Abstract
Many homeodomain transcription factors function in organogenesis and cell differentiation. The Nkx family illustrates these functions especially well, and the Nkx6 subfamily controls differentiation in the central nervous system and pancreas. Nkx6.3, a recent addition to this subfamily, overlaps Nkx6.1 and Nkx6.2 in expression in the hindbrain and stomach. Nkx6.3 transcripts localize in the epithelium of the most distal stomach region, the antrum and pylorus; expression in the adult intestine is lower and confined to the proximal duodenum. Nkx6.3−/− mice develop and grow normally, with a grossly intact stomach and duodenum. These mice show markedly reduced gastrin mRNA, many fewer gastrin-producing (G) cells in the stomach antrum, hypogastrinemia, and increased stomach luminal pH, with a corresponding increase in somatostatin mRNA levels and antral somatostatin-producing (D) cells. They express normal levels of other transcription factors required for gastric endocrine cell differentiation, Pdx1, Pax6, and Ngn3; conversely, Ngn3−/− mice, which also show reduced gastrin levels, express Nkx6.3 normally. These studies implicate Nkx6.3 as a selective regulator of G- and D-cell lineages, which are believed to derive from a common progenitor, and suggest that it operates in parallel with Ngn3.
Many homeodomain transcription factors play pivotal roles in development and cell differentiation. Among homeobox genes, the Nkx family is second in size to the clustered Hox genes, and its 18 members serve diverse functions in the development of the heart, pancreas, lungs, gastrointestinal (GI) tract, central nervous system, and skeleton (15, 27, 32, 44-46). The pervasive importance of Nkx genes has led to speculation about a developmental “Nkx code,” which proposes that particular combinations of coexpressed Nkx genes regulate cell differentiation in many tissues (44).
Nkx genes are grouped by subfamilies according to sequence homology, and the two founding paralogs of the Nkx6 subfamily, Nkx6.2 (previously called Gtx) and Nkx6.1, are expressed specifically and required in the developing central nervous system and pancreas (17, 38). The proteins they encode have in common a DNA sequence preference, transcriptional repression activity, and dynamically regulated expression in the mouse embryonic hindbrain and spinal cord (28, 29, 34). Early in pancreas development, Nkx6.1 and Nkx6.2 are coexpressed in undifferentiated epithelium; the two genes subsequently mark distinct endocrine cell populations (9). Nkx6.1-deficient mice show a profound, cell-autonomous failure to expand β-cell precursors, without an increase in other pancreatic endocrine cell types (40). Nkx6.1 and Nkx6.2 compound-null mutants carry more severe defects in the pancreas, with even fewer β cells and reduced numbers of glucagon-producing α cells (9). Thus, Nkx6 subfamily function in the pancreas centers on endocrine cell differentiation.
The sequence of Nkx6.3, the newest member (1, 33), is closest to that of Drosophila Nk6, which implies that it may be the ancestral founder of the vertebrate Nkx6 subfamily (33). Like its paralogs, Nkx6.3 contains an Engrailed homology domain, which may mediate interaction with transcriptional corepressors (29). In embryonic day 12.5 (E12.5) mouse embryos, Nkx6.3 expression is reported to be restricted to endoderm-derived cells in the gastric antrum, pyloric sphincter, and proximal duodenum and to a subset of V2 interneurons in the caudal hindbrain (1), but it is excluded from the pancreas (1, 33). Its expression overlaps that of Nkx6.1 in the brain and that of Nkx6.2 in the stomach.
In rodents, a stratified squamous epithelium extends from the esophagus to line the fundus or dome of the stomach, followed by the glandular mucosa of the corpus and antrum, which carries four principal specialized cell types (14). Surface mucous (also known as pit or foveolar) cells line the lumen; H/K-ATPase-expressing parietal (oxyntic) and pepsinogen-producing chief (zymogenic) cells occupy the bulk of each glandular unit. Enteroendocrine cells are the least abundant and encompass a number of sublineages, most of which produce a single hormone (41). Ghrelin-, serotonin-, somatostatin-, and histamine-secreting cells are scattered throughout the glandular stomach epithelium, whereas those that secrete gastrin are confined to the distal stomach, the antral-pyloric segment (36). While much is known about the morphology and physiology of stomach epithelial cells, few transcriptional regulators that govern their differentiation have been characterized (11, 12, 21, 23, 35). Intestinal enteroendocrine differentiation is attributed to an axis of three transcription factors, Math1, Ngn3, and NeuroD (12, 48), which progressively help determine cell fates. Less is known about the transcriptional regulation of stomach endocrine cells, although Ngn3 seems to have a prominent role (12, 23). Gastrin, the principal secreted product of G cells, induces acid secretion in the stomach by stimulating enterochromaffin-like cells to release histamine, which activates parietal-cell H2 receptors to signal HCl release (47). Gene-targeting studies implicate the transcription factors Pdx1, Pax6, and Ngn3 in G-cell differentiation through mechanisms that remain unclear (12, 20, 21, 23).
To determine the in vivo requirements for Nkx6.3, we defined the gastric expression domain in detail and used homologous recombination to inactivate the mouse gene. Mutant mice lack discernible defects in gross or cellular morphology in the foregut but reveal a profound reduction in the number of gastrin-producing G cells and in levels of gastrin mRNA. Somatostatin-producing D cells are consistently increased in number, whereas other stomach endocrine cell lineages are unaffected. Thus, Nkx6.3 seems to function late in enteroendocrine differentiation, likely influencing the identity of G versus D cells.
MATERIALS AND METHODS
Nkx6.3 gene targeting and genotyping.
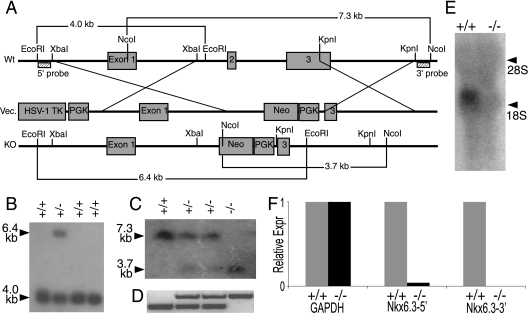
Our targeting strategy was designed to delete exons 2 and 3, resulting in complete excision of the homeodomain. A strain 129/Sv bacterial artificial chromosome clone (Invitrogen, Carlsbad, CA) containing the Nkx6.3 locus was digested with XbaI or KpnI to isolate 3.5- and 2.2-kb fragments corresponding to the desired 5′ and 3′ homology arms, respectively. These fragments were cloned into a pPNT-derived vector, and the targeting construct was electroporated into strain 129/Sv-derived TC1 embryonic stem (ES) cells. Clones were selected for growth in the presence of G418 and ganciclovir, and homologous recombination was identified by Southern analysis. Two recombinant clones were injected into C57BL/6 blastocysts to generate independent lines of Nkx6.3-null mice, which were maintained on a mixed C57BL/6-129/Sv genetic background.
For Southern analysis, genomic DNA was digested with EcoRI to monitor 5′ recombination and with NcoI to assess 3′ recombination. Membranes were hybridized with 283-bp to 338-bp probes derived from genomic sequences outside the homology arms (see Fig. 2A) amplified with primers AAAAGGATGATGAATCCCTTGAGGC and CCTGCTGCGAGCAGACTCAC (5′ probe) and primers GGGGTGTGGGCACGTAAAGG and CTTAGACTTAGTCTC CAAGT (3′ probe). With these restriction enzymes and probes, homologous recombination is predicted to replace a 4.0-kb band in the wild-type allele with a 6.4-kb band in the mutant allele (5′) and, at the 3′ end, a 7.3-kb band in the wild-type allele with a 3.7-kb band in the mutant allele. To distinguish alleles by PCR, three primers were used simultaneously: WTKO-F (AGGGGGTCACTACAACATGC, which corresponds to sequences 5′ of exon 2), WT-R (TTCCTCCCCACTTCCTTTCT, complementary to a fragment within exon2), and KO-R (TCGCCTTCTTGACGAGTTCT, which corresponds to a sequence in the neomycin resistance gene). WTKO-F and WT-R amplify a 0.4-kb fragment of wild-type exon 2; WTKO-F and KO-R amplify a 0.7-kb fragment in the recombined allele (see Fig. 2D).
FIG. 2.
Targeted disruption of the mouse Nkx6.3 gene. (A) Gene-targeting strategy to replace exons 2 and 3 in the mouse Nkx6.3 locus with a PGK-Neor cassette. Also shown are the locations of the 5′ and 3′ flanking probes used for Southern analysis and predicted band sizes in EcoRI- or NcoI-digested DNA. Wt, wild type; Vec., vector; KO, knockout. (B) Southern blot assay of ES cell DNA digested with EcoRI and interrogated with the 5′ probe. (C) Southern blot assay of mouse tail DNA digested with NcoI and interrogated with the 3′ probe. (D) PCR analysis of mouse tail DNA to distinguish Nkx6.3 wild-type (+/+), heterozygote (+/−), and mutant (−/−) animals. (E) Northern analysis of RNA from the distal stomachs of adult wild-type and Nkx6.3 knockout mice, demonstrating the absence of Nkx6.3 mRNA in the latter. 18S and 28S RNA species are visible on the blot and confirmed equal loading. (F) qRT-PCR of cDNA from adult wild-type and Nkx6.3-null stomachs, showing that Nkx6.3 mRNA is virtually undetectable with primers from the 5′ and 3′ ends. Expr, expression.
RNA expression studies.
For real-time quantitative reverse transcription (qRT)-PCR, total RNA was extracted from adult GI organs with Trizol (Invitrogen) and reverse transcribed with oligo(dT) primer and Superscript III enzyme (Invitrogen). qPCR primers, designed with the help of Primer3 software (37), were applied with SYBR green mastermix (Applied Biosystems, Foster City, CA) in an ABI Prism 7500 thermal cycler, and the results were analyzed with 7300 System Sequence Detection Software (Applied Biosystems). Results were normalized to the level of GAPDH mRNA.
For Northern analysis, we used a membrane carrying total RNA from multiple adult mouse tissues (Seegene, Seoul, South Korea; Fig. 1A) or one loaded with RNA extracted from adult intestine segments or distal stomach tissue with Trizol reagent (Fig. 1A and 2E). Membranes were hybridized at 65°C with a 32P-labeled 700-bp probe corresponding to the 3′ coding region of mouse Nkx6.3 and washed with 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate.
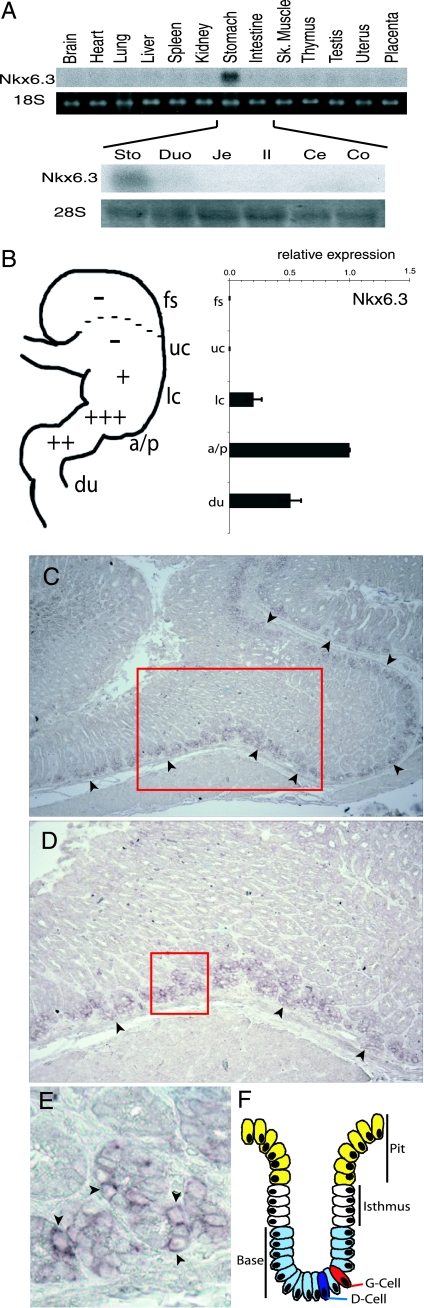
FIG. 1.
Expression of Nkx6.3 in the stomach. (A) Northern analysis of Nkx6.3 expression in adult mouse organs (top), showing the highest levels in the stomach, and in adult stomach and intestinal segments (bottom). The data confirm stomach (Sto) expression and reveal trace levels in the duodenum (Duo) but absence in the jejunum (Je), ileum (Il), cecum (Ce), or colon (Co). 18S or 28S RNA serves as a loading control. Sk., skeletal. (B) Relative expression of Nkx6.3 mRNAs in isolated forestomach (fs), upper (uc) and lower (lc) corpus, antrum-pylorus (a/p), and duodenum (du) samples from adult wild-type mice, as measured by qRT-PCR. All results were normalized to the level of GAPDH mRNA, and each segment is represented relative to an assigned wild-type antrum expression value of 1.0. The diagram depicts the anatomy and results schematically. (C) In situ hybridization on adult wild-type stomach tissue localizes Nkx6.3 transcripts in the epithelial compartment, especially in cells at the bases of antral gland units. Arrowheads mark the continuous line of Nkx6.3 expression along the base of the antral mucosa. (D) Higher resolution of the boxed area in panel C, showing Nkx6.3 expression in epithelial cells at the bottoms of glands. Arrowheads point to groups of Nkx6.3-expressing cells; expression is absent in the submucosa and the upper regions of glands. (E) Magnified image of the boxed area in panel D, showing nests of epithelial cells at the gland base, bordering the submucosa. Arrowheads point to cells with the highest Nkx6.3 signal level. (F) Graphic depiction of an antral gland unit where G and D cells occupy the base.
For in situ hybridization, tissues were fixed overnight in 4% paraformaldehyde at 4°C and sections were prepared as described below for immunohistochemistry. Sections were hybridized overnight at 65°C with a digoxigenin-labeled riboprobe transcribed from the Nkx6.3 sequence cloned into Topo II (Invitrogen); constructs were confirmed by DNA sequencing. Slides were washed with 2× and 0.2× SSC, blocked with 10% fetal bovine serum, and incubated with 1:1,000 alkaline phosphatase-conjugated digoxigenin antibody (Roche, Indianapolis, IN). Staining was visualized with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (BCIP). Images were captured with an Olympus BX41 compound microscope with an attached charge-coupled device camera and processed with Photoshop 7.0 (Adobe) to match the microscopic view.
Immunohistochemistry and tissue staining.
Adult mice were sacrificed between 2 and 10 months, and their GI organs were fixed overnight in 4% paraformaldehyde for in situ hybridization and some immunohistochemistry (gastrin) applications or in Bouin's solution (Sigma, St. Louis, MO) for histochemistry and other immunohistochemical applications (chromogranins, somatostatin, ghrelin, and serotonin). After dehydration in a graded ethanol series and embedding in paraffin, 4- to 6-μm-thick tissue specimens were rehydrated, treated with 0.5% hydrogen peroxide for 30 min, and blocked with 10% fetal bovine serum for 1 h at ambient temperature. The primary antibodies used were against chromogranins A and B (Abcam, Cambridge, MA), gastrin (E5 [Cure Digestive Diseases Research Center, University of California Los Angeles] and NCL-GASp [Vision Biosystems]), somatostatin (gift from R. Lechan, Tufts University), ghrelin (gift from K. Kangawa, Osaka, Japan), serotonin (ImmunoStar), and H+K+-ATPase (2B6; Medical and Biological Laboratories, Nagoya, Japan). Antibodies were applied overnight at 4°C at the following dilutions: chromogranins, 1:200; gastrin, 1:6,000 for E5 and 1:1,000 for NCL-GASp; somatostatin, 1:400; ghrelin, 1:25,000; serotonin, 1:10,000; H+K+-ATPase, 1:1,000. Secondary antibodies were biotin-conjugated anti-mouse or anti-rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA). Reactions were completed with the Vectastain avidin-biotin-peroxidase complex staining kit (Vector) and diaminobenzidine substrate (Sigma, St. Louis, MO). Slides were examined on an Olympus BX41 compound microscope, and results were captured with a charge-coupled device camera with Photoshop 7.0 software.
Fixed, paraffin-embedded tissues were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), or Alcian blue according to standard protocols. Tissue sections (4 to 6 μm) were hydrated in a graded ethanol series and stained with Gill's H&E for 3 min and 30 s, respectively; with Alcian blue for 30 min, followed by 1 min Nuclear Fast Red counterstaining; or with PAS reagent for 10 min and 5 min, respectively, followed by counterstaining in Harris's hematoxylin for 3 min.
Cells were counted in multiple 250-μm-long (A-P axis) areas of vertically aligned tissue sections from the antrums of several adult mice. D cells in the corpus were counted in multiple vertical glands closest to the squamous forestomach, and density was expressed per high-power microscopic field (data not shown). Unpaired t tests were conducted with GraphPad software (http://www.graphpad.com/quickcalcs/ttest1.cfm).
Transmission electron microscopy.
Adult mice (8 to 12 weeks old) were euthanized after starvation for 3 h. The esophagus and distal duodenum were clamped, and fixative (2.5% paraformaldehyde, 5% glutaraldehyde, 0.06% picric acid, 0.1 M cacodylate, 0.06% CaCl2) was infused into the stomach for 2 min before these organs were isolated and fixed overnight at 4°C in the same solution. Samples were postfixed in OsO4 and embedded in Epon 812. After light microscope inspection of toluidine blue-stained 0.5-μm sections, appropriate thin sections were stained with uranyl acetate and lead citrate and examined in a JEOL 1200 electron microscope at an accelerating voltage of 80 kV.
Overexpression studies.
293TD cells were cultured to semiconfluence in Dulbecco's modified Eagle medium supplemented with antibiotics and 10% fetal bovine serum and transfected overnight with 1 μg of Nkx6.3 expression plasmid or 1 μg of pCIG vector with Transfectin (Bio-Rad). Total RNA was extracted 48 h later and analyzed by qRT-PCR.
pH and serum gastrin measurements.
Age-matched mice, 5 to 17 months old, were fasted overnight with access to water. To determine gastric pH, mice were anesthetized and, after ligating the gastroesophageal and gastroduodenal junctions, the stomach lumen was flushed with a 140 mM NaCl solution (2 ml). The pH of the effluent was measured with Corning pH meter 320. Serum gastrin was quantified by radioimmunoassays in duplicate at the Diagnostic Center for Population and Animal Health, Michigan State University. Statistical analysis was done by unpaired t tests with GraphPad software (http://www.graphpad.com/quickcalcs/ttest1.cfm).
RESULTS AND DISCUSSION
Nkx6.3 expression in the GI tract.
In an expression screen of ∼1,400 TF genes in the developing mouse gut (3), we recognized a novel homeobox gene to be expressed much more abundantly in stomach than in the intestine. Its homeodomain sequence is most closely related to those in the Nkx6 subfamily, and the new gene is accordingly called Nkx6.3 (1, 30, 33). Northern analysis of multiple adult mouse organs (Fig. 1A) reinforced the view that Nkx6.3 RNA levels are especially high in the stomach and revealed its exclusion from other sites, including the brain and small intestine, organs where regionally restricted expression is evident in developing embryos (1, 33). This result may reflect reduced expression in the adult, combined with the fact that local areas of expression constitute a minor fraction of that in the whole organ. Indeed, Northern analysis of adult mouse gut segments revealed high expression in the stomach and trace levels in the duodenum but none in the jejunum, ileum, cecum, or colon (Fig. 1A).
The mouse stomach is lined by stratified squamous epithelium over the rostral one-third to one-half of its surface and by glandular mucosa over the remainder. To refine the Nkx6.3 expression domain, we first examined discrete adult stomach regions by real-time qRT-PCR. Nkx6.3 expression was undetectable in the squamous forestomach or upper half of the gastric corpus, and levels were highest in the antral-pyloric segment, where the mucosa has a distinctive glandular architecture; compared to the antrum, expression was fivefold lower in the distal corpus and at least twofold lower in the proximal duodenum (Fig. 1B). In situ hybridization localized Nkx6.3 mRNA exclusively within the epithelial compartment of the adult antrum, near the bases of individual gastric units (Fig. 1C to F). This regional distribution in the adult stomach matches that reported in developing and newborn mice (1) and reveals Nkx6.3 as an antral marker. Furthermore, spatial restriction to the bases of individual gastric units, away from the zone of immature proliferating cells in the isthmus, suggests that only differentiated migrant cells express Nkx6.3.
These results identify Nkx6.3 as an epithelial cell product in the distal stomach, which differs from the remaining mucosa in that it lacks acid-producing (parietal) and zymogenic cells and is the exclusive residence for the endocrine G-cell population that secretes gastrin (36). Notably, Nkx6.3 expression overlaps that of its homolog Nkx6.2, which is also expressed in undifferentiated pancreatic epithelium and mature insulin-producing β cells (9, 33). Nkx6.3 expression is especially restricted and hence suggestive of specific regional functions.
Targeted disruption of the mouse Nkx6.3 gene.
To determine Nkx6.3 functions in vivo, we designed a targeting construct to delete exons 2 and 3 in ES cells, thus replacing the complete homeodomain and flanking regions with a neomycin resistance cassette (Fig. 2A). We screened G418-resistant clones by Southern analysis (Fig. 2B), identified five recombinants, and chose two clones to generate independent lines of knockout mice. The null allele was transmitted in Mendelian proportions, as judged by Southern analysis of tail DNA: with a probe from the 3′ aspect of the locus, NcoI digests revealed a 3.7-kb mutant band in place of a 7.3-kb wild-type band (Fig. 2C). PCR analysis of genomic DNA returned the expected 700-bp product corresponding to the mutant allele and a 400-bp wild-type product (Fig. 2D).
Northern analysis verified the absence of the full-length 2.1-kb RNA or other RNAs (Fig. 2E). qRT-PCR of antral stomach mRNA indicated a complete absence of transcripts from 3′ portions (exons 2 and 3) of the Nkx6.3 gene, whereas a fragment amplified from exon 1 could be detected at <5% of wild-type levels (Fig. 2F). These data confirm the generation of a null allele and loss of the residual transcript, probably by nonsense-mediated mRNA decay.
Nkx6.3-null mice show a selective and severe reduction in G-cell numbers.
Nkx6.3-null mutants gained weight at the same rate as littermates, lacked overt defects, and were fertile. Their digestive organs showed normal relationships, dimensions, and histology in both E14.5 embryos (data not shown) and 8- to 12-week-old adults (Fig. 3). As the Nkx6.3 expression domain spans the distal stomach and proximal duodenum and overlaps that of regional transcription factor Pdx1 (20, 31), we asked first if it influences local tissue boundaries. Brünner's glands constitute a submucosal network of bicarbonate- and mucus-secreting units that are confined to the proximal duodenum (8) and resemble mucus-secreting cells found only in the distal gastric epithelium (5). We therefore tested whether Brünner's glands are a particular venue for Nkx6.3 function.
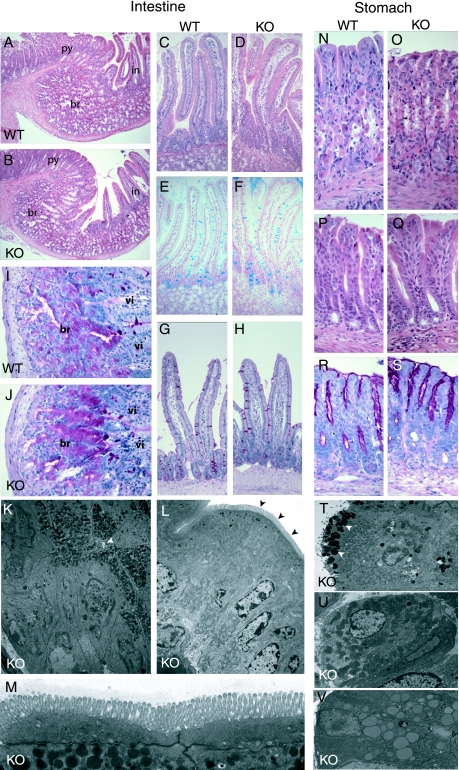
FIG. 3.
Normal features of the gastro-duodenal junction, duodenum, and stomach in Nkx6.3-deficient adult (8- to 12-week-old) mice. (A, B) H&E staining at the pyloric junction of wild-type (WT) (A) and knockout (KO) (B) adult mice shows normal tissue architecture and morphology in Nkx603-null mice. py, pylorus; in, intestine, br, Brünner's glands. (C to H) H&E (C, D), Alcian blue (E, F), and PAS (G, H) staining of control (C, E, G) and knockout (D, F, H) mouse duodenums reveals normal organization and goblet cell frequency. (I, J) PAS staining demonstrates normal morphology in wild-type (I) and mutant (J) Brünner's glands. br, Brünner's glands; vi, villus. (K to M) Transmission electron micrographs of Brünner's glands cells (K, several cells facing a small central lumen pointed to by the white arrowhead), enterocytes (L), and the intestinal apical brush border (labeled with black arrowheads and magnified in panel M), revealing normal features of each compared to wild-type samples (not shown). Mucosal glands in the gastric corpus (N, O) or antrum (P to S) of the adult wild-type (N, P, R) or Nkx6.3-null (O, Q, S) stomach, stained with H&E (N to Q) or PAS (R, S) to show the normal glandular organization and dominant epithelial cell lineages. (T to V) Transmission electron micrographs of representative foveolar (T), parietal (U), and chief (V) cells in Nkx6.3-deficient mice, which show normal ultrastructural features compared to wild-type specimens (not shown). The white arrowheads in panel T point to mucin-laden granules at the apex of a foveolar (pit) cell.
Stained tissue sections revealed a normal transition between the gastric and intestinal mucosa, with sharp demarcation (Fig. 3A and B) and no mixing between stomach glands and intestinal villi; transmission electron microscopy confirmed these impressions (data not shown). Duodenal villi showed normal architecture (Fig. 3C and D), with normal numbers and appearance of goblet (Fig. 3E to H) and Paneth (data not shown) cells. Brünner's glands extended the same length along the duodenum in mutant and wild-type mice and were similar in organization (Fig. 3I and J) and ultrastructure (Fig. 3K). Duodenal enterocytes in Nkx6.3−/− adults showed wild-type morphology (Fig. 3L), with intact apical brush borders (Fig. 3M). Thus, the proximal duodenum, a site of low Nkx6.3 expression, seems to differentiate normally in its absence. In areas of high Nkx6.3 expression, the mucosa and submucosa showed normal morphology in the distal body (Fig. 3N and O) and antrum (Fig. 3P and Q) of the mutant stomach. PAS staining indicated normal amounts and distribution of glycosylated neutral mucins (Fig. 3R and S), and H&E staining revealed the typical appearance and frequency of foveolar, parietal, and chief cells (data not shown), findings that we confirmed by transmission electron microscopy (Fig. 3T to V).
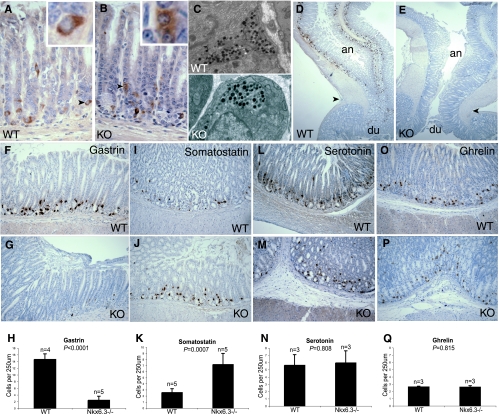
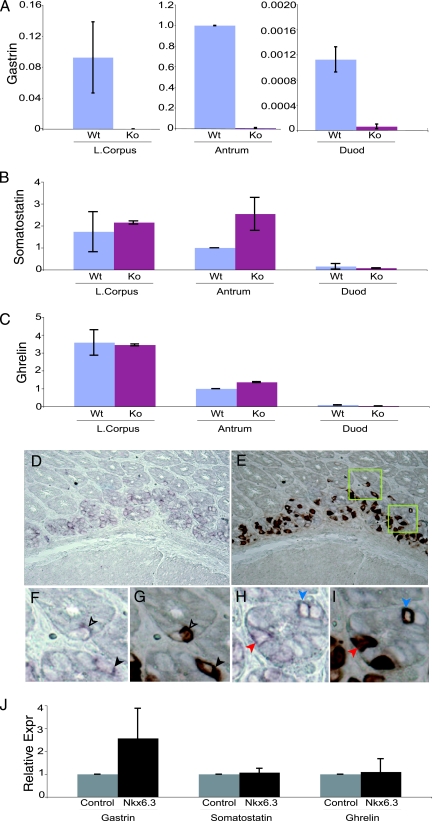
In situ hybridization places Nkx6.3 mRNA predominantly at the bases of antral gland units (Fig. 1C to E); similar results obtained with newborn mice (1) had led another group to speculate that Nkx6.3 is expressed in chromogranin A-positive gastric endocrine cells. At first glance, we observed no anomalies in chromogranin expression or endocrine-cell ultrastructure in Nkx6.3-null mice (Fig. 4A to C). However, immunohistochemical scrutiny of individual gastric endocrine markers revealed a dramatic decrease in the number of gastrin-producing G cells in all mutant animals (Fig. 4D to H). Conversely, there was a consistent approximately threefold increase in the number of somatostatin-expressing cells in the antrum and no change in that of ghrelin- or serotonin-producing cells, which are distributed throughout the gastric glandular epithelium (Fig. 4I to Q). These results were carefully quantified by morphometric analysis of vertical tissue sections (Fig. 4H, K, N, and Q). Consistent with the reduced number of G cells, we observed a marked reduction in serum gastrin levels and a proportional elevation in the stomach luminal pH (Table 1). In contrast to gastrin−/− mice (6, 16), circulating gastrin was reduced but not absent. Nevertheless, as expected from the effects of hypogastrinemia and resulting hypochlorhydria, aged Nkx6.3−/− mice (>5 months old) showed a variable degree of parietal cell atrophy and, occasionally, a notable expansion of basal mucous cells (data not shown).
FIG. 4.
Decreased number of gastric G cells in the absence of Nkx6.3. (A, B) Immunostaining for chromogranins A and B in stomach glands from wild-type (WT) (A) and knockout (KO) (B) mice. Arrowheads point to the single cells shown at a higher resolution in each inset. (C) Transmission electron micrographs of the cytoplasm, including secretory granules, of representative wild-type (top) and Nkx6.3−/− (bottom) gastric endocrine cells. (D to H) Gastrin immunostaining shows that the Nkx6.3-null antrum is virtually devoid of G cells, as confirmed by cell counts averaged from multiple animals (H). Low-magnification views (D, E) illustrate the antrum (an), with the duodenal (du) junction marked by arrowheads. (I to Q) Immunostaining for somatostatin (I to K), serotonin (L to N), and ghrelin (O to Q), with cell counts from identical vertical tissue sections represented in bar graphs. Photographs were taken from antral glands, and all counts were obtained in the antrum, the region with the highest Nkx6.3 expression level. Somatostatin-expressing D cells are increased about threefold in the Nkx6.3−/− antrum, whereas serotonin- and ghrelin-producing cell numbers are normal. Bar graphs represent means ± standard deviations; P values were obtained by unpaired t test. All analyses were conducted with adult mice between 8 and 12 weeks of age.
TABLE 1.
Circulating gastrin levels and stomach luminal pH in Nkx6.3−/− and control animalsa
| Mice | Mean serum gastrin concn (ng/liter) ± SEM (no. of mice) | P value | Mean gastric pH ± SEM (no. of mice) | P value |
|---|---|---|---|---|
| Wild type | 87.1 ± 11.0 (7) | 0.0075 | 3.9 ± 0.3 (16) | 0.0032 |
| Knockout | 37.7 ± 10.8 (7) | 5.5 ± 0.3 (7) |
Values were determined in adult mice between 5 and 17 months of age that were matched for the two genotypes as described in Materials and Methods. Statistical significance was determined with the unpaired t test.
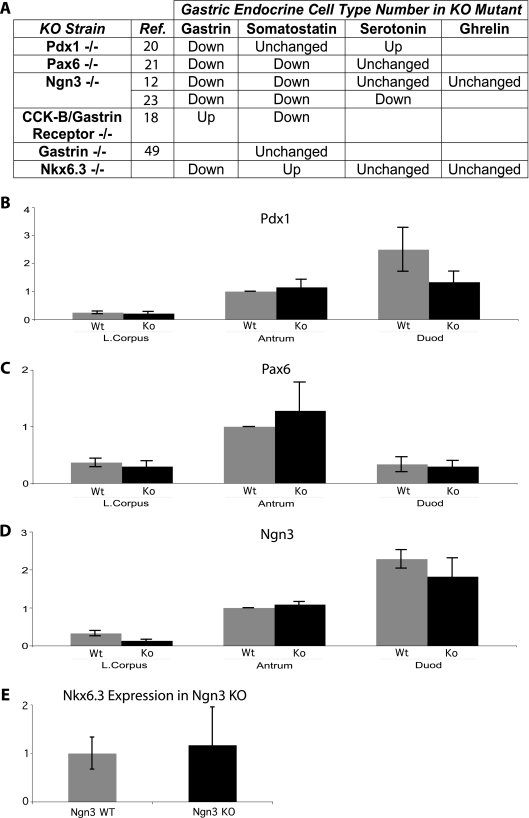
Nullizygous mice with mutations in other transcription factor genes, Pdx1, Ngn3, and Pax6, also have reduced G-cell numbers (12, 20, 21, 23). However, the proteins encoded by the latter genes affect additional hormonal lineages in the stomach and other organs, suggesting early or broad roles in endocrine cell differentiation. Nkx6.3 seems to have a more restricted role.
Nkx6.3 functions in G-cell differentiation are likely cell autonomous.
The foregoing results suggest that Nkx6.3 is required for G-cell differentiation. In qRT-PCR analysis of the antrum, the region with the highest endogenous expression of both gastrin (25, 36) and Nkx6.3 (Fig. 1B), and the duodenum, gastrin mRNA was barely detectable in Nkx6.3−/− mice (Fig. 5A); it is hence unlikely that loss of gastrin immunostaining results from altered posttranscriptional events. We consistently observed ∼2.5-fold higher somatostatin transcript levels with no change in ghrelin in the Nkx6.3−/− antrum (Fig. 5B and C). Alterations in gastrin and somatostatin mRNAs thus correspond to the markedly reduced G-cell and modestly increased D-cell numbers. Although the observed changes could result from direct or indirect effects of Nkx6.3 on the expression of these genes, we suspect that the major role of Nkx6.3 is to regulate G- versus D-cell fate.
FIG. 5.
gastrin mRNA expression is abrogated in the Nkx6.3-null antrum, and Nkx6.3 and gastrin colocalize in antral epithelial cells. (A to C) Relative expression of gastrin, somatostatin, and ghrelin mRNAs in the isolated lower (L.) corpus, antrum, and duodenum (Duod) from adult (8- to 12-week-old) wild-type (Wt) and Nkx6.3 mutant (knockout [Ko]) mice, as measured by qRT-PCR. All results were normalized to the level of GAPDH mRNA, and each segment is represented relative to an assigned wild-type antral expression value of 1.0; high gastrin levels in the wild-type antrum necessitated the use of three different y-axis scales in panel A. gastrin expression is dramatically reduced in the Nkx6.3-null antrum (A), whereas somatostatin expression is increased 2.5-fold (B) and ghrelin levels are unchanged (C). (D to I) Nkx6.3 in situ hybridization (D, F, H) followed by gastrin immunostaining (E, G, I) on the same sections. Almost all G cells coexpress Nkx6.3; conversely, cells with especially high Nkx6.3 signals coexpress gastrin, as appreciated best in high-resolution photomicrographs (F to I). Matching arrowhead colors designate Nkx6.3 in situ hybridization (F, H) and gastrin immunostaining (G, I) on the same cells. (J) qRT-PCR analysis of gastrin, somatostatin, and ghrelin mRNA levels in 293TD epithelial cells in response to forced Nkx6.3 expression (Expr) by plasmid transfection. The results represent a composite of five data points from two independent experiments. All bars (A to C, J) reflect the mean ± the standard deviation.
Individual endocrine lineages are defined according to the hormone they produce; there are as yet no additional accepted criteria (36), and G cells, by definition, are recognized on the basis of gastrin expression. It is formally possible that the Nkx6.3−/− mouse antrum harbors immature progenitors that fail to develop into functional G cells, but the lack of pre-G-cell markers precludes definitive resolution of this uncertainty. Candidate markers such as gastrin-releasing peptide receptor or somatostatin receptor 2 are not confined to G cells or their precursors but are expressed in many gastric mucosal cell types (2, 4).
Although gastrin and Nkx6.3 both localize at the bases of antral gland units, loss of gastrin gene expression in Nkx6.3−/− mice could reflect a cell-autonomous role in G cells or an effect exerted by neighboring cells. We therefore defined the Nkx6.3 expression domain in greater detail by following Nkx6.3 in situ hybridization with immunostaining for gastrin on the same tissue sections from wild-type adult mice. About 54% of Nkx6.3+ cells showed gastrin expression, whereas nearly every gastrin-stained cell also expressed Nkx6.3; most Nkx6.3+ gastrin− cells displayed a relatively weak Nkx6.3 signal. G cells thus constitute a prominent subpopulation of Nkx6.3+ cells, particularly those with the highest mRNA levels (Fig. 5D to I), suggesting a cell-autonomous function for Nkx6.3. To support this idea further, we expressed Nkx6.3 in 293TD kidney epithelial cells, which do not normally express gut hormones. In these heterologous cells, Nkx6.3 consistently induced modest expression of gastrin but not somatostatin or ghrelin transcripts (Fig. 5J), suggesting that Nkx6.3 is able to increase gastrin gene expression specifically. There are no data to indicate if this effect is direct or indirect.
In gastrin−/− (49), gastrin/cholecystokinin receptor-null (18), and other knockout mice with G-cell deficiency (Fig. 6A), hypochlorhydria is associated with normal or reduced D-cell numbers, often reflecting a physiologic response to sustained elevation of the gastric pH (26, 36). Nkx6.3−/− mice differ notably in that D-cell numbers increase in spite of hypogastrinemia and hypochlorhydria. Moreover, physiologic D-cell changes in response to the gastric pH appear throughout the stomach, including the corpus, which has many D (but no G) cells and lacks Nkx6.3 expression (24); gastrin infusion into gastrin−/− mice, for example, reduces D-cell density throughout the stomach (49). By contrast, somatostatin mRNA levels in Nkx6.3−/− mice are elevated only in the antrum and are similar to those of wild-type controls in the corpus (Fig. 5B). Corpus D-cell numbers are also similar in wild-type and mutant mice (43.4 ± 11 versus 45.3 ± 11 per high-power microscopic field, P = 0.81; data not shown). These results imply that D-cell numbers increase in the absence of Nkx6.3 by a mechanism other than a feedback effect of reduced gastrin or hypochlorhydria, and they point to a gastrin-independent effect on G-cell versus D-cell differentiation.
FIG. 6.
Nkx6.3 and Ngn3 affect G-cell differentiation through independent pathways. (A) Summary table of mutant mouse strains reported to have altered G-cell numbers and their effects on other gastric endocrine cell lineages. Empty boxes represent absence of published data. KO, knockout; Ref. reference. Pdx1, Pax6, and Ngn3 also function in organs other than the stomach. Effects on D-cell density in mice with gastrin or its receptor knocked out are opposite to those in Nkx6.3−/− mice. (B to D) Relative expression levels of Pdx1 (B), Pax6 (C), and Ngn3 (D) mRNAs are unchanged in adult Nkx6.3−/− GI segments compared to those in wild-type (Wt) mice, as measured by qRT-PCR. Results were normalized to the level of GAPDH mRNA, and each segment is represented relative to an assigned wild-type antrum expression value of 1.0. (E) Nkx6.3 mRNA levels are unchanged in the neonatal Ngn3-null antrum compared to that in littermate controls. Bar graphs show the mean ± the standard deviation. L., lower; Duod, duodenum.
Nkx6.3 and antral G-cell differentiation.
Larsson and colleagues have postulated that asymmetric division of bipotential G/D precursors in the isthmus of gastric glands generates gastrin- and somatostatin-producing daughter cells that remain in physical contact (19, 22). Genetic analyses have led to related inferences for other cell types; for example, Ngn3−/− mice have excess goblet cells and lack all endocrine lineages (12), whereas Math1−/− and Hes1−/− intestines have complementary phenotypes with respect to enterocyte and secretory-cell numbers (13, 48). Thus, multipotent gut epithelial progenitors readily adopt alternative fates when the absence of a lineage-restricted transcription factor prohibits certain choices. Our results are consistent with the notion of bipotential G/D-cell progenitors and hint that Nkx6.3 expression levels may determine G-cell identity in part. In one possible model, undifferentiated endocrine precursors in the gastric antrum require a threshold level of Nkx6.3 to differentiate into G cells, whereas those with low Nkx6.3 expression become D cells. Loss of Nkx6.3 would thus have opposite effects on gastrin- and somatostatin-producing cells, as we observed. The presence of Nkx6.3 mRNA in mature G cells (Fig. 5D to I) but not the isthmus of gastric glands (Fig. 1C and D) may further indicate that its expression is activated in the course of differentiation and is absent from early progenitors. Cells expressing the mutant Nkx6.3 gene may represent such immature cells, but the abundance of this transcript is low (Fig. 2F), either because it is unstable or because there are few progenitors. In the future, recombination-based cell lineage tracing in crosses between ROSA26 reporter (43) and Nkx6.3Cre mice could help establish and refine the proposed model.
Gastrin and somatostatin have opposing effects on stomach acid release: gastrin induces HCl secretion, while somatostatin inhibits it (10, 39, 42). In pathological or drug-induced states of sustained hypochlorhydria, G-cell numbers increase at the expense of D cells (36). Although molecular mechanisms of G- and D-cell differentiation are poorly understood, our results raise the possibility that antral endocrine progenitors may respond to physiologic needs to alter G/D-cell proportions by modulating cellular Nkx6.3 levels.
Nkx6.3 functions in G-cell differentiation appear to be independent of other known transcription factors.
Previous studies implicate Ngn3, Pdx1, and Pax6 as transcriptional regulators of G-cell differentiation (12, 20, 21, 23), although additional endocrine defects in mice lacking these factors are distinct from those we observed in the absence of Nkx6.3 (Fig. 6A). Nevertheless, the partial overlap of endocrine phenotypes suggests that Nkx6.3 could function in a pathway shared with other G-cell factors, and we determined mRNA expression levels in Nkx6.3-null stomach tissue by qRT-PCR. There was no statistically significant difference in Ngn3, Pdx1, or Pax6 mRNA levels in adult Nkx6.3−/− and littermate control stomachs (Fig. 6B to D), indicating that Nkx6.3 is not required for their expression. We also assessed Nkx6.3 expression in Ngn3-null newborns, which die within 2 days after birth (7, 23); Nkx6.3 mRNA levels were similar in Ngn3-null and littermate control stomachs (Fig. 6E). These data suggest that Nkx6.3 acts independently of Ngn3; both transcription factors are necessary, but neither alone is sufficient to enable gastrin gene expression and G-cell differentiation.
The difference between the narrow Nkx6.3−/− phenotype and the diverse endocrine effects of loss of other transcription factors (Fig. 6A) suggests that the latter operate early in endocrine cell ontogeny or independently in multiple lineages. In contrast, our results reveal Nkx6.3 as a selective regulator that localizes in mature cells at the bases of antral gland units and appears to influence G- versus D-cell identity in a cell-autonomous fashion.
Acknowledgments
This study was supported in part through the generosity of the Caring for Carcinoid Foundation; grants R01DK61139 (to R.A.S.), R01DK43673, and R01DK67166 (to A.B.L.) from the NIH; and a Research Scholar Award from the American Gastroenterological Association (to M.Y.C.).
We are grateful to R. Lechan and K. Kangawa for providing antibodies, Klaus Kaestner for sharing Ngn3-deficient mice, and Abdul Khan and Flore Celestin for expert assistance.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Alanentalo, T., F. Chatonnet, M. Karlen, R. Sulniute, J. Ericson, E. Andersson, and U. Ahlgren. 2006. Cloning and analysis of Nkx6.3 during CNS and gastrointestinal development. Gene Expr. Patterns 6162-170. [DOI] [PubMed] [Google Scholar]
- 2.Allen, J. P., A. J. Canty, S. Schulz, P. P. Humphrey, P. C. Emson, and H. M. Young. 2002. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J. Comp. Neurol. 454329-340. [DOI] [PubMed] [Google Scholar]
- 3.Choi, M. Y., A. I. Romer, M. Hu, M. Lepourcelet, A. Mechoor, A. Yesilaltay, M. Krieger, P. A. Gray, and R. A. Shivdasani. 2006. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development 1334119-4129. [DOI] [PubMed] [Google Scholar]
- 4.Ferris, H. A., R. E. Carroll, D. L. Lorimer, and R. V. Benya. 1997. Location and characterization of the human GRP receptor expressed by gastrointestinal epithelial cells. Peptides 18663-672. [DOI] [PubMed] [Google Scholar]
- 5.Friend, D. S. 1965. The fine structure of Brunner's glands in the mouse. J. Cell Biol. 25563-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friis-Hansen, L., F. Sundler, Y. Li, P. J. Gillespie, T. L. Saunders, J. K. Greenson, C. Owyang, J. F. Rehfeld, and L. C. Samuelson. 1998. Impaired gastric acid secretion in gastrin-deficient mice. Am. J. Physiol. 274G561-G568. [DOI] [PubMed] [Google Scholar]
- 7.Gradwohl, G., A. Dierich, M. LeMeur, and F. Guillemot. 2000. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 971607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman, M. L. 1958. The glands of Brunner. Physiol. Rev. 38675-690. [DOI] [PubMed] [Google Scholar]
- 9.Henseleit, K. D., S. B. Nelson, K. Kuhlbrodt, J. C. Hennings, J. Ericson, and M. Sander. 2005. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development 1323139-3149. [DOI] [PubMed] [Google Scholar]
- 10.Holst, J. J., C. Orskov, and S. Seier-Poulsen. 1992. Somatostatin is an essential paracrine link in acid inhibition of gastrin secretion. Digestion 5195-102. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen, C. M., N. Narita, M. Bielinska, A. J. Syder, J. I. Gordon, and D. B. Wilson. 2002. Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev. Biol. 24134-46. [DOI] [PubMed] [Google Scholar]
- 12.Jenny, M., C. Uhl, C. Roche, I. Duluc, V. Guillermin, F. Guillemot, J. Jensen, M. Kedinger, and G. Gradwohl. 2002. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 216338-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen, J., E. E. Pedersen, P. Galante, J. Hald, R. S. Heller, M. Ishibashi, R. Kageyama, F. Guillemot, P. Serup, and O. D. Madsen. 2000. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2436-44. [DOI] [PubMed] [Google Scholar]
- 14.Karam, S. M., and C. P. Leblond. 1992. Identifying and counting epithelial cell types in the “corpus” of the mouse stomach. Anat. Rec. 232231-246. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, S., Y. Hara, T. Pineau, P. Fernandez-Salguero, C. H. Fox, J. M. Ward, and F. J. Gonzalez. 1996. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1060-69. [DOI] [PubMed] [Google Scholar]
- 16.Koh, T. J., J. R. Goldenring, S. Ito, H. Mashimo, A. S. Kopin, A. Varro, G. J. Dockray, and T. C. Wang. 1997. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology 1131015-1025. [DOI] [PubMed] [Google Scholar]
- 17.Komuro, I., M. Schalling, L. Jahn, R. Bodmer, N. A. Jenkins, N. G. Copeland, and S. Izumo. 1993. Gtx: a novel murine homeobox-containing gene, expressed specifically in glial cells of the brain and germ cells of testis, has a transcriptional repressor activity in vitro for a serum-inducible promoter. EMBO J. 121387-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langhans, N., G. Rindi, M. Chiu, J. F. Rehfeld, B. Ardman, M. Beinborn, and A. S. Kopin. 1997. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112280-286. [DOI] [PubMed] [Google Scholar]
- 19.Larsson, L. I. 2000. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc. Res. Tech. 48272-281. [DOI] [PubMed] [Google Scholar]
- 20.Larsson, L. I., O. D. Madsen, P. Serup, J. Jonsson, and H. Edlund. 1996. Pancreatic-duodenal homeobox 1—role in gastric endocrine patterning. Mech. Dev. 60175-184. [DOI] [PubMed] [Google Scholar]
- 21.Larsson, L. I., L. St-Onge, D. M. Hougaard, B. Sosa-Pineda, and P. Gruss. 1998. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech. Dev. 79153-159. [DOI] [PubMed] [Google Scholar]
- 22.Larsson, L. I., J. E. Tingstedt, and D. M. Hougaard. 1995. Coexpression of the gastrin and somatostatin genes in differentiating and neoplastic human cells. Histochem. Cell Biol. 104139-144. [DOI] [PubMed] [Google Scholar]
- 23.Lee, C. S., N. Perreault, J. E. Brestelli, and K. H. Kaestner. 2002. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 161488-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, H., R. Hakanson, A. Karlsson, H. Mattsson, and F. Sundler. 1992. Lansoprazole and omeprazole have similar effects on plasma gastrin levels, enterochromaffin-like cells, gastrin cells and somatostatin cells in the rat stomach. Digestion 51125-132. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenberger, L. M., J. Lechago, and L. R. Johnson. 1975. Depression of antral and serum gastrin concentration by food deprivation in the rat. Gastroenterology 681473-1479. [PubMed] [Google Scholar]
- 26.Lundell, L., A. E. Bishop, S. R. Bloom, K. Carlsson, H. Mattsson, J. M. Polak, and B. Ryberg. 1988. Gastrin and somatostatin in the rat antrum. The effect of removal of acid-secreting mucosa. Regul. Pept. 2377-87. [DOI] [PubMed] [Google Scholar]
- 27.Lyons, I., L. M. Parsons, L. Hartley, R. Li, J. E. Andrews, L. Robb, and R. P. Harvey. 1995. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 91654-1666. [DOI] [PubMed] [Google Scholar]
- 28.Mirmira, R. G., H. Watada, and M. S. German. 2000. Beta-cell differentiation factor Nkx6.1 contains distinct DNA binding interference and transcriptional repression domains. J. Biol. Chem. 27514743-14751. [DOI] [PubMed] [Google Scholar]
- 29.Muhr, J., E. Andersson, M. Persson, T. M. Jessell, and J. Ericson. 2001. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell 104861-873. [DOI] [PubMed] [Google Scholar]
- 30.Nelson, S. B., C. Janiesch, and M. Sander. 2005. Expression of Nkx6 genes in the hindbrain and gut of the developing mouse. J. Histochem. Cytochem. 53787-790. [DOI] [PubMed] [Google Scholar]
- 31.Offield, M. F., T. L. Jetton, P. A. Labosky, M. Ray, R. W. Stein, M. A. Magnuson, B. L. Hogan, and C. V. Wright. 1996. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122983-995. [DOI] [PubMed] [Google Scholar]
- 32.Pabst, O., R. Zweigerdt, and H. H. Arnold. 1999. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development 1262215-2225. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen, J. K., S. B. Nelson, M. C. Jorgensen, K. D. Henseleit, Y. Fujitani, C. V. Wright, M. Sander, and P. Serup. 2005. Endodermal expression of Nkx6 genes depends differentially on Pdx1. Dev. Biol. 288487-501. [DOI] [PubMed] [Google Scholar]
- 34.Qiu, M., K. Shimamura, L. Sussel, S. Chen, and J. L. Rubenstein. 1998. Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech. Dev. 7277-88. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey, V. G., J. M. Doherty, C. C. Chen, T. S. Stappenbeck, S. F. Konieczny, and J. C. Mills. 2007. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134211-222. [DOI] [PubMed] [Google Scholar]
- 36.Rindi, G., A. B. Leiter, A. S. Kopin, C. Bordi, and E. Solcia. 2004. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann. N. Y. Acad. Sci. 10141-12. [DOI] [PubMed] [Google Scholar]
- 37.Rozen, S., and H. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 38.Rudnick, A., T. Y. Ling, H. Odagiri, W. J. Rutter, and M. S. German. 1994. Pancreatic beta cells express a diverse set of homeobox genes. Proc. Natl. Acad. Sci. USA 9112203-12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs, G., N. Zeng, and C. Prinz. 1997. Physiology of isolated gastric endocrine cells. Annu. Rev. Physiol. 59243-256. [DOI] [PubMed] [Google Scholar]
- 40.Sander, M., L. Sussel, J. Conners, D. Scheel, J. Kalamaras, F. Dela Cruz, V. Schwitzgebel, A. Hayes-Jordan, and M. German. 2000. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 1275533-5540. [DOI] [PubMed] [Google Scholar]
- 41.Schonhoff, S. E., M. Giel-Moloney, and A. B. Leiter. 2004. Minireview: development and differentiation of gut endocrine cells. Endocrinology 1452639-2644. [DOI] [PubMed] [Google Scholar]
- 42.Schubert, M. L., N. F. Edwards, and G. M. Makhlouf. 1988. Regulation of gastric somatostatin secretion in the mouse by luminal acidity: a local feedback mechanism. Gastroenterology 94317-322. [DOI] [PubMed] [Google Scholar]
- 43.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 2170-71. [DOI] [PubMed] [Google Scholar]
- 44.Stanfel, M. N., K. A. Moses, R. J. Schwartz, and W. E. Zimmer. 2005. Regulation of organ development by the NKX-homeodomain factors: an NKX code. Cell. Mol. Biol. (Noisy-le-grand) 51(Suppl.)OL785-OL799. [PubMed] [Google Scholar]
- 45.Sussel, L., J. Kalamaras, D. J. Hartigan-O'Connor, J. J. Meneses, R. A. Pedersen, J. L. Rubenstein, and M. S. German. 1998. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 1252213-2221. [DOI] [PubMed] [Google Scholar]
- 46.Tribioli, C., and T. Lufkin. 1999. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development 1265699-5711. [DOI] [PubMed] [Google Scholar]
- 47.Waldum, H. L., A. K. Sandvik, E. Brenna, and H. Petersen. 1991. Gastrin-histamine sequence in the regulation of gastric acid secretion. Gut 32698-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, Q., N. A. Bermingham, M. J. Finegold, and H. Y. Zoghbi. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 2942155-2158. [DOI] [PubMed] [Google Scholar]
- 49.Zavros, Y., G. Rieder, A. Ferguson, L. C. Samuelson, and J. L. Merchant. 2002. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am. J. Physiol. Gastrointest. Liver Physiol. 282G175-G183. [DOI] [PubMed] [Google Scholar]