Abstract
Birdsong is a species-typical vocal signal that facilitates reproduction and deters competitors. Song production is regulated by a clearly defined and specialized neural circuitry in which high concentrations of catecholamines are present. The nuclei within the song control circuit receive projections from catecholaminergic cell populations involved in attention, arousal and motivation, including periaqueductal gray (PAG), ventral tegmental area (VTA), locus coeruleus (LoC) and sub coeruleus (SC). Here, we examine whether catecholamine-containing neurons in these regions exhibit the immediate early gene, ZENK, during spontaneous, undirected song production in male zebra finches (Taeniopygia guttata). Males were assigned to “singing” or “silent” groups based on the total duration of spontaneous, undirected song produced within a 30 min period. We quantified the number of cells expressing both ZENK-ir and tyrosine hydroxylase (TH)-ir within the VTA, PAG, LoC and SC. The number of cells expressing co-localized ZENK and TH-ir was significantly elevated within the PAG in males that were singing compared to silent males. The number of cells expressing ZENK-ir alone was also elevated in the VTA and SC in singing males compared to silent males. Although ZENK expression is elevated in singing birds it does not positively correlate with the amount of singing produced. It is therefore likely that catecholaminergic PAG neurons are involved in motivational or attentional components of vocal expression rather than vocal motor output. Overall, our study is consistent with the hypothesis that PAG catecholamine-containing neurons as well as VTA and SC neurons play a role in vocal communication of male songbirds.
Keywords: birdsong, immediate early gene, tyrosine hydroxylase, vocal communication
Vocal communication in songbirds is regulated by a specialized neural system that is essential for the production, learning and development of song [1-4]. This specialized system is clearly modulated by other neural systems that project to the song control system so that song production is adjusted as a function of the social context and other variable environmental events the signaler may encounter [5-6]. The catecholaminergic system is one such system. It is involved in the modulation of state-dependent sensory processing [7], and processing of motor information [8] as well as cognitive and affective processes such as motivation [9] attention [10-12], and arousal [13-14]. Furthermore, catecholamine neurons, which include dopaminergic and noradrenergic cells, regulate social behavior in a wide array of taxa [15-20], and exhibit distinctive profiles of responsiveness depending on the nature of the social interaction [20]. Consequently, catecholaminergic cells are strong candidates to substantially contribute to the regulation of social behaviors such as the production of vocal communication signals. Here, we examine the role of catecholaminergic cell groups during vocal communication in a male songbird species, the zebra finch (Taeniopygia guttata).
Male songbirds produce structurally complex, species-typical vocal signals that are essential for courtship and territory defense [21-22]. Songbirds have evolved a specialized neural system referred to as the song control system. This system includes telencephalic, diencephalic, mesencephalic and myencephalic nuclei that regulate learning and production of these vocal signals (see fig.1a). Catecholamines and their receptors are located throughout the song control system in adult male songbirds and to a lesser extent in females, at least in species which exhibit sex differences in vocal output [24-31, 19, 32].
Figure 1.
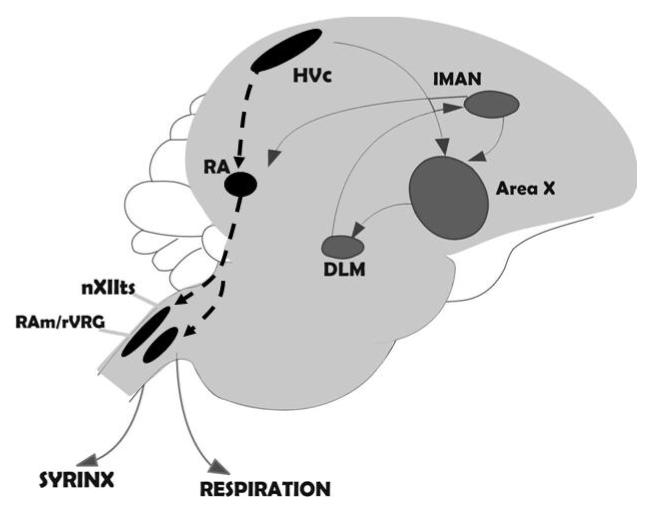
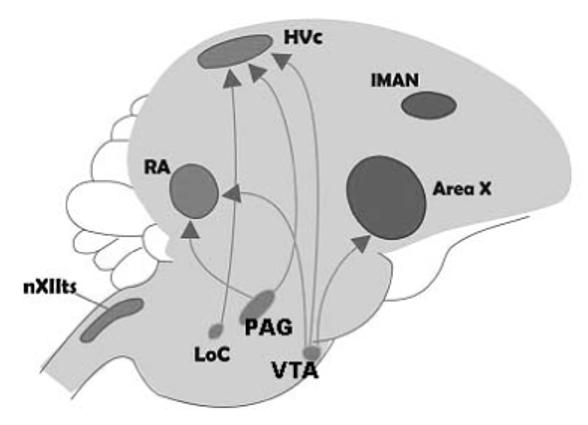
A. Simple illustration of the specialized circuitry involved in the songbird vocal control system. One forebrain pathway (black dashed line) is involved in vocal motor output. The anterior forebrain pathway (solid gray line) is involved in song learning during development and maintenance of song during adulthood. B. Generalized illustration of the brainstem catecholaminergic cell groups that project to the forebrain song control nuclei. The LoC and SC send predominately noradrenergic projections and the VTA and PAG send mainly dopaminergic projections. Abbreviations: RA = robust nucleus of the arcopallium; nXIIts = tracheosyringeal subdivision of the motor nucleus of cranial nerve XII; lMAN = lateral region of the magnocellular nucleus of the anterior neostriatum; ICo - nucleus intercollicularis; DLM - dorsolateral thalamic nucleus; VTA = ventral tegmental area; PAG = periaqueductal gray; LoC = locus coeruleus; SC = sub coeruleus.
Four populations of catecholaminergic cell groups are known to send projections into the song control nuclei. These include the ventral tegmental area (VTA; previously referred to as the area ventralis of Tsai; group A10 in the nomenclature [reviewed in 33]), the periaqueductal gray (PAG; also referred to as midbrain central gray; group A11), locus coeruleus (LoC; group A6) and sub coeruleus (SC; group A6). These cell populations send dopaminergic and/or noradrenergic projections into the song control system. The LoC and SC are primarily noradrenergic in nature and send projections into Area X [34], HVC and RA. VTA and PAG are primarily dopaminergic in nature. PAG sends direct projections to HVC and RA whereas VTA sends direct projections to Area X and to a lesser extent to HVC and RA [16 & 32 for review; 30, 35] (see fig.1b).
In the present study we address questions that are central to understanding the contribution of catecholamines in the production of song. We examined the expression of an immediate early gene (IEG), known as ZENK that is an acronym for the same gene that has been named zif-268, egr-1, NGFI-A, or Krox-24 in other species [36]. We asked whether ZENK induction occurs in dopaminergic (VTA and PAG) or noradrenergic (LoC and SC) cell groups during undirected, spontaneous song production in male zebra finches. We also examine whether ZENK induction is expressed within catecholamine-containing cells in these brain regions, as measured by the presence of tyrosine hydroxylase (TH), the rate-limiting enzyme necessary for catecholamine synthesis. In addressing these questions, we examined cells expressing both ZENK-ir and TH-ir or ZENK-ir alone.
Methods
Housing
Male zebra finches were obtained from a local supplier and kept in 49 × 95 × 51cm cages (four to six males per cage) at Johns Hopkins University, Baltimore, MD. All birds were fed finch food and provided water ad libitum. Birds were maintained on 14h light:10h dark. Sixteen males were placed in controlled acoustic environments overnight (Industrial Acoustic Company, Bronx N.Y). Lights were automatically turned on in the morning. At this time the males began to sing spontaneous, undirected songs within the acoustic chambers. The total duration of spontaneous, undirected songs was recorded with 16-bit resolution at 20 kHz (Iotech DaqBoard 2000) using custom software written by Amish Dave.
Group Assignment
Male zebra finches were assigned to either the “singing” group or the “silence” group based on the number of seconds a male spent singing during a 30 min trial period (i.e. total duration of song). Total song duration of the males assigned to the singing group ranged from 13 - 315 sec. The mean song duration was 138 sec ± 29.7 / 30 min. Removing the male that only sang 13 sec of song did not alter the results of our analyses, therefore we kept this male in the song group (N = 11). Males that were assigned to the silence group sang 1 sec of song or less (N = 5). The mean song duration in this group is 0.36 sec ± 0.36. All procedures were approved by Johns Hopkins University IACUC.
Tissue Processing and Immunocytochemistry
The amount of singing a male produced was recorded for 30 min, as described above. One hour after this period ended, males were sacrificed via rapid decapitation. The brain was removed and fixed in 5 % acrolein and cryoprotected in 30% sucrose until cut into three series of 40μm coronal sections. One tissue series was used to perform two sequential free-floating immunocytochemical reactions (ICC) following standard avidin-biotin (ABC) complex procedures, as described in [37-38, 5]. In brief, tissue was incubated in ZENK antibody (1:2000 dilution of Egr-1 raised in rabbit; Santa Cruz Biotechnology, Santa Cruz, CA) at 4° C for 48 h. Tissue was then incubated in biotinylated goat anti-rabbit secondary antibody (1: 250 dilution Vector Laboratories, Burlingame, Ca.) for 1 h at room temperature. Antibody bound to ZENK protein was then visualized using a 1: 200 dilution of Vectastain ABC Elite kit (Vector Laboratories) and nickel enhanced 3,3′-diaminobezidene tetrahydrochloride chromagen, yielding black reaction product (DAB; Polysciences, Warrington, Pa.). Immediately following the visualization of antibody bound to ZENK protein, the tissue was incubated in TH antibody (1:10,000 dilution of TH antibody raised in mouse; Immunostar, Hudson, WI.) at 4° C for 48 h. Tissue was then incubated in biotinylated horse anti-mouse secondary antibody (1:250 dilution Vector Laboratories) for 1 h at room temperature. Antibody bound to TH was visualized as above with the exception of an incubation in DAB without nickel enhancement, yielding brown reaction product. The tissue was then mounted onto slides and coverslipped.
Quantification
Three immunoreactive patterns were quantified; the number of cells immunoreactive for both ZENK and TH (ZENK / TH -ir; fig. 2), the number of cells immunoreactive for ZENK alone (ZENK-ir; fig. 2) and the number of cells immunoreactive for TH alone (TH-ir; fig. 2). These proteins were quantified in four regions which will be referred to according to the revised avian neuroanatomical nomenclature recommended by Reiner et al. [23]. These include the ventral tegmental area (VTA; fig. 3a), locus coeruleus (LoC; fig.3b), sub coeruleus (SC; fig. 3b), and periaqueductal gray (PAG; figs.3c,d). A Zeiss Axioskop (Carl Zeiss, Thornwood, NY) bright-field microscope projected 20x (ZENK-ir) and 40x (ZENK / TH -ir and TH-ir) images to a CCD camera (Sony Model XC-77, Japan). Openlab 3.1.7 (Improvision, Lexington, Ma.) software was used to capture photomicrographs and protein expression was counted manually on photomicrographs to ensure no cell (or ZENK protein) was counted twice. All sections containing SC or LoC were quantified. VTA and PAG are larger nuclei and therefore, these were quantified by counting every other section. Sections that were folded or damaged were excluded. ZENK-ir and ZENK / TH -ir cells were counted across the entire surface area of each brain region. TH-ir was counted from random samples of 40x photomicrographs (1 sample for LoC and SC; 2-3 samples for VTA and PAG). Openlab software was also used to measure the surface area of each region. We used a t-test to examine whether there were significant differences in the surface area of any of the brain regions between singing and non-singing males. There were no significant differences in surface area between the two groups in any of the brain regions we examined (VTA; P = 0.38, PAG; P = 0.64; LoC; P = 0.24; SC; P = 0.23) therefore, we standardized ZENK / TH -ir and ZENK -ir cells per mm2.
Figure 2.
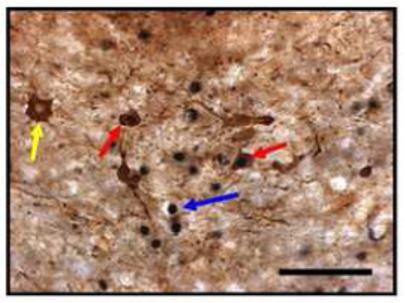
Photomicrograph of the three cell types quantified. Red arrows indicate ZENK/TH-ir positive cells. Blue arrows indicate ZENK-ir positive cells. Yellow arrows indicate TH-ir positive cells. Scale bar = 50 μm.
Figure 3.
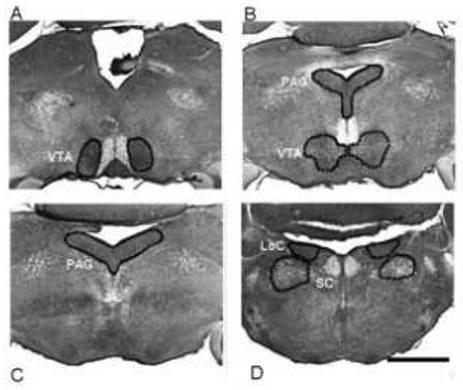
Photomicrograph of four catecholaminergic brainstem regions. Panel A shows rostral ventral tegmental area (VTA). Panel B shows the locus coeruleus (LoC) with the sub coeruleus (SC). Panel C shows the rostral region of the periaqueductal gray (PAG) and the caudal VTA. Panel D shows the caudal region of the PAG. Scale bar = 1mm
Statistical Analysis
Data were analyzed using unpaired t-tests. In each analysis, the dependent variable analyzed was either ZENK / TH-ir expression, ZENK-ir expression alone or TH-ir expression. Separate tests were done for each of these dependent variables. Because we analyzed two brain regions for each catecholaminergic cell type (dopaminergic cell groups include VTA and PAG and noradrenergic cell groups include LoC and SC) we used a Bonferroni correction for multiple comparisons. The alpha value for these comparisons is P ≤ 0.025. There was one case with unequal variances (ZENK-ir alone in SC), and therefore, the student t-test assuming unequal variances was used.
We used linear regression to examine whether total song duration significantly predicted the number of TH-containing neurons that expressed ZENK-ir or ZENK-ir alone.
There were noticeably more cells that were ZENK / TH -ir in caudal PAG relative to rostral PAG. Therefore, we divide the PAG into rostral and caudal sections to examine this difference statistically. Total ZENK / TH -ir counts between the caudal and rostral PAG was compared using a paired t-test. Alpha level was set at ≤ 0.05 for this analysis. All means are reported ± S.E.M. for all analyses.
Results
TH -ir expression
No brain region had a significantly different number of TH -ir cells between singing and silent males (VTA; DF = 14; t = 1.52 P = 0.15; PAG; t = 1.73 P = 0.11; LoC; t = 0.47 P = 0.64; SC; t = 1.56 P = 0.14). This result indicates that differences in our other dependent variables (i.e. ZENK / TH -ir and ZENK- ir) are not explained by differences in the number of TH-ir cells between singing and silent males. Therefore, our dependent variables are expressed as total number of -ir cells per mm2.
ZENK / TH -ir expression
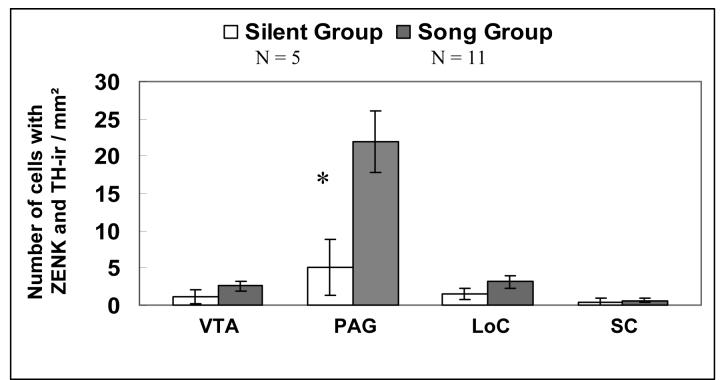
The comparison of ZENK / TH-ir in dopaminergic cell groups (i.e. VTA and PAG) revealed that a significantly greater number of cells were immunoreactive for both ZENK and TH in males that were singing compared to males that were silent only within the PAG (DF = 14; t = 2.54; P = 0.024) whereas there was no statistically significant difference in ZENK / TH -ir between singing and silent males in the VTA (DF = 14; t = 1.2; P = 0.25; fig. 4).
Figure 4.
Number of cells with co-localized ZENK / TH-ir within four catecholaminergic nuclei in male zebra finch singing spontaneous, undirected song and males that did not sing. All means are reported ± S.E.M. Asterisks denote significant differences between groups in a pairwise comparison (t-test; P ≤ 0.025).
Comparison of ZENK / TH-ir in noradrenergic cell groups (i.e. LoC and SC) revealed no significant difference in the number of cells immunoreactive for both ZENK and TH in males that were singing compared to males that were silent (LoC; DF = 14; t = 1.25; P = 0.23; SC; DF = 14; t = 0.29; P = 0.78; fig. 4)
ZENK- ir expression
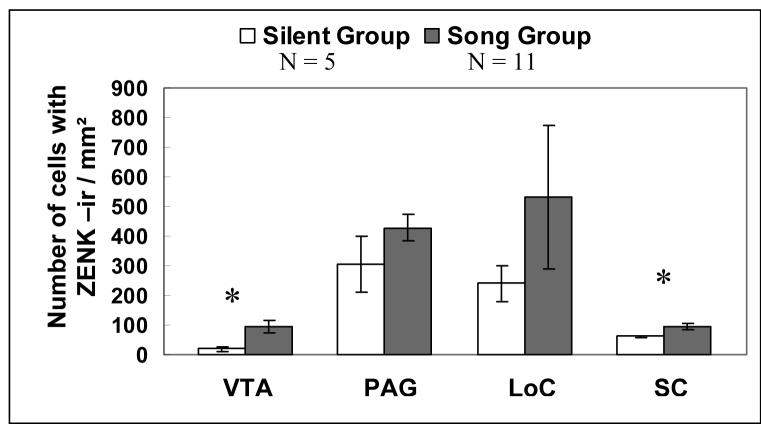
A significantly greater number of cells immunoreactive for ZENK alone was found in the VTA in males that were singing as compared to males that were not singing (DF = 14; t = 2.5; P = 0.025) whereas there was no difference in ZENK-ir between singing and silent males in the PAG (DF = 14; t = 1.39; P = 0.19; fig. 6).
Figure 6.
Number of ZENK-ir cells within four catecholaminergic nuclei in male zebra finch singing spontaneous, undirected song and males that did not sing. All means are reported ± S.E.M. Asterisks denote significant differences between groups in a pairwise comparison (t-test; P ≤ 0.025).
A significantly greater number of cells immunoreactive for ZENK alone was found in the SC (DF = 11.2; t = 3.43; P = 0.005) whereas there was no difference in ZENK-ir between singing and silent males in the LoC (DF = 14; t = 0.80; P = 0.44; fig. 6).
ZENK / TH -ir in PAG
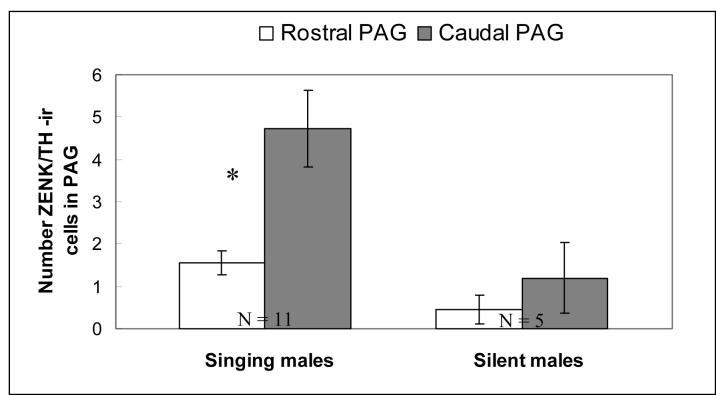
In male zebra finches that were singing, there were significantly more cells that were both ZENK-ir and TH-ir in the caudal region of PAG as opposed to the rostral region of PAG (fig. 5; t = 2.22, DF = 10; P = 0.05). In males that were not singing, however, there was no significant difference in the number of cells with ZENK / TH -ir in the caudal regions of PAG as compared to the rostral regions of PAG (DF = 4; t = 1.34; P = 0.25). The caudal region of PAG also exhibited a significantly greater density of TH cells and a significantly smaller surface area in males that sang (TH cell number: DF = 10; t = 3.37; P = 0.007; surface area: DF = 10; t = 8.15; P < 0.001) as well as males that did not sing (TH cell number: DF = 4; t = 4.3; P = 0.01; surface area: DF = 4; t = 8.2; P = 0.001).
Figure 5.
Number of cells with co-localized ZENK / TH-ir between the rostral and caudal regions of the PAG. All means are reported ± S.E.M. Asterisks denote significant differences between groups in a pairwise comparison (t-test; P ≤ 0.05).
Total Song Duration
Within the group of males that sang (N = 11), there were no significant correlations between total duration of song and ZENK / TH-ir expression (VTA: R = 0.23, P = 0.48; PAG: R = 0.18, P = 0.58; LoC: R = 0.31; P = 0.35; SC: R = 0.30, P = 0.37) or the expression of ZENK-ir alone (VTA: R = 0.32, P = 0.34; PAG: R = 0.03, P = 0.93; LoC: R = 0.27; P = 0.42; SC: R = 0.43, P = 0.18) within this group.
Discussion
The goal of this study was to investigate whether catecholamine cell groups known to project to the song system can be linked to song production. We used the induction of the protein product of the immediate early gene ZENK as a measure of the importance of these cell groups in relation to song behavior. Previous studies had shown that ZENK induction assessed either based on the measurement of the mRNA or the protein product was driven by the act of singing in song nuclei such as HVC and RA [39]. This was apparent based on the fact that ZENK was upregulated in singing birds even when they were deaf and that the number of cells expressing ZENK positively correlates with the number of songs produced [39]. In this study we extended the use of ZENK expression to catecholaminergic cell groups that project to HVC, RA and other song nuclei. We found that there was a significant increasing in the number of TH-containing cells also expressing the ZENK protein in singing male zebra finches as compared to non-singing males within the PAG. Additionally, in other catecholaminergic cell groups, we found an increase in the number of cells within these nuclei expressing ZENK in singing males as opposed to non-singing males but this increase was not associated with TH cells. Both of these findings provide us with valuable information about the role of these cells groups might play in the control of birdsong. We will discuss each type of finding in turn.
Several catecholaminergic brain regions did not exhibit significantly elevated ZENK expression in males that sang as opposed to males that did not sing. This result should not be interpreted to mean that the catecholamine cells in those areas are not involved in song production or motivation to sing. Cells may be physiologically active without expressing ZENK or alternatively, other genes may be expressed instead of ZENK. It is also possible ZENK expression in these areas may be context-dependent. Consequently, the absence of elevated gene expression in some catecholaminergic regions does not imply that those cells are not involved in song production.
There are two possibilities for the role of the PAG in vocal communication in male songbirds. First, it is possible that this group of TH cells within the PAG exhibit motor-driven ZENK expression. Alternatively, it is also possible that this population of TH cells is involved in motivational and/or emotional components of vocal expression. For instance, in mammals, the PAG is considered to be a part of an emotional-motor system [40]. It is essential for the precise timing of respiratory, laryngeal and oral muscle activity required for species-typical vocalizations in many vertebrates [41-42]. In other vertebrates, PAG activity also correlates with temporal patterning of vocalizations among cells that project to motor neurons [43-45]. However, TH-containing cells within PAG have not been reported to be involved in the neural control of vocal motor output in birds. In contrast, a separate population of cells lateral to the PAG, referred to as the dorsomedial (DM) portion of the nucleus intercollicularis (ICo), receives efferent projections from RA and in turn, sends projections to nXIIts in the songbird. Based on studies of non-songbird species in the galliform and columbiform orders, these are the cells that are reportedly involved in the neural control of avian vocal motor behaviors [46-49]. Consequently, it is the DM cell group that is lateral to PAG, rather than PAG cell group per se, that corresponds to a midbrain cell group that is involved in the neural control of vocal motor output.
Further evidence is consistent with the hypothesis that PAG catecholamine-containing neurons are involved in the motivational and/or emotional components of vocal expression. In songbirds, the PAG receives projections from the medial preoptic nucleus (POM [50]) which is involved in the induction of sexually motivated song in male songbirds [51]. A circuitry has been described in squirrel monkeys in which brain structures involved in motivation control, such as the medio-basal amygdala, anterior cingulate cortex and hypothalamus, project to the PAG [52], and it is thought that this circuit is involved in motivational and emotional components of vocal expression in this system. Finally, if ZENK induction within PAG were motor-driven or were driven by motor output in our study, one would expect the number of TH cells expressing ZENK to be positively associated with the number of songs produced and/or the total amount of vocal motor output, as was found for ZENK expression associated with song output measured in HVC and RA of singing male zebra finches [39]. Our data reveal that the total amount of song produced is not correlated with either the number of cells that are ZENK / TH -ir or ZENK-ir alone in any of the brain regions we examined. Thus, it is possible that catecholaminergic cells within PAG are involved in the induction of song or motivation to sing.
In male birds of several species, several sociosexual behaviors are associated with IEG induction in catecholaminergic neurons within the PAG and the VTA. For example, FOS induction in TH cells within both the PAG and VTA is associated with sexual interactions in male Japanese quail (Coturnix japonica) [53]. In male zebra finches, engaging in reproductive behaviors as well as agonistic behaviors induce FOS-ir in TH cells within the PAG. The TH cells within the VTA, however, exhibit a significantly elevated FOS induction after the occurrence of agonistic encounters as compared to sexual behaviors [20]. Sexual interactions in male zebra finch induced FOS-ir expression in brainstem catecholaminergic cell groups as well, including the rostral LoC and the substantia nigra [20]. IEG expression in these catecholamine cell groups indicates that they play an active role in the neural circuitry underlying sociosexual behaviors, including agnostic encounters, in several species of male birds.
Within the PAG, TH cells tend to be significantly clustered in the caudal part of this large brain region. These TH-ir cells are the ones within the PAG that appear to exhibit ZENK induction when a male zebra finch sings. It is possible that this reflects a functional topographical organization within PAG. For example, in mammals, the PAG is organized in relation to affective expression such as defensive reactions and autonomic regulation [54] including sexual responses [55]. It is possible that this localized response is indicative of a similar topographic organization in birds but more studies concerning the functional topographic organization of the PAG in birds are needed to clarify the validity of this hypothesis.
ZENK-ir expression within the VTA is elevated in singing males as compared to silent males. However, this song-associated induction of ZENK was not observed within TH cells. It is possible that ZENK induction within the VTA occurs within GABA-ergic neurons as reported recently by Hara et al. [6]. In addition, Maney and Ball [56] reported elevated fos-like immunoreactivity in VTA of male song sparrows (Melospiza melodia) during territorial song production [55]. However, these males were provoked to sing by song playback and these males also performed territorial flight displays while singing. In male European starlings (Sturnus vulgaris) the number of fos labeled neurons in the VTA correlates positively to the number of songs produced [57]. This positive correlation was only observed during the breeding season and not outside of a breeding context, suggesting that the relationship between the amount of song and fos expression in the VTA is context-dependent. Together, these studies indicate a role for VTA in regulating aggressive and sexually motivated vocal communication in songbirds.
Significant induction of ZENK in singing birds was also observed in the noradrenergic cell group, the SC. We did not detect significant ZENK induction in the LoC itself though. The exact role of the SC cell group in vocal communication is largely unknown. However, noradrenergic neurons in the LoC and the SC project to several song nuclei [34-35]. Furthermore, lesions of noradrenergic neurons using a noraderenergic-specific neurotoxin N-(2-chloroethyl)-N-ethyl 2-bromobenzylamine, (DSP-4) caused male zebra finches to increase their latency to sing to a female. Once singing commenced, however, the songs were normal species-typical songs [58]. In male zebra finches, DSP-4 abolishes context-dependent motor-driven gene expression in Area X [5]. Consequently, it is possible that these noradrenergic cells play a role in mediating the effects of environmental context on song behavior [16 for review; 5].
In summary, this study reveals a significant role for PAG catecholamine-containing neurons in vocal communication in male songbirds. We suggest that these PAG neurons are likely involved in the induction of song and are thus related to motivational components of vocal expression rather than vocal motor output per se. In addition, neurons within the VTA (predominately dopaminergic region) and the SC (predominately noradrenergic region) exhibit elevated ZENK-ir in males that sing spontaneously, suggesting that cell groups within the VTA and SC are also involved in the induction of song.
Acknowledgements
We thank Andrea Wallace and Vincent Zhang for technical assistance. This study was supported by a grant (NIH/NINDS RO1 NS 35467) to GFB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen S. Lynch, Johns Hopkins University Department of Psychological and Brain Sciences, 3400 North Charles Street, Baltimore, MD 21211 (410) 516- 4030 lynchks@jhu.edu.
Bettina Diekamp, Johns Hopkins University Department of Psychological and Brain Sciences, 3400 North Charles Street, Baltimore, MD 21211 (410) 516- 0228 b.diekamp@jhu.edu.
Gregory F. Ball, Johns Hopkins University Department of Psychological and Brain Sciences, 3400 North Charles Street, Baltimore, MD 21211 (410) 516- 7910 gball@jhu.edu
References
- [1].Nottebohm F, Stokes T, Leonard M. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- [2].Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian songsystem. J. Neurobio. 1997;33:495–500. [PubMed] [Google Scholar]
- [3].Jarvis ED. Learned birdsong and the neurobiology of human language. Ann. N.Y. Acad. Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nottebohm F. The neural basis of birdsong. Pub. Lib. Sci. 2005;3:759–761. doi: 10.1371/journal.pbio.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castelino CB, Ball GF. The role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur. J. Neurosci. 2005;25:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- [6].Hara H, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur. J. Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cardin J, Schmidt M. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J. Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Solis MM, Perkel DJ. Noradrenergic modulation of activity in a vocal control nucleus in vitro. J. Neurophysiol. 2006;95:2265–2276. doi: 10.1152/jn.00836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:1–12. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- [10].Aston-Jones G, Rajkowski J, Kubiak P, Tatiana A. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Granon S, Passetti F, Thomas K, Dalley J, Everitt B, Robbins T. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1206–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- [13].Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga M, editor. The cognitive neurosciences. MIT; Cambridge MA: 1995. pp. 703–720. [Google Scholar]
- [15].Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards Horm. Behav. 2001;40:483–489. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]
- [16].Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone. Multiple sites of action and role of ascending catecholamine projections. Ann. N.Y. Acad. Sci. 2003;1007:211–231. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- [17].Endepols H, Schul J, Gerhardt HC, Walkowiak W. 6-hydroxydopamine lesions in anuran amphibians: a new model system for Parkinson’s disease? J. Neurobio. 2004;60:395–410. doi: 10.1002/neu.20047. [DOI] [PubMed] [Google Scholar]
- [18].Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- [19].Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J. Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neurosci. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kroodsma DE, Byers BE. The functions of bird song. Amer. Zool. 1991;31:318–328. [Google Scholar]
- [22].Collins S. Vocal fighting and flirting: the functions of birdsong. In: Marler P, Slabbekoorn H, editors. Natures Music: The science of birdsong. Elsevier; London: 2004. pp. 39–79. [Google Scholar]
- [23].Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised Nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to Area X in the zebra finch. J. Comp. Neurol. 1981;196:347–357. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- [25].Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Beh. Evol. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- [26].Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- [27].Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J. Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- [28].Ball GF. Neurochemical specializations associated with vocal learning and production in songbirds and budgerigars. Brain Beh. Evol. 1994;44:234–246. doi: 10.1159/000113579. [DOI] [PubMed] [Google Scholar]
- [29].Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J. Neurobio. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [30].Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tiss. Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- [31].Riters LV, Ball GF. Sex differences in the densities of α2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J. Chem. Neuroanat. 2002;23:269–277. doi: 10.1016/s0891-0618(02)00005-4. [DOI] [PubMed] [Google Scholar]
- [32].Ball GF, Balthazart J. The neuroendocrinology and neurochemistry of birdsong. In: Lajtha A, editor. Handbook of neurochemistry and molecular neurobiology 3rd ed. Springer; New York: 2007. pp. 420–457. [Google Scholar]
- [33].Reiner A, Karle E, Jerson KD, Medina L. In: Catecholaminergic perikarya and fibers in the avian nervous system. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Smeets WJAJ, Reiner A, editors. Cambridge Univ. Press; Cambridge UK: 1994. pp. 135–181. [Google Scholar]
- [34].Castelino CB;, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus area X of the medial striatum in male zebra finches (Taeniopygia guttata) J. Comp. Neurol. 2007;502:544–562. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- [35].Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J. Comp. Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- [36].Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc. Natl. Acad. Sci. USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J. Comp. Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [38].Duffy DL, Bentley GE, Ball GF. Does sex or photoperiodic condition influence ZENK induction in response to song in European starlings? Brain Res. 1999;844:78–82. doi: 10.1016/s0006-8993(99)01915-0. [DOI] [PubMed] [Google Scholar]
- [39].Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc. Nat. Acad. Sci. USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dubbeldam JL, den-Boer-Visser AM. The central mesencephalic grey in birds: Nucleus intercollicularis and substantia grisea centralis. Brain Res. Bull. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- [41].Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobio. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- [42].Davis PJ, Zhang SP, Bandler R. Midbrain and medullary regulation of respiration and vocalization. Prog. Brain Res. 1996;107:315–325. doi: 10.1016/s0079-6123(08)61873-7. [DOI] [PubMed] [Google Scholar]
- [43].Larson CR. On the relation of PAG neurons to laryngeal and respiratory muscles during vocalization in the monkey. Brain Res. 1991;552:77–86. doi: 10.1016/0006-8993(91)90662-f. [DOI] [PubMed] [Google Scholar]
- [44].Jürgens U. Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 2000;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- [45].Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J. Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- [46].Cohen J, Cheng MF. The role of the midbrain in courtship behavior of the female ring dove (Streptopelia risoria): evidence from radiofrequency lesion and hormone implant studies. Brain Res. 1981;207:279–301. doi: 10.1016/0006-8993(81)90365-6. [DOI] [PubMed] [Google Scholar]
- [47].Cohen J. Hormones and midbrain mediation of courtship behavior in the male ringdove (Streptopelia risoria) J. Comp. Physiol. Psychol. 1981;95:512–528. doi: 10.1037/h0077797. [DOI] [PubMed] [Google Scholar]
- [48].Cohen J, Cheng MF. Effects of testosterone metabolites and estrogen in the midbrain control of courtship behavior in the male ring dove (Streptopelia risoria) Neuroendocrinol. 1982;34:64–74. doi: 10.1159/000123279. [DOI] [PubMed] [Google Scholar]
- [49].Shaw BK. Involvement of a midbrain vocal nucleus in the production of both the acoustic and postural components of crowing behavior in Japanese quail. J. Comp. Physiol. A. 2000;186:747–757. doi: 10.1007/s003590000128. [DOI] [PubMed] [Google Scholar]
- [50].Riters LV, Algers SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- [51].Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm. Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- [52].Dujardin E, Jürgens U. Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 2005;1034:114–131. doi: 10.1016/j.brainres.2004.11.048. [DOI] [PubMed] [Google Scholar]
- [53].Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes C-FOS and ZENK (EGR-1) in catecholaminergic neurons of male Japanese quail. Neurosci. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- [54].Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- [55].Schwarz-Giblen S, McCarthy MM. A sexual column in the PAG. Trends Neurosci. 1995;18:129. doi: 10.1016/0166-2236(95)93889-6. [DOI] [PubMed] [Google Scholar]
- [56].Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J. Neurobio. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- [57].Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulation motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobio. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- [58].Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol. Biochem. Behav. 1996;53:213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]