Abstract
A core group of transcriptional regulatory factors regulate circadian rhythms in mammalian cells. While the suprachiasmatic nucleus in the brain serves as the central core circadian oscillator, circadian clocks also exist within peripheral tissues and cells. A growing body of evidence has demonstrated that >20% of expressed mRNAs in bone and adipose tissues oscillate in a circadian manner. The current manuscript reports evidence of the core circadian transcriptional apparatus within primary cultures of murine and human bone marrow-derived mesenchymal stem cells (BMSCs). Exposure of confluent, quiescent BMSCs to dexamethasone synchronized the oscillating expression of the mRNAs encoding the albumin D binding protein (dbp), brain-muscle arnt-like 1 (bmal1), period 3 (per3), rev-erb α, and rev-erb β. The genes displayed a mean oscillatory period of 22.2 to 24.3 hours. The acrophase or peak expression of mRNAs encoding “positive” (bmal1) and “negative” (per3) transcriptional regulatory factors were out of phase with each other by ∼8-12 hours, consistent with in vivo observations. In vivo, glycogen synthase kinase 3β (GSK3β) mediated phosphorylation regulates the turnover of per3 and core circadian transcriptional apparatus. In vitro addition of lithium chloride, a GSK3β inhibitor, significantly shifted the acrophase of all genes by 4.2-4.7 hours oscillation in BMSCs; however, only the male murine BMSCs displayed a significant increase in the length of the period of oscillation. We conclude that human and murine BMSCs represent a valid in vitro model for the analysis of circadian mechanisms in bone metabolism and stem cell biology.
Keywords: Bone Marrow Mesenchymal Stem Cells, Circadian, Dexamethasone, Lithium Chloride, Rev-erb α
Introduction
Circadian mechanisms regulate physiologic processes in organisms ranging from algae to man (28, 43). Conserved transcription regulatory factors belonging to the basic Helix-Loop-Helix and/or Period-Arnt-Singleminded (bHLH-PAS) domain families regulate the oscillatory expression of genes according to a circadian rhythm (28). These include the heterodimeric partners, Brain Muscle Arnt-Like 1 (Bmal1) and Clock, which positively regulate transcription, and their counter-regulators, Cryptochrome (Cry) and Period (Per), which repress transcription(9). In addition, a number of their immediate downstream targets have been identified, including albumin D Binding Protein (DBP) (39), Rev-erbα, and Rev-erbβ; the latter two belong to the nuclear hormone receptor family and are related to the estrogen and glucocorticoid receptors (36). The enzyme glycogen synthase kinase 3β (GSK3β) mediates a critical event necessary to maintain the 24 hour nature of the circadian clock, namely, the phosphorylation of the Period and Rev-erbα proteins and their subsequent targeting to the proteasome for turnover and degradation (6, 29, 33, 55). Lithium chloride (LiCl) can be used to inhibit the actions of GSK3β and to modulate and lengthen the oscillatory circadian period in animal models (24, 26, 55).
While the mammalian circadian clock is located centrally in the suprachiasmatic nucleus of the brain, recent studies have demonstrated that the circadian transcriptional apparatus can be found in peripheral tissues throughout the body, including adipose tissue and bone (22, 27, 44, 56). A number of observations are consistent with a circadian regulatory role in the metabolism of adipose tissue and bone. The levels of circulating biomarkers of adipose (adiponectin, leptin) (15, 41, 45, 54) and bone (osteocalcin, C-telopeptide) (21, 32, 48) activity display circadian rhythmicity in animal experiments and human clinical studies. Murine mutants deficient in the Clock or Per genes exhibit metabolic abnormalities. The Clock-/- mice show increased incidence of obesity (51) while Cry-/- and Per-/- mice display increased bone mass relative to wild type controls (14). Microarray analyses of murine adipose tissue depots and calvarial bone have found that >20% of transcripts are expressed according to a circadian oscillatory rhythm (38, 57, 58). In addition to the conserved transcriptional regulatory factors associated with the core circadian apparatus (Bmal1, Clock, Cry, Per, Rev-erb), these included genes expressing critical proteins in oxidative phosphorylation, gluconeogenesis, lipogenesis, and steroid metabolism (38, 57, 58). Together, these findings suggest that circadian mechanisms are integral to the fundamental biochemical processes necessary for adipose and bone formation.
The majority of mammalian circadian studies have required the use of intact animals. Pioneering work by the Schibler and Dunlap laboratories have demonstrated that circadian rhythms can be studied in vitro, using cell models (2-4, 8, 13). This group has demonstrated that fibroblasts from both the rat and man can express the core circadian transcriptional apparatus genes in an oscillatory manner. This response can be elicited following cell exposure to high concentrations of fetal bovine serum or glucocorticoid (3, 4, 8). Based on this literature, we hypothesized that circadian mechanisms contribute to the metabolism and differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). In this manuscript, we report that both murine and human BMSCs respond to dexamethasone with an oscillatory expression of genes encoding circadian-associated transcriptional regulatory factors. These responses can be further modulated by the addition of LiCl. These observations suggest that BMSC may serve as an alternative to in vivo models for the study of circadian processes.
Materials and Methods
Materials
All materials were obtained from Sigma/Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
MSC isolation and expansion
All protocols for animal use were reviewed and approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee. Cohorts of 20-30 C57BL/6 male or female mice (age 6-12 weeks) were euthanized by carbon dioxide asphyxiation and the femur and tibia removed under aseptic conditions. The marrow cavities were flushed with sterile medium using a #25 gauge needle and cultured established in T25 flasks in Stromal Medium (DMEM/F-12 Ham's with 10% FBS and 100 U penicillin/100 μg streptomycin/0.25 μg Fungizone) with little modification of previously described protocols(19). After 24 to 48 hours of culture at 5% CO2 and 37°C, the non-adherent cells were removed by gentle rinsing with sterile, prewarmed PBS, and the cells maintained in Stromal Medium until confluent. Cells were harvested by typsin/EDTA digestion and seeded at a density of 5 X 103 per cm2 through two subsequent passages and were designated murine bone marrow MSCs (muBMSCs). Human bone marrow MSCs (huBMSCs) from normal donors (one female, age 25, and two males, ages 32) were obtained from the Tulane Center for Distribution of Adult Stem Cells (wolfe@tulane.edu) as previously described (10, 37). These samples were obtained under a protocol reviewed and approved by the Tulane University Institutional Review Board. Cryopreserved vials of huBMSCs at passage 5-7 were thawed, expanded in Stromal Medium, and plated after 1-2 passages in 24 well plates at a density of 5 X 103 per cm2; thus, the huBMSCs from the three individual donors were at passages 6-9 at the time of the experiment.
Murine Bone Marrow MSC Differentiation
Confluent cultures of primary murine bone marrow-derived MSCs (passage 3) were maintained in Stromal Medium alone for fibroblast assessment; induced to undergo osteogenesis by continuous maintenance in Stromal Medium supplemented with 10 nM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/ml sodium ascorbate2-phosphate; or induced to undergo adipogenesis by replacing the stromal media with adipocyte induction medium composed of DMEM/F-12 with 3% FBS, 33 μM biotin, 17 μM pantothenate, 1 μM bovine insulin, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX), 5 μM rosiglitazone, and 100 U penicillin/100 μg streptomycin/0.25 μg Fungizone (30). After three days, adipocyte induction medium was changed to an adipocyte maintenance medium that was identical except for the deletion of both IBMX and rosiglitazone. Cells were maintained in culture for up to 10 days, with 90% of the maintenance media replaced every three days. Fibroblast cultures were rinsed with PBS, fixed in formalin, and stained for 20 minutes with 0.1% Toluidine Blue in formalin. Osteogenic cultures were rinsed in 0.9% NaCl, fixed in 70% ethanol, and stained with Alizarin Red for detection of o calcium phosphate mineralization.
Flow Cytometry
Flow cytometry was performed on representative murine bone marrow derived MSCs cultured from passages 4 (30). All cells were cryopreserved at concentrations of 1.0 million cells per ml in 80% fetal bovine serum, 10% DMSO, and 10% DMEM/F12 Ham's medium for a period ∼1 month prior to analysis. Prior to flow cytometric analysis, individual vials of cells were rapidly thawed in a 37°C water bath (1-2 minutes of agitation), resuspended in 5 to 10 ml of Stromal Medium, centrifuged at room temperature, and expanded in culture for a period 4 days prior to utilization for immunostaining. The MSCs were trypsin digested, assessed for viability by trypan blue exclusion, and analyzed using the following mouse monoclonal antibodies conjugated to phycoerythrin (with the exception of CD45, conjugated to Per CP) directed against the indicated antigen: from BD Pharmingen CD44 (Cat. # 553134), CD45 (Cat.# 553093), CD73 (Cat.# 550741), CD90 (Cat.#554898), CD117 (Cat.# 553869), Sca1 (Cat.# 553336), IgG1κ control (Cat.# 554680); from eBiosciences CD14 (Cat.# 12-0141-81), CD29 (Cat.# 12-0291-81), CD105 (Cat.# 12-1051-81). 10,000 events were acquired per antibody set on a Becton Dickinson FACSCaliber flow cytometer using CELLQuest acquisition software (Becton Dickinson). Data analysis was performed using Flow Jo analysis software (Tree Star).
Dexamethasone Exposure and Core Circadian Transcription Factor mRNA Analysis
The medium was removed from confluent cultures of undifferentiated murine or human MSCs in 12 well plates and replaced with DMEM/Ham's F12 medium supplemented with 2 % FCS and 100 U penicillin/100 μg streptomycin/0.25 μg Fungizone alone or further supplemented with 1 μM dexamethasone. The MSCs were exposed to the dexamethasone for 2 hrs. After 2 hrs, the medium was replaced with DMEM/Ham's F12 medium and 100 U penicillin/100 μg streptomycin/0.25 μg Fungizone alone. The cells did not receive any further medium changes from this point onward until the time of harvest. Individual plates were harvested for total RNA at 4-hr intervals up to 52 hrs following the initial exposure. When present, lithium chloride was added at a concentration of 20 mM and was present during both the 2 hr induction period and the subsequent 2% FCS replacement medium (53).
Semi-Quantitative Real-time RT-PCR
Total RNA was purified from tissues using TriReagent (Molecular Research Center) according to the manufacturer's specifications. Approximately 2μg of total RNA was reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (MMLV-RT; Promega), with Oligo dT at 42°C for 1 hr in a 20μL reaction. Primers for genes of interest (listed in Table 1) were identified using Primer Express software (Applied Biosystems). Real time RT-PCR was performed on diluted cDNA samples with SYBR® Green PCR Master Mix (Applied Biosystems) using the 7900 Real Time PCR system (Applied Biosystems) under universal cycling conditions (95°C for 10 min; 40 cycles of 95°C for 15 sec; then 60°C for 1 min). The RT-PCR for all primer pairs had been validated and determined to display single peaks in their dissociation curves. All results were normalized relative to a Cyclophilin B expression control (20, 57).
Table 1.
Primer Sequences
| Specie | Gene | Accession Number | Forward | Reverse |
|---|---|---|---|---|
| Mu | Bmal1 | AF010305 | AACCTTCCCGCAGCTAACAG | AGTCCTCTTTGGGCCACCTT |
| Mu | Cry1 | NM_053208 | AGGAGGACAGATCCCAATGGA | GCAACCTTCTGGATGCCTTCT |
| Mu | CycloB | NM_010431 | GGTGGAGAGCACCAAGACAGA | GCCGGAGTCGACAATGATG |
| Mu | DBP | NM_053207 | GGAACTGAAGCCTCAACCAAT | CTCCGGCTCCAGTACTTCTCA |
| Mu | Per1 | NM_011065 | CCAGATTGGTGGAGGTTACTGAGT | GCGAGAGTCTTCTTGGAGCAGTAG |
| Mu | Per3 | AF050182 | CCGCCCCTACAGTCAGAAAG | GCCCCACGTGCTTAAATCCT |
| Mu | Rev-erbα | NM_145434 | CCCTGGACTCCAATAACAACACA | GCCATTGGAGCTGTCACTGTAG |
| Mu | Rev-erbβ | MMU09504 | GGAACGGACCGTCACCTTT | TCCCCTGCTCCCATTGAGT |
| Hu | Bmal1 | NM_001178 | GTACCAACATGCAACGCAATG | TGTGTATGGATTGGTGGCACC |
| Hu | CycloB | M60857 | GGAGATGGCACAGGAGGAAA | CGTAGTGCTTCAGTTTGAAGTTCTCA |
| Hu | DBP | NM_001352 | CCAATCATGAAGAAGGCAAGAAA | GGCTGCCTCGTTGTTCTTGT |
| Hu | Per3 | NM_016831 | AGATGTCCTGGCGTCTTCTCA | TCATACCGTGCAGCTCTTTGG |
| Hu | Rev-erbα | X72631 | GTTTGCCAAACACATCCCG | AAGCAAAGCGCACCATCAG |
| Hu | Rev-erbβ | NM_005126 | AACAAGCAAATCGAGTGCACC | TCCATAGTGGAATCCTGACGC |
Periodicity Analysis
Periodicity of the circadian data obtained by RT-PCR was tested with Time Series Analysis-Single Cosinor v. 6.0 software (Expert Soft Technologie). Each data set was fitted to a general cosine equation model A cos(2p t/T) + B sin(2p t/T) + M, where A is the amplitude, T is the period (24 hrs), and M is the MESOR (Midline Estimating Statistic Of Rhythm) (5, 31), providing the percentage of data points that behave in a rhythmic manner, and the r2 value for the fit of the data set to the model curve. The model was also tested for validity at the 0.950 probability level (ANOVA). The period was determined using the chronobiometric ellipse test. The mean ± the standard deviation of the acrophase (peak) gene expression levels were compared using the Student's t-test.
Results
In the initial experiments, muBMSCs were isolated from the marrow of female FEV mice. The immunophenotype and differentiation potential of representative cultures were determined (Figure 1). Flow cytometry demonstrated that the muBMSCs expressed the following stromal-associated surface antigens: CD29, CD44, Sca1, >90% positive; CD73, >60% positive; and CD105, >40% positive (Figure 1 A). In contrast, the hematopoietic marker, CD45, was expressed on ∼10% of the cell population. When maintained in the presence of Stromal Medium, the muBMSCs displayed fibroblast morphology (Figure 1 B). Nine days following exposure to adipogenic factors, the muBMSCs exhibited vacuoles staining Oil Red O+ for neutral lipids while the presence of osteogenic medium for the same period led to positive staining with Alizarin Red for calcium phosphate mineralization.
Figure 1.
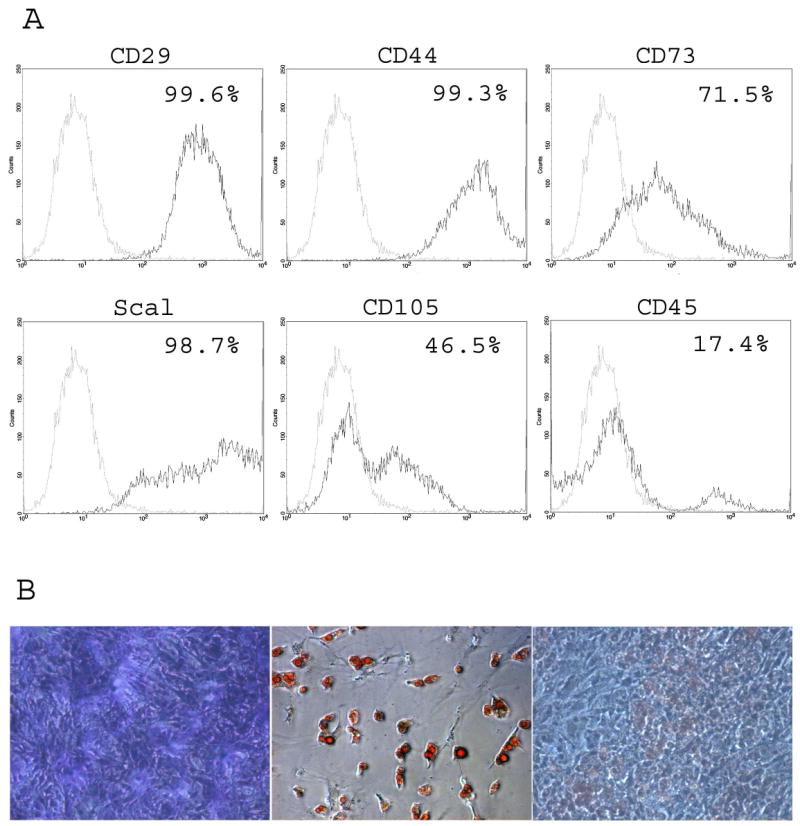
Characterization of muBMSCs. (A) Flow cytometry assessment of immunophenotype. Representative muBMSC cultures were examined for the following surface antigen expression: CD14 (LPS receptor), CD29 (integrin β1), CD44 (hyaluronate receptor), CD45 (leukocyte common antigen), CD73 (5′ ectonucleotidase), CD90 (Thy1), CD105 (endoglin), CD117 (c-kit), Sca1 (stem cell antigen). The black lines represent the histogram determined with the antigen specific antibody while the gray lines represent the histogram determined with the isotype-matched non-specific antibody control. (B) Histochemical assessment of morphology. Primary cultured muBMSCs (passage 3) were maintained in Stromal Medium (a) or induced to undergo adipogenesis (b) or osteogenesis (c) for 6 to 9 days. Individual wells were fixed and stained for (a) fibroblasts (Toluidine Blue), (b) adipocytes (Oil Red O), or (c) mineralization (Alizarin Red).
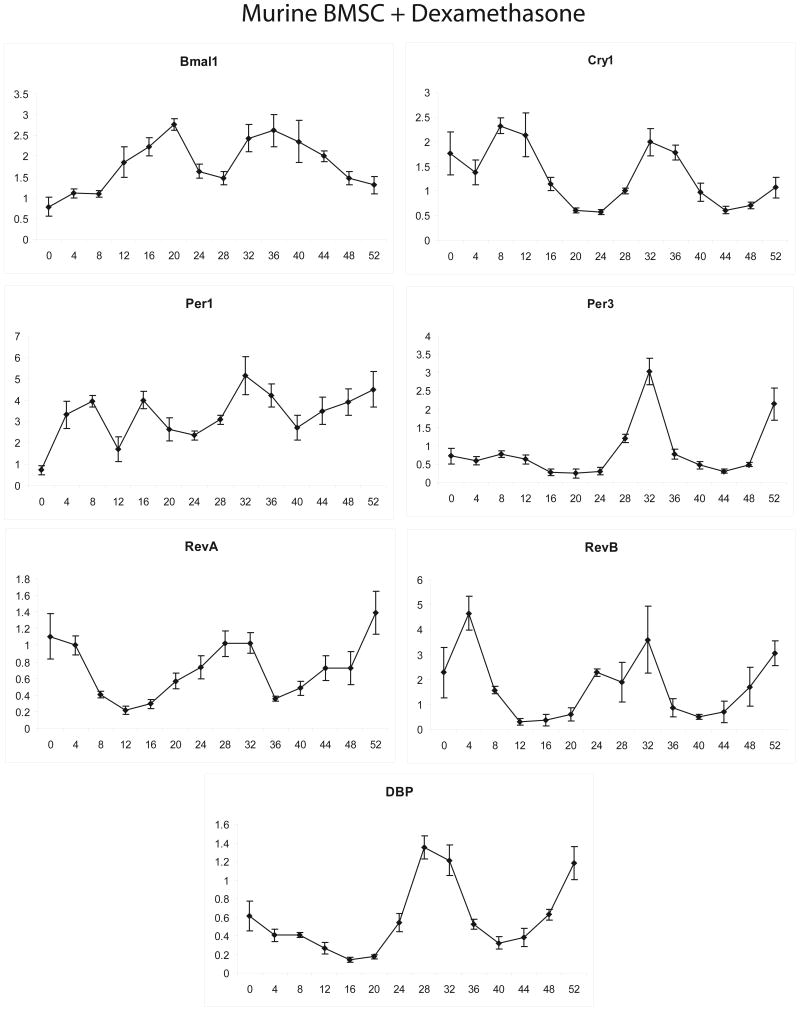
In the initial studies, confluent and quiescent muBMSCs (passage 3) were exposed to 1 μM dexamethasone for 2 hours and then converted to medium alone. Individual plates were harvested at 4 hour intervals for total RNA isolation and subsequent real time PCR analysis of mRNA expression. The expression of representative positive (bmal1), negative (cry1, per1, per3), and downstream target genes (dbp, rev-erbα, rev-erbβ) associated with the core circadian transcriptional apparatus were determined and normalized relative to cyclophilin B (Figure 2). Our previous studies on murine tissues have documented these genes to display a robust circadian oscillation and determined cyclophilin to be an appropriate control for normalizing circadian analyses (38). Dexamethasone exposure synchronized an oscillating expression profile of the mRNAs as a function of time. Of note is the fact that the peak expression or “acrophase” of bmal1 was 5.9, 11.0 and 12.6 hours out of phase with those of cry1, per1, and per3, respectively. This is consistent with in vivo observations, where the expression of the mRNAs encoding the “positive” and “negative” arms of the core circadian transcriptional apparatus are expressed in anti-phase relative to one another (28).
Figure 2.
Expression profile of mRNAs encoding transcriptional regulatory factors relating to the core circadian oscillator following dexamethasone exposure of muBMSCs. Confluent, quiescent cultures of muBMSCs isolated from FEB female mice were exposed to 1 μM dexamethasone for 2 hrs and subsequently harvested at 4 hr intervals for isolation of total RNA. Real time PCR analysis was performed to examine expression of mRNAs Albumin D binding protein (DBP), Brain-Muscle Arnt-Like 1 (bmal1), Cryptochrome 1 (cry1), Period 1 (per1), Period 3 (per3), Rev-erb α., and Rev-erb β, all normalized relative to the control, cyclophilin B. Assays were preformed in triplicate and display the mean ± S.D.
Further studies compared muBMSCs isolated from male and female mice in response to dexamethasone exposure in the absence and presence of 20 mM lithium chloride, an inhibitor of GSK3β known to modulate the circadian rhythm in vivo (24, 55) (Figure 3). A subset of the circadian-associated genes was selected for further analysis; bmal1 and per3 served as “positive” and “negative” representatives while dbp, rev-erbα and rev-erbβ served as downstream targets. Cosinor analysis was used to evaluate the datasets statistically. The acrophase for each gene in response to dexamethasone alone was not significantly different as a function of gender (Table 2). Consistent with the previous experiment, the bmal1 acrophase (13.1-13.8 hrs) was phase shifted 12.2-13.3 hrs relative to the acrophase of per3 (2.0-2.4 hrs). The period of oscillation for the five circadian-associated genes following exposure to dexamethasone alone ranged between 22.0 to 26.4 hrs for the male mice and 19.3 to 28.3 hrs for the female mice (Table 2). Making the assumption that the period of each gene was subject to regulation by a similar mechanism, we determined the mean period length for all five genes as 24.3 hrs (male) and 22.2 hrs (female); there was no significant difference between genders (Table 2). With the exception of per3 in the female mice, the addition of lithium chloride to muBMSCs in the presence of dexamethasone increased the oscillation period of gene expression for each of the five circadian-associated genes (Table 2). It should be noted that there was a wide range in the period lengths among the individual genes in the presence of lithium chloride. Despite this variability, the mean period length for the male muBMSCs increased significantly by 7.5 hrs; however, the increase of 1.4 hrs for mean period length for the female muBMSCs did not achieve significance. In contrast, the presence of lithium chloride had a more consistent effect on the acrophase of the individual genes, shifting the time of peak expression by 2.3 to 6.9 hrs. Again, assuming the existence of a shared common mechanism, we determined that the mean shift in the acrophase for all genes was 4.2 hrs in the males and 4.7 hrs in the females. These values were not significantly different between genders.
Figure 3.
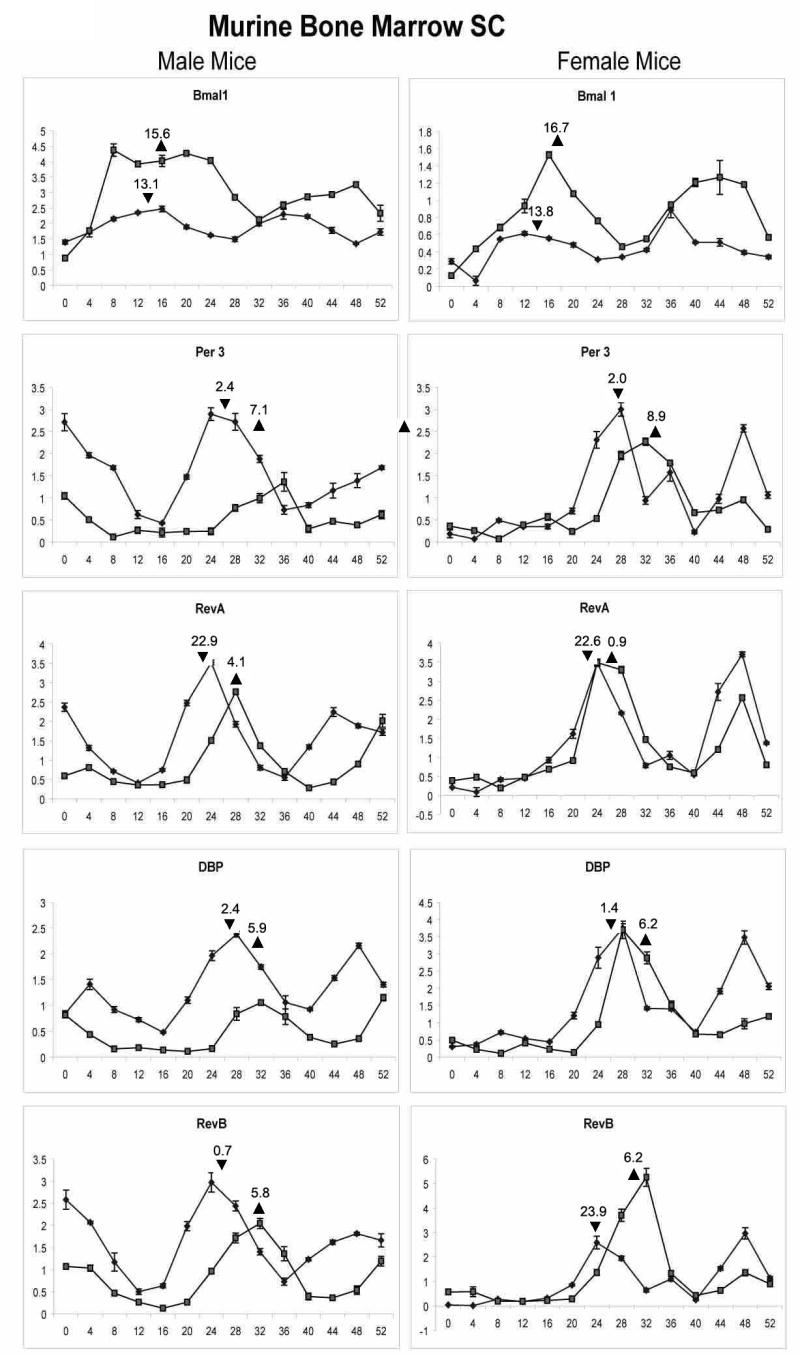
Influence of lithium chloride on dexamethasone exposure of male and female derived muBMSCs. Confluent, quiescent cultures of muBMSCs isolated from male (left panel) and female (right panel) C57BL/6 mice were exposed to 1 μM dexamethasone for 2 hrs in the absence (diamond symbol) or continuous presence (square symbol) of 20 mM lithium chloride and subsequently harvested at 4 hr intervals for isolation of total RNA. Real time PCR analysis was performed to examine expression of mRNAs bmal1, DBP, Per3, Rev-erb α., and Rev-erb β, all normalized relative to the control, cyclophilin B. Assays were preformed in triplicate and represent the mean ± S.D. Calculation of mean acrophase of gene expression. The acrophase is expressed in Circadian Time hours and a single acrophase is displayed following dexamethasone synchronization in the absence (triangle facing up) or presence (triangle facing down) of LiCl. The mean shift is the difference calculated between the acrophase in the absence and presence of LiCl.
Table 2.
Cosinor Analysis of Male and Female Murine BMSC mRNA Response to Dexamethasone ± Lithium Chloride Exposure (Figure 3)
| Gene | Gender | Period | Acrophase | Acrophase shift | ||
|---|---|---|---|---|---|---|
| Dex | Dex + LiCl | Dex | Dex + LiCl | |||
| Bmal1 | M | 24.4 | 38.7 | 13.1 | 15.6 | 2.5 |
| Bmal1 | F | 24.0 | 27.3 | 13.8 | 16.7 | 2.9 |
| Per3 | M | 26.4 | 35 | 2.4 | 7.1 | 4.7 |
| Per3 | F | 19.3 | 17.9 | 2.0 | 8.9 | 6.9 |
| Rev-erbα | M | 24.2 | 27.5 | 22.9 | 4.1 | 5.2 |
| Rev-erbα | F | 28.3 | 32.4 | 22.6 | 0.9 | 2.3 |
| DBP | M | 22.0 | 27.9 | 2.4 | 5.9 | 3.5 |
| DBP | F | 19.5 | 20.4 | 1.4 | 6.2 | 4.8 |
| Rev-erbβ | M | 24.6 | 29.7 | 0.7 | 5.8 | 5.1 |
| Rev-erbβ | F | 19.8 | 20.2 | 23.9 | 6.2 | 6.3 |
| Mean | M | 24.3±1.6a | 31.8±4.9 a,b | 4.2±1.1 | ||
| Mean | F | 22.2±3.9 | 23.6±6.0b | 4.7±2.0 | ||
Acrophase values are reported based on Circadian Time following dexamethasone exposure. There was a significant difference in the mean period of male BMSCs in the absence and presence of LiCl (a p = 0.009) and between the periods of male and female in the presence of LiCl (b p = 0.044). There was no statistical difference between genders in the period of the dexamethasone alone response nor in the acrophase shift (one tailed Student's t-test).
The response of huBMSCs (n = 3 donors) to dexamethasone in the absence and presence of lithium chloride was evaluated in comparable studies (Figure 4). The huBMSCs exhibited a time dependent oscillation in their expression of bmal1, DBP, per3, rev-erbα, and rev-erbβ mRNAs. As observed in the murine model, the acrophase of bmal1 in response to dexamethasone alone (14.1 hrs) was out of phase with that of per3 (2.4 hrs) by approximately 12 hrs (Table 3). The mean period of oscillation for all genes considered together with dexamethasone alone (22.8 hrs) was not significantly different that in the presence of lithium chloride (23.6 hrs). Nevertheless, the addition of lithium chloride resulted in a significant shift in the acrophase of each mRNA, with a mean value for all genes considered together of 5.0 ± 1.2 hrs.
Figure 4.
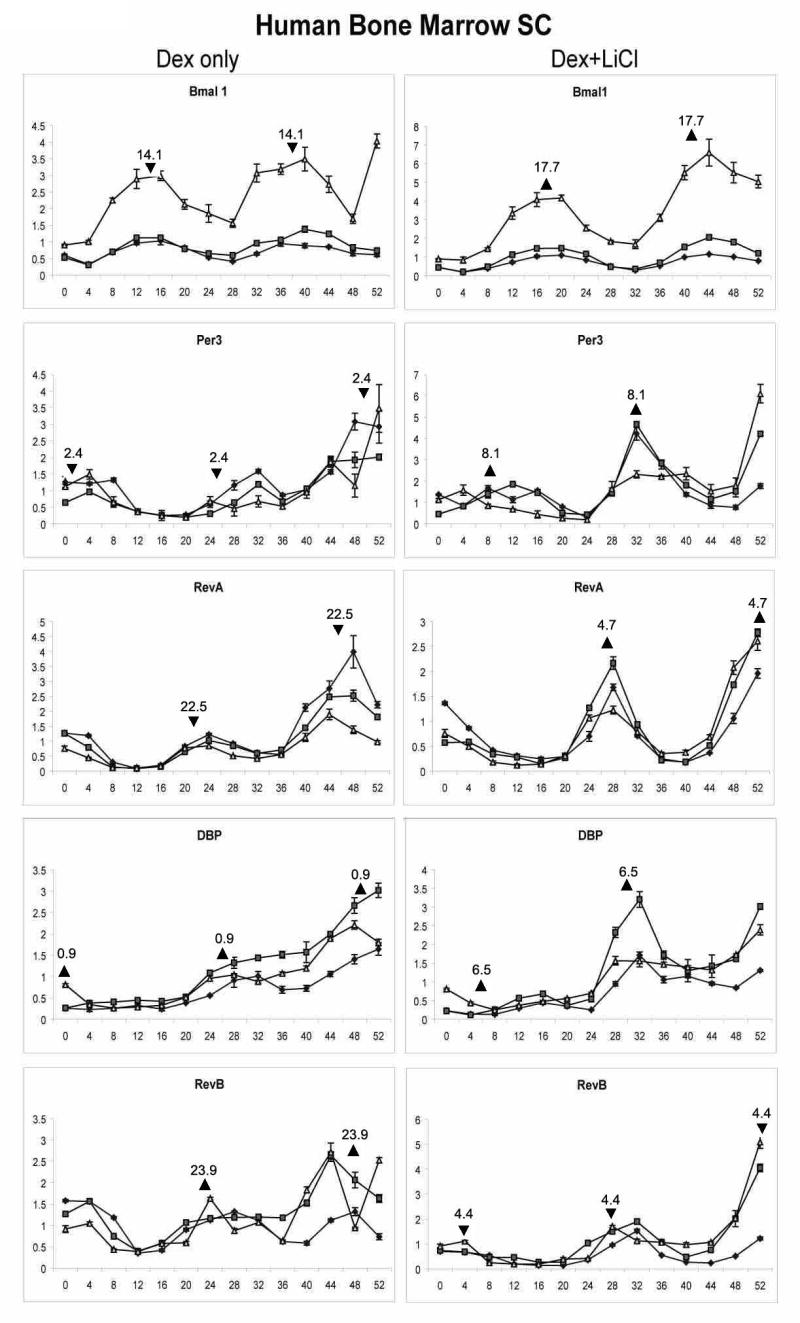
Effect of dexamethasone and lithium chloride exposure on mRNA expression in huMSCs. Confluent, quiescent cultures of huBMSCs isolated from n = 3 normal bone marrow donors (represented by closed circle, closed square, open triangle, respectively) were exposed to 1 μM dexamethasone for 2 hrs in the absence (left panel) or continuous presence (right panel) of 20 mM lithium chloride and subsequently harvested at 4 hr intervals for isolation of total RNA. Real time PCR analysis was performed to examine expression of mRNAs bmal1, DBP, Per3, Rev-erb α., and Rev-erb β, all normalized relative to the control, cyclophilin B. Assays were preformed in triplicate and represent the mean ± S.D. Calculation of mean acrophase of gene expression. The acrophase is expressed in Circadian Time hours. Each acrophase within the two period cycle is displayed after dexamethasone synchronization in the absence (triangle facing up) or presence (triangle facing down) of LiCl. The mean shift is the difference calculated between the acrophase in the absence and presence of LiCl.
Table 3.
Cosinor Analysis of Human BMSC mRNA Response to Dexamethasone ± Lithium Chloride Exposure
| Gene | Treatment | Period | P value | Acrophase | Acrophase shift | P value |
|---|---|---|---|---|---|---|
| Bmal1 | Dex | 25.6±1.9 | 0.051 | 14.1±1.1 | 3.6 | 0.002 |
| Bmal1 | Dex/ LiCl | 27.5±0.5 | 17.7±1.5 | |||
| Per3 | Dex | 22.4±1.3 | 0.106 | 2.4±0.5 | 5.7 | 0.001 |
| Per3 | Dex/ LiCl | 21.3±0.7 | 8.1±1.5 | |||
| Rev-erbα | Dex | 24.4±0.1 | 0.050 | 22.5±0.6 | 6.2 | 0.001 |
| Rev-erbα | Dex/ LiCl | 26.0±0.9 | 4.7±1.2 | |||
| DBP | Dex | 21.1±3.6 | 0.227 | 0.9±1.7 | 5.6 | 0.039 |
| DBP | Dex/ LiCl | 18.7±0.9 | 6.5±3.6 | |||
| Rev-erbβ | Dex | 22.8±1.1 | 0.077 | 23.9±1.9 | 4.5 | 0.011 |
| Rev-erbβ | Dex/ LiCl | 24.6±1.9 | 4.4±1.3 | |||
| Mean | Dex | 22.8±1,.2 | 5.0±1.2 | |||
| Dex/LiCl | 23.6±3.6 |
Acrophase values are reported based on Circadian Time following dexamethasone exposure. There was no significantly difference in the mean period length in the absence and presence of LiCl (p = 0.26, one way Student's t-test).
Discussion
The current data indicate that murine and human BMSCs can serve as in vitro models for the circadian analysis of gene expression profiles. We report that dexamethasone exposure synchronizes an oscillatory expression profile in representative mRNAs involved in the core circadian transcriptional apparatus. This is consistent with previous in vitro studies performed using rat and human skin derived fibroblasts (2-4, 8, 13). In these models, exposure to dexamethasone or serum shock initiated the oscillatory expression of mRNAs associated with the core circadian transcriptional apparatus over a 48 hrs period (2-4, 8, 13). Using a sophisticated in vitro luciferase reporter assays and in vivo wheel running analyses, Brown et al. (8) compared the circadian period in different murine strains. Based on activity measurements in vivo, two murine strains displayed periods between the ranges of 22.6 to 23.8 hrs. The in vitro period following dexamethasone synchronization in fibroblasts from these same murine strains ranged between 20-23.4 hrs as determined by bmal1 promoter/luciferase reporter construct (8). Our in vitro analyses in male and female muBMSCs, based on mRNA measurements of multiple genes, determined a period range of 19.5-28.3 hrs. Using the same in vitro approach, Brown et al. (8) found that dexamethasone synchronized response of human dermal fibroblasts isolated from foreskin, buttock, or abdomen of 19 donors had a period range of 22.7 to 26.2 hrs, with a mean of 24.5 ±0.75 hrs. Based on our analysis of multiple mRNA expression profiles in human BMSCs, we observed a mean period of 22.8 ± 1.2 hrs, albeit in a smaller population of donors. Just as has been reported for human dermal fibroblasts with respect to circadian profiles (8), there is evidence in the literature indicating that BMSCs isolated from different human donors or murine strains are not identical with respect to osteogenic capability (34, 35). Thus, it is likely that some donor to donor variability will exist in circadian measurements in vitro.
In addition, we found that the lithium chloride shifted the acrophase of multiple genes in both murine and human BMSCs by >4 hrs. We postulate that lithium chloride modulates the acrophase of gene expression by inhibiting GSK3β-mediated phosphorylation of period and related target proteins, further studies will be necessary to document such a mechanistic explanation. In the male muBMSCs, where the presence of lithium chloride significantly lengthened the period of oscillation, we hypothesize that this same mechanism may be responsible; however, in the female muBMSCs and huBMSCs, the period increase did not achieve significance. This suggests that there may be gender specificity to the actions of lithium chloride as well as inter-specie differences in the behavior of the circadian apparatus. Nevertheless, the lithium chloride-dependent lengthening of the oscillatory period in male muBMSCs and the acrophase shift observed in all BMSCs are consistent with our observations in human adipose-derived stem cells (huASCs) (53). Like the BMSCs, dexamethasone exposure of huASCs (n = 4) synchronized the oscillatory expression profile in mRNAs encoding the core circadian transcriptional apparatus. Similar to the current study, the presence of lithium chloride significantly increased the oscillatory period of per3 and rev-erbα, but not bmal1, by ∼5 hrs in huASCs (53). In addition to ourselves, (25), De Ugarte et al. (12), Huang et al. (23), Romanov et al. (40), Sakaguchi et al. (42), and Wagner et al. (52) have compared the differentiation potential and biochemical properties of human adipose and bone marrow-derived stem cells. The current findings provide another example of the similarities between these cell populations.
Circadian mechanisms have been implicated in the physiology and metabolism of adipose tissue and bone. The mRNAs encoding the protein components of the core circadian transcriptional apparatus display an oscillatory expression profile in multiple adipose tissue depots (1, 38, 57). Consistent with this, small interfering RNAs for bmal1 inhibited adipogenesis of 3T3-L1 cells pre-adipocytes in vitro (46). The bmal1/clock heterodimers were found to bind to cis elements within the promoters of adipocyte-associated genes such as SREBP1(46). Likewise, in murine calvarial bone, the mRNA expression of osteocalcin and related osteogenic biomarkers were found to display a circadian rhythmicity in microarray analyses of (58). Analyses of an osteocalcin promoter luciferase reporter transgenic mice lend support to this observation by demonstrating circadian rhythmicity in the level of bioluminescence detected in the skeletal tissues (manuscript in preparation: Y. Gafni, A. Ptitsyn, G. Peled, J.M. Gimble, D. Gazit). To date, the core circadian transcriptional apparatus has received little attention in the context of the stromal component of the bone marrow microenvironment. Instead, a greater level of scrutiny has been focused on the circadian rhythmicity of hematopoietic stem cells and their progeny (7, 11, 47, 49, 50). Hematopoietic cell models provide evidence that the core circadian transcriptional apparatus can modulate cell cycle and cell division (16-18). These findings from the literature imply that circadian mechanisms have the potential to modulate the two defining hallmarks of all stem cells, namely, their ability to self-renew and their multipotentiality.
The current manuscript provides a rationale supporting the use of primary human and murine BMSCs as in vitro models for the analysis of circadian mechanisms. The use of in vitro cultures reduces the need for costly and variable animal models or the need to perform multiple biopsies on a single human donor within a relatively short period of time. The outcomes from such studies will have potential relevance to bone physiology, the differentiation of BMSCs for tissue engineering applications, and fundamental mechanisms relating to stem cell biology.
Acknowledgments
The authors gratefully acknowledge the assistance of Randall Mynatt, PhD, and Dieyun Ding in the Transgenic Core Facility, Pennington Biomedical Research Center, for providing mice from their breeding colony. The authors acknowledge support from the Pennington Biomedical Research Foundation (X.W., J.M.G.) and NIH grants DK072476 (G.Y., J.M.G.) and DE016371 (F.G., D.G., J.M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Rhythmic mRNA Expression of Clock Genes and Adipocytokines in Mouse Visceral Adipose Tissue. Endocrinology. 2005 doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 2.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 3.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–37. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 4.Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–4. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 5.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 6.Blau J. A new role for an old kinase: CK2 and the circadian clock. Nat Neurosci. 2003;6:208–10. doi: 10.1038/nn0303-208. [DOI] [PubMed] [Google Scholar]
- 7.Bourin P, Ledain AF, Beau J, Mille D, Levi F. In-vitro circadian rhythm of murine bone marrow progenitor production. Chronobiol Int. 2002;19:57–67. doi: 10.1081/cbi-120002677. [DOI] [PubMed] [Google Scholar]
- 8.Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SA, Ripperger J, Kadener S, Fleury-Olela F, Vilbois F, Rosbash M, Schibler U. PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science. 2005;308:693–6. doi: 10.1126/science.1107373. [DOI] [PubMed] [Google Scholar]
- 10.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Hondt L, McAuliffe C, Damon J, Reilly J, Carlson J, Dooner M, Colvin G, Lambert JF, Hsieh CC, Habibian H, Stencel K, Quesenberry PJ. Circadian variations of bone marrow engraftability. J Cell Physiol. 2004;200:63–70. doi: 10.1002/jcp.20032. [DOI] [PubMed] [Google Scholar]
- 12.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 13.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–7. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 14.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–15. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 16.Gery S, Gombart AF, Yi WS, Koeffler C, Hofmann WK, Koeffler HP. Transcription profiling of C/EBP targets identifies Per2 as a gene implicated in myeloid leukemia. Blood. 2005;106:2827–36. doi: 10.1182/blood-2005-01-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–82. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 18.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007 doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- 19.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–94. [PubMed] [Google Scholar]
- 20.Goh BC, W X, Ann E, Evans AE, Meagan L, Johnson ML, Molly R, Hill MR, G J. Food Entrainment of Circadian Gene Expression Altered in PPARα-/- Brown Fat and Heart. Biochemical and Biophysical Research Communications. 2007;360:828–833. doi: 10.1016/j.bbrc.2007.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab. 1985;60:736–9. doi: 10.1210/jcem-60-4-736. [DOI] [PubMed] [Google Scholar]
- 22.Hogenesch JB, Panda S, Kay S, Takahashi JS. Circadian transcriptional output in the SCN and liver of the mouse. Novartis Found Symp. 2003;253:171–80. discussion 52-5, 102-9, 180-3 passim. [PubMed] [Google Scholar]
- 23.Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383–9. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 24.Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, Hamada T, Shibata S. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2004;19:2281–7. doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 25.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1286–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J. 2001;20:7128–36. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–79. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell JB, M K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Halvorsen YD, Storms RW, Goh B, Kilroy GS, Wu X, Gimble JM. The immunophenotype of human adipose derived cells: Temporal changes in stromal- and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 31.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–23. [PubMed] [Google Scholar]
- 32.Nielsen HK, Laurberg P, Brixen K, Mosekilde L. Relations between diurnal variations in serum osteocalcin, cortisol, parathyroid hormone, and ionized calcium in normal individuals. Acta Endocrinol (Copenh) 1991;124:391–8. doi: 10.1530/acta.0.1240391. [DOI] [PubMed] [Google Scholar]
- 33.Padiath QS, Paranjpe D, Jain S, Sharma VK. Glycogen synthase kinase 3beta as a likely target for the action of lithium on circadian clocks. Chronobiol Int. 2004;21:43–55. doi: 10.1081/cbi-120027981. [DOI] [PubMed] [Google Scholar]
- 34.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 35.Phinney DG, Kopen G, Righter W, Webster S, Tremain N, Prockop DJ. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–36. [PubMed] [Google Scholar]
- 36.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 37.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–6. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 38.Ptitysn AA, Z S, Conrad SA, Scott LK, Mynatt ML, Gimble JM. Circadian Clocks are Resounding in Peripheral Tissues. PLoS Computational Biology. 2006;2:e16. doi: 10.1371/journal.pcbi.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–89. [PMC free article] [PubMed] [Google Scholar]
- 40.Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–43. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 41.Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, Boyadjian R, Steil GM. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–9. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 43.Schibler U. The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep. 2005;6 doi: 10.1038/sj.embor.7400424. Spec No:S9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–60. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 45.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–7. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–6. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smaaland R, Sothern RB, Laerum OD, Abrahamsen JF. Rhythms in human bone marrow and blood cells. Chronobiol Int. 2002;19:101–27. doi: 10.1081/cbi-120002594. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava AK, Bhattacharyya S, Li X, Mohan S, Baylink DJ. Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice. Bone. 2001;29:361–7. doi: 10.1016/s8756-3282(01)00581-6. [DOI] [PubMed] [Google Scholar]
- 49.Tsinkalovsky O, Rosenlund B, Laerum OD, Eiken HG. Clock gene expression in purified mouse hematopoietic stem cells. Exp Hematol. 2005;33:100–7. doi: 10.1016/j.exphem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Tsinkalovsky O, Smaaland R, Rosenlund B, Sothern RB, Hirt A, Steine S, Badiee A, Abrahamsen JF, Eiken HG, Laerum OD. Circadian variations in clock gene expression of human bone marrow CD34+ cells. J Biol Rhythms. 2007;22:140–50. doi: 10.1177/0748730406299078. [DOI] [PubMed] [Google Scholar]
- 51.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–16. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Wu X, Z S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, Eckel RH, Mynatt RL, Gimble JM. Circadian Gene Expression in Human Subcutaneous Adipose-derived Stem Cells: Induction by Dexamethasone, Serum, and Thiazolidinedione in the Undifferentiated and Adipogenic States. Obesity. 2007;15:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- 54.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–9. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–5. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 56.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–70. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 58.Zvonic S, Ptitsyn AA, Kilroy G, Wu X, Conrad SA, Scott LK, Guilak F, Pelled G, Gazit D, Gimble JM. Circadian Oscillation of Gene Expression in Murine Calvarial Bone. J Bone Miner Res. 2007;22:357–365. doi: 10.1359/jbmr.061114. [DOI] [PubMed] [Google Scholar]