Abstract
The target of rapamycin (TOR) signaling regulates the nucleocytoplasmic shuttling of transcription factors in yeast. Whether the mammalian counterpart of TOR (mTOR) also regulates nucleocytoplasmic shuttling is not known. Using a phospho-specific monoclonal antibody, we demonstrate that mTOR phosphorylates Ser168,170 of endogenous NFATc4, which are conserved gate-keeping Ser residues that control NFAT subcellular distribution. The mTOR acts as a basal kinase during the resting state to maintain NFATc4 in the cytosol. Inactivation and nuclear export of NFATc4 are mediated by rephosphorylation of Ser168,170, which can be a nuclear event. Kinetic analyses demonstrate that rephosphorylation of Ser168,170 of endogenous NFATc4 is mediated by mTOR and, surprisingly, by extracellular signal-regulated kinase 5 (ERK5) mitogen-activated protein kinase as well. Ablation of ERK5 in the Erk5−/− cells ascertains defects in NFATc4 rephosphorylation and nucleocytoplasmic shuttling. In addition, phosphorylation of NFATc4 by ERK5 primes subsequent phosphorylation mediated by CK1α. These results demonstrate that distinct protein kinases are integrated to phosphorylate the gate-keeping residues Ser168,170 of NFATc4, to regulate subcellular distribution. These data also expand the repertoire of physiological substrates of mTOR and ERK5.
Target of rapamycin (TOR) signaling contributes to cell growth and differentiation in organisms ranging from yeast to mammals (18, 23, 31, 37). For example, TOR plays a prominent role in protein translation by regulating ribosome biogenesis. Downstream effectors of TOR signaling include the translation initiation factor 4E-binding protein (4E-BP1) and ribosomal S6 kinase-1 (S6K-1). In Saccharomyces cerevisiae, TOR also regulates subcellular localization of transcription factors upon stress (4). Whether the mammalian counterpart of TOR (mTOR) also regulates subcellular localization of transcription factors is not known.
Extracellular signal-regulated kinase 5 (ERK5) is a member of the mitogen-activated protein kinase (MAPK) family and is activated by mitogenic or stressful stimuli (26, 36). These stimuli include epidermal growth factor, oxidative damage, and osmotic stress. The physiological role of ERK5 signaling is unclear, in part because little is known about the substrates for ERK5. Connexin 43, p90 RSK, and MEF2C can be phosphorylated by ERK5 (7, 19, 29). Further identification of novel substrates will shed new insights into ERK5 function.
Transcription factor NFAT is highly phosphorylated and is located in the cytosol of resting cells (12, 17). Dephosphorylation mediated by calcineurin phosphatase promotes NFAT nuclear accumulation. Indeed, replacement of conserved Ser residues with Ala at a Ser-rich region (SRR) (e.g., Ser172 of NFATc1, Ser168 of NFATc2, Ser163,165 of NFATc3, and Ser168,170 of NFATc4) increased NFAT nuclear localization (3, 10, 11, 40). Replacement of other conserved Ser residues with Ala in the SRR facilitates but is not sufficient to cause NFAT nuclear accumulation (1, 2, 16). Therefore, the conserved Ser residues in the SRR function as “gate keepers,” and dephosphorylation of these residues is a prerequisite for NFAT nuclear accumulation. However, unresolved questions in understanding NFAT phosphorylation and subcellular distribution include: what are the protein kinases that mediate basal phosphorylation of NFAT at resting state; are these protein kinases also involved in rephosphorylation upon NFAT inactivation; and where does the rephosphorylation of NFAT take place? Given the critical role of the gate-keeping Ser residues in regulating NFAT subcellular distribution, further investigation of these conserved residues in the SRR shall elucidate the unanswered questions.
The purpose of this study was to examine basal phosphorylation and rephosphorylation of the gate-keeping residues Ser168,170 of NFATc4. Here, we report that using a phospho-specific monoclonal antibody, we uncovered the integration of the protein kinase mTOR in mediating basal phosphorylation of Ser168,170 of endogenous NFATc4. Upon transcription termination and rephosphorylation, Ser168,170 of endogenous NFATc4 are phosphorylated by mTOR and, surprisingly, by ERK5 as well. Rephosphorylation of NFATc4 can be a nuclear event. Thus, distinct protein kinases mediate phosphorylation of Ser168,170 of NFATc4 at the resting state and upon stimulation. These data also expand the repertoire of physiological substrates of mTOR and ERK5.
MATERIALS AND METHODS
Reagents.
Monoclonal phospho-antibody against Ser168,170 of NFATc4 was generated with the phosphopeptide GGAFFpSPpSPGSSS as antigen, using standard hybridoma techniques. Antibodies for NFATc4 (catalog no. sc13036), p38 (catalog no. 9212), P-p38 (catalog no. 9211), S6K (catalog no. sc230), and P-S6K (catalog no. sc8416) were obtained from Santa Cruz Biotechnology and/or Cell Signaling. Recombinant CK1 and GSK3β were obtained from Upstate Biotechnology. Tubulin antibody was obtained from a monoclonal antibody facility (University of Iowa). Leptomycin was obtained from Sigma. Various protein kinase inhibitors and phosphatase inhibitors were obtained from Calbiochem, Sigma, and/or Fisher Scientific.
Cell culture.
COS cells were cultured in Dulbecco's modified Eagle's medium. BHK cells were cultured in minimal essential medium. All media were supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 mg/ml) (Invitrogen). Cells were transfected by using Lipofectamine (Invitrogen). Primary mouse embryonic fibroblasts (MEFs) were isolated from C57BL/6, Nfatc4−/−, and Erk5−/− embryos and cultured as described previously (35, 39).
Protein kinase assays.
Expression plasmids for mTOR (0.2 μg) and ERK5 (0.2 μg) were transfected into COS cells with and without constitutive active phosphoinostide-3 kinase (PI3K) p110 (0.2 μg) or MEK5DD (0.2 μg), respectively. Cell extracts were prepared, and immunoprecipitation was performed to isolate mTOR or ERK5. Immune complex kinase assays were performed with recombinant NFATc4 proteins (1 μg) as the substrates. Phosphorylation of Ser168,170 of NFATc4 was determined by immunoblotting analysis using phospho-NFATc4 antibody. The incorporation of [32P]ATP into recombinant NFATc4 proteins was also determined by immune complex kinase assay and autoradiography. Tandem protein kinase assays was performed using immunoprecipitated ERK5 or recombinant CK1 in the presence of ATP (1 μM) in a priming reaction (30°C, 30 min). Subsequent kinase assays were performed in the presence of [32P]ATP with CK1, GSK3β, or ERK5 as consecutive kinases. The phosphorylation of recombinant NFATc4 proteins was examined by autoradiography.
Coimmunoprecipitation analysis.
The expression plasmid for NFATc4 (0.5 μg) was cotransfected with Myc-tagged mTOR (0.5 μg) or FLAG-tagged ERK5 (0.5 μg) into BHK cells. Cell extracts prepared using Triton-lysis buffer were incubated (5 h at 4°C) with 20 μl of protein-G Sepharose and 10 μl of NFATc4 rabbit polyclonal antibody (Santa Cruz Biotechnology). After three washes with Triton lysis buffer, the bound proteins (mTOR or ERK5) were detected by protein immunoblotting analysis.
Subcellular fractionation.
Cytoplasmic and nuclear extracts were prepared as described previously (40). In brief, harvested cells were gently resuspended in cytoplasmic buffer (10 mM HEPES [pH 7.9], 50 mM NaCl, 0.5 M sucrose, 1 mM EDTA, 0.25 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 mM benzamidine, and 5 μg/ml leupeptin) containing 5% NP-40. After 10 min on ice, nuclei were pelleted at 3,000 rpm for 5 min at 4°C and cytoplasmic extract was collected. Isolated nuclei were resuspended in nuclear extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM spermidine, 0.15 mM spermine, 25% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 mM benzamidine, and 5 μg/ml leupeptin) for 30 min, with periodic vortexing every 5 min. The sample was spun at 15,000 rpm for 15 min at 4°C to collect nuclear extracts.
Luciferase assays.
An NFAT expression vector (0.1 μg) was cotransfected with an NFAT-luciferase reporter plasmid (0.3 μg) and the control plasmid pRSV β-galactosidase (0.2 μg) into BHK cells. Plasmids expressing PI3K p110 (0.1 μg) and mTOR (0.1 μg) or MEK5DD (0.1 μg) and ERK5 (0.1 μg) were also cotransfected, as indicated. Luciferase and β-galactosidase activities were measured at 36 h after transfection. The data were presented as the relative luciferase activity, calculated as the ratio of the activity of luciferase to the activity of β-galactosidase (values are means ± standard deviations [SD]; n = 4).
Immunofluorescence analysis.
Expression plasmids for Flag-tagged NFATc4 (0.3 μg) or calcineurin (CnAα and CnB; 0.2 μg) were transfected into BHK cells. NFATc4 was detected by immunofluorescence analysis with phospho-NFATc4 mouse monoclonal antibody (1:100) or with NFATc4 rabbit polyclonal antibody (1:100; Santa Cruz Biotechnology). The secondary antibody was Texas Red-conjugated anti-mouse or anti-rabbit immunoglobulin antibody (1:100; Jackson Immunoresearch), and nuclei were visualized with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma).
RESULTS
Specificity of the phospho-NFATc4 monoclonal antibody against phospho-Ser168,170.
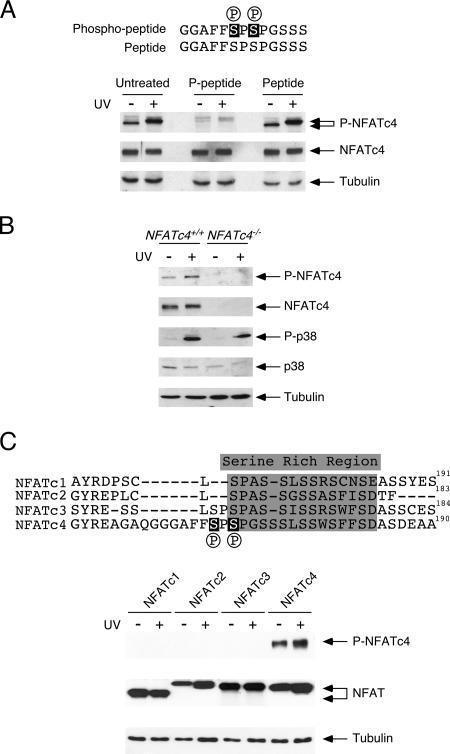
To elucidate the phosphorylation of the gate-keeping residues Ser168,170 in the SRR of NFATc4 in vivo, we developed a phospho-specific monoclonal antibody. Immunoblotting analyses demonstrated basal phosphorylation at Ser168,170 of endogenous NFATc4, which increased in intensity upon UV irradiation (Fig. 1A). Interestingly, UV irradiation also decreased the electrophoretic mobility of NFATc4, suggesting additional phosphorylation at NFATc4. Accumulative phosphorylation at NFATc4 supports the current models in which phosphorylation at Ser168,170 of NFATc4 might provide a priming effect and facilitate subsequent additional phosphorylation by CK1α (27, 41).
FIG. 1.
Specificity of the phospho-NFATc4 monoclonal antibody against phospho-Ser168,170. (A) The phospho-NFATc4 peptide blocks detection by a phospho-NFATc4 monoclonal antibody. MEFs were irradiated (+) or not (−) with UV light, and phosphorylation of endogenous NFATc4 Ser168,170 was determined by immunoblotting analysis using a phospho-NFATc4 monoclonal antibody (P-NFATc4). The expression of total NFATc4 was also determined (NFATc4). The expression of tubulin was used as a loading control. The specificity of the phospho-NFATc4 antibody was determined by incubation with either the antigenic phospho-Ser168,170 NFATc4 peptide (P-peptide) or the peptide without phosphorylation at Ser168,170 (Peptide) before immunoblotting analysis. (B) Determining the specificity of phospho-NFATc4 monoclonal antibody using Nfatc4−/− cells. Nfatc4+/+ and Nfatc4−/− MEFs were irradiated (+) or not (−) with UV light, and the phosphorylation at Ser168,170 of endogenous NFATc4 was determined by a phospho-NFATc4 monoclonal antibody (P-NFATc4) in immunoblotting analysis. Activation of p38 MAPK was determined by phospho-specific p38 antibody (P-p38). The expression levels of NFATc4, p38 MAPK, and tubulin were also shown. (C) Phospho-NFATc4 monoclonal antibody exhibits no cross-reactivity with other NFAT members. COS cells transiently transfected with NFATc1, NFATc2, NFATc3, or NFATc4 were irradiated (+) or not (−) with UV light. Cell lysate was probed with phospho-NFATc4 monoclonal antibody (P-NFATc4). Similar expression levels of different NFAT isoforms and of tubulin were also shown.
Next, we ascertained the specificity of the phospho-NFATc4 monoclonal antibody (Fig. 1). Competition with the antigenic peptide contained by phospho-Ser168,170 of NFATc4, but not the unphosphorylated peptide, abrogated detection of endogenous NFATc4 (Fig. 1A). Notably, NFATc4 phosphorylation was detected only in Nfatc4+/+ but not in Nfatc4−/− cells (Fig. 1B). In addition, other NFAT isoforms (NFATc1, NFATc2, and NFATc3) were not recognized by the phospho-NFATc4 monoclonal antibody (Fig. 1C). These data established the specificity of the phospho-NFATc4 monoclonal antibody.
Phosphorylation at Ser168,170 of NFATc4 by p38 MAPK.
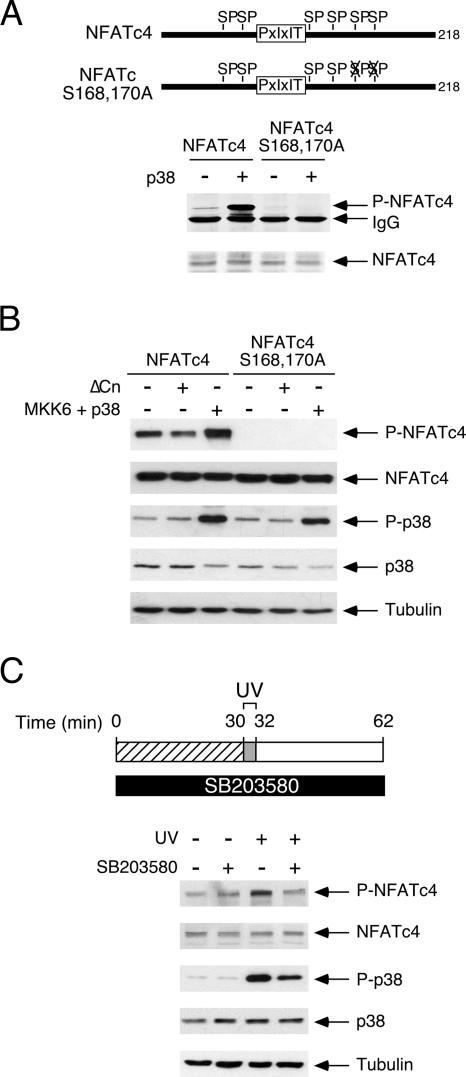
Previous studies demonstrated that the p38 MAPK phosphorylates Ser168,170 of NFATc4 (40). We confirmed the phosphorylation of Ser168,170 of NFATc4 using immune complex kinase assays (Fig. 2A). In vitro kinase assays indicated that phosphorylation of Ser168,170 of NFATc4 occurred upon activation of the p38 MAPK (Fig. 2A). Activation of the p38 MAPK by coexpression of the upstream kinase MKK6 also increased phosphorylation of Ser168,170 of NFATc4 in vivo (Fig. 2B). Notably, the coexpression of constitutively active calcineurin reduced phosphorylation of Ser168,170 of NFATc4 (Fig. 2B). The replacement of Ser168,170 of NFATc4 with Ala, however, abrogated the detection of NFATc4 (Fig. 2A and B). In addition, phosphorylation of Ser168,170 of endogenous NFATc4 by p38 MAPK was sensitive to SB203580 (Fig. 2C), which specifically inhibits p38 MAPK. Together, these data demonstrate that the phospho-NFATc4 monoclonal antibody specifically recognizes Ser168,170 of NFATc4. These data also confirm that p38 MAPK mediates the phosphorylation of Ser168,170 of NFATc4.
FIG. 2.
Phosphorylation at Ser168,170 of NFATc4 by p38 MAPK. (A) Phospho-NFATc4 monoclonal antibody detects p38 MAPK-mediated phosphorylation at Ser168,170 of NFATc4. Recombinant proteins containing amino acids 1 to 218 of the wild type and Ala168,170 of NFATc4 were phosphorylated by p38 MAPK upon activation (+) by upstream MAP2K MKK6 in immune complex kinase assays. Phosphorylation at Ser168,170 of NFATc4 was detected by phospho-NFATc4 monoclonal antibody (P-NFATc4). Similar amounts of NFATc4 recombinant protein were also shown. (B) Calcineurin phosphatase mediates dephosphorylation, while MKK6/p38 MAPK signaling mediates phosphorylation at Ser168,170 of NFATc4. COS cells transiently transfected with the full-length wild type or Ala168,170 of NFATc4 were coexpressed (+) or not (−) with constitutively active calcineurin (ΔCn) or MKK6 and p38 MAPK. Phosphorylation of Ser168,170 of NFATc4 was assessed by phospho-NFATc4 monoclonal antibody (P-NFATc4) in immunoblotting analyses. Activation of p38 MAPK (P-p38) and expression of NFATc4, p38 MAPK, and tubulin was also shown. (C) p38 MAPK inhibitor SB203580 blocks NFATc4 phosphorylation upon UV irradiation. MEFs were preincubated (+) or not (−) with SB203580, as indicated, before UV irradiation (2 min). Cells were recovered for 30 min before they were harvested. The extent of phosphorylation at Ser168,170 of endogenous NFATc4 was determined by immunoblotting analyses (P-NFATc4). The activation of p38 MAPK and the expression of NFATc4, p38 MAPK, and tubulin were also shown.
Identification of the protein kinases that target NFATc4 Ser168,170 at the resting state.
At the resting state, the calcineurin phosphatase inhibitor cyclosporine A (CsA) or the tacrolimus/FK506 analog FK520 increased phosphorylation of Ser168,170 of endogenous NFATc4 (Fig. 3A). Notably, calcineurin inhibition also led to a decrease in the electrophoretic mobility of NFATc4 (Fig. 3A), supporting increased NFATc4 phosphorylation. Neither okadaic acid nor calyculin A affected NFATc4 phosphorylation at Ser168,170 (Fig. 3A). These data indicate that the tonic protein kinase(s) activity is present to maintain basal phosphorylation at Ser168,170 of NFATc4 at the resting state, which is counteracted by the phosphatase calcineurin. Although p38 MAPK phosphorylates Ser168,170 of NFATc4 upon UV irradiation, p38 MAPK does not contribute to the basal phosphorylation of NFATc4 (40) (Fig. 2). One or more novel NFATc4 kinases, therefore, are present to counteract the phosphatase calcineurin at resting state.
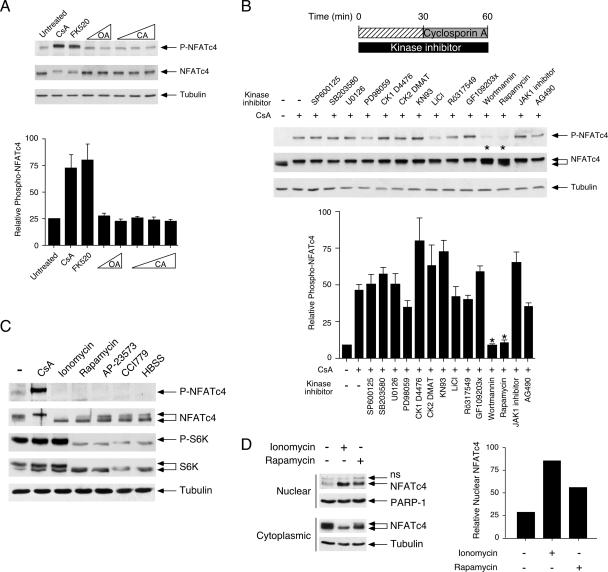
FIG. 3.
Identification of protein kinases targeting the NFATc4 Ser168,170 at the resting state. (A) Calcineurin inhibitors increase phosphorylation at Ser168,170 of NFATc4. MEFs were serum starved for 2 h before they were incubated with calcineurin inhibitor CsA (5 μM) or tacrolimus/FK506 analog FK520 (200 nM) for 30 min. Cells were also treated with various concentrations of okadaic acid (OA; 100 pM and 100 nM) or calyculin A (CA; 1 nM, 2 nM, and 1 μM). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The expression of NFATc4 and tubulin is also shown. The relative intensity of NFATc4 phosphorylation (P-NFATc4/NFATc4) was quantified by ImageQuant software and presented (mean ± SD [n = 3]). (B) Rapamycin or wortmannin blocks CsA-mediated phosphorylation at Ser168,170 of NFATc4. MEFs were serum starved for 2 h before incubation with various protein kinase inhibitors as indicated. Protein kinase inhibitor-treated cells were stimulated with CsA for 30 min before being harvested. Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The expression of NFATc4 and tubulin is also shown. The relative intensity of NFATc4 phosphorylation (P-NFATc4/NFATc4) was determined by ImageQuant software (mean ± SD [n = 3]). Protein kinase inhibitors administered include JNK inhibitor SP600125 (50 nM), p38 MAPK inhibitor SB203580 (1 μM), MEK inhibitor U0126 (5 μM), MEK1/2 inhibitor PD98059 (2 μM), CK1 inhibitor D4476 (1 μM), CK2 inhibitor DMAT (400 nM), CaMK inhibitor KN93 (3 μM), GSK inhibitor LiCl (20 mM), PKC inhibitor Rö317549 (200 nM), PKC inhibitor GF109203x (24 nM), PI3K inhibitor wortmannin (10 nM), mTOR kinase inhibitor rapamycin (100 nM), JAK1 inhibitor (100 nM), and Tyr kinase inhibitor AG490 (100 μM). (C) The effect of mTOR inhibition on basal phosphorylation of NFATc4. MEFs were serum starved for 2 h before incubation with rapamycin (100 nM) or ionomycin (5 μM) for 60 min. The effect of rapamycin analogs AP-23573 (10 nM) and CCI779 (50 nM) is also shown. Amino acid starvation of MEFs was carried out in Hanks' buffered salt solution (HBSS) for 60 min before being harvested. Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The electrophoretic mobility of NFATc4 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The effect of mTOR inhibition was determined by phosphorylation of S6K (P-S6K). The expression of S6K (S6K) was also shown as a control. (D) The effect of rapamycin on the subcellular distribution of NFATc4. MEFs were serum starved for 2 h before incubation with rapamycin (100 nM) or ionomycin (5 μM) for 60 min. The amount of NFATc4 in nuclear and cytoplasmic fractions was revealed by SDS-PAGE. The relative intensity of nuclear NFATc4 (nucleocytoplasmic plus nuclear) was quantified by ImageQuant software and presented. The results are representative of three independent experiments. ns, nonspecific cross-reactivity.
Next, we determined whether protein kinase inhibitors would affect basal phosphorylation at Ser168,170 of endogenous NFATc4 (Fig. 3B). Coupling with various protein kinase inhibitors would diminish tonic activity of the NFAT kinase(s), reset the unbalance elicited by the calcineurin phosphatase inhibitor, and dissect signaling pathways that were involved in the basal phosphorylation of NFATc4. Among the protein kinase inhibitors tested, wortmannin and rapamycin blocked increased phosphorylation at Ser168,170 of NFATc4 elicited by CsA (Fig. 3B). Notably, wortmannin and rapamycin also attenuated the decrease in electrophoretic mobility of NFATc4 mediated by CsA (Fig. 3B). Rapamycin binds to FKBP12 binding protein and specifically inhibits mTOR protein kinase (18, 23, 31, 37). Wortmannin blocks PI3K activation and thus inhibits mTOR activation (8, 32). Therefore, the mTOR signaling pathway likely accounts for the basal phosphorylation at Ser168,170.
Furthermore, we determined the extent of phosphorylation and the subcellular distribution of endogenous NFATc4 upon inhibition of mTOR by rapamycin (Fig. 3C and D). Administration of rapamycin increased the electrophoretic mobility of NFATc4 (Fig. 3C). Rapamycin-treated NFATc4, indeed, exhibited electrophoretic mobility similar to that of the dephosphorylated NFATc4 elicited by calcium ionophore ionomycin (Fig. 3C), which activates calcineurin to promote dephosphorylation. NFATc4 phosphorylation at Ser168,170 was also reduced in the presence of ionomycin or rapamycin (Fig. 3C). Phosphorylation of S6K, however, was sensitive only to rapamycin treatment (Fig. 3C). In addition, two different rapamycin analogs, AP-23573 and CCI779, also elicited NFATc4 and S6K dephosphorylation (Fig. 3C). Amino acid starvation, which inhibits mTOR, caused dephosphorylation of NFATc4 and S6K, as well (Fig. 3C). Notably, NFATc4 dephosphorylation was concomitant with the increase in nuclear accumulation of NFATc4 upon rapamycin treatment (Fig. 3D). These data demonstrate that the inhibition of mTOR by rapamycin leads to dysregulated phosphorylation and increased nuclear accumulation of NFATc4.
Identification of protein kinases targeting the NFATc4 Ser168,170 upon rephosphorylation.
In addition to regulating basal phosphorylation at the resting state, mTOR might participate in the rephosphorylation of Ser168,170 of NFATc4 upon inactivation. To assess rephosphorylation of endogenous NFATc4, we exploited the reversibility and kinetics of NFAT phosphorylation (Fig. 4A). Ionomycin was used to activate calcineurin, which dephosphorylates endogenous NFATc4, including Ser168,170 (Fig. 4A). Notably, the phospho-NFATc4 monoclonal antibody failed to detect phosphorylation at Ser168,170 in the presence of ionomycin (Fig. 4A). Termination of calcineurin activation and rephosphorylation of NFATc4 were initiated by removing ionomycin from and subsequently washing the cells. Immunoblotting analysis demonstrated rephosphorylation at Ser168,170 of NFATc4 after cells were washed (Fig. 4A). Indeed, the electrophoretic mobility of the rephosphorylated NFATc4 was similar to that of the basal state (Fig. 4A).
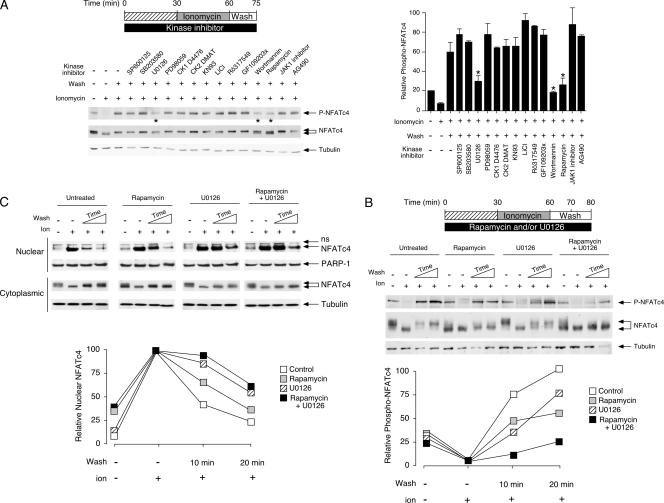
FIG. 4.
Identification of protein kinases targeting NFATc4 Ser168,170 upon rephosphorylation. (A) Rapamycin, wortmannin, or U0126 blocks rephosphorylation at Ser168,170 of NFATc4. MEFs were serum starved for 2 h before incubation with various protein kinase inhibitors as indicated. Cells were then stimulated with ionomycin for 30 min before the removal of ionomycin and subsequent wash (15 min). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The expression of NFATc4 and tubulin is also shown. The relative intensity of NFATc4 phosphorylation (P-NFATc4/NFATc4) was quantified by ImageQuant software and presented (mean ± SD [n = 3]). See the legend to Fig. 3 for the concentration of protein kinase inhibitors administered. (B and C) The effects of rapamycin and U0126 in NFATc4 rephosphorylation and nuclear export. MEFs were serum starved for 2 h before incubation with rapamycin (100 nM) and/or U0126 (5 μM), as indicated. Treated cells were stimulated with ionomycin for 30 min before the removal of ionomycin and subsequent wash (10 or 20 min). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4) (B). The electrophoretic mobility of NFATc4 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (B). The levels of NFATc4 in nuclear and cytoplasmic fractions are also shown (C). The relative intensities of phospho-NFATc4 and nuclear NFATc4 (nucleocytoplasmic plus nuclear) were quantified by ImageQuant software and presented. The results are representative of three independent experiments. ns, nonspecific cross-reactivity.
In the presence of wortmannin or rapamycin, used to inhibit mTOR activity, rephosphorylation at Ser168,170 of endogenous NFATc4 was attenuated (Fig. 4A). Notably, NFATc4 was found to have increased electrophoretic mobility (Fig. 4A). These data indicate that, in addition to basal phosphorylation at the resting state, mTOR also regulates rephosphorylation of Ser168,170 of NFATc4 upon inactivation.
Next, we asked whether one or more other protein kinases were involved in the rephosphorylation of Ser168,170 of NFATc4. Pretreatment of cells with the p38 MAPK inhibitor SB203580 did not affect the rephosphorylation of NFATc4 (Fig. 4A), despite the fact that p38 MAPK clearly phosphorylated Ser168,170 of endogenous NFATc4 upon UV irradiation (Fig. 2). Surprisingly, U0126, but not PD98059, also blocked rephosphorylation at Ser168,170 of endogenous NFATc4 (Fig. 4A). Given that U0126 and PD98059 inhibit MEK/ERK activation (including that of MEK1-2 and MEK5) and the higher selectivity of PD98059 on MEK1-2 activation (24), we surmised that the MEK5/ERK5 MAPK signaling might be involved in the rephosphorylation of Ser168,170 of NFATc4.
We ascertained the effect of rapamycin and/or U0126 on the rephosphorylation of endogenous NFATc4 by kinetic analysis (Fig. 4B and C). Ionomycin treatment increased the electrophoretic mobility (Fig. 4B) and promoted nuclear localization of NFATc4 (Fig. 4C). The phospho-NFATc4 monoclonal antibody failed to detect phosphorylation at Ser168,170 in the presence of ionomycin (Fig. 4B). Upon inactivation, the bulk of NFATc4 was rephosphorylated and exhibited decreased electrophoretic mobility (Fig. 4B). Less than 40% of NFATc4 remained in the nucleus after a 10-min wash (Fig. 4C). There was a concomitant increase in NFATc4 phosphorylation at Ser168,170 (Fig. 4B). By 20 min, rephosphorylated NFATc4 in the cytosol was more apparent (Fig. 4C). Notably, rephosphorylated NFATc4 exhibited electrophoretic mobility similar to that found in the resting state (Fig. 4B), indicating the reversibility of NFATc4 phosphorylation.
In the presence of rapamycin, used to inhibit mTOR, the electrophoretic mobility of endogenous NFATc4 was increased even at the resting state (Fig. 4B), along with an increase in nuclear accumulation of NFATc4 (Fig. 4C). Ionomycin treatment further increased NFATc4 nuclear accumulation (Fig. 4C). Ionomycin treatment, however, did not further affect the electrophoretic mobility of NFATc4 in rapamycin-treated cells (Fig. 4B). Upon inactivation, only a modest increase in NFATc4 phosphorylation at Ser168,170 and a modest decrease in the electrophoretic mobility of NFATc4 were found (Fig. 4B), indicating partial impairment of rephosphorylation upon mTOR inhibition. Indeed, in the presence of rapamycin, ∼60% of NFATc4 remained in the nucleus even after a 10-min wash (Fig. 4C). Hence, mTOR, in part, mediates NFATc4 rephosphorylation.
Next, we determined the effect of U0126 on the kinetics of the rephosphorylation of NFATc4 (Fig. 4B and C). Unlike rapamycin treatment, the administration of U0126 did not affect the basal phosphorylation (Fig. 4B) and the nuclear accumulation of NFATc4 (Fig. 4C). In the presence of ionomycin, dephosphorylated NFATc4 with increased electrophoretic mobility were found in the nucleus (Fig. 4B). Upon inactivation, some NFATc4 exhibited decreased electrophoretic mobility, while some NFATc4 remained dephosphorylated, indicating impairment of rephosphorylation in the presence of U0126 (Fig. 4B). A modest increase in rephosphorylation at Ser168,170 was also found (Fig. 4B). The impairment in rephosphorylation in the presence of U0126 was correlated with the delayed nuclear export of NFATc4 after cells were washed (Fig. 4C). Indeed, over 80% of NFATc4 remained in the nucleus after a 10-min wash (Fig. 4C). These data indicate that the MEK/ERK MAPK signaling, likely the MEK5/ERK5 cascade, also partly mediates NFATc4 rephosphorylation.
Given the partial rephosphorylation of NFATc4 upon mTOR inhibition or administration of U0126, we examined the combined effects of rapamycin and U0126 (Fig. 4B and C). Similar to the effect of rapamycin alone, treatment with rapamycin plus U0126 indicated that basal phosphorylation of NFATc4 was dysregulated (Fig. 4B). Upon inactivation, the electrophoretic mobility of NFATc4 was unaffected (Fig. 4B). Notably, the modest decrease in electrophoretic mobility of NFATc4 upon inhibiting mTOR alone was blocked (Fig. 4B). Rephosphorylation of NFATc4 at Ser168,170 was also markedly reduced after cells were washed (Fig. 4B). In addition, the nuclear export of NFATc4 was delayed in the presence of rapamycin plus U0126 (Fig. 4C). Together, these data indicate that rapamycin and U0126 block rephosphorylation and attenuate nuclear export of NFATc4.
Protein kinases mTOR and ERK5 bind to and phosphorylate Ser168,170 of NFATc4.
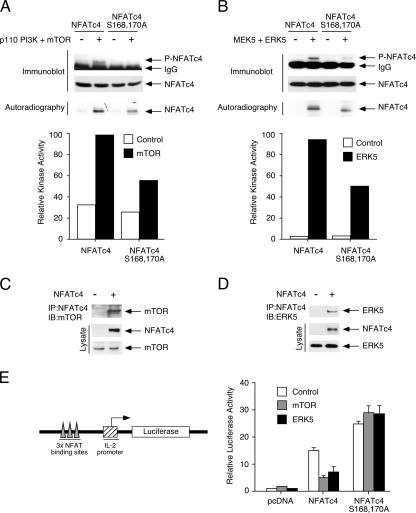
Next, we determined whether mTOR or ERK5 phosphorylated Ser168,170 of NFATc4 by using immune complex kinase assays (Fig. 5A and B). The activation of mTOR or ERK5 increased phosphorylation at Ser168,170 (Fig. 5A and B). Replacement of Ser168,170 with Ala reduced mTOR- or ERK5-mediated phosphorylation (Fig. 5A and B). Phosphorylation of NFATc4 by mTOR or ERK5 protein kinases was also confirmed by the incorporation of [32P]ATP (Fig. 5A and B). These data indicate that mTOR or ERK5 phosphorylates NFATc4, including the gate-keeping residues Ser168,170.
FIG. 5.
Protein kinases mTOR and ERK5 bind to and phosphorylate Ser168,170 of NFATc4. (A and B) Phosphorylation of Ser168,170 of NFATc4 by mTOR and ERK5. Recombinant proteins containing amino acids 1 to 218 of the wild-type and by Ala168,170 of NFATc4 were phosphorylated by mTOR upon activation (+) by constitutive active PI3K p110 (A) or by ERK5 upon activation (+) by upstream MAP2K MEK5 (B). Phosphorylation of Ser168,170 of NFATc4 was detected by phospho-NFATc4 monoclonal antibody (P-NFATc4) by immunoblotting analyses. A similar amount of NFATc4 recombinant protein is shown. Autoradiography and the extent of phosphorylation quantitated by PhosphorImager are also presented. The results are representative of three independent experiments. (C and D) NFATc4 interacts with mTOR and ERK5. Cells expressing NFATc4 and mTOR (C) or ERK5 (D) were immunoprecipitated (IP) with M2 monoclonal antibody, which recognizes NFATc4. The presence of mTOR or ERK5 in the NFATc4 precipitates was determined by immunoblotting (IB) analysis. The expression of NFATc4, mTOR, and ERK5 is also shown. (E) Activation of mTOR or ERK5 inhibits NFATc4-mediated reporter gene activity. BHK cells were transfected with an NFAT-luciferase reporter plasmid together with an expression vector for the wild-type or the Ala168,170 NFATc4. Cells were also cotransfected with PI3K p110 and mTOR or MEK5 and ERK5. The cells were harvested 36 h after transfection, and luciferase activity was normalized to β-galactosidase activity and presented (mean ± SD [n = 4]).
Furthermore, we determined whether mTOR or ERK5 interacted with NFATc4 (Fig. 5C and D). Coimmunoprecipitation assays indicated the presence of mTOR or ERK5 in the NFATc4 precipitates (Fig. 5C and D). These data indicate that mTOR or ERK5 interacts with NFATc4.
Phosphorylation at the gate-keeping Ser residues affects subcellular localization and subsequent NFAT-mediated gene transcription (3, 10, 11, 40). We asked whether mTOR or ERK5 activation affected NFAT-mediated gene transcription (Fig. 5E). The expression of NFATc4 increased NFAT-mediated gene transcription (Fig. 5E). Coexpression with the mTOR upstream activator p110 PI3K reduced NFATc4-mediated gene transcription (Fig. 5E). Similarly, activation of ERK5 reduced NFATc4-mediated gene transcription (Fig. 5E). Expression of the Ala168,170 of NFATc4 led to further increase in NFATc4-mediated gene transcription (Fig. 5E). Neither mTOR nor ERK5, however, reduced the gene transcription mediated by the Ala168,170 of NFATc4 (Fig. 5E), indicating transcription inhibition was mediated by phosphorylating Ser168,170 of NFATc4. Together, these data demonstrate that mTOR and ERK5 negatively regulate NFATc4-mediated gene transcription by binding to and phosphorylating Ser168,170 of NFATc4.
ERK5 regulates NFATc4 phosphorylation and nucleocytoplasmic shuttling.
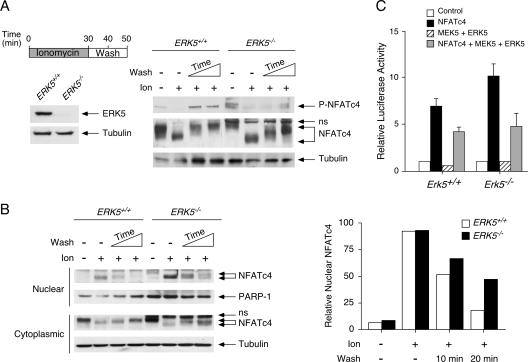
Given that U0126 inhibits both ERK1-2 and ERK5 signaling, we ascertained the role of ERK5 in the rephosphorylation of Ser168,170 of endogenous NFATc4, using the Erk5−/− cells (Fig. 6A and B). Immunoblotting analysis confirmed the lack of ERK5 in the Erk5−/− cells (Fig. 6A). At the resting state, NFATc4 was found mainly in the cytoplasmic compartments of the Erk5+/+ and Erk5−/− cells (Fig. 6B). Less than 10% of the NFATc4 protein in the Erk5+/+ or Erk5−/− cells was found in the nuclear fraction at the resting state. Ionomycin treatment increased the electrophoretic mobility (Fig. 6A) and the nuclear accumulation (Fig. 6B) of NFATc4 in Erk5+/+ and Erk5−/− cells. Removing the ionomycin and subsequent cell washing promoted rephosphorylation and nuclear export of NFATc4 in the Erk5+/+ cells (Fig. 6A and B). Less than 40% and 10% of nuclear NFATc4 were found in the Erk5+/+ cells after a 10- and 20-min wash, respectively (Fig. 6B). In the Erk5−/− cells, however, the kinetics of NFATc4 rephosphorylation (Fig. 6A) and nuclear export (Fig. 6B) were reduced, as indicated by the decreased phosphorylation of NFATc4 at Ser168,170 (Fig. 6A) and the increased accumulation of nuclear NFATc4 (Fig. 6B). Notably, the electrophoretic mobility of NFATc4 in the Erk5−/− cells was increased compared to that in the Erk5+/+ cells (Fig. 6A). In addition, over 40% of NFATc4 remained in the nuclear fraction (Fig. 6B), along with a modest increase in phosphorylation at Ser168,170 (Fig. 6A) in the Erk5−/− cells, even after a 20-min wash. The partial rephosphorylation and delayed nuclear export of NFATc4 in the Erk5−/− cells resemble the effect of U0126 on the Erk5+/+ cells (Fig. 4B and C). Rescue of the Erk5−/− cells with exogenous ERK5 also led to partial rephosphorylation of NFATc4 (data not shown). These data demonstrate that ERK5 regulates rephosphorylation and nucleocytoplasmic shuttling of NFATc4.
FIG. 6.
ERK5 regulates NFATc4 phosphorylation and nucleocytoplasmic shuttling. (A) Ablation of ERK5 affects electrophoretic mobility of NFATc4. Erk5+/+ and Erk5−/− MEFs were stimulated with ionomycin for 30 min before ionomycin withdrawal and then washed (10 or 20 min). Phosphorylation at Ser168,170 of endogenous NFATc4 was determined by phospho-NFATc4 monoclonal antibody (P-NFATc4). The electrophoretic mobility of NFATc4 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The expression of tubulin is also shown. The expression of ERK5 in Erk5+/+ and Erk5−/− MEFs is shown to confirm the genotype. (B) ERK5 regulates the nucleocytoplasmic shuttling of NFATc4. Erk5+/+ and Erk5−/− MEFs were stimulated with ionomycin for 30 min before ionomycin withdrawal and then washed (10 or 20 min). The amounts of NFATc4 in cytoplasmic and nuclear fractions were determined by immunoblotting analysis. The expression of tubulin and PARP-1 is shown. The relative intensity of nuclear NFATc4 (nucleocytoplasmic plus nuclear) was quantified by ImageQuant software and presented. The results are representative of three independent experiments. (C) ERK5 regulates NFATc4-mediated gene transcription. The Erk5+/+ or Erk5−/− MEFs were transiently transfected with an NFAT-luciferase reporter plasmid together with an expression vector for wild-type NFATc4. Cells were also cotransfected with MEK5 and ERK5 as indicated. Cells were harvested 36 h after transfection, and luciferase activity was normalized to β-galactosidase activity and presented (mean ± SD [n = 4]).
Dysregulation of nucleocytoplasmic shuttling in the Erk5−/− cells would affect NFAT-mediated gene transcription. We therefore determined NFAT-mediated gene transcription in the Erk5−/− cells, using luciferase reporter assays (Fig. 6C). Expression of NFATc4 increased NFAT-mediated gene transcription in Erk5+/+ cells (Fig. 6C). In the Erk5−/− cells, dysregulation of NFATc4 subcellular localization led to a further increase in NFAT-mediated gene transcription (Fig. 6C). Coexpression of MEK5 and ERK5 reduced NFAT reporter activity in Erk5+/+ cells and, importantly, in the Erk5−/− cells as well (Fig. 6C). Together, these data demonstrate that MEK5/ERK5 signaling negatively affects NFAT-mediated gene transcription.
Cytoplasmic-nuclear distribution of mTOR and ERK5.
One unresolved question was where did the rephosphorylation of Ser168,170 of NFATc4 take place. Phosphorylation of Ser168,170 of NFATc4 might take place in the nucleus before nuclear export. Notably, both mTOR and ERK5 were present in the nucleus (6, 20-22). Alternatively, nuclear export might precede phosphorylation at Ser168,170 of NFATc4. Hence, mTOR and ERK5 phosphorylate Ser168,170 of NFATc4 in the cytosol.
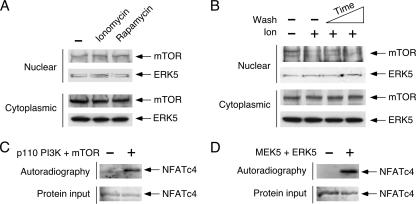
Next, we determined whether the differential subcellular distribution of mTOR and ERK5 might account for the location of NFATc4 rephosphorylation. We performed subcellular fractionation and determined the subcellular distribution of mTOR and ERK5 in the presence of ionomycin or rapamycin (Fig. 7). Similar amounts of nuclear and cytoplasmic mTOR and ERK5 were detected in the presence and absence of ionomycin (Fig. 7A). Rapamycin also did not affect the subcellular distribution of mTOR or of ERK5 (Fig. 7A). Upon inactivation, subcellular distribution of mTOR or ERK5 in the nucleus and cytoplasm was also similar (Fig. 7B). Indeed, nuclear mTOR or ERK5 phosphorylated NFATc4 as expected (Fig. 7C and D). These data indicate that the subcellular distribution of mTOR and ERK5 is not affected by ionomycin or rapamycin, which elicits dephosphorylation and nuclear accumulation of NFATc4.
FIG. 7.
Nucleocytoplasmic distribution of mTOR and ERK5. (A) The effects of ionomycin and rapamycin on subcellular distribution of mTOR and ERK5. MEFs were serum starved for 2 h before incubation with ionomycin (5 μM) or rapamycin (100 nM) for 60 min. The amounts of mTOR and ERK5 in nuclear and cytoplasmic fractions were revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (B) Subcellular distribution of mTOR and ERK5 during NFAT phosphorylation cycle. Serum-starved (2 h) MEFs were stimulated with ionomycin for 30 min before removal of ionomycin and subsequently washed (10 or 20 min). The levels of mTOR and ERK5 in nuclear and cytoplasmic fractions is shown. (C and D) Phosphorylation of NFATc4 by nuclear mTOR and ERK5. Nuclear extracts prepared from cells transfected with mTOR (C) or ERK5 (D) were immunoprecipitated, and immune complex protein kinase assays were performed using recombinant proteins containing amino acids 1 to 218 of NFATc4. Phosphorylation of NFATc4 was detected by autoradiography. Similar amounts of NFATc4 recombinant protein are shown.
Rephosphorylation of Ser168,170 of NFATc4 in the nucleus.
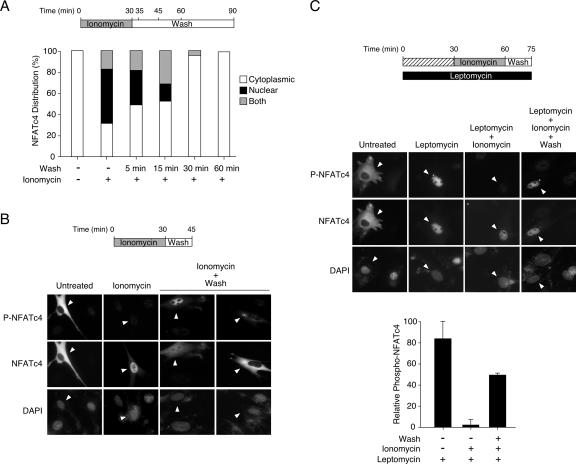
Furthermore, we addressed the question of where the rephosphorylation of Ser168,170 of NFATc4 takes place by using the phospho-NFATc4 monoclonal antibody in immunofluorescence analyses (Fig. 8). At the resting state, NFATc4 was present in the cytosol (Fig. 8A and B). Cytosolic NFATc4 containing phosphorylated Ser168,170 was revealed by staining the cells with the phospho-NFATc4 monoclonal antibody. Ionomycin stimulation activated calcineurin, and ∼75% of cells exhibited NFATc4 in the nucleus (Fig. 8A). Phosphorylation of Ser168,170, however, was abolished in nuclear NFATc4, as only negligible background staining was detected using the phospho-NFATc4 antibody (Fig. 8B). Upon inactivation, both nuclear and cytoplasmic NFATc4 were detected within 15 min (Fig. 8B). In some cells, NFATc4 and phospho-NFATc4 were detected in the nuclei, suggesting rephosphorylation of Ser168,170 of NFATc4 in the nucleus before nuclear export (Fig. 8B). In other cells, cytoplasmic NFATc4 with diffuse staining of phospho-NFATc4 around the nucleus was found (Fig. 8B). It is possible that rapid nuclear export obscured the location where the phosphorylation of Ser168,170 of NFATc4 took place.
FIG. 8.
Rephosphorylation of Ser168,170 of NFATc4 in the nucleus. (A) Kinetic analysis of NFATc4 subcellular distribution. BHK cells transiently transfected with NFATc4 were stimulated (+) or not (−) with ionomycin for 30 min to elicit NFATc4 nuclear accumulation. Treated cells were washed twice with medium and fixed with methanol for the times indicated after the wash. Immunofluorescence was performed, and cytoplasmic and nuclear staining of NFATc4 was scored with a microscope (n = 100). (B) Determining the rephosphorylation of NFATc4 by using a phospho-NFATc4 monoclonal antibody. BHK cells transiently transfected with NFATc4 were stimulated or not with ionomycin for 30 min before removal of the ionomycin and subsequent wash (15 min). Untreated cells, ionomycin-treated cells, and ionomycin-treated and subsequently washed cells were stained with phospho-NFATc4 monoclonal antibody (P-NFATc4), NFATc4 antibody, and DAPI and subjected to immunofluorescence analysis. Cells expressing NFATc4 are shown (arrowheads). (C) Rephosphorylation of NFATc4 at Ser168,170 can be a nuclear event. BHK cells transiently transfected with NFATc4 were pretreated or not with leptomycin, as indicated, before they were stimulated with ionomycin (30 min). Ionomycin-treated cells were subjected to washing (15 min), and phosphorylation of NFATc4 was determined by immunofluorescence analysis. Untreated cells, leptomycin-treated cells, leptomycin-plus-ionomycin-treated cells, and leptomycin-plus-ionomycin-treated and subsequently washed cells were stained with phospho-NFATc4 monoclonal antibody (P-NFATc4), NFATc4 antibody, and DAPI. Cells expressing NFATc4 are shown (arrowheads). The relative intensity of phospho-NFATc4 in the nucleus (P-NFATc4/NFATc4) was quantified by using ImageQuant software and presented (mean ± SD [n = 3]).
To circumvent the rapid nuclear export of rephosphorylated NFATc4 and ascertain that rephosphorylation of NFATc4 could be a nuclear event, we exploited the nuclear export inhibitor leptomycin (Fig. 8C). Leptomycin blocks Crm-mediated nuclear export and confines cytoplasmic nuclear shuttling proteins to the nucleus. In the presence of leptomycin, NFATc4 was restricted in the nucleus (Fig. 8C). Notably, phospho-NFATc4 was found in the nucleus, even at the resting state (Fig. 8C). Upon stimulation with ionomycin, phosphorylation at Ser168,170 of NFATc4 was abolished (Fig. 8C). Removing ionomycin and subsequent washing, however, led to rephosphorylation at Ser168,170 of NFATc4 in the nucleus (Fig. 8C). These data demonstrate that rephosphorylation at Ser168,170 of NFATc4 can be a nuclear event.
ERK5 as a priming protein kinase of NFATc4.
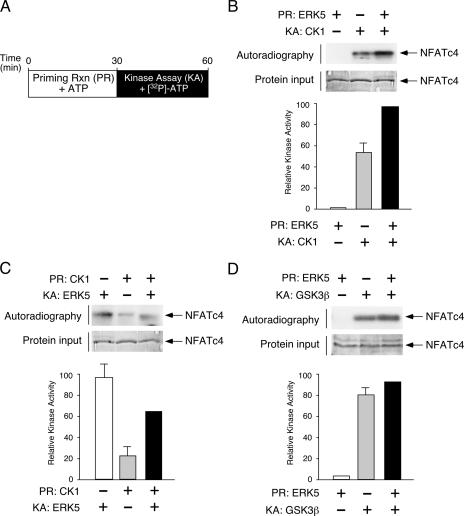
Previous studies demonstrated that the protein kinase CK1α also phosphorylated the SRR (27, 41), where Ser168,170 of NFATc4 are located. Current models suggested that phosphorylation at Ser168,170 might provide a priming effect and facilitate subsequent additional phosphorylation by CK1α. Hence, a combination of different kinases phosphorylates SRR and provides an accumulative effect to mediate nuclear export of NFAT.
To test the effect of priming upon phosphorylation at Ser168,170, we performed tandem kinase assays with unlabeled ATP in a priming reaction, followed by kinase assays of the presence of [32P]ATP (Fig. 9A). Immunoprecipitated ERK5 and recombinant CK1 were used as the priming kinase and the consecutive kinase to phosphorylate NFATc4, respectively (Fig. 9B). Although CK1 phosphorylated NFATc4, the extent of NFATc4 phosphorylation was further increased in the presence of ERK5 in the priming reaction (Fig. 9B). Reversed experiments using CK1 as the priming kinase, however, did not increase the consecutive phosphorylation mediated by ERK5 (Fig. 9C). These data indicate that the phosphorylation of ERK5 exerts a positive effect on subsequent phosphorylation by CK1 but not vice versa.
FIG. 9.
ERK5 as the priming protein kinase of NFATc4. (A) Schematic illustration of tandem protein kinase assays to determine the effect of the priming reaction (PR) on NFATc4 phosphorylation. Recombinant proteins containing amino acids 1 to 218 of NFATc4 were phosphorylated in the presence and absence of priming protein kinase for 30 min using unlabeled ATP as the substrate. Primed NFATc4 was further phosphorylated in the presence and absence of consecutive protein kinase (KA) using [32P]ATP as substrate. Phosphorylation of NFATc4 was detected by autoradiography and quantitated by PhosphorImager. (B) The effect of ERK5 as the priming protein kinase in CK1-mediated phosphorylation of NFATc4 is shown. NFATc4 recombinant proteins were primed (PR) using immune complex kinase assays in the presence of ERK5 and unlabeled ATP. Primed NFATc4 was subjected to phosphorylation in the presence of [32P]ATP and CK1 as the consecutive protein kinase (KA). Phosphorylation of NFATc4 was detected by autoradiography and was quantitated by PhosphorImager. Similar amounts of NFATc4 recombinant protein are shown. (C) The effect of CK1 as the priming protein kinase is shown. NFATc4 recombinant proteins were primed (PR) by recombinant CK1 in the presence of unlabeled ATP. Primed NFATc4 was subjected to phosphorylation in the presence of [32P]ATP and immunoprecipitated ERK5 as the consecutive protein kinase (KA). Phosphorylation of NFATc4 was detected by autoradiography and quantitated by PhosphorImager. A similar amount of NFATc4 recombinant protein is shown. (D) Effect of ERK5 as the priming protein kinase on GSK3β-mediated phosphorylation of NFATc4. NFATc4 recombinant proteins were primed (PR) using immune complex kinase assays in the presence of ERK5 and unlabeled ATP. Primed NFATc4 was subjected to phosphorylation in the presence of [32P]ATP and GSK3β as the consecutive protein kinase (KA). Phosphorylation of NFATc4 was detected by autoradiography and quantitated by PhosphorImager. A similar amount of NFATc4 recombinant protein is shown.
We also determined whether ERK5 influences GSK3β-mediated phosphorylation (Fig. 9C). Although GSK3β phosphorylated NFATc4, the extent of NFATc4 phosphorylation was not affected in the presence of ERK5 in the priming reaction (Fig. 9C). These data indicate a specific role for ERK5 in priming CK1-mediated phosphorylation of NFATc4.
DISCUSSION
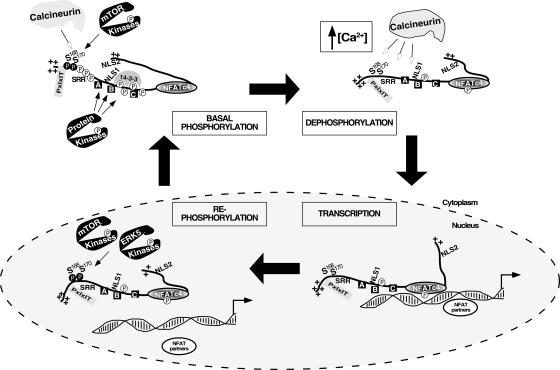
In this report, we have demonstrated that mTOR mediates basal phosphorylation and rephosphorylation of the gate-keeping residues Ser168,170 to regulate the subcellular distribution of endogenous NFATc4. Surprisingly, MEK5/ERK5 signaling also mediates rephosphorylation at Ser168,170 and, thus, nucleocytoplasmic shuttling of NFATc4. These results provide detailed mechanistic insight into NFATc4 phosphorylation and nucleocytoplasmic shuttling (Fig. 10). At the resting state, a balance of tonic activity from calcineurin phosphatase and mTOR protein kinase is required. This balance can be affected by the calcineurin inhibitor CsA or the mTOR-specific inhibitor rapamycin, which alters NFATc4 nuclear accumulation. Upon increased intracellular calcium, activated calcineurin dephosphorylates NFATc4, including the gate-keeping amino acid residues Ser168,170, to promote nuclear translocation. Once in the nucleus, NFATc4 interacts with NFAT partners and coregulators to mediate gene transcription. Transcription termination requires rephosphorylation of NFATc4, which is mediated by the protein kinase mTOR or ERK5. Rephosphorylation at Ser168,170 of NFATc4 can be a nuclear event. Phospho-Ser168,170 facilitates the subsequent additional phosphorylation of NFATc4 mediated by CK1α (27, 41), providing an accumulative effect to mediate nuclear export of NFAT.
FIG. 10.
Schematic illustration of mTOR and ERK5 in basal phosphorylation and rephosphorylation of NFATc4. At the resting state, a balance between the tonic activity from calcineurin phosphatase and the mTOR protein kinase is required to maintain cytosolic localization of NFATc4. Upon an increase in intracellular calcium, activated calcineurin dephosphorylates NFATc4, including the gate-keeping amino acid residues Ser168,170, to promote nuclear translocation. Once in the nucleus, NFATc4 interacts with NFAT partners and coregulators to mediate gene transcription. Transcription termination promotes rephosphorylation of NFATc4, which is mediated by the protein kinases mTOR and ERK5. Rephosphorylation of Ser168,170 of NFATc4 can be a nuclear event. Additional protein kinases also target other phosphorylation sites, either in the nucleus (e.g., DYRK) or in the cytosol (e.g., GSK3β and CK1α) to facilitate nuclear export and/or cytoplasmic retention to complete the NFATc4 phosphorylation cycle.
Additional protein kinases also phosphorylate other conserved Ser residues, either in the nucleus (e.g., DYRK) (1, 16) or in the cytosol (e.g., GSK3β) (3), to facilitate NFATc4 phosphorylation and cytoplasmic localization. It is possible that similar cooperation by DYRK in the priming of phosphorylation to facilitate subsequent GSK3β-mediated phosphorylation is in place. Such integration of distinct protein kinases to phosphorylate different domains may allow effective regulation of NFAT, which participates in critical biological processes including cell growth, differentiation, and development.
Here, we also ascertained phosphorylation of Ser168,170 of endogenous NFATc4 by p38 MAPK. Phosphorylation of Ser168,170 by p38 MAPK, however, does not contribute to basal phosphorylation or rephosphorylation of NFATc4. Given that p38 MAPK is activated by inflammatory stress (e.g., stimulation by interleukin 1 or tumor necrosis factor α) or upon UV irradiation, phosphorylation of Ser168,170 of NFATc4 by p38 MAPK may be dependent on the pathophysiological state of the cells. Nonetheless, the integration of three distinct protein kinases (mTOR at the resting state, ERK5 upon rephosphorylation, and p38 MAPK under stress) supports the critical “gate-keeping” function of the phosphorylation of Ser168,170 of NFATc4, which facilitates cytoplasmic retention and/or nuclear export to terminate NFAT-mediated gene transcription.
Both ERK5 and p38 MAPK belong to a similar group of protein kinases and are activated by upstream MAPK kinases, including MAP2K and MAP3K (25, 28, 36). MEK5 mediates the activation of ERK5, whereas MKK3, MKK4, and MKK6 modulate p38 MAPK activation. Previous studies indicated that a specific docking domain is required for MAPK interaction (15, 34). Recruitment of ERK5, however, seems to be independent of p38 MAPK (data not shown), suggesting that their interactions with NFATc4 are not mutually exclusive. Alternatively, the avidity of NFATc4 binding is different from that of ERK5 and p38 MAPK, despite the similar docking motifs that may be required. It is possible that the interaction and subsequent phosphorylation between NFATc4 and ERK5, NFATc4 and p38 MAPK, and NFATc4 and mTOR is dependent on the pathophysiological state of the cells.
Dysregulated mTOR signaling plays an important role in tumorigenesis (13). Recently, the mTOR inhibitor rapamycin has been in clinical trials for cancer therapy and use by transplant patients (5). It is likely that the mTOR-NFAT network is physiologically relevant. For example, the mTOR-NFAT network may contribute to angiogenesis. Previous studies demonstrated that NFAT contributed to the myocardial-endocardial transition, in part, via the repression of VEGF expression (9). Mutations in the TSC1 or TSC2 gene, which exhibits hyperactivation of mTOR signaling, lead to increased vascularization. Indeed, the expression of VEGF is elevated in Tsc1−/− and Tsc2−/− cells (14). It is tempting to speculate that constitutive activation of mTOR, frequently found in cancer cells, causes cytoplasmic retention of NFAT, which in part contributes to increased VEGF expression and vascular formation.
The ERK5-NFAT network may also contribute to angiogenesis and vascular formation. Previous studies demonstrated that target disruption of ERK5 is embryonic lethal, in part, due to problems in angiogenesis (30, 33, 38). The mechanism for the role of ERK5 in angiogenesis is unclear. Given that ERK5 mediates NFATc4 nuclear export and nuclear NFAT negatively regulates expression of VEGF, it is likely that ERK5 deficiency causes nuclear accumulation of NFAT, which represses VEGF gene transcription.
In summary, mTOR and ERK5 phosphorylate the gate-keeping residues Ser168,170 and regulate the nucleocytoplasmic shuttling of NFATc4. Regulating the subcellular distribution of NFATc4 represents a novel physiological function mediated by the mTOR and ERK5 signaling pathways.
Acknowledgments
We thank Melanie Cobb, Kun-Liang Guan, and Jonathan Backer for reagents. We also thank Jonathan Backer, Fernando Macian, Alan Whitmarsh, and members of our laboratories for their critical reading of the manuscript.
This research is supported, in part, by grants from the National Institutes of Health, the American Diabetes Association, and the American Heart Association (to C.-W.C.).
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Arron, J. R., M. M. Winslow, A. Polleri, C. P. Chang, H. Wu, X. Gao, J. R. Neilson, L. Chen, J. J. Heit, S. K. Kim, N. Yamasaki, T. Miyakawa, U. Francke, I. A. Graef, and G. R. Crabtree. 2006. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441595-600. [DOI] [PubMed] [Google Scholar]
- 2.Beals, C. R., N. A. Clipstone, S. N. Ho, and G. R. Crabtree. 1997. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev. 11824-834. [DOI] [PubMed] [Google Scholar]
- 3.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 2751930-1934. [DOI] [PubMed] [Google Scholar]
- 4.Beck, T., and M. N. Hall. 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402689-692. [DOI] [PubMed] [Google Scholar]
- 5.Buhaescu, I., H. Izzedine, and A. Covic. 2006. Sirolimus—challenging current perspectives. Ther. Drug Monit. 28577-584. [DOI] [PubMed] [Google Scholar]
- 6.Buschbeck, M., and A. Ullrich. 2005. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J. Biol. Chem. 2802659-2667. [DOI] [PubMed] [Google Scholar]
- 7.Cameron, S. J., S. Malik, M. Akaike, N. Lerner-Marmarosh, C. Yan, J. D. Lee, J. Abe, and J. Yang. 2003. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation. J. Biol. Chem. 27818682-18688. [DOI] [PubMed] [Google Scholar]
- 8.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 2961655-1657. [DOI] [PubMed] [Google Scholar]
- 9.Chang, C. P., J. R. Neilson, J. H. Bayle, J. E. Gestwicki, A. Kuo, K. Stankunas, I. A. Graef, and G. R. Crabtree. 2004. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 118649-663. [DOI] [PubMed] [Google Scholar]
- 10.Chow, C. W., C. Dong, R. A. Flavell, and R. J. Davis. 2000. c-Jun NH2-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol. Cell. Biol. 205227-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow, C. W., M. Rincon, J. Cavanagh, M. Dickens, and R. J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 2781638-1641. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.)S67-S79. [DOI] [PubMed] [Google Scholar]
- 13.Easton, J. B., and P. J. Houghton. 2006. mTOR and cancer therapy. Oncogene 256436-6446. [DOI] [PubMed] [Google Scholar]
- 14.El-Hashemite, N., V. Walker, H. Zhang, and D. J. Kwiatkowski. 2003. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 635173-5177. [PubMed] [Google Scholar]
- 15.Enslen, H., and R. J. Davis. 2001. Regulation of MAP kinases by docking domains. Biol. Cell 935-14. [DOI] [PubMed] [Google Scholar]
- 16.Gwack, Y., S. Sharma, J. Nardone, B. Tanasa, A. Iuga, S. Srikanth, H. Okamura, D. Bolton, S. Feske, P. G. Hogan, and A. Rao. 2006. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441646-650. [DOI] [PubMed] [Google Scholar]
- 17.Hogan, P. G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 172205-2232. [DOI] [PubMed] [Google Scholar]
- 18.Inoki, K., H. Ouyang, Y. Li, and K. L. Guan. 2005. Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Biol. Rev. 6979-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato, Y., V. V. Kravchenko, R. I. Tapping, J. Han, R. J. Ulevitch, and J. D. Lee. 1997. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 167054-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, J. E., and J. Chen. 2000. Cytoplasmic-nuclear shuttling of FKBP12-rapamycin-associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc. Natl. Acad. Sci. USA 9714340-14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondoh, K., K. Terasawa, H. Morimoto, and E. Nishida. 2006. Regulation of nuclear translocation of extracellular signal-regulated kinase 5 by active nuclear import and export mechanisms. Mol. Cell. Biol. 261679-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, H., C. K. Tsang, M. Watkins, P. G. Bertram, and X. F. Zheng. 2006. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature 4421058-1061. [DOI] [PubMed] [Google Scholar]
- 23.Mamane, Y., E. Petroulakis, O. LeBacquer, and N. Sonenberg. 2006. mTOR, translation initiation and cancer. Oncogene 256416-6422. [DOI] [PubMed] [Google Scholar]
- 24.Mody, N., J. Leitch, C. Armstrong, J. Dixon, and P. Cohen. 2001. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 50221-24. [DOI] [PubMed] [Google Scholar]
- 25.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 1991-118. [DOI] [PubMed] [Google Scholar]
- 26.Nishimoto, S., and E. Nishida. 2006. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 7782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura, H., C. Garcia-Rodriguez, H. Martinson, J. Qin, D. M. Virshup, and A. Rao. 2004. A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol. 244184-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raman, M., W. Chen, and M. H. Cobb. 2007. Differential regulation and properties of MAPKs. Oncogene 263100-3112. [DOI] [PubMed] [Google Scholar]
- 29.Ranganathan, A., G. W. Pearson, C. A. Chrestensen, T. W. Sturgill, and M. H. Cobb. 2006. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch. Biochem. Biophys. 4498-16. [DOI] [PubMed] [Google Scholar]
- 30.Regan, C. P., W. Li, D. M. Boucher, S. Spatz, M. S. Su, and K. Kuida. 2002. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc. Natl. Acad. Sci. USA 999248-9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiling, J. H., and D. M. Sabatini. 2006. Stress and mTORture signaling. Oncogene 256373-6383. [DOI] [PubMed] [Google Scholar]
- 32.Richardson, C. J., S. S. Schalm, and J. Blenis. 2004. PI3-kinase and TOR: PIKTORing cell growth. Semin. Cell Dev. Biol. 15147-159. [DOI] [PubMed] [Google Scholar]
- 33.Sohn, S. J., B. K. Sarvis, D. Cado, and A. Winoto. 2002. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J. Biol. Chem. 27743344-43351. [DOI] [PubMed] [Google Scholar]
- 34.Tanoue, T., and E. Nishida. 2003. Molecular recognitions in the MAP kinase cascades. Cell. Signal. 15455-462. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., K. G. Finegan, A. C. Robinson, L. Knowles, R. Khosravi-Far, K. A. Hinchliffe, R. P. Boot-Handford, and C. Tournier. 2006. Activation of extracellular signal-regulated protein kinase 5 downregulates FasL upon osmotic stress. Cell Death Differ. 132099-2108. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X., and C. Tournier. 2006. Regulation of cellular functions by the ERK5 signalling pathway. Cell. Signal. 18753-760. [DOI] [PubMed] [Google Scholar]
- 37.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124471-484. [DOI] [PubMed] [Google Scholar]
- 38.Yan, L., J. Carr, P. R. Ashby, V. Murry-Tait, C. Thompson, and J. S. Arthur. 2003. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev. Biol. 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, T. T., H. Y. Suk, X. Yang, O. Olabisi, R. Y. Yu, J. Durand, L. A. Jelicks, J. Y. Kim, P. E. Scherer, Y. Wang, Y. Feng, L. Rossetti, I. A. Graef, G. R. Crabtree, and C. W. Chow. 2006. Role of transcription factor NFAT in glucose and insulin homeostasis. Mol. Cell. Biol. 267372-7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, T. T., Q. Xiong, H. Enslen, R. J. Davis, and C. W. Chow. 2002. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol. Cell. Biol. 223892-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, J., F. Shibasaki, R. Price, J. C. Guillemot, T. Yano, V. Dotsch, G. Wagner, P. Ferrara, and F. McKeon. 1998. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell 93851-861. [DOI] [PubMed] [Google Scholar]