 39 instances (∼8%), with 33 of them containing the sequence l·l·F·F and six containing l·l·Y·F followed by the polar and charged residues. Our alignments were not reliable to the single-residue level, because there was very little sequence conservation outside of motif IV in this region of the proteins, which was partly a reflection of the insertions or deletions that occurred. Therefore, it was possible that the second phenylalanine was in the position equivalent to that of the phenylalanine in the other sequences.
FIG. 1.
Sequence alignments and consensus for motif IV. (A) Five hundred unique sequences were aligned as described in the text (BLAST-500). The frequency of occurrence of the predominant residues and the derived consensus are shown, where capital letters are residues, h is hydrophobic, + is positively charged, c is charged, and p is polar. F405 is boxed. The double-headed arrows indicate the structural context of the motif based on the solved crystal structures. S, β-sheet; L, loop; H, helix. The residues interacting with the RNA substrate are indicated. (B) Consensus sequences for motif IV from other families of SF2 helicases and from the UvrD family of SF1. Hel308 is classified as an Ski2-like protein, but it had little sequence homology to yeast Ski2. The nomenclature is the same as that described above; in addition, l is aliphatic, o is alcohol, − is negatively charged, and x is any residue. An asterisk indicates that there are no solved crystal structures in the Protein Data Bank. A superscript circle indicates that it is ambiguous whether the G or the T is in a position equivalent to that of the F.
Motif IV sequence in ribosomal biogenesis helicases.
The majority of the sequences with a tyrosine were found to have sequence similarity with the DEAD-box protein Spb4 in yeast and other fungi in the SwissProt database (www.expasy.ch/). We did another BLAST search, using Spb4 as bait, to obtain 76 new sequences, of which 74 contained an unusual motif IV. The vast majority of the sequences with a tyrosine (a total of 12 l·l·Y·F and 7 l·l·Y·l sequences) were Spb4-like proteins in lower eukaryotes, while the two adjacent phenylalanines (l·l·F·F) were Ddx55-like proteins (an Spb4 homolog) in higher eukaryotes. Some of the others were identified as DEAD-box proteins Has1 (Ddx18), Dbp4 (Ddx10), Drs1, and Dpb7. The alignments were unambiguous for this subfamily of proteins, because a conserved lysine or, less frequently, an arginine was found two residues upstream of motif IV (as defined in Fig. 1A), and a conserved aromatic group (tyrosine or, less frequently, a phenylalanine or tryptophan) was found three residues downstream. On this basis, we were able to determine that, of the 76 total sequences with two adjacent phenylalanines, six sequences were l·F·F·l rather than l·l·F·F. Thus, all of the identified proteins with an unusual motif IV were implicated in ribosomal biogenesis.
Motif IV sequence in other helicase families.
We noted previously that some of the other SF2 helicase families have l·l·F as the first part of the consensus sequence for motif IV (46), but we were interested in determining how widely this was true. We did additional BLAST searches as described in Materials and Methods and shown in Fig. 1B. Although a conserved phenylalanine and, less frequently, a tyrosine generally were found, they were not universally conserved among the SF2 helicases: the UvrB family has a threonine at the position equivalent to that of the phenylalanine, while the SecA family has either a glycine or threonine. Interestingly, the dengue and yellow fever subfamily of viral NS3 proteins had a tryptophan preceding the phenylalanine (V/A·W·F·V·P·S·I·K·A). Comparisons to SF1 helicases were less obvious because of the sequence divergence, particularly within motifs. Nevertheless, the UvrD family of helicases, which includes PcrA and Rep, had a motif IV (called IVa in SF1) sequence that consisted of l·l·Y followed by polar groups (Fig. 1B).
Severe growth phenotypes of motif IV mutants.
We randomly mutagenized the codon for F405 in DED1, transformed yeast cells with the DNA, selected the transformed cells on SD-Leu plates, and then streaked the resulting colonies on 5-FOA plates to eliminate cells retaining the wild-type copy of the DBP1 gene, which complements the DED1 deletion when overexpressed. About 460 colonies were tested on 5-FOA plates at 18, 30, and 36°C. More than 60% of the clones failed to grow, but we obtained about 22% wild-type or pseudo-wild-type colonies, a little more than 2% of which grew poorly at high temperatures and about 14% that grew slowly at all temperatures (Fig. 2A). The plasmid DNA was recovered from representative colonies, retransformed into yeast, replated on SD-Leu plates, and restreaked on 5-FOA plates to verify the phenotypes. This was done to ensure that the phenotypes were a result of the plasmid-encoded mutations and not due to recombination between plasmids. We sequenced 15 of the recovered wild-type and pseudo-wild-type plasmids, five conditional mutants, and 11 plasmids that did not support growth. This study was not intended as an exhaustive screen; we were mostly interested in obtaining conditional mutations for subsequent genetic analyses.
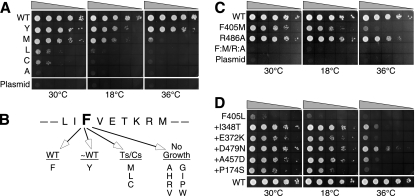
FIG. 2.
Growth phenotypes of mutants and suppressors. Plasmids bearing mutations of DED1 were transformed into yeast, and then drops of serially diluted cultures (by factors of 10) were spotted onto 5-FOA plates to eliminate cells retaining the wild-type (WT) copy of the gene. The plasmid control consisted of the vector alone. Plates were incubated at the indicated temperatures. (A) Random and site-specific mutagenesis of the highly conserved F405 in motif IV. (B) Summary of the growth phenotypes. Ts, heat sensitive; Cs, cold sensitive. (C) The combination of the viable motif-IV-F405M and motif-VI-R486A mutations was nonviable. (D) Intragenic suppressors of the conditional F405L mutation.
Of the Ded1 plasmids sequenced that supported growth to about the same level as the wild type, 10 plasmids contained phenylalanine and 5 contained tyrosine in the third position of motif IV. The cells containing the latter plasmids grew slightly less well than those containing phenylalanine, and they were classified as pseudo-wild type (Fig. 2B). The plasmids of the two colonies that grew poorly at 36°C both contained methionine in place of phenylalanine, and of the colonies that grew poorly at 18 or 36°C, two of the plasmids encoded leucine and one encoded cysteine. The colonies that failed to grow contained plasmids encoding a variety of small, bulky, or polar residues in place of the phenylalanine. Different codons for each residue were obtained, indicating that the oligonucleotides were sufficiently randomized. However, we failed to obtain any clones containing either tryptophan or histidine, which might have been expected to substitute for phenylalanine. Therefore, we did site-specific mutagenesis to obtain these two mutants; neither one supported growth on 5-FOA plates at any temperature. The results are summarized in Fig. 2B. None of the mutants inhibited growth (i.e., showed a dominant-negative phenotype) in the presence of wild-type Ded1. The effects were not due to degradation or altered protein expression, as determined by a Western blot analysis of the mutants in the wild-type strain (data not shown).
Motif VI arginine stacks on the motif IV phenylalanine.
In the solved crystal structures of 10 different DEAD-box proteins and 18 different crystals, the conserved phenylalanine always is seen forming van der Waals stacking interactions on the first arginine of motif VI (the equivalent of R486 for Ded1). This arginine has various interactions with other residues, but typically it is seen interacting with other residues of motif IV, with conserved residues located between motifs V and VI, and occasionally with other residues in motif VI (Fig. 3C). These extensive interactions, and the possible role they play in communicating between the motifs, suggested that the F405 mutations actually were disrupting the critical role of R486 in Ded1. Therefore, we mutated the arginine into an alanine in a site-specific manner and tested it in vivo (Fig. 2C). The resulting colonies grew nearly as well as those with wild-type Ded1. This indicated that, despite the interactions seen with the equivalent of R486 in the crystal structures, it was F405 that was playing the more important role. However, when we combined the R486 mutation with the thermosensitive F405M mutation, the cells were no longer able to grow at any temperature (Fig. 2C). Other R486 mutations show more severe phenotypes (J. Banroques, unpublished).
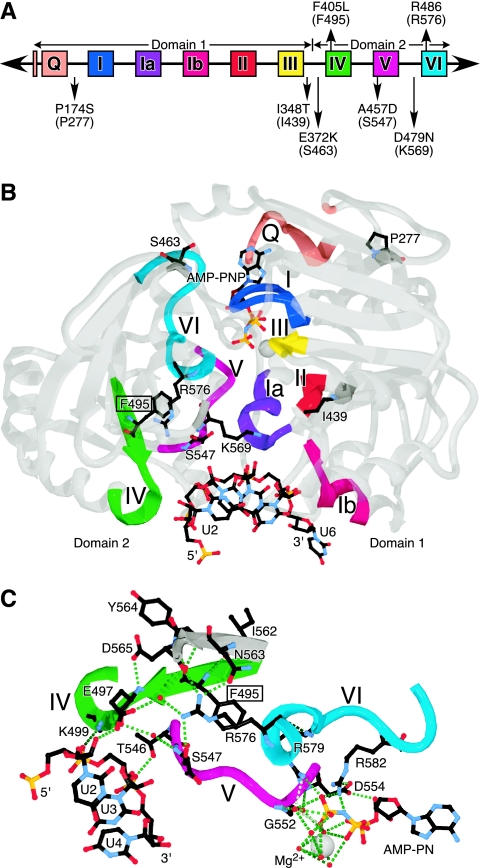
FIG. 3.
(A) Positions of the mutated residues relative to the conserved motifs. The original mutations are shown above the line, and the suppressors of the F405L mutant are shown below. The Vasa equivalents are shown in parentheses. The figure is not drawn to scale. (B) Solved crystal structure of the Ded1-related Vasa protein with a bound RNA substrate and the nucleotide analog AMP-PNP (Protein Data Bank identification number 2DB3). The motifs are colored as shown in panel A, and the gray regions are shown with 50% transparency to facilitate the viewing of internal structures. The protein is shown from the backside of its typical presentation. Five nucleotides bound to the protein are resolved. The interactions are nonspecific and mostly involve the phosphodiester backbone and the 2′ hydroxyl. The residues shown are the Vasa equivalents of the residues shown in panel A, and the conserved phenylalanine is boxed. (C) Details of the network of interactions centered around the conserved phenylalanine of motif IV (boxed). Only the interactions of domain 2 with the AMP-PNP, the RNA, and with the conserved phenylalanine of motif IV and arginine of motif VI are shown. Additional interactions with the RNA come from R528 of the QxxR sequence and the upstream G521, as well as from domain 1. The small red spheres are water molecules. The images shown in panels B and C were made with SwissPdbViewer (18) and were rendered with POV-Ray 3.6 (www.povray.org).
Intragenic suppressors of the phenylalanine mutant grow better.
We decided to investigate gene-encoded (intragenic) changes that might reveal something about other residues that potentially interact with the phenylalanine. We chose the ded1-F405L mutant, because cells transformed with this plasmid grew very poorly under all conditions. We mutagenized the plasmid containing the mutation by either PCR or UV mutagenesis. Five independent reactions were done by PCR and four by UV irradiation using an F405L mutation with the codon CTG; this was done to reduce the appearance of revertants to phenylalanine or tyrosine. We obtained three independent colonies from the UV treatment and five from the PCR mutagenesis that grew significantly better than the F405L mutant alone. The recovered plasmids then were sequenced. In one plasmid, the 405L had mutated to 405M; two others had multiple changes and were discarded. The remaining clones had single changes corresponding to P174S, I348T, E372K, A457D, and D479N in addition to the original F405L mutation (Fig. 2D).
The intragenic mutations were able to suppress the F405L phenotype to various extents, particularly at higher temperatures. The majority of the mutations were between motifs and often involved conserved functional groups (Fig. 3A). The P174S mutation changed a highly conserved proline to a polar group (80% P and 1% S in the BLAST-500-aligned sequences), I348T replaced a conserved aliphatic group with a polar residue (88% l and 1% T), E372K changed a conserved polar group (27% E, 9% K, and 76% polar), A457D restored a highly conserved aspartic acid in motif V (6% A, 76% D, and 93% polar), and D479N changed a conserved polar group (33% D, 18% N, and 94% polar). Most of the changes mapped close to the cleft separating the two RecA-like domains in the solved crystal structures of Vasa (Fig. 3B), but only A457 lies close enough to be able to make contacts with R486. We dissociated the suppressor mutations from the original F405L mutation, and we tested yeast cells transformed with the plasmids. All of the isolated suppressor mutants grew as well as cells containing the wild-type Ded1 at all temperatures on 5-FOA plates (data not shown).
Motif IV phenylalanine essential in other DEAD-box proteins.
Many of the suppressor changes already are present in other yeast DEAD-box proteins. If the suppressors were compensating for structural perturbations caused by the motif IV phenylalanine mutation, then we would expect this mutation to be less severe in those proteins. Dbp2 and eIF4A are two essential yeast proteins in which the positions corresponding to the suppressor mutations A457D and D479N in Ded1 already are aspartic acid and asparagine, respectively, for both proteins. We changed the conserved phenylalanine to leucine in plasmid-borne copies of both genes (dbp2-F366L and tif2-F266L), transformed yeast cells, selected them on SD-Leu plates, and then restreaked them on 5-FOA plates to eliminate cells retaining the wild-type copy of the gene. In both cases, the mutated plasmids failed to support growth under all conditions tested (data not shown). Thus, the severity of the phenylalanine-to-leucine phenotype varied between helicases, which perhaps reflected the relative importance of the enzymatic activity of the different proteins in vivo.
Reduced in vitro ATPase activity of mutants.
We subcloned representative ded1 mutants into a pET expression vector containing a carboxyl-terminal His6 tag. This permitted us to express the proteins in E. coli and to purify them on nickel affinity columns. We were able to successfully obtain the wild-type Ded1 and a number of mutated Ded1 proteins to high purity and yield (Fig. 4A and data not shown). We then tested the proteins for their capacity to hydrolyze ATP in the presence of RNA by using a molybdate-malachite green assay to monitor the production of free phosphate. We used saturating concentrations of total yeast RNA, as previously described, because the in vivo substrate for Ded1 is unknown (11). In our initial survey, we did time courses to determine the initial reaction velocities with 1 mM ATP (Fig. 4B). All of the mutated proteins of F405 had 10% or less of the wild-type ATPase activity, including the F405Y mutant that supported pseudo-wild-type growth in vivo. Although the effects were more pronounced in vitro than in vivo, there was a strong correlation between the ATPase activities and the growth phenotypes. Mutations that failed to support growth in yeast (F405A and F405W) had a background level of activity of less than 1% of that of the wild type. It was unlikely that these effects were due to protein denaturation or misfolding, because they all behaved about the same during purification (according to chromatography, solubility, and yield), with the possible exception of F405A, which was more difficult to purify; it was more likely to be found in the inclusion bodies of E. coli or to precipitate during purification. Nevertheless, we could not rule out more localized perturbations.
FIG. 4.
Purification and ATPase assays. (A) Proteins were purified as described in the text, about 1 μg of the purified proteins was separated on a 10% Laemmli SDS-polyacrylamide gel, and then the gel was stained with Coomassie blue. (B) Initial reaction rates as a percentage of the wild-type ATPase activity of the different mutants in the presence of 1 mM ATP and 300 ng/μl of total yeast RNA. Velocities were normalized for the same protein concentration (1 nM). (C) Initial reaction velocities of the intragenic suppressors of the F405L mutation and of the suppressors in isolation. M, molecular size marker, in kilodaltons; WT, wild type.
Although the motif VI mutation R486A supported nearly wild-type levels of growth, it had only about 25% of the initial reaction velocity, and when it was combined with the F405M mutation the activity was further reduced to less than 2% of that of the wild type (Fig. 4B). None of the suppressors of the F405L mutation supported wild-type levels of growth; consistent with this, the reaction velocities were increased only 2.7- to 5.3-fold in vitro (Fig. 4C). The F405L suppressors showed a good correlation between the ATPase activities and the in vivo growth profiles at 30°C, but some divergence was noted at higher growth temperatures, which implied that some of the mutated proteins were less stable. Most of the suppressors alone, when dissociated from the F405L mutation, had less ATPase activity than the wild type, including the A457D mutation that restored the canonical consensus sequence of motif V (Fig. 4C). However, we obtained nearly wild-type activity when it was combined with the D457N suppressor, although the synergistic effects were relatively small. An interesting result was that the I348T suppressor, which changed a highly conserved aliphatic group into a polar group, was 40% more active than the wild type (Fig. 4C).
We did time courses with variable ATP concentrations to determine the kinetic parameters of some of the more active mutants. We also analyzed the various suppressors (Table 1). The results again showed a strong correlation between the in vitro ATPase activity and the in vivo phenotypes. A minimum of 9% enzymatic performance (kcat/Km) in vitro, relative to that of the wild type, was needed for pseudo-wild-type growth, while 1% activity barely supported growth. Interestingly, the suppressors needed to enhance the activity by only two- to fivefold to significantly enhance growth. The mutations and suppressors primarily affected ATP hydrolysis (kcat) rather than ATP binding (Km). The F405L mutation bound ATP about 2.5-fold less well than the wild type, but the suppressors of the mutation showed only slightly better binding in most cases and worse binding in the case of P174S. However, this latter mutation was located between the Q motif and motif I, which are used to bind the ATP (11, 45). Interestingly, the motif V A457D suppressor, separated from the F405L mutation, had a twofold higher affinity for ATP and a nearly fourfold reduced catalysis, while the isolated I348T suppressor mutant had about 60% of the kcat and more than fivefold higher binding affinity.
TABLE 1.
Kinetic parameters for RNA-dependent ATPase activitiesa
| Protein | Km (mM) | kcat (min−1)b | kcat/Km (M−1 min−1)c | % WTd |
|---|---|---|---|---|
| WT | 0.37 ± 0.03 | 280 ± 30 | 740,000 ± 70,000 | 100 |
| F405L | 0.92 ± 0.06 | 7.1 ± 0.7 | 7,700 ± 800 | 1.0 |
| F405Y | 0.50 ± 0.02 | 33 ± 3 | 66,000 ± 7,000 | 8.9 |
| F405L+D479N | 0.76 ± 0.05 | 19 ± 2 | 25,000 ± 3,000 | 3.3 |
| F405L+A457D | 0.73 ± 0.05 | 21 ± 2 | 28,000 ± 3,000 | 3.8 |
| F405L+P174S | 1.30 ± 0.01 | 19 ± 2 | 14,000 ± 1,000 | 1.9 |
| F405L+I348T | 0.84 ± 0.01 | 34 ± 3 | 40,000 ± 4,000 | 5.4 |
| A457D | 0.19 ± 0.04 | 78 ± 8 | 420,000 ± 90,00 | 57 |
| I348T | 0.069 ± 0.003 | 170 ± 17 | 2,500,000 ± 250,000 | 330 |
Values were derived from nonlinear fits to the Michaelis-Menten equation. The standard errors of estimation were derived from the curve fits. WT, wild type.
A minimum of 10% error was assumed for the protein concentrations.
The largest errors of kcat or Km were carried forward.
The enzymatic performance (kcat/Km) relative to the wild-type protein.
We were not able to obtain reliable kinetic values for the equivalent motif IV mutations in eIF4A and Dbp2. The wild-type eIF4A protein had a low intrinsic ATPase activity, with an enzymatic performance (kcat/Km) that was more than 200-fold less than that for wild-type Ded1. Dbp2 was extremely toxic to E. coli when expressed, even at low levels, and we were unable to purify the protein. We previously showed that we could UV cross-link ATP to eIF4A, and that the cross-linking efficiency was strongly correlated with the ATPase activity (45). We obtained threefold less UV cross-linking of ATP with the lethal eIF4A F266L mutant than with the wild-type protein (data not shown). This was consistent with the mutation having a more profound effect on ATP hydrolysis than on ATP binding in eIF4A, as was seen with the Ded1 mutants.
Reduced strand displacement activity of mutants.
We carried out strand displacement assays of the purified proteins as previously described (11). We used a 44-nucleotide-long RNA (R1) transcribed in vitro with T7 polymerase and hybridized to a 16-nucleotide-long DNA oligonucleotide (Hyb1) to form a 3′ RNA-DNA duplex with a 5′ single-stranded RNA (11) and another 44-nucleotide-long RNA (K01) that hybridized with Hyb1 to form a 5′ RNA-DNA duplex with a 3′ single-stranded RNA (Fig. 5A). Although the duplexes had the same calculated free energy (ΔG° = −19.8 kcal/mol) and predicted melting temperatures under the reaction conditions used (melting temperatures of ∼53°C), the actual levels of stability were lower because both RNAs could form intramolecular hairpins that could compete with or block Hyb1 binding. R1 was predicted to form a weakly stable hairpin of −2.7 kcal/mol, while K01 could form a more stable hairpin of −6.4 kcal/mol. Nevertheless, the duplexes were stable under our reaction conditions, and the oligonucleotides would rapidly reanneal if displaced. Therefore, we used two different oligonucleotide traps to block the reformation of the radioactively labeled duplex. In the first trap, we used a 25-fold excess of cold Hyb1, which resulted in the formation of an unlabeled duplex (ca. 4% labeled duplex at equilibrium). In the second trap, we added a 25-fold excess of an 18-nucleotide-long DNA (α-Hyb1) that was complementary to Hyb1 (Fig. 5A). This second trap resulted in a fully single-stranded RNA during the displacement reaction.
Representative results are shown in Fig. 5B. The wild-type Ded1 could displace the top strand of a duplex at a 25- to 50-fold excess of substrate to enzyme during a 60-min incubation at either 30 or 37°C. Both trap systems gave effectively the same results with the wild-type protein, although the α-Hyb1 trap had slightly higher background displacement (Fig. 5C and data not shown). This indicated that the limiting step in the reaction was binding the single-stranded region of the RNA, and that this was largely independent of the presence of a duplex. Thus, free RNA was an effective competitor in the reaction. Although Ded1 had no ATP-dependent affinity for a DNA oligonucleotide (see the following section on RNA binding assays), it did have a weak ATP-independent affinity; the DNA traps probably acted as weak competitive inhibitors. Both the 5′ and 3′ duplexes gave similar results, but the 5′ duplex was more readily displaced by the various Ded1 proteins, as predicted from the free energies; there was no observed directionality. In nearly all cases, there was a close correlation between the ATPase activities and the strand displacement activities. A possible exception was the isolated A457D suppressor mutation that had slightly higher strand displacement activity than the wild type but less ATPase activity. However, this mutant had a twofold higher affinity for ATP than the wild type, which suggested that ATP binding plays a more prominent role in the reaction than ATP hydrolysis.
Wild-type Ded1 has a higher affinity for RNA in the presence of ATP.
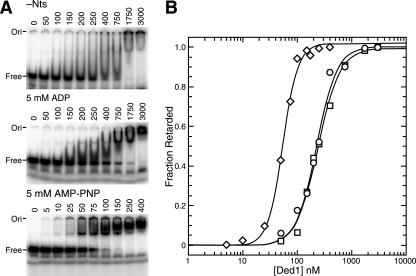
Because motif IV is involved in RNA binding (1, 4, 41), we decided to investigate the substrate binding properties of the various mutants. We previously showed that Ded1 has a higher affinity for the RNA substrate in the presence of AMP-PNP, which is a nonhydrolyzable analog of ATP, than in the absence of a nucleotide or in the presence of ADP by using EMSA; moreover, we showed that mutants with reduced binding constants for ATP had reduced RNA affinities (11). We expanded on this earlier work by using different RNA substrates and by determining the binding constants for half saturation (K1/2). We used the 44-nucleotide-long R1 RNA and two 25-nucleotide-long RNAs that either were pyrimidine rich (RNA01) or purine rich (RNA02) (Table 2). To facilitate the analyses, we used a constant RNA concentration and varied the protein concentration, but essentially the same results were obtained with a fixed protein concentration and variable RNA concentrations. The data were fit to the Hill equation; the best fits were for a single binding site. We obtained slightly positive cooperativity at very high protein concentrations, but this was probably the result of molecular exclusion or Manning condensation effects. We have no evidence that Ded1 was functionally active as a multimer. The results are shown in Fig. 6, and they are summarized in Table 2.
FIG. 6.
EMSA of wild-type Ded1. (A) Various concentrations of wild-type Ded1 (shown in nanomolar) were incubated with 5 nM of the 32P-labeled 25-mer RNA01 and the indicated cofactors (5 mM) as described in the text and then separated on a 6% nondenaturing polyacrylamide gel. −Nts, no nucleotide added. (B) Quantification of the results shown in panel A. Data were fit to the Hill equation. ○, no added nucleotide; □, ADP added; ⋄, AMP-PNP added.
In the presence of AMP-PNP, we obtained several discrete, slower-migrating bands that increased in intensity with increasing protein concentrations. In contrast, much higher protein concentrations were needed to get band shifting when there was no added nucleotide, and the retarded RNA tended to smear on the gel. At very high concentrations of Ded1, the radioactively labeled RNA often was trapped in the well of the polyacrylamide gel. Similarly, high Ded1 concentrations were needed in the presence of ADP, but there were qualitative differences in the results from those obtained without nucleotide. In general, the ADP-dependent band shifts formed more discreet bands than the nucleotide-independent band shifts. Moreover, the ADP-dependent affinity for the RNA was reproducibly slightly less than that for the nucleotide-independent affinity, and both were five- to eightfold less than that for AMP-PNP. The actual differences probably were greater, because the radioactivity in the well was counted as retarded even though it might have been precipitated. Nevertheless, the protein-RNA complexes were soluble and at equilibrium in solution; this was based on the ability to compete the bound labeled RNA with added unlabeled RNA after the initial incubation.
These results indicated that there were at least three different conformational forms of the Ded1 protein that depended on the presence or absence of the nucleotide. It is known that AMP-PNP is an imperfect mimic of ATP, and consequently the interactions between the protein and the analog may be slightly different from those with ATP (36). Therefore, it was possible that ATP would have given higher levels of substrate binding. However, EMSA with ATP gave results similar to those with ADP; this was probably the result of ATPase activity during the course of the experiment (data not shown).
There was little difference in the binding affinities of the pyrimidine-rich RNA01 and the purine-rich RNA02; this was expected, because the vast majority of the interactions seen in the solved crystal structures with a bound substrate involve the phosphodiester backbone (1, 4, 41). About a third of the interactions involve the 2′ hydroxyl of the sugar. Consistently with this, DNA oligonucleotides and double-stranded RNA both show weak, nucleotide-independent binding (data not shown). The pKi of Ded1 was calculated to be between 7.7 and 8.2, so the protein could have a slight net-positive charge under our reaction conditions. Thus, some of the apparent binding could be due to nonspecific interactions. The proteins showed somewhat higher affinity for the 44-nucleotide-long R1 RNA than the 25-nucleotide-long oligonucleotides; this may have been because of the larger target size and because multiple proteins could interact. These experiments do not formally distinguish between a modification of a single site or the use of multiple sites. However, deletions of the carboxyl-terminal domain of Ded1 gave similar profiles, albeit with greatly reduced affinities, and other yeast DEAD-box proteins showed similar properties, including eIF4A (N. K. Tanner, unpublished). Thus, our data were most consistent with a single binding site that was altered with ATP or ADP binding.
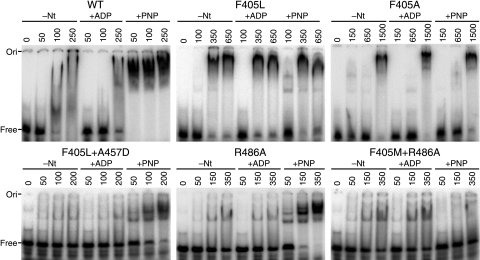
F405 mutants lose ATP-dependent affinities for RNA.
The EMSA results for the mutants closely followed the ATPase and strand displacement activities (Fig. 7, Table 2, and data not shown). The F405L mutant had only slightly higher affinity for the RNA in the presence of AMP-PNP than in its absence, and this capacity was lost entirely with the F405A mutant. Thus, the binding affinity was completely dissociated from the added nucleotide. This mutant showed very weak binding in general, which suggested that it had an altered conformation. Interestingly, many of the F405 mutants showed more discrete bands than the wild type in the absence of a nucleotide, even though the calculated binding affinities were similar. Both the R486A and the F405M mutants showed only slightly reduced AMP-PNP-dependent binding affinities, but the mutant combining the mutations largely lost the ability to distinguish between the nucleotide-bound and nucleotide-free states (Fig. 7). All four suppressors partially restored the ability of the F405L mutant to bind RNA with a higher affinity in the presence of AMP-PNP (Fig. 7 and data not shown). Although the suppressors in isolation have AMP-PNP-dependent binding that was similar to that of the wild type, the ADP-dependent and free forms tended to form more discrete bands, which indicated that the binding sites were altered (data not shown).
FIG. 7.
EMSA of Ded1 mutants. Various concentrations of wild-type (WT) and mutant Ded1 (shown in nanomolar) were incubated with 5 nM of the 32P-labeled 44-nucleotide-long R1 and the indicated cofactors (5 mM) as described in the text and then separated on a 6% nondenaturing polyacrylamide gel. The proteins had a somewhat higher affinity for this longer RNA, which increased the sensitivity of the assay. −Nt, no nucleotide added.
Cation-π interactions of the phenylalanine.
The phenotypes, both in vivo and in vitro, of the mutants were much more pronounced than would be expected from the simple substitutions of the mutants. Aliphatic side chains (I, L, and V) occur far more frequently in β-sheets than phenylalanine, and all of the residues listed in Fig. 2B occur with significant frequency (52). Consistently with this, we made the corresponding substitutions in the solved crystal structure of Vasa with the Swiss-PdbViewer, and we found that they all were tolerated, with the exception of proline, which showed some steric clash. It was possible that the substitutions were promoting alternative conformations, but the similar ATP-independent RNA binding affinities of the mutant and wild-type proteins, the similar properties during purification, and the fact that the suppressor mutations of F405L mapped in both RecA-like domains made this unlikely. A possible exception was the F405A mutant that had a four- to fivefold lower ATP-independent affinity for RNA and that was more likely to precipitate during purification. Oddly, alanine substitution often is considered to be neutral in mutagenic studies because it has a small, nonpolar side chain and because it is unlikely to alter or disrupt the peptide backbone (12). Nevertheless, the primary effects of all of the mutants were on ATP-dependent RNA binding and ATP hydrolysis. Finally, the trypsin digestion of the various proteins under nondenaturing conditions showed the same core cleavage patterns, although flanking sequence elements (outside the two RecA-like domains) (Fig. 3A) showed some enhanced (more rapid) cleavage; we determined this by analyzing the major cleavage products of the wild type and F405L mutant by nano-liquid chromatography-ESI mass spectroscopy (data not shown). Therefore, phenylalanine and, to a lesser extent, tyrosine must have intrinsic and unique properties that are essential for DEAD-box protein activity.
The π systems of aromatic residues are known to form electrostatic potentials on the face of the ring, because the sp2 carbons in the π system are more electronegative than the hydrogens; consequently, they can bind positively charged residues (recently reviewed in reference 14). It is common to find cation-π interactions in proteins, and they typically involve arginine or lysine interacting with the π systems of phenylalanine, tyrosine, or tryptophan. They can contribute 2 to 4 kcal/mol in binding energy in addition to other (e.g., van der Waals) interactions, and they are commonly used by proteins for ligand recognition and catalysis (51). They also enhance the stability of protein-nucleic acid complexes (49). Therefore, we analyzed the 18 solved crystal structures of the DEAD-box proteins in the protein data bank using the CaPTURE program (for cation-π trends using realistic electrostatics; http://capture.caltech.edu/), which predicts these interactions based on energetic calculations and on the distances between the π systems and the positively charged residues (16).
All of the structures, except eIF4A (Protein Data Bank identification numbers 1fuu and 1fuk), showed moderately stable cation-π interactions between the conserved phenylalanine of motif IV and a highly conserved arginine of motif VI (data not shown). For Vasa, the cation-π interaction contributed −1.79 ± 0.08 kcal/mol of binding energy for the four proteins in the unit cell, and these values were in the range of the other crystal structures as well. The calculated van der Waals binding energies were consistently more favorable (−2.63 ± 0.08 kcal/mol for Vasa). The actual energies are undoubtedly different, because the crystal structures do not have high enough resolution to accurately determine the distances. It was probably for this reason that the eIF4A structure fell below the cutoff limit (>−1 kcal/mol). Interestingly, eIF4A also has a partially unresolved motif VI in the crystal structure (1fuu, 1fuk) (6). There were predicted cation-π interactions in other regions of the proteins between arginine or lysine with phenylalanine, tyrosine, or tryptophan that often were more stable. Some were partially conserved between the different proteins, but none of the other interactions involved highly conserved residues (data not shown). Thus, the cation-π interactions between the phenylalanine in motif IV and the first arginine of motif VI probably are defining characteristics of DEAD-box proteins.
Cation-π interactions of other helicase families.
We analyzed 21 solved crystal structures of other families of SF2 helicases and of the UvrD family of SF1. Only the viral NS3 proteins showed a strong correlation between the presence of a phenylalanine in motif IV and a cation-π interaction with arginine of motif VI, as in the DEAD-box family. The XPB and Hef families of DNA repair proteins have highly conserved phenylalanines and arginines in the same positions, but in the solved crystal structures the arginines are oriented differently from the DEAD-box family (2fwr, 2flz, 1wp9) (15, 35). In contrast, the archaebacterial DNA repair protein Hel308 has a highly conserve methionine in place of the arginine in motif VI; this methionine forms van der Waals contacts with the conserved phenylalanine, but this interaction would not be revealed by the CaPTURE program (2p6u, 2p6r) (5). Finally, none of the solved crystal structures with a tyrosine in motif IV showed any interactions, even though tyrosine should have binding energies with arginine that are similar to those with phenylalanine (14).
DISCUSSION
Motif IV of SF2 helicases typically is characterized by two aliphatic groups, a phenylalanine (or, less often, a tyrosine), and then a series of charged and polar groups (Fig. 1B). The structural and functional conservation of motif IV is high even though only the phenylalanine (tyrosine) is conserved on the amino acid level (Fig. 1). The polar and charged residues following the phenylalanine interact with the sugars and phosphate backbones of bound RNA or DNA substrates in the solved crystal structures, although the residues involved vary among helicase families (1, 4, 5, 29, 41). It is not clear what the roles of the other polar and charged residues are, but they may be needed at different phases of substrate binding or for interactions with cofactors.
All of the domain 2 RecA-like cores, which included the β-sheet-loop-helix structure of motif IV, of the DEAD-box proteins were largely superimposable (see Fig. S1 in the supplemental material). Most of the variability was found in the carboxyl-terminal sequences of motifs V and VI, which form interactions with the ATP bound on domain 1 when it is present. Moreover, domain 2 of all of the other families of SF2 helicases with solved structures show the same RecA-like architecture. Comparisons with SF1 were more difficult, but it appears that motif IVa in SF1 families likewise has the same structural context, and the motif probably serves roles similar to those it serves in SF2 families.
The role of the phenylalanine (or tyrosine) is something of an enigma. Most of the SF2 families examined have this residue, but only the DEAD-box and viral NS3 proteins showed van der Waals and cation-π interactions with a conserved arginine of motif VI. These interactions are expected to be highly specific because of the distance constraints and to be very stable (−4.42 ± 0.15 kcal/mol in the case of Vasa). Additional stability would come from van der Waals interactions with other residues. Therefore, it is intriguing that the Hel308 family of DNA helicases (5), with a bound DNA (2p6r), has a conserved methionine in place of the arginine, and that the sulfur interacts with the edge of the benzene ring of phenylalanine, which should have a slightly positive electrostatic charge around the ring hydrogens (33); in contrast, the structure without a substrate (2p6u) shows van der Waals interactions between the face of the benzene ring and the alkane carbons of the methionine. This suggests that these sorts of interactions are in the conformational repertoire of the other helicase families as well. Nevertheless, care must be taken in all of these cases because of the uncertainty associated with interpreting the density maps of the defraction patterns.
Our results revealed the importance of the highly conserved phenylalanine in motif IV of DEAD-box proteins. Changing the phenylalanine to leucine in Ded1 strongly inhibited yeast growth, while equivalent changes in the yeast proteins Dbp2 and eIF4A failed to support growth. It is likely that this is true for most other DEAD-box helicases as well, although the severity of the mutations will undoubtedly depend on the enzymatic properties of each protein in vivo. Previous studies showed that mutations of the conserved phenylalanine (F442) in the essential yeast spliceosome protein Prp28 either were wild type (F442H) or temperature sensitive (F442G and F442S) (7). However, the enzymatic activity of Prp28 may not have been needed under the experimental conditions used, because the requirement for Prp28 can be eliminated entirely by alterations of the U1-C protein or of the U1 RNA that destabilizes the U1 to 5′ splice site (8). The only other examples of mutations of the conserved phenylalanine were made in the related DEAH family of helicases. The mutant F309A in the spliceosome protein Prp43 has a slow-growth and highly temperature-sensitive phenotype (44). In contrast, the mutant F697A in the spliceosome protein Prp22 was unable to grow at 30°C or less (39). However, this rather odd result can be understood in the sense that Prp22 catalyzes product release, which might be facilitated, ipso facto, at higher temperatures (37°C) in vivo. Indeed, the F697A mutant has no strand displacement activity in vitro, and it is unable to catalyze mRNA release in spliceosome assays (39).
Our in vitro results point to the central role played by the motif IV phenylalanine in linking ATP binding and hydrolysis with RNA binding. All of the phenylalanine mutations had reduced affinities for ATP and strongly reduced hydrolysis. However, the most striking effect was the dissociation of the cooperative binding of ATP and RNA. The more profound the mutation (i.e., the less phenylalanine-like), the weaker the ATP-dependent affinity for the RNA. These results are in contrast to those previously obtained for the DEAH-box protein Prp22; the equivalent mutation in this protein (F697A) showed no ATP-dependent loss of RNA binding on mobility shift assays (39). However, these experiments were done in the absence of Mg2+ to prevent the hydrolysis of the ATP; many of the interactions of motifs II, V, and VI with the ATP would be expected to be lost under these conditions, because they occur through the coordinated Mg2+ and bound water molecules.
The suppressors compensated for the F405L mutation in different ways by affecting the relationship between Km and kcat (Table 1). As shown in Fig. 3A and B, the suppressors are located in various positions in the protein, but they all are in close proximity to the various motifs responsible for ATP and RNA binding. Thus, P277 in Vasa (equivalent to P174S in Ded1) is close to residues of the Q motif and the conserved, isolated, upstream phenylalanines, which are involved in adenine recognition. I439 (I348T) is close to motif II, which is involved in binding the phosphates of ATP, and to motif III, which is involved in intradomain interactions. S463 (E372K) is close to motif I, which is involved in phosphate binding, and conserved residues of unknown function located between motifs III and IV. S547 (A457D) is part of motif V, which is involved in RNA binding. Finally, K569 (D479N) is located close to motifs II, III, and VI. Therefore, the suppressors could modify either RNA binding or ATP binding and hydrolysis, either by enhancing or reducing the affinities, and thereby they could fine-tune the relationship between ATP and substrate binding, enzymatic activity, and product release. The net result was that all four suppressors of F405L had enhanced ATP-dependent binding of RNA (Fig. 7, Table 2, and data not shown), enhanced RNA-dependent ATPase activity (Fig. 4C, Table 1), and enhanced strand displacement activity (Fig. 5 and data not shown), although none approached the wild-type levels.
The phenylalanine of motif IV and the first arginine of motif VI are at the center of a network of interactions between motifs IV, V, and VI, and thereby they are ideally suited for communicating the states of the RNA and ATP binding sites (Fig. 3C). At first glance, the arginine seems to be having the more extensive and more important interactions. This does not seem to be the case for Ded1, because the R486A mutation had nearly wild-type growth and good enzymatic activity. However, under laboratory conditions, the absence of interactions may be less detrimental than the wrong ones. Consistently with this, it appears that the alanine mutation is unusually benign (J. Banroques, unpublished). This would explain why the tryptophan and histidine mutants were not viable, because the mutations would significantly alter the cation-π interactions and subsequently the orientation of the arginine. Likewise, charged or bulky residues would be expected to perturb the positioning of the arginine. However, this explanation is insufficient to explain the strong phenotypes of the other mutants and the high conservation of the phenylalanine.
The phenylalanine also must play an essential role in maintaining the structural integrity of the RecA-like domain 2 by preventing structural bulging or distortions with RNA binding, especially because motifs IV and V, and the QxxR sequence, make contacts with the RNA. This is consistent with the temperature sensitivity of the mutants, because the structures would be expected to be less stable at the higher temperatures. In the same sense, a mutation of the aliphatic residue (L403S) in the β-sheet preceding the phenylalanine also is temperature sensitive (R. Bock, unpublished data). This is true for other protein families; for example, a cysteine-to-phenylalanine mutation in the β-hairpin of βB2-crystallin, which occurs naturally in βγ-crystallins, results in enhanced conformational stability and reduced rates of unfolding (32). Domains 1 and 2 could function as two rigid bodies that are twisted and pulled together with substrate (ATP and RNA) binding. This might explain why ribosomal processing helicases often have two aromatic groups in motif IV, because the RNA environment might be much larger and additional stabilization might be required. This interpretation also may explain why a tryptophan precedes phenylalanine in the dengue and yellow fever subfamily of NS3 helicases. Thus, the phenylalanine could function as a molecular anchor to prevent the distortion of the RecA-like domain. In at least the DEAD-box and viral NS3 helicases, it also could be used to orient the motif VI arginine and ensure the correct network of interactions between motifs.
Supplementary Material
Acknowledgments
We thank Marc Dreyfus, Beate Schwer, and Tommaso Villa for looking over the manuscript. We thank Ronald Bock and Pascal Gurtner for the initial motif IV screen, Pierre-Alain Braillard for the motif VI mutant, Ronald Bock for plasmids, Sam Landry for his structural insight, and Dennis Dougherty for advice on cation-π interactions. We are very much indebted to Costa Georgopoulos for his undying support of our endeavors.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), by the Swiss National Science Foundation, and by the Canton of Geneva. J.B. also was supported by a Short Term European Molecular Biology Organization Fellowship.
Footnotes
Published ahead of print on 10 March 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Andersen, C. B., L. Ballut, J. S. Johansen, H. Chamieh, K. H. Nielsen, C. L. Oliveira, J. S. Pedersen, B. Séraphin, H. Le Hir, and G. R. Andersen. 2006. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science 3131968-1972. [DOI] [PubMed] [Google Scholar]
- 2.Askjaer, P., R. Rosendahl, and J. Kjems. 2000. Nuclear export of the DEAD box An3 protein by CRM1 is coupled to An3 helicase activity. J. Biol. Chem. 27511561-11568. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot, K., M. Muldoon, L. Rajkowitsch, J. Hughes, and J. E. McCarthy. 2004. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 51987-1001. [DOI] [PubMed] [Google Scholar]
- 4.Bono, F., J. Ebert, E. Lorentzen, and E. Conti. 2006. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell 126713-725. [DOI] [PubMed] [Google Scholar]
- 5.Büttner, K., S. Nehring, and K. P. Hopfner. 2007. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 14647-652. [DOI] [PubMed] [Google Scholar]
- 6.Caruthers, J. M., E. R. Johnson, and D. B. McKay. 2000. Crystal structure of yeast initiation factor 4A, a DEAD-box RNA helicase. Proc. Natl. Acad. Sci. USA 9713080-13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, T. H., L. J. Latus, Z. Liu, and J. M. Abbott. 1997. Genetic interactions of conserved regions in the DEAD-box protein Prp28p. Nucleic Acids Res. 255033-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. Y., L. Stands, J. P. Staley, R. R. Jackups, Jr., L. J. Latus, and T. H. Chang. 2001. Specific alterations of U1-C protein or U1 small nuclear RNA can eliminate the requirement of Prp28p, an essential DEAD box splicing factor. Mol. Cell 7227-232. [DOI] [PubMed] [Google Scholar]
- 9.Chuang, R. Y., P. L. Weaver, Z. Liu, and T. H. Chang. 1997. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science 2751468-1471. [DOI] [PubMed] [Google Scholar]
- 10.Cordin, O., J. Banroques, N. K. Tanner, and P. Linder. 2006. The DEAD-box protein family of RNA helicases. Gene 36717-37. [DOI] [PubMed] [Google Scholar]
- 11.Cordin, O., N. K. Tanner, M. Doere, P. Linder, and J. Banroques. 2004. The newly discovered Q motif of DEAD-box RNA helicases regulates RNA-binding and helicase activity. EMBO J. 232478-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham, B. C., and J. A. Wells. 1989. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science 2441081-1085. [DOI] [PubMed] [Google Scholar]
- 13.de la Cruz, J., I. Iost, D. Kressler, and P. Linder. 1997. The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 945201-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougherty, D. A. 2007. Cation-pi interactions involving aromatic amino acids. J. Nutr. 1371504S-1517S. [DOI] [PubMed] [Google Scholar]
- 15.Fan, L., A. S. Arvai, P. K. Cooper, S. Iwai, F. Hanaoka, and J. A. Tainer. 2006. Conserved XPB core structure and motifs for DNA unwinding: implications for pathway selection of transcription or excision repair. Mol. Cell 2227-37. [DOI] [PubMed] [Google Scholar]
- 16.Gallivan, J. P., and D. A. Dougherty. 1999. Cation-pi interactions in structural biology. Proc. Natl. Acad. Sci. USA 969459-9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbalenya, A. E., and E. V. Koonin. 1993. Helicases: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 3419-429. [Google Scholar]
- 18.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 182714-2723. [DOI] [PubMed] [Google Scholar]
- 19.Guthrie, C., and G. R. Fink (ed.). 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, San Diego, CA.
- 20.Halls, C., S. Mohr, M. Del Campo, Q. Yang, E. Jankowsky, and A. M. Lambowitz. 2007. Involvement of DEAD-box proteins in group I and group II intron splicing. Biochemical characterization of Mss116p, ATP hydrolysis-dependent and -independent mechanisms, and general RNA chaperone activity. J. Mol. Biol. 365835-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Högbom, M., R. Collins, S. van den Berg, R. M. Jenvert, T. Karlberg, T. Kotenyova, A. Flores, G. B. Hedestam, and L. H. Schiavone. 2007. Crystal structure of conserved domains 1 and 2 of the human DEAD-box helicase DDX3X in complex with the mononucleotide AMP. J. Mol. Biol. 372150-159. [DOI] [PubMed] [Google Scholar]
- 22.Iost, I., M. Dreyfus, and P. Linder. 1999. Ded1p, a DEAD-box protein required for translation initiation in Saccharomyces cerevisiae, is an RNA helicase. J. Biol. Chem. 27417677-17683. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson, D. J., and J. D. Beggs. 1991. A suppressor of yeast spp81/ded1 mutations encodes a very similar putative ATP-dependent RNA helicase. Mol. Microbiol. 5805-812. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson, D. J., B. Rahe, J. Pringle, and J. D. Beggs. 1991. A suppressor of a yeast splicing mutation (prp8-1) encodes a putative ATP-dependent RNA helicase. Nature 349715-717. [DOI] [PubMed] [Google Scholar]
- 25.Jankowsky, E., and H. Bowers. 2006. Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases. Nucleic Acids Res. 344181-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowsky, E., and M. E. Fairman. 2007. RNA helicases—one fold for many functions. Curr. Opin. Struct. Biol. 17316-324. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone, O., R. Deuring, R. Bock, P. Linder, M. T. Fuller, and P. Lasko. 2005. Belle is a Drosophila DEAD-box protein required for viability and in the germ line. Dev. Biol. 27792-101. [DOI] [PubMed] [Google Scholar]
- 28.Kawamukai, M. 1999. Isolation of a novel gene, moc2, encoding a putative RNA helicase as a suppressor of sterile strains in Schizosaccharomyces pombe. Biochim. Biophys. Acta 144693-101. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. L., K. A. Morgenstern, J. P. Griffith, M. D. Dwyer, J. A. Thomson, M. A. Murcko, C. Lin, and P. R. Caron. 1998. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure 689-100. [DOI] [PubMed] [Google Scholar]
- 30.Linder, P. 2006. Dead-box proteins: a family affair-active and passive players in RNP-remodeling. Nucleic Acids Res. 344168-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linder, P., P. F. Lasko, M. Ashburner, P. Leroy, P. J. Nielsen, K. Nishi, J. Schnier, and P. P. Slonimski. 1989. Birth of the D-E-A-D box. Nature 337121-122. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald, J. T., A. G. Purkiss, M. A. Smith, P. Evans, J. M. Goodfellow, and C. Slingsby. 2005. Unfolding crystallins: the destabilizing role of a beta-hairpin cysteine in betaB2-crystallin by simulation and experiment. Protein Sci. 141282-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mecozzi, S., A. P. West, Jr., and D. A. Dougherty. 1996. Cation-pi interactions in aromatics of biological and medicinal interest: electrostatic potential surfaces as a useful qualitative guide. Proc. Natl. Acad. Sci. USA 9310566-10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumberg, D., R. Muller, and M. Funk. 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156119-122. [DOI] [PubMed] [Google Scholar]
- 35.Nishino, T., K. Komori, D. Tsuchiya, Y. Ishino, and K. Morikawa. 2005. Crystal structure and functional implications of Pyrococcus furiosus hef helicase domain involved in branched DNA processing. Structure 13143-153. [DOI] [PubMed] [Google Scholar]
- 36.Polach, K. J., and O. C. Uhlenbeck. 2002. Cooperative binding of ATP and RNA substrates to the DEAD/H protein DbpA. Biochemistry 413693-3702. [DOI] [PubMed] [Google Scholar]
- 37.Rocak, S., and P. Linder. 2004. DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 5232-241. [DOI] [PubMed] [Google Scholar]
- 38.Rost, B., G. Yachdav, and J. Liu. 2004. The PredictProtein server. Nucleic Acids Res. 32W321-W326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider, S., E. Campodonico, and B. Schwer. 2004. Motifs IV and V in the DEAH box splicing factor Prp22 are important for RNA unwinding, and helicase-defective Prp22 mutants are suppressed by Prp8. J. Biol. Chem. 2798617-8626. [DOI] [PubMed] [Google Scholar]
- 40.Schwer, B. 2001. A new twist on RNA helicases: DExH/D box proteins as RNPases. Nat. Struct. Biol. 8113-116. [DOI] [PubMed] [Google Scholar]
- 41.Sengoku, T., O. Nureki, A. Nakamura, S. Kobayashi, and S. Yokoyama. 2006. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125287-300. [DOI] [PubMed] [Google Scholar]
- 42.Silverman, E., G. Edwalds-Gilbert, and R. J. Lin. 2003. DExD/H-box proteins and their partners: helping RNA helicases unwind. Gene 3121-16. [DOI] [PubMed] [Google Scholar]
- 43.Singleton, M. R., M. S. Dillingham, and D. B. Wigley. 2007. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 7623-50. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka, N., and B. Schwer. 2006. Mutations in PRP43 that uncouple RNA-dependent NTPase activity and pre-mRNA splicing function. Biochemistry 456510-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanner, N. K., O. Cordin, J. Banroques, M. Doere, and P. Linder. 2003. The Q motif: a newly identified motif in DEAD box helicases may regulate ATP binding and hydrolysis. Mol. Cell 11127-138. [DOI] [PubMed] [Google Scholar]
- 46.Tanner, N. K., and P. Linder. 2001. DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol. Cell 8251-262. [DOI] [PubMed] [Google Scholar]
- 47.Thuillier, V., S. Stettler, A. Sentenac, P. Thuriaux, and M. Werner. 1995. A mutation in the C31 subunit of Saccharomyces cerevisiae RNA polymerase III affects transcription initiation. EMBO J. 14351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Hippel, P. H. 2004. Helicases become mechanistically simpler and functionally more complex. Nat. Struct. Mol. Biol. 11494-496. [DOI] [PubMed] [Google Scholar]
- 49.Wintjens, R., J. Lievin, M. Rooman, and E. Buisine. 2000. Contribution of cation-pi interactions to the stability of protein-DNA complexes. J. Mol. Biol. 302395-410. [DOI] [PubMed] [Google Scholar]
- 50.Yang, Q., M. E. Fairman, and E. Jankowsky. 2007. DEAD-box-protein-assisted RNA structure conversion towards and against thermodynamic equilibrium values. J. Mol. Biol. 3681087-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zacharias, N., and D. A. Dougherty. 2002. Cation-pi interactions in ligand recognition and catalysis. Trends Pharmacol. Sci. 23281-287. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, H., and W. Braun. 1999. Sequence specificity, statistical potentials, and three-dimensional structure prediction with self-correcting distance geometry calculations of beta-sheet formation in proteins. Protein Sci. 8326-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.