Abstract
Developmental disabilities (e.g. attention deficit disorder; cerebral palsy) are frequently associated with deviations of the typical pattern of motor skill maturation. Neurophysiologic tools, such as transcranial magnetic stimulation (TMS), which probe motor cortex function, can potentially provide insights into both typical neuromotor maturation and the mechanisms underlying the motor skill deficits in children with developmental disabilities. These insights may set the stage for finding effective interventions for these disorders. We review the literature pertaining to the use of TMS in pediatrics. Most TMS-evoked parameters show age-related changes in typically developing children and some of these are abnormal in a number of childhood-onset neurological disorders. Although no TMS-evoked parameters are diagnostic for any disorder, changes in certain parameters appear to reflect disease burden or may provide a measure of treatment-related improvement. Furthermore, TMS may be especially useful when combined with other neurophysiologic modalities (e.g. fMRI). However, much work remains to be done to determine if TMS-evoked parameters can be used as valid and reliable biomarkers for disease-burden, the natural history of neurological injury and repair, and the efficacy of pharmacological and rehabilitation interventions.
Keywords: Transcranial Magnetic Stimulation, Maturation, Children, Motor Cortex Function, Developmental Disorders
Introduction
Transcranial magnetic stimulation (TMS) has proved to be a useful probe of motor function in adults. In this article we review the utility of TMS as a probe of motor function in both typically developing children and in those with anomalous motor development. First, we briefly review normal neuromotor maturation and give an overview of those aspects of the central nervous system that undergo structural changes during childhood. Following this we discuss methods used to assess brain function focusing on TMS. Finally, we review studies which have examined the utility of TMS to gain insights into the neural substrates of developmental disorders (such as cerebral palsy and attention deficit disorder) and the potential of TMS-evoked parameters as biomarkers for disease-burden and treatment response.
Clinical Measures of Neuromotor Development
Motor cortex function plays an important role in the acquisition of new motor skills – a capacity which is present throughout life. Typically the rate of skill acquisition is greatest during early childhood when motor cortex function is also undergoing rapid maturational changes. A working knowledge of neuromotor maturation is, therefore, an important prelude to understand the role that TMS might have in probing motor cortex function and its relationship to motor skill acquisition in children
Neuromotor development follows a predictable, though highly variable, pattern in the majority of children. Assessment tools which track motor maturation examine not only the temporal acquisition of skills but also the quality and stability of skills over time and serve to detect those children whose performance falls outside the expected range.
Assessment of the motor system begins in the newborn and infant with an examination of spontaneous movements (Einspieler and Prechtl, 2005). Rudimentary gross and fine motor skills are acquired during the first year of life and become stable through the pre-school years (Illingsworth, 1983). Fine motor skills (e.g. drawing and writing skills, manipulating objects such as scissors) make noticeable gains through the early school years (Croce et al., 2001; Frankenburg et al., 1992; Majnemer and Snider, 2005; Plubrukarn and Theeramanoparp, 2003; Yule et al., 1967). As children get older, hand dexterity continues to improve in a more subtle way (increased rhythmicity, speed, and coordination of movements) reaching a plateau during mid- to late-adolescence depending on the complexity of the motor skill (more complex motor skills reaching maturity later) (Denckla, 1973; Denckla, 1974; Largo et al., 2001a; Largo et al., 2001b; Wolff et al., 1998).
Associated movements (synkinesis, mirror movements, etc.) are present in most children under 10 years of age and their intensity and frequency vary according to the complexity of the motor task. By the time puberty is reached, associated movements have diminished in intensity although they continue to accompany complex or forceful movements throughout adult life (Largo et al., 2003; Lazarus, 1992).
Mechanisms underlying the establishment of cortical asymmetry during development are unclear. Hand preference in typically developing children becomes apparent as early as two years of age and is firmly established by 4 years of age (Cavill and Bryden, 2003; Illingsworth, 1983). Anomalous patterns of hand preference have been found in children with developmental disorders even in the absence of obvious structural abnormalities of either hemisphere (Hauck and Dewey, 2001; Niederhofer, 2005).
Structural changes in the brain during neurodevelopment
Structural and functional maturation of the motor cortex, intracortical white matter, and corpus callosum occur along the same time trajectory as maturation of fine motor skills. Thus, it is possible that, in maturity, they form the neural substrate of dexterous fine motor skills. Here we briefly review what is known regarding the maturational changes that occur in the motor system in the typically developing child.
Post-natal central nervous system maturation is a complex process which can be traced using both post mortem histology and in vivo neuroimaging. In the first 2 years of post-natal life there is over-production of synapses. This is followed by a longer process, lasting through mid-adolescence, where pruning of excessive synapses and activity-dependent refinement of synaptic connections take place (Johnston, 2003; Johnston, 2004). The visual cortex completes this process of pruning earlier than the motor cortex (Huttenlocher and Dabholkar, 1997).
Changes in gyral morphology also occur during neuromaturation. This factor is especially relevant to TMS since activation of pyramidal tract neurons depends on the orientation of their axons relative to the electric field induced by the magnetic coil (Amassian et al., 1992). However, since the ontogeny of gyrification is virtually complete by the end of the neonatal period, the effect of gyral maturation on TMS-evoked parameters would primarily be seen in the first six weeks of post-natal life (Armstrong et al., 1995).
Myelination is virtually complete in the corticospinal tracts by the end of the second year of post-natal life. In contrast, myelination of intracortical and callosal white matter is not complete until early adulthood (Yakovlev and Lecours, 1967). Using standard magnetic resonance imaging (MRI) sequences, the grey-white matter contrast appears “adult-like” as early as 18 months of age (Hayakawa et al., 1991). For this reason, quantitative evaluation of MR images is necessary to detect the more subtle changes in central myelination (Giedd et al., 1999) which continue until the middle of the third decades of post-natal life (see Paus and colleagues (Paus et al., 2001) for an overview of the uses of MRI in understanding neurodevelopment). Diffusion tensor imaging (DTI) is becoming an important tool in the evaluation of the brain development, but much work is required to refine the technique and establish norms (Barnea-Goraly et al., 2005; Ben Bashat et al., 2005).
TMS in Children – General Considerations
A large number of studies have used TMS to examine motor cortex function in children. Most examine a specific disorder while others examine age-related changes in TMS-evoked parameters in typically developing children. The focus of this review is to highlight insights gained from both type of studies and to compare the findings in these studies to those in similar studies in adults.
However, is it valid to compare differences in TMS-evoked parameters between children and adults when the same coil size has been used in both populations? This concern arises from a study showing that, when the same sized coil is used to stimulate brains of different volumes, TMS-efficacy is reduced in animals whose brain volume is smaller than that of the adult human (Weissman et al., 1992). All but one study examining TMS-evoked parameters in infants and children have used coils of a similar size to those used in adults. Since children have smaller head circumferences than adults, it could be argued that any differences in TMS-evoked parameters between children and adults reflect reduced TMS-efficacy rather than a dissimilarity of brain function.
Interestingly, despite the smaller head circumferences in children, brain volume in humans remains remarkably similar from 6 years of age onwards with only a small reduction in volume for infants and children under 6 years of age (Bartholomeusz et al., 2002). It is, therefore, reasonable to assume that age-related differences in TMS-evoked parameters in children primarily reflect maturational changes of cerebral and corticospinal myelination (Mukherjee and McKinstry, 2006; Yakovlev and Lecours, 1967), intracortical synaptic and neuronal maturation (Huttenlocher and Dabholkar, 1997; Johnston, 2003) and, in early infancy, gyrification (Armstrong et al., 1995).
TMS-evoked Parameters in Typically Developing Children
Since age-related changes in brain structure and in motor behaviors are apparent, it would be reasonable to assume that function also changes with age during development. Studies using TMS in children seek to determine those TMS-evoked parameters which best reflect the functional changes that occur during neurodevelopment.
Motor Evoked Potential (MEP) Threshold
Compared to adults, children under 10 years of age have higher motor evoked potential (MEP) thresholds (see figure 1). By mid-adolescence, thresholds have decreased to adult levels (Garvey et al., 2003; Moll et al., 1999; Nezu et al., 1997). In children, as in adults, the MEP threshold is higher when the target muscle is at rest (resting motor threshold) than when there is background muscle activation (active motor threshold) (Garvey et al., 2003). It may not be possible to elicit, even using maximal stimulator output, reliable MEP responses when muscles are at rest in children younger than 6 years of age (Koh and Eyre, 1988). When the target muscle is active, MEP responses can be elicited even in neonates (Eyre et al., 2001).
Figure 1. Age-Related Changes in the Motor Evoked Potential (MEP).
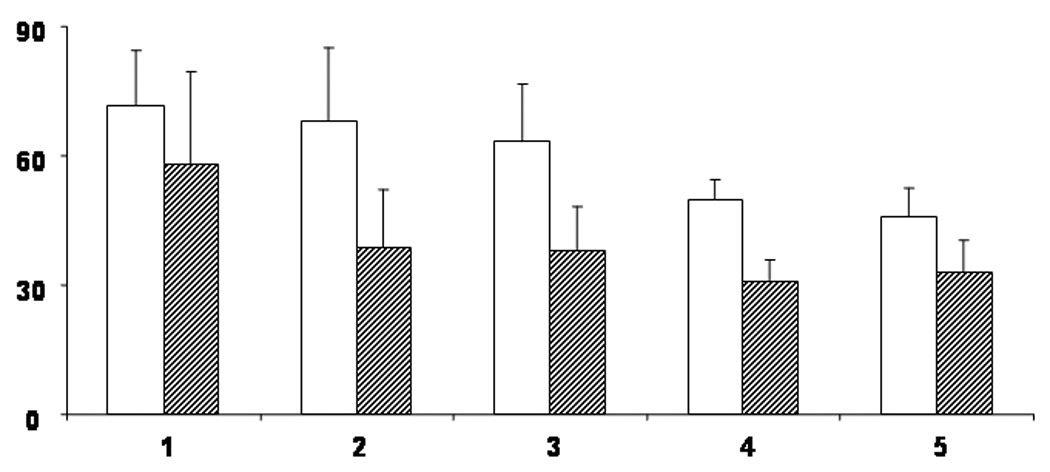
MEP threshold was obtained using a focal coil both when the target muscle (first dorsal interosseus [FDI]) was at rest (RMT) and when there was background muscle activation (AMT). Percentage of maximum stimulator intensity is represented on the Y axis. Age group is represented on the X axis. Group 1 = 6 to 7 year old children (n=5); Group 2 = 8 to 9 year old children (n=8); Group 3 = 10 to 11 year old children (n=10); Group 4 = 12 to 13 year old children (n=8); Group 5 = adults (n=9). Open boxes represent RMT, hatched boxes represent AMT. Error bars represent 1 standard deviation from the mean. Note that thresholds get lower with age and that, for all age groups, lower stimulation intensities are required to obtain the AMT. From Garvey MA & Gilbert DL, Transcranial Magnetic Stimulation in Children, Eur J Paediatr Neurol 2004; 8: 7–19.
MEP latency
The latency of a TMS-evoked MEP provides an estimate of central motor conduction time (CMCT). The developmental pattern of CMCT when muscles are at rest (resting-CMCT) is distinct from the pattern when muscles are active (active-CMCT). Active-CMCT reaches maturity in children within the first 3 to 5 years of post-natal life; in contrast, resting-CMCT does not reach maturity until early adolescence (Eyre et al., 1991; Fietzek et al., 2000; Koh and Eyre, 1988; Müller et al., 1994). In adults, the “latency jump” (i.e. the difference between MEP latencies evoked at rest and those evoked with background muscle activation) is thought to reflect transsynaptic activation of cortical motoneurons via interneurons and recruitment of faster pyramidal tract neurons at higher levels of muscle activation (Abbruzzese and Trompetto, 2002; Rossini et al., 1994). The “latency jump” is 4 times greater in pre-school children than in adults and it gradually decreases in magnitude until it reaches maturity in mid-adolescence. Mechanisms responsible for the gradual decrease of this latency jump in children are still unclear, but may include neuronal and synaptic maturation within the motor cortex, maturation of central myelination, and developmental aspects of central motoneuronal recruitment which are at present unknown (Caramia et al., 1993).
Cortical Maps
Cortical maps give an estimate of the somatotopic representations of muscles within the motor cortex. By stimulating at a number of different scalp positions (using a stimulus intensity close to MEP threshold) and measuring the amplitude of the MEP at each site, it is possible to assess the location of the optimal position for stimulation and the center-of-gravity, which defines the mean position of the map (Abbruzzese and Trompetto, 2002; Chen, 2000). Only one study has examined cortical maps in a small group of typically developing children, 6 to 14 years of age, as a comparison for children with cerebral palsy (Maegaki et al., 1999). Cortical representation sites for the tibialis anterior, biceps brachialis, and abductor pollicis brevis muscle were identified between 1 to 4 cm, 4 to 6 cm, and 5 to 8 cm lateral to the cranial vertex, respectively; the authors did not report the optimal stimulation site or the center of gravity. These preliminary data provide evidence that the mature motor homunculus is established early in life.
Ipsilateral MEP
Stimulation of the motor cortex with TMS may evoke an MEP in the homologous target muscle ipsilateral to the stimulation (iMEP). This iMEP indicates the presence of an ipsilateral corticofugal motor projection that may be the means by which the motor cortex controls ipsilateral movements in healthy subjects (Ziemann et al., 1999). It may also mediate the enhanced participation of the less affected hemisphere after injury to the developing brain (see below) (Carr et al., 1993; Staudt et al., 2002). It is possible to identify different types of TMS-evoked ipsilateral projections by comparing the characteristics of the iMEP with those of the contralateral MEP. Cross-correlation analysis of electromyographic (EMG) activity or, in distal muscles, the long latency reflex, can provide information regarding the origin of the ipsilateral projections (Carr et al., 1993; Eyre et al., 2001; Mayston et al., 1997; Staudt et al., 2002).
Cortical Inhibition
TMS can also provide a window into the inhibitory functions of the motor cortex. Two paradigms have been used in children: the paired pulse paradigm and the silent period.
Paired pulse studies
Intracortical excitability and inhibition can be assessed by delivering two stimuli in a condition-test paradigm (Kujirai et al., 1993). Here the investigator delivers two pulses with varying time intervals between the pulses, ranging from 1 to 70 ms. Several paradigms using different interstimulus intervals to assess intracortical inhibition have been described in adult subjects. The short inter-stimulus intracortical inhibition (SICI) paradigm (which has been shown to be of intracortical origin (Kujirai et al., 1993; Mall et al., 2004; Nakamura et al., 1997; Ziemann et al., 1996) and is mediated by GABAA– receptors (Ziemann, 2004)]) is the only paradigm that has been used in children.
A recent study examined maturation of ICI in subjects ranging in age from 6 to 34 years of age (Mall et al., 2004). The paradigm was limited to testing inhibition using a 2 ms inter-stimulus interval (ISI) since this ISI is thought to approximate GABA-mediated intracortical inhibition (Ziemann et al., 2001). The study demonstrated that intracortical inhibition is nearly 4 times greater in adults compared with children less than 10 years of age. This difference remains whether the intensity of test-stimulus is fixed according to the level of the motor threshold (as is prescribed in the original paradigm) or is varied according to the amplitude of the MEP evoked by test-stimulus. Previous studies have shown that decreased levels of SICI are associated with increased practice-dependent plasticity (Ziemann et al.) which raises the question as to whether the increased plasticity following central nervous system injury in children (as has been postulated elsewhere (Kennard, 1936)) is in some way related to the decreased levels of intracortical inhibition in this population.
A two-coil conditioned-test paradigm has also been used to study inter-cortical inhibition (Ferbert et al., 1992). Here, a conditioning stimulus is delivered to the motor cortex of one hemisphere and the test stimulus is delivered to the homologous motor cortex of the other hemisphere. Although there is a clear pattern of inhibition and facilitation in neurologically intact adult subjects when inter-stimulus intervals are set between 5 and 15 ms (with maximal inhibition at 7 ms), no developmental pattern is present in typically developing children (Mayston et al., 1999). This is surprising since both intracortical and interhemipsheric inhibition show robust developmental trajectories (Garvey et al., 2003; Mall et al., 2004). This may indicate a disparity in the maturation rates of the various cortical inhibitory circuits since the ipsilateral silent period (iSP) and two coil paradigm are thought to test two different types of interhemipsheric inhibition. However, the disparity may also reflect methodological differences, such as a variation in the populations studied: two-coil paradigm was tested in children who were all pre-pubertal, while SICI and iSP were tested in both children and adolescents. In addition, more recently, concerns have been raised regarding the effect of physical interactions between electrical fields on neuronal excitation when stimulating distant areas of the brain using two coils (Cincotta et al., 2005). This may decrease the ability of the two coil paradigm to accurately reflect intercortical inhibition and may be more of a concern in children due to their smaller head size.
Silent periods
The ontogeny of the iSP is thought to reflect maturation of cortical inhibitory neurons and myelination of the mid-body of the corpus callosum. The iSP is absent in pre-school children and can be first consistently evoked in 6 to 7 year old children (Heinen et al., 1998b). At this point, latency is delayed and duration shortened compared with the mature iSP. Over the ensuing years latency gets shorter and duration gets longer; both are close to maturity by early adolescence (Garvey et al., 2003) (see figure 2).
Figure 2. Maturation of the Ipsilateral Silent Period (iSP).
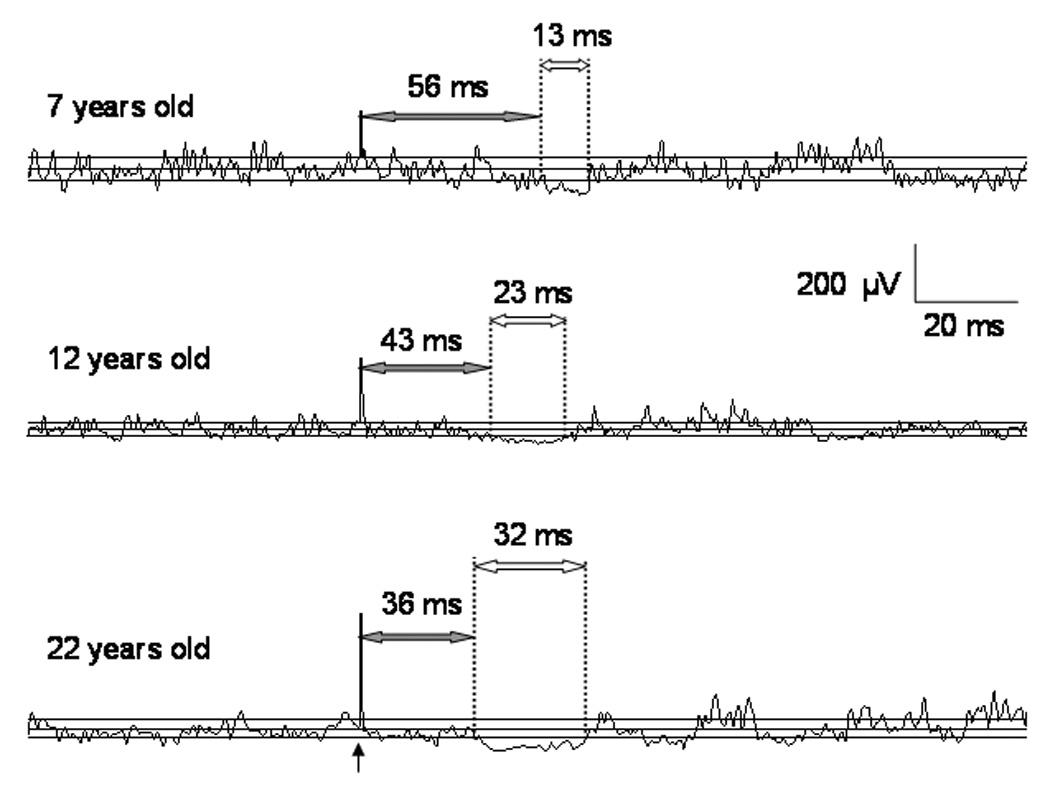
The figure shows three EMG tracings of the iSP from a 7 year old boy (upper), a 12 year old girl (middle), and a 22 year old man (lower). Each tracing is an average of 10 single-trial rectified sweeps. The vertical arrow represents the stimulus which occurred 100 ms after the start of each sweep. There are three horizontal solid lines in each tracing: the middle line represents the mean EMG amplitude (µV) during the pre-stimulus period; the upper and lower lines represent the upper and lower 95% variation limits of the mean pre-stimulus EMG amplitude. These limits were calculated using statistical process control. The vertical dotted lines represent the onset latency and end of the iSP. For each tracing, the shaded arrow heads and accompanying number indicates the onset latency, the open arrow heads and accompanying number indicates the iSP duration. Note that latency is longest and duration shortest in the 7 year old child; latency and duration are intermediate in the 12 year old child; and latency is shortest and duration is longest in the adult subject. From Garvey MA & Gilbert DL, Transcranial Magnetic Stimulation in Children, Eur J Paediatr Neurol 2004; 8: 7–19.
Unlike the ipsilateral silent period, the contralateral silent period (CSP) is present in pre-school children (Heinen et al., 1998b) but it is not clear whether the maturational profile is complete at this age (Garvey et al., 2003; Moll et al., 1999). Because of the large variability in this TMS-evoked parameter, future studies should examine its maturational trajectory with larger sample sizes.
Age-related changes in asymmetry
The most extensively studied phenomenon of cortical asymmetry is the MEP threshold. In young adults, a mean difference of 5% (ranging between −10 and +15%) has been consistently found between the dominant (lower) and non-dominant MEP threshold (Triggs et al., 1997; Triggs et al., 1994; Triggs et al., 1999). Asymmetry appears to be present in cortical maps (larger maps on the dominant hemisphere)(Cicinelli et al., 1997); the CSP (shorter duration when the dominant motor cortex is stimulated)(Priori et al., 1999); and interhemispheric inhibition using the two-coil paired pulse paradigm (greater inhibition when the conditioning stimulus is delivered to the dominant hemisphere)(Netz et al., 1995). Reports show conflicting data as to whether there is functional asymmetry of SICI/ICF (Cicinelli et al., 2000; Civardi et al., 2000; Ilic et al., 2004; Maeda et al., 2002).
Few TMS studies have examined cortical asymmetry in children. Side-to-side differences in MEP threshold (lower in the dominant motor cortex) and duration of CSP (longer when evoked by stimulating the non-dominant hemisphere) are similar to those seen in adults. However, the side-to-side difference in MEP threshold is 5 times larger in 6 to 8 year old children than in adults, getting gradually smaller as children get older (Garvey et al., 2003) (see figure 3). This decrease in side-to-side asymmetry in children appears to be the start of a process which continues throughout life: no significant asymmetry of MEP threshold is found in elderly subjects (Matsunaga et al., 1998).
Figure 3. Asymmetry of Motor Evoked Potential Thresholds.
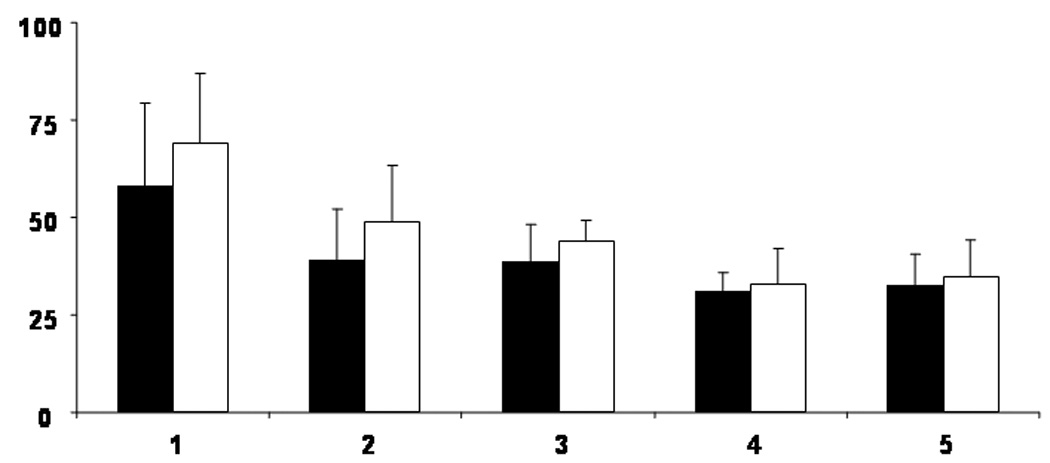
The active MEP thresholds (AMT) in a group of right-handed subjects obtained using a focal coil with background muscle activation of the first dorsal interosseus [FDI]. Percentage of maximum stimulator intensity is represented on the Y axis. Age group is represented on the X axis. Group 1 = 6 to 7 year old children (n=5); Group 2 = 8 to 9 year old children (n=8); Group 3 = 10 to 11 year old children (n=10); Group 4 = 12 to 13 year old children (n=8); Group 5 = adults (n=9). Filled boxes represent the AMT when the left (dominant) cortex is stimulated; open boxes represent the AMT when the right cortex is stimulated. Error bars represent 1 standard deviation from the mean.
Note that AMT requires higher stimulation intensities when the right (non-dominant) cortex is stimulated. Asymmetry in the AMT is greater in the youngest children (group 1) and smallest in the adults (group 5). From Garvey MA & Gilbert DL, Transcranial Magnetic Stimulation in Children, Eur J Paediatr Neurol 2004; 8: 7–19.
Maturation of the iSP also shows side-to-side differences. In six-year-old children, stimulation of the dominant hemisphere evokes an iSP more consistently than stimulation of the non-dominant hemisphere. When both are present, duration of the dominant iSP is shorter than the non-dominant iSP, which is coherent with the asymmetry of the CSP (Garvey et al., 2003). This supports the presence of enhanced cortical inhibitory mechanisms in the non-dominant cortex (Priori et al., 1999). However, unlike the CSP which shows no age-related differences in asymmetry, functional asymmetry of the iSP is present only in younger children. Therefore, other factors, such as side-to-side differences in silent period threshold (Lo and Fook-Chong, 2005), may also be responsible for asymmetry in the various inhibitory TMS-evoked parameters (Garvey et al., 2003).
Relationship between TMS-evoked parameters and Neuromotor Function
It is reasonable to assume that TMS-evoked parameters in some way reflect motor cortical functions that take place during voluntary brain activity. This can be seen in the similarities found between the maturational profiles of behavioral measures (e.g. finger tapping speed) and the later-maturing TMS-evoked parameters of iSP latency and duration and motor threshold (Fietzek et al., 2000; Heinen et al., 1998a; Müller and Hömberg, 1992). However, it is important to realize that the relationship between individual TMS-evoked parameters and specific voluntary motor behaviors may not be straightforward. For this reason, before it is possible to use these parameters as biological markers for these motor behaviors it is important to examine the relationships in a more systematic way. For instance, multiple regression models which include “age” as a variable (Kleinbaum et al., 1998) indicate that finger tapping speed is related to iSP latency, but not to motor threshold or iSP duration (Garvey et al., 2003). Likewise, in young adults, inter-hemispheric asymmetry in MEP threshold correlates strongly with inter-manual asymmetry of fine motor dexterity but not strength (Triggs et al., 1997) and, in patients with multiple sclerosis, changes in CMCT appear to reflect abnormalities of phasic movements while variations in the TMS-evoked measures of the triple stimulation technique may more accurately reflect deficits in muscle strength (Humm et al., 2006; Vanderkamp et al., 1991).
Developmental neuromotor “anomalies” (e.g. slower and more clumsy fine motor skills and an increased degree of associated movements) decrease with age (Denckla, 1973; Denckla, 1974; Largo et al., 2001a; Largo et al., 2001b). While maturation of certain aspects of intracortical and interhemispheric inhibition (Garvey et al., 2003; Mall et al., 2004) may contribute to the disappearance of these movements, developmental changes in the wider network of areas involved in motor control may also play a role. A small number of investigators have used functional imaging to examine these motor networks. Unfortunately, these studies have shown no clear consensus regarding the maturational trajectory of these networks. In one study, children who performed a simple motor task showed an increased amount of activation outside the primary motor cortex compared to adults who performed the same task (Halder et al., 2007; Muller et al., 1998) while in two others children showed a relative decrease in the amount of activation (Guzzetta et al., 2007; Mall et al., 2005). The discrepancy between the studies may result from methodological differences including the task chosen and the age-range of the subjects. However, these studies demonstrate that cross-modality studies will be necessary to fully understand the age-related changes in cortical networks associated with neuromotor maturation. Activation in functional MRI provides a window into the wider networks of motor control but it does not easily differentiate between inhibition and excitation (Almeida and Stetter, 2002; Tagamets and Horwitz, 2001); TMS-evoked parameters clearly distinguish between the two but their effects principally reflect activity within the primary motor cortex.
TMS-evoked Parameters in Developmental Disorders
It appears to be reasonable to assume that motor skill deficits in children with developmental disabilities will be associated with structural and/or functional abnormalities of motor circuits within the central nervous system. TMS-evoked parameters are abnormal in a number of these disorders which lends support to this assumption. In this section, we review those studies that have examined motor cortex function in neurological disorders which have their onset in childhood.
Cerebral Palsy
Functional recovery may be quite good in a sizable group of children who suffer perinatal insults. Unfortunately, this is by no means universal and, at times, the outcome can be devastating (Johnston) There are many factors which may determine this variability of outcomes, including the type, the extent, and the timing of the process that caused the original lesion (Staudt et al., 2004)
One of the more common disorders which arise from perinatal insults is cerebral palsy. Cerebral palsy is a group of disorders characterized by injuries to the developing brain which affect the motor system; other areas of the brain may also be affected (Bax et al., 2005). Although children with cerebral palsy may manifest different functional deficits, they tend to fall into three or four different subgroups based on the topography of the motor deficits. Within these groups it may also be possible to identify children who share a common etiology. Studies of a group of individuals with cerebral palsy who have comparable motor dysfunction or similar etiologies may be especially helpful in determining the association between the underlying pattern of brain reorganization which occurred in response to the initial injury and functional outcome.
Enhanced plasticity of the developing brain
Certain children with hemiplegic cerebral palsy show prominent mirror movements of the paretic hand when they voluntarily move their less affected hand. The presence of these mirror movements suggests that the contra-lesional hemisphere plays an important role in the motor control of the paretic hand. Neurophysiologic tools, including TMS, have shown that there may be enhanced participation of the contra-lesional hemisphere in motor control of the paretic hand in the absence of prominent mirror movements (Carr et al., 1993; Eyre et al., 2001; Staudt et al., 2002). When used in conjunction with each other, TMS, cross-correlation analysis, and distal long-latency reflexes help to elucidate the origin of the projections by which the contra-lesional hemisphere participates in the motor control of paretic hand muscles. Thus, TMS-evoked ipsilateral projections which appear to originate from branched pyramidal tract neurons in the primary motor cortex of the contra-lesional hemisphere are associated with prominent mirror movements while those projections which appear to arise from areas other than the primary motor cortex are not associated with mirror movements (Carr et al., 1993; Eyre et al., 2001; Staudt et al., 2002).
Altered cortical maps
Periventricular leukomalacia (PVL) arising from ischemia of the central white matter (especially the motor and sensory fibers of the lower extremities (Hoon et al., 2002; Volpe, 1998)) is not uncommon in prematurely born infants. As children with PVL get older, they may manifest a bilateral, spastic type of cerebral palsy (quadriplegia or diplegia). Cortical maps of the lower extremity muscles are laterally displaced in children born prematurely who have PVL and spastic diplegia. In contrast, maps of lower extremity muscles are normally placed in individuals born at term who have spastic diplegia and in those with a bilateral dyskinetic form cerebral palsy, neither of which are associated with abnormalities resulting from ischemia of central white matter (Maegaki et al., 1999).
Transcallosal Inhibition
Children with spastic diplegia resulting from PVL may also show severe structural deficits of the corpus callosum. One group of investigators examined the iSP (as a measure of callosal function) in a small group of adolescents with spastic diplegia and spastic paraparesis. The iSP was present in both typically developing adolescents and in the adolescents with spastic paraplegia, but was absent in the subjects with spastic diplegia (Heinen et al., 1999). The iSP is mediated via the mid-body of the corpus callosum which carries fibers connecting the motor areas of both hemispheres (Meyer et al., 1995). It would be interesting to determine if the iSP is present but abnormal in children with mild spastic diplegia (providing an accurate reflection of callosal structural deficits in these children (Meyer et al., 1999)) and if they are related to severity of their clinical neuromotor deficits.
Attention Deficit Hyperactivity Disorder
Children with attention deficit hyperactivity disorder (ADHD) have difficulty inhibiting impulsive and off-task behaviors which contribute to the impulsive behavior and motor anomalies that are characteristic features of this disorder (Barkley, 1997). The characteristic feature of these anomalous behaviors in ADHD is that they are qualitatively similar, but more prominent, than those seen in younger, typically developing children (Denckla and Rudel, 1978; Mostofsky et al., 2003). For example, the motor anomalies in ADHD, which manifest as slowed finger tapping and overflow movements, are similar to the associated movements in seen typically developing children but are more prominent than would be expected for a given age and do not disappear until late adolescence. For this reason, it is postulated that cortical systems involved in automatic inhibition are immature in children with ADHD, a hypothesis also supported by evidence from neuroimaging studies (Rubia et al., 2000).
Abnormal intracortical inhibition (using the standard paired pulse paradigm) appears to be related to the severity of ADHD symptoms (Gilbert et al., 2004; Gilbert et al., 2005; Moll et al., 2000). This finding is present in individuals who only have ADHD and in those with co-morbid tic disorders. Interestingly, in these latter individuals, decreased intracortical inhibition correlates with ADHD symptoms while a shortened cortical silent period appears to correlate with tic severity and distribution (Moll et al., 2006; Moll et al., 2001; Ziemann et al., 1997). Although these findings are not specific to either disorder they have provided preliminary insights into their neural substrates and suggest that, although ADHD and Tourette syndrome are frequently co-morbid, they are associated with distinct abnormalities of cortical inhibition.
One study has shown that drugs used to control ADHD symptoms “normalize” (that is, enhance) intracortical inhibition in children with ADHD. This is in contrast to their effect in neurologically normal adults in whom they have been shown to consistently decrease inhibition (see (Gilbert et al., 2006) for a meta-analysis). It is possible that decreased cortical inhibition in individuals with ADHD is a compensatory response which is further augmented by stimulant and norepinephrine (NE) reuptake inhibitor medications which control the ADHD symptoms. However, further studies are warranted to examine the effect of drugs on intracortical inhibition in persons with ADHD and to determine if cortical inhibition will be a useful surrogate for treatment response.
Two studies have found abnormalities of the ipsilateral silent period in children with ADHD (Buchmann et al., 2003; Garvey et al., 2005). In one study, although latency of the iSP was closely correlated with finger speed in typically developing children, a similar correlation was absent in age-matched children with ADHD, suggesting that abnormal interhemispheric interactions may be related, in a complex manner, to the neuromotor anomalies present in children with ADHD (see figure 4). Since the neural mechanisms of overflow movements are thought to be closely related to those of response inhibition, it is unlikely that the two forms of cortical inhibition reflect distinct aspects of the ADHD symptom complex (Mostofsky et al., 2003). TMS may provide a window into the relationship between the neural mechanisms responsible for response inhibition and those which give rise to overflow movements.
Figure 4. Age-related changes in onset latency of the ipsilateral silent period (iSP).
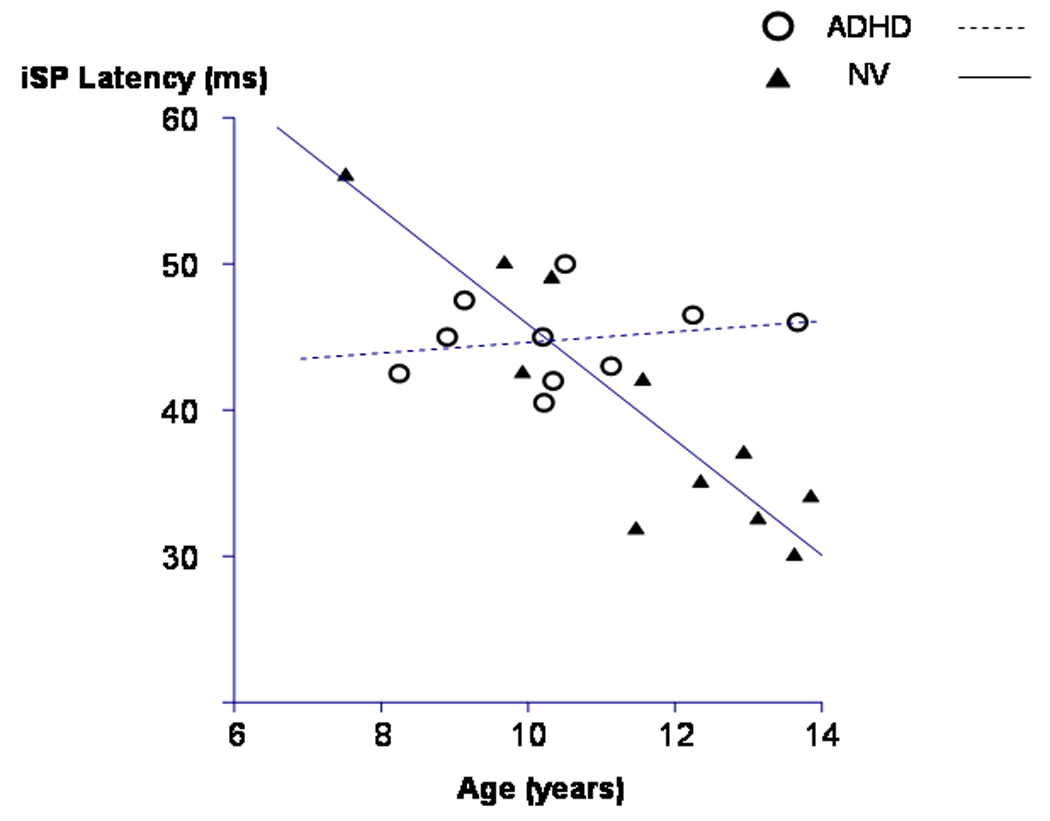
This figure shows age-related changes in the onset latency of the ipsilateral silent period. Typically developing boys (control group) are represented by the filled triangles and their regression slope is represented by the solid line. Boys with ADHD are represented by the open circles and their regression slope is represented by the dashed line. The regression slopes for the two groups were significantly different from each other. Only the typically developing boys showed a significant age-related decrease in iSP latency. From Garvey MA, et al, Clin Neurophys, 2005;116: 1889–96.
Other Neurological Disorders in Children
To date, only one disorder, Rett syndrome, has shown unique abnormalities of TMS-evoked parameters. Children during the rapid destructive stage of Rett syndrome (1–3 years of age) show abnormally short resting-CMCT. Although this finding is non-localizing, is has not been found in any other disorder and suggests the presence of abnormal synaptic organization within the motor cortex or abnormalities of cortical or spinal motoneurons (Eyre et al., 1990; Heinen and Korinthenberg, 1996; Nezu et al., 1998). Since CMCT is of normal latency in children who are in the pseudo-stationary stage of Rett Syndrome (15 years and older), it may reflect an underlying dynamic process which burns itself out over time. The CMCT abnormalities may not be of significant diagnostic use in most typical cases of Rett syndrome who all appear to have mutations in the X-linked methyl CpG binding protein 2 gene (MeCP2) (Dunn, 2001; Hoffbuhr et al., 2001). However, they are likely to be of use in atypical cases or those children who present with the Rett phenotype but do not have the gene mutation. They may also offer valuable insights into the pathophysiologic process underlying the rapid destructive phase of the disorder.
TMS-evoked parameters appear to be a marker of the disease burden in certain neurological disorders and may be sensitive to changes that occur after treatment. For example, prolonged CMCT is present in the early stages of Freidreich ataxia (Cruz Martinez and Anciones, 1992) and disease progression is accompanied by further prolongation of CMCT is related to disease progression (Cruz-Martinez and Palau, 1997). In Wilson’s disease and in adrenomyeloneuropathy, treatment-related clinical improvement correlates with improvement of CMCT abnormalities (Hitomi et al., 2005; Hitomi et al., 2003; Meyer et al., 1991).
TMS-evoked CMCT measures may be abnormal before the onset of clinical neurological symptoms of mucolipidosis III, adrenoleukodystrophy, and malnutrition (Nezu et al., 1996a; Tamer et al., 1997; Toscano et al., 1998). They do not appear to have a direct relationship with disease burden or treatment in Pelizaeus-Merzbacher disease (Nezu, 1995; Nezu et al., 1996b). Epilepsy research may benefit from the insights which single and paired-pulse TMS studies can offer on seizure prediction (Wright et al., 2006), the pathophysiology of catamenial seizures(Hattemer et al., 2006; Hattemer et al., 2007), the increased seizure threshold during sleep deprivation (Scalise et al., 2006) and the pharmacological properties of anti-epileptic drugs (Ziemann et al., 1998).
Future Studies
The potential of TMS as a neurophysiologic tool in children has not yet been fully explored. Although many investigators have published small case reports or case series, few have gone on to fully characterize the neurophysiology of childhood neurological disorders. TMS studies may also be helpful in characterization of different phenotypes of the same genetic disorder. In addition, TMS also has potential for assessing medication compliance and therapeutic effect in disorders treated with neuroactive drugs (Ziemann, 2004).
Acknowledgments
While preparing this manuscript, Dr Garvey received funding from NINDS grant 1K22 NS042680-01A1 and Dr Volker Mall received funding from Deutsche Forschungsgemeinschaft DFG: MA 3306/1 and Allergan, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marjorie A. Garvey, Neuroscience Research Center, National Rehabilitation Hospital, 102 Irving Street, NW, Washington, DC 20010.
Volker Mall, Division of Neuropediatrics and Muscular Disorders, Department of Pediatrics and Adolescent Medicine, University Hospital Freiburg, Mathildenstraβe 1, D - 79104 Freiburg.
References
- Abbruzzese G, Trompetto C. Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol. 2002;19:307–321. doi: 10.1097/00004691-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Almeida R, Stetter M. Modeling the link between functional imaging and neuronal activity: synaptic metabolic demand and spike rates. Neuroimage. 2002;17:1065–1079. [PubMed] [Google Scholar]
- Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33:239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–576. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21:503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Buchmann J, Wolters A, Haessler F, Bohne S, Nordbeck R, Kunesch E. Disturbed transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) Clin Neurophysiol. 2003;114:2036–2042. doi: 10.1016/s1388-2457(03)00208-6. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Desiato MT, Cicinelli P, Iani C, Rossini PM. Latency jump of "relaxed" versus "contracted" motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalogr Clin Neurophysiol. 1993;89:61–66. doi: 10.1016/0168-5597(93)90086-5. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(Pt 5):1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Cavill S, Bryden P. Development of handedness: comparison of questionnaire and performance-based measures of preference. Brain Cogn. 2003;53:149–151. doi: 10.1016/s0278-2626(03)00098-8. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Bassi A, Scivoletto G, Rossini PM. Interhemispheric differences of hand muscle representation in human motor cortex. Muscle Nerve. 1997;20:535–542. doi: 10.1002/(sici)1097-4598(199705)20:5<535::aid-mus1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Oliveri M, Palmieri MG, Filippi MM, Pasqualetti P, et al. Intracortical excitatory and inhibitory phenomena to paired transcranial magnetic stimulation in healthy human subjects: differences between the right and left hemisphere. Neurosci Lett. 2000;288:171–174. doi: 10.1016/s0304-3940(00)01216-7. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Borgheresi A, Jung P, Balestrieri F, Giovannelli F, Zaccara G, et al. Physical interactions between induced electrical fields can have substantial effects on neuronal excitation during simultaneous TMS of two brain areas. Clin Neurophysiol. 2005;116:1733–1742. doi: 10.1016/j.clinph.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin Neurophysiol. 2000;111:624–629. doi: 10.1016/s1388-2457(99)00301-6. [DOI] [PubMed] [Google Scholar]
- Croce RV, Horvat M, McCarthy E. Reliability and concurrent validity of the movement assessment battery for children. Percept Mot Skills. 2001;93:275–280. doi: 10.2466/pms.2001.93.1.275. [DOI] [PubMed] [Google Scholar]
- Cruz Martinez A, Anciones B. Central motor conduction to upper and lower limbs after magnetic stimulation of the brain and peripheral nerve abnormalities in 20 patients with Friedreich's ataxia. Acta Neurol Scand. 1992;85:323–326. doi: 10.1111/j.1600-0404.1992.tb04051.x. [DOI] [PubMed] [Google Scholar]
- Cruz-Martinez A, Palau F. Central motor conduction time by magnetic stimulation of the cortex and peripheral nerve conduction follow-up studies in Friedreich's ataxia. Electroencephalogr Clin Neurophysiol. 1997;105:458–461. doi: 10.1016/s0924-980x(97)00047-7. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Dev Med Child Neurol. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of motor co-ordination in normal children. Dev Med Child Neurol. 1974;16:729–741. doi: 10.1111/j.1469-8749.1974.tb03393.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Anomalies of motor development in hyperactive boys. Ann Neurol. 1978;3:231–233. doi: 10.1002/ana.410030308. [DOI] [PubMed] [Google Scholar]
- Dunn HG. Importance of Rett syndrome in child neurology. Brain Dev. 2001;23 Suppl 1:S38–S43. doi: 10.1016/s0387-7604(01)00335-7. [DOI] [PubMed] [Google Scholar]
- Einspieler C, Prechtl HF. Prechtl's assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11:61–67. doi: 10.1002/mrdd.20051. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Kerr AM, Miller S, O'Sullivan MC, Ramesh V. Neurophysiological observations on corticospinal projections to the upper limb in subjects with Rett syndrome. J Neurol Neurosurg Psychiatry. 1990;53:874–879. doi: 10.1136/jnnp.53.10.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: Investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek UM, Heinen F, Berweck S, Maute S, Hufschmidt A, Schulte-Mönting J, et al. Development of the corticospinal system and hand motor function: central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42:220–227. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91–97. [PubMed] [Google Scholar]
- Garvey MA, Barker CA, Bartko JJ, Denckla MB, Wassermann EM, Castellanos FX, et al. The ipsilateral silent period in boys with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2005;116:1889–1896. doi: 10.1016/j.clinph.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Bartko JJ, Denckla MB, Barker CA, Wassermann EM. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114:1662–1670. doi: 10.1016/s1388-2457(03)00130-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, et al. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome. Mov Disord. 2004;19:416–425. doi: 10.1002/mds.20044. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Ridel KR, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Comparison of the inhibitory and excitatory effects of ADHD medications methylphenidate and atomoxetine on motor cortex. Neuropsychopharmacology. 2006;31:442–449. doi: 10.1038/sj.npp.1300806. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM. Transcranial magnetic stimulation-evoked cortical inhibition: a consistent marker of attention-deficit/hyperactivity disorder scores in tourette syndrome. Biol Psychiatry. 2005;57:1597–1600. doi: 10.1016/j.biopsych.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Staudt M, Petacchi E, Ehlers J, Erb M, Wilke M, et al. Brain representation of active and passive hand movements in children. Pediatr Res. 2007;61 doi: 10.1203/pdr.0b013e3180332c2e. In press. [DOI] [PubMed] [Google Scholar]
- Halder P, Brem S, Bucher K, Boujraf S, Summers P, Dietrich T, et al. Electrophysiological and hemodynamic evidence for late maturation of hand power grip and force control under visual feedback. Hum Brain Mapp. 2007;28:69–84. doi: 10.1002/hbm.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattemer K, Knake S, Reis J, Oertel WH, Rosenow F, Hamer HM. Cyclical excitability of the motor cortex in patients with catamenial epilepsy: a transcranial magnetic stimulation study. Seizure. 2006;15:653–657. doi: 10.1016/j.seizure.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hattemer K, Knake S, Reis J, Rochon J, Oertel WH, Rosenow F, et al. Excitability of the motor cortex during ovulatory and anovulatory cycles: a transcranial magnetic stimulation study. Clin Endocrinol (Oxf) 2007;66:387–393. doi: 10.1111/j.1365-2265.2007.02744.x. [DOI] [PubMed] [Google Scholar]
- Hauck JA, Dewey D. Hand preference and motor functioning in children with autism. J Autism Dev Disord. 2001;31:265–277. doi: 10.1023/a:1010791118978. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Konishi Y, Kuriyama M, Konishi K, Matsuda T. Normal brain maturation in MRI. Eur J Radiol. 1991;12:208–215. doi: 10.1016/0720-048x(91)90074-6. [DOI] [PubMed] [Google Scholar]
- Heinen F, Fietzek UM, Berweck S, Hufschmidt A, Deuschl G, Korinthenberg R. Fast corticospinal system and motor performance in children: conduction proceeds skill. Pediatr Neurol. 1998a;19:217–221. doi: 10.1016/s0887-8994(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Heinen F, Glocker FX, Fietzek U, Meyer B-U, Lücking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998b;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- Heinen F, Kirschner J, Fietzek U, Glocker FX, Mall V, Korinthenberg R. Absence of transcallosal inhibition in adolescents with diplegic cerebral palsy. Muscle Nerve. 1999;22:255–257. doi: 10.1002/(sici)1097-4598(199902)22:2<255::aid-mus14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Heinen F, Korinthenberg R. Does transcranial magnetic stimulation allow early diagnosis of Rett syndrome? Neuropediatrics. 1996;27:223–224. doi: 10.1055/s-2007-973794. [DOI] [PubMed] [Google Scholar]
- Hitomi T, Mezaki T, Tomimoto H, Ikeda A, Shimohama S, Okazaki T, et al. Long-term effect of bone marrow transplantation in adult-onset adrenoleukodystrophy. Eur J Neurol. 2005;12:807–810. doi: 10.1111/j.1468-1331.2005.01055.x. [DOI] [PubMed] [Google Scholar]
- Hitomi T, Mezaki T, Tsujii T, Kinoshita M, Tomimoto H, Ikeda A, et al. Improvement of central motor conduction after bone marrow transplantation in adrenoleukodystrophy. J Neurol Neurosurg Psychiatry. 2003;74:373–375. doi: 10.1136/jnnp.74.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbuhr K, Devaney JM, LaFleur B, Sirianni N, Scacheri C, Giron J, et al. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology. 2001;56:1486–1495. doi: 10.1212/wnl.56.11.1486. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Jr, Lawrie WT, Jr, Melhem ER, Reinhardt EM, Van Zijl PC, Solaiyappan M, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology. 2002;59:752–756. doi: 10.1212/wnl.59.5.752. [DOI] [PubMed] [Google Scholar]
- Humm AM, Z'Graggen WJ, Buhler R, Magistris MR, Rosler KM. Quantification of central motor conduction deficits in multiple sclerosis patients before and after treatment of acute exacerbation by methylprednisolone. J Neurol Neurosurg Psychiatry. 2006;77:345–350. doi: 10.1136/jnnp.2005.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Jung P, Ziemann U. Subtle hemispheric asymmetry of motor cortical inhibitory tone. Clin Neurophysiol. 2004;115:330–340. doi: 10.1016/j.clinph.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Illingsworth RS. The Development of the Infant and Young Child: Normal and Abnormal. Edinburgh: Churchill Livingstone; 1983. [Google Scholar]
- Johnston MV. Injury and plasticity in the developing brain. Exp Neurol. 2003;184 Suppl 1:S37–S41. doi: 10.1016/s0014-4886(03)00355-8. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26:73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- Kennard MA. Age and other factors in motor recovery from precentral lesions in monkeys. American Journal of Physiology. 1936;115:137–146. [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariate Methods. Pacific Grove: Brooks/Cole Publishing Company; 1998. [Google Scholar]
- Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol. 2001a;43:444–453. doi: 10.1017/s0012162201000822. [DOI] [PubMed] [Google Scholar]
- Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L, et al. Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol. 2001b;43:436–443. doi: 10.1017/s0012162201000810. [DOI] [PubMed] [Google Scholar]
- Largo RH, Fischer JE, Rousson V. Neuromotor development from kindergarten age to adolescence: developmental course and variability. Swiss Med Wkly. 2003;133:193–199. doi: 10.4414/smw.2003.09883. [DOI] [PubMed] [Google Scholar]
- Lazarus JC. Associated movement in hemiplegia: the effects of force exerted, limb usage and inhibitory training. Arch Phys Med Rehabil. 1992;73:1044–1449. [PubMed] [Google Scholar]
- Lo YL, Fook-Chong S. The silent period threshold as a measure of corticospinal inhibition. J Clin Neurophysiol. 2005;22:176–179. [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Ishii S, Eda I, Ohtagaki A, Kitahara T, et al. Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr Res. 1999;45:559–567. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- Majnemer A, Snider L. A comparison of developmental assessments of the newborn and young infant. Ment Retard Dev Disabil Res Rev. 2005;11:68–73. doi: 10.1002/mrdd.20052. [DOI] [PubMed] [Google Scholar]
- Mall V, Berweck S, Fietzek UM, Glocker FX, Oberhuber U, Walther M, et al. Low level of intracortical inhibition in children shown by transcranial magnetic stimulation. Neuropediatrics. 2004;35:120–125. doi: 10.1055/s-2004-815834. [DOI] [PubMed] [Google Scholar]
- Mall V, Linder M, Herpers M, Schelle A, Mendez-Mendez J, Korinthenberg R, et al. Recruitment of the sensorimotor cortex--a developmental FMRI study. Neuropediatrics. 2005;36:373–379. doi: 10.1055/s-2005-873077. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Uozumi T, Tsuji S, Murai Y. Age-dependent changes in physiological threshold asymmetries for the motor evoked potential and silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;109:502–507. doi: 10.1016/s1388-2457(98)00020-0. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain. 1997;120(Pt 7):1199–1216. doi: 10.1093/brain/120.7.1199. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Britton TC, Benecke R. Wilson's disease: normalisation of cortically evoked motor responses with treatment. Journal of Neurology. 1991;238:327–330. doi: 10.1007/BF00315332. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Röricht S, Schmierer K, Irlbacher K, Meierkord H, Niehaus L, et al. First diagnostic applications of transcallosal inhibition in diseases affecting callosal neurones (multiple sclerosis, hydrocephalus, Huntington's disease) Electroencephalogr Clin Neurophysiol Suppl. 1999;51:233–242. [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Gevensleben H, Rothenberger A. Tic distribution and inhibitory processes in the sensorimotor circuit during adolescence: a cross-sectional TMS study. Neurosci Lett. 2006;403:96–99. doi: 10.1016/j.neulet.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett. 2000;284:121–125. doi: 10.1016/s0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott GE, Wirth S, Bock N, Rothenberger A. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: evidence for additive inhibitory deficits within the motor system. Ann Neurol. 2001;49:393–396. [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Wischer S, Tergau F, Paulus W, Rothenberger A. Motor system excitability in healthy children: developmental aspects from transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:243–249. [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Percept Mot Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, McKinstry RC. Diffusion Tensor Imaging and Tractography of Human Brain Development. Neuroimaging Clinics of North America Advanced Pediatric Imaging. 2006;16:19–43. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Müller K, Ebner B, Hömberg V. Maturation of fastest afferent and efferent central and peripheral pathways: no evidence for a constancy of central conduction delays. Neurosci Lett. 1994;166:9–12. doi: 10.1016/0304-3940(94)90828-1. [DOI] [PubMed] [Google Scholar]
- Müller K, Hömberg V. Development of speed of repetitive movements in children is determined by structural changes in corticospinal efferents. Neurosci Lett. 1992;144:57–60. doi: 10.1016/0304-3940(92)90715-j. [DOI] [PubMed] [Google Scholar]
- Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT. Developmental changes of cortical and cerebellar motor control: a clinical positron emission tomography study with children and adults. J Child Neurol. 1998;13:550–556. doi: 10.1177/088307389801301105. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498(Pt 3):817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Nezu A. Neurophysiological study in Pelizaeus-Merzbacher disease. Brain Dev. 1995;17:175–181. doi: 10.1016/0387-7604(95)00028-a. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Kobayashi T, Sekiguchi H, Ikuta K, Matsuyama S, et al. Transcranial magnetic stimulation in an adrenoleukodystrophy patient. Brain Dev. 1996a;18:327–329. doi: 10.1016/0387-7604(96)00011-3. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Osaka H, Kobayashi T, Ohtsuki N. Effect of digitalis on conduction dysfunction in Pelizaeus-Merzbacher disease. J Neurol Sci. 1996b;141:49–53. doi: 10.1016/0022-510x(96)00134-7. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Takeshita S, Tanaka M. Characteristic response to transcranial magnetic stimulation in Rett syndrome. Electroencephalogr Clin Neurophysiol. 1998;109:100–103. doi: 10.1016/s0924-980x(97)00081-7. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Uehara S, Kobayashi T, Tanaka M, Saito K. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176–180. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- Niederhofer H. Hand preference in attention deficit hyperactivity disorder. Percept Mot Skills. 2005;101:808–810. doi: 10.2466/pms.101.3.808-810. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Plubrukarn R, Theeramanoparp S. Human figure drawing test: validity in assessing intelligence in children aged 3–10 years. J Med Assoc Thai. 2003;86 Suppl 3:S610–S617. [PubMed] [Google Scholar]
- Priori A, Oliviero A, Donati E, Callea L, Bertolasi L, Rothwell JC. Human handedness and asymmetry of the motor cortical silent period. Exp Brain Res. 1999;128:390–396. doi: 10.1007/s002210050859. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Scalise A, Desiato MT, Gigli GL, Romigi A, Tombini M, Marciani MG, et al. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–1598. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Tagamets MA, Horwitz B. Interpreting PET and fMRI measures of functional neural activity: the effects of synaptic inhibition on cortical activation in human imaging studies. Brain Res Bull. 2001;54:267–273. doi: 10.1016/s0361-9230(00)00435-4. [DOI] [PubMed] [Google Scholar]
- Tamer SK, Misra S, Jaiswal S. Central motor conduction time in malnourished children. Arch Dis Child. 1997;77:323–325. doi: 10.1136/adc.77.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano E, Perretti A, Balbi P, Silvestro E, Andria G, Parenti G. Detection of subclinical central nervous system abnormalities in two patients with mucolipidosis III by the use of motor and somatosensory evoked potentials. Neuropediatrics. 1998;29:40–42. doi: 10.1055/s-2007-973532. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Calvanio R, Levine M. Transcranial magnetic stimulation reveals a hemispheric asymmetry correlate of intermanual differences in motor performance. Neuropsychologia. 1997;35:1355–1363. doi: 10.1016/s0028-3932(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Calvanio R, Macdonell RA, Cros D, Chiappa KH. Physiological motor asymmetry in human handedness: evidence from transcranial magnetic stimulation. Brain Res. 1994;636:270–276. doi: 10.1016/0006-8993(94)91026-x. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Subramanium B, Rossi F. Hand preference and transcranial magnetic stimulation asymmetry of cortical motor representation. Brain Res. 1999;835:324–329. doi: 10.1016/s0006-8993(99)01629-7. [DOI] [PubMed] [Google Scholar]
- Vanderkamp W, Denoordhout AM, Thompson PD, Rothwell JC, Day BL, Marsden CD. Correlation of Phasic Muscle Strength and Cortico-Motor-Neuron Conduction Time in Multiple-Sclerosis. Annals of Neurology. 1991;29:6–12. doi: 10.1002/ana.410290104. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–151. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- Weissman JD, Epstein CM, Davey KR. Magnetic brain stimulation and brain size: relevance to animal studies. Electroencephalogr Clin Neurophysiol. 1992;85:215–219. doi: 10.1016/0168-5597(92)90135-x. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Kotwica K, Obregon M. The development of interlimb coordination during bimanual finger tapping. Int J Neurosci. 1998;93:7–27. doi: 10.3109/00207459808986408. [DOI] [PubMed] [Google Scholar]
- Wright MA, Orth M, Patsalos PN, Smith SJ, Richardson MP. Cortical excitability predicts seizures in acutely drug-reduced temporal lobe epilepsy patients. Neurology. 2006;67:1646–1651. doi: 10.1212/01.wnl.0000242729.85335.a3. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A-R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Yule W, Lockyer L, Noone A. The reliability and validity of the Goodenough-Harris drawing test. Br J Educ Psychol. 1967;37:110–111. doi: 10.1111/j.2044-8279.1967.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518(Pt 3):895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Steinhoff BJ, Tergau F, Paulus W. Transcranial magnetic stimulation: its current role in epilepsy research. Epilepsy Res. 1998;30:11–30. doi: 10.1016/s0920-1211(97)00079-x. [DOI] [PubMed] [Google Scholar]


