Abstract
The adipose-derived plasma protein, adiponectin (APN), has various protective effects on cardiovascular diseases. In this study, we show that endogenous APN is required for full cyclooxygenase-2 (COX-2) induction by ischemia-reperfusion injury in the heart in vivo. In rat neonatal cardiac myocytes, APN-induced COX-2 expression was reduced by treatment with a sphingosine kinase-1 (SphK-1) inhibitor or siRNA targeting SphK-1. Treatment with a sphingosine-1-phosphate (S1P) receptor antagonist also diminished COX-2 expression in response to APN stimulation. These findings suggest that APN is a physiological regulator of COX-2 signaling in the heart and that this regulation occurs in part via a SphK-1–S1P receptor dependent mechanism in cardiac myocytes.
Keywords: adiponectin, cyclooxygenase-2, sphingosine kinase-1, cardiac myocytes
1. Introduction
Obesity-related diseases are closely associated with the development of cardiovascular diseases. Adiponectin (APN) is a plasma protein that is primarily produced by adipose tissue [1]. Plasma APN concentrations are decreased in association with obesity, hypertension and ischemic heart diseases. APN displays various protective properties in the context of obesity-related metabolic, inflammatory and cardiovascular diseases [2–6]. We have reported that APN protects the heart from various injuries [5, 7] and that it inhibits myocardial ischemia-reperfusion injury through both AMP-activated protein kinase (AMPK)- and cyclooxygenase-2 (COX-2)- dependent mechanisms [8].
COX-2, the key enzyme regulating the production of prostaglandins, plays a variety of roles in the cardiovascular system. COX inhibitors are widely used as non-steroidal anti-inflammatory drugs in chronic inflammatory diseases [9]. However, COX-2 selective inhibitors have been associated with an increased risk of cardiovascular events [10]. It has been reported that COX-2 exerts cardioprotective effects via the production of prostaglandin (PG) E2 synthesis in a model of myocardial ischemia-reperfusion injury [11]. Furthermore, we have recently reported that APN suppresses lipopolysaccharide-induced tumor necrosis factor (TNF)-α production in cardiac cells through COX-2-PGE2-dependent pathways which are independent of AMPK [8]. However, the molecular mechanisms by which APN regulates COX-2 expression in cardiac cells remain unknown.
Sphingosine-1-phosphate (S1P) is generated from sphingosine through phosphorylation by sphingosine kinase (SphK) [12]. S1P has various bioactivities, including the stimulation of angiogenesis and the suppression of apoptosis [13]. It has been reported that S1P increases COX- 2 expression in vascular endothelial cells [14] or smooth muscle cells [15]. These findings led us to speculate that SphK acts as a modulator for APN-COX-2 signaling axis in cardiac myocytes. In this study, we investigated whether APN regulates COX-2 expression through SphK-dependent pathway.
2. Materials and methods
2.1 Mouse model of myocardial ischemia-reperfusion
Wild-type (WT) and APN-deficient (APN-KO) mice in C57/BL6 background were used for this study. Study protocols were approved by the Institutional Animal Care and Use Committee in Boston University. Mice at the ages of 10 to 12 weeks were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). A left thoracotomy was performed, and the left anterior descending artery (LAD) was visualized under a microscope and ligated with 8-0 silk suture using snare occluder. Mice were subjected to 30 min of LAD ligation, followed by 48 h of reperfusion [8]. Mice were then sacrificed and the hearts were extracted for Western blot analysis.
2.2 Cell culture and treatment
Primary neonatal rat ventricular cardiac myocytes were isolated and cultured as previously described [16]. In brief, cardiac myocytes were treated with serum-free Dulbecco’s Modified Eagle medium (DMEM) for 24 hours prior to each experiment, and then treated with or without human recombinant APN (30 µg/ml) for 18 hours or sphingosine-1-phosphate (1.0µM) for 6 hours (BIOMOL International, L.P., PA, USA). Recombinant human APN from baculovirus-insect cell expression system was obtained from Nosan Corp. [6]. Preincubation with 10µM sphingosine kinase inhibitor (SKI) (Cayman, MI, USA), 0.01, 0.1 and 1.0µM VPC23019 (Avanti Polar Lipids, AL, USA), an antagonist of S1P receptors 1 and 3, or vehicle was performed for 1 hour prior to APN treatment.
2.3 RNA interference experiments
The siRNA directed to SphK-1 and non-targeting siRNA control were purchased from Dharmacon (siGENOME SMART Pool and ON-TARGETplus siCONTROL Non-Targeting siRNA Pool, CO, USA), and transfection with this agent was performed as previously reported [6]. Briefly, the cardiac myocytes were transfected for 24 hours with 100 nM of each siRNA by using RNAiMAX reagent (Invitrogen, CA, USA) in OptiMEM (Invitrogen, CA, USA) according to the manufacturer’s instructions, and then medium was changed to fresh serum-free DMEM. Experiments with cardiac myocytes transfected with siRNA were performed 48 hours later after siRNA transfection.
2.4 Measurements of the levels of mRNA expression
Total RNA was extracted from cardiac myocytes with RNeasy micro kit (QIAGEN, CA, USA). cDNA synthesis from total RNA was performed using a cDNA synthesis kit (QuantiTect Reverse Transcription Kit, QIAGEN, CA, USA) according to the manufacturer’s instructions. Transcript expression levels of SphK-1 and GAPDH were quantified by iCycler iQ Real-Time PCR Detection Systems (BIO-RAD, CA, USA) using SYBR Green Master Mix (Applied Biosystems, CA, USA). Transcript levels of SphK-1 were adjusted relative to the expression of GAPDH. The sequence of PCR primer are as follows; 5’-GCAAGGCTCTGAAGCTCTTT-3’ and 5’-CCCAGTACCCAGTTCTTCT-3’ for SphK-1 and 5’-TCAAGAAGGTGGTGAAGCAG- 3’ and 5’-AGGTGGAAGAATGGGAGTTG -3’ for GAPDH.
2.5 Western blotting
Protein was extracted from cardiac myocytes with cell lysis buffer (Cell Signaling, MA, USA). The method of protein extraction from mice hearts was previously described in detail [17]. Whole cell lysate were resolved on SDS polyacrylamide gels (BIO-RAD, CA, USA), followed by electrophoretic transfer PVDF membranes (Hybond™-ECL, Amersham Biosciences, NJ, USA). Immunoreactive bands were visualized using ECL or ECL-PLUS chemiluminescence reagent (Amersham Biosciences, NJ, USA). The membranes were exposed to COX-2 antibody (Cayman, MI, USA) and α-tubulin antibody (Calbiochem, CA, USA), and then exposed to anti-rabbit or anti-mouse secondary antibodies conjugated with horseradish peroxidase, respectively. The band density was quantified by using Image J 1.38X (http://rsb.info.nih.gov/ij/). All of them were normalized to α-tubulin.
2.6 Statistical analysis
Data are presented as mean ± SEM. Statistical analysis was performed by a 2-tailed Student’s t test or ANOVA analysis. A value of P < 0.05 was accepted as statistically significant.
3. Results
3.1 APN signaling is required for full COX-2 induction following acute ischemic injury in the heart
WT and APN-KO mice underwent myocardial ischemia-reperfusion injury or sham surgery, and COX-2 protein levels in the heart were assessed. Little or no expression of COX-2 protein could be detected by Western blotting in hearts of WT and APN-KO mice that had undergone sham surgery. Ischemia-reperfusion markedly increased the expression of COX-2 in WT mice hearts, but this upregulation was attenuated in the APN-KO hearts (Fig. 1A).
Fig. 1.
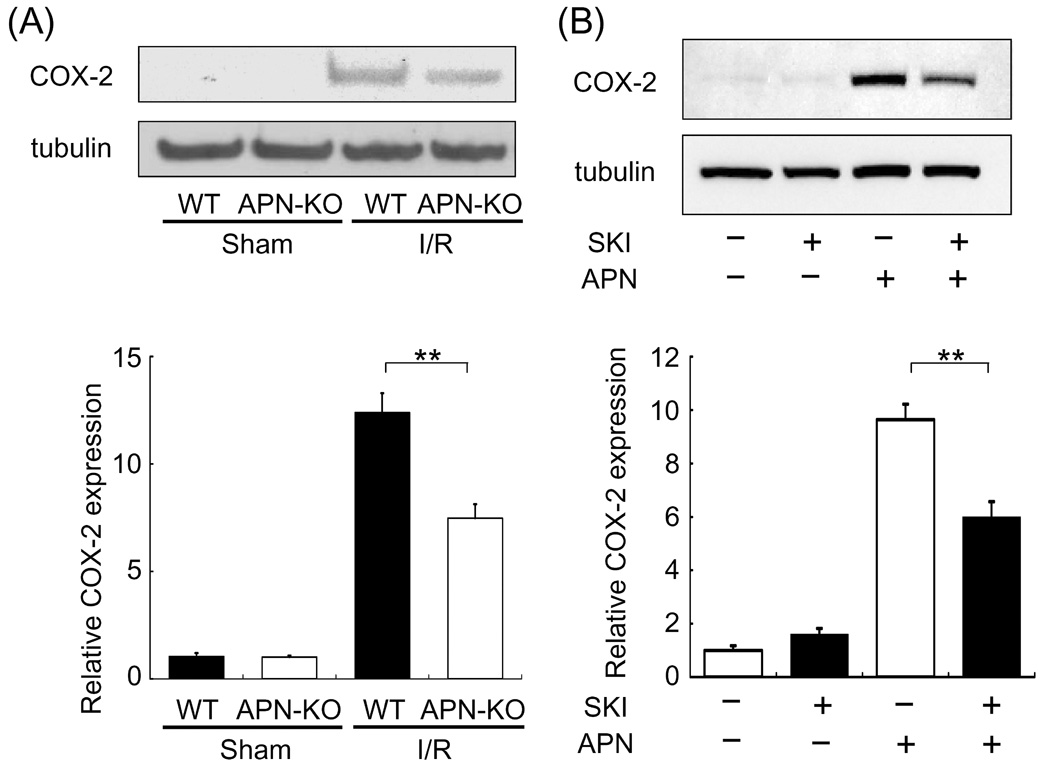
APN regulation of COX-2 in the heart and cultured cardiac myocytes. (A) APN is required for full induction of COX-2 by ischemia-reperfusion in heart. The expression of COX-2 protein in heart tissues from WT and APN-KO mice at 48 h after sham operation or ischemia-reperfusion was analyzed by Western blotting. The expression levels of COX-2 were quantified and expressed relative to sham-operated WT mice. Values are expressed as means ± SEM. **P<0.01, n=4 in each group. (B) SphK-1 inhibition reduces COX-2 induction by APN in cardiac myocytes. Cardiac myocytes were pretreated with 10 µM SKI for 1 hour prior to APN treatment. Cells were then treated with APN (30 µg/ml) or vehicle for 18 hours. Cell extracts were analyzed in Western blotting. Values are expressed relative to the vehicle-treated cultures and expressed as means ± SEM.. *P<0.01, n=8 in each group.
3.2 APN-induced COX-2 upregulation is reduced by SphK-1 inhibition
To elucidate the mechanism of COX-2 regulation by APN, cultured cardiac myocytes were treated with recombinant human APN. APN significantly increased COX-2 expression in neonatal rat cardiac myocytes [8] (Fig. 1B). To test whether APN augments COX-2 expression through a SphK-1-dependent pathway, cardiac myocytes were pretreated with a SphK-1 inhibitor (SKI) followed by incubation with APN or vehicle. While no differences in COX-2 expression were observed between the presence or absence of SKI under basal conditions, APN-induced COX-2 upregulation was diminished by 38% by preincubation with SKI (Fig.2). To corroborate these findings, knockdown experiments were performed using siRNA against SphK-1. SphK-1 mRNA levels were reduced by 68% by treatment with siRNA for SphK-1 compared to control RNA (Fig. 2A). Knockdown of SphK-1 suppressed APN-stimulated COX-2 expression by 43% compared to control RNA (Fig. 2B).
Fig. 2.
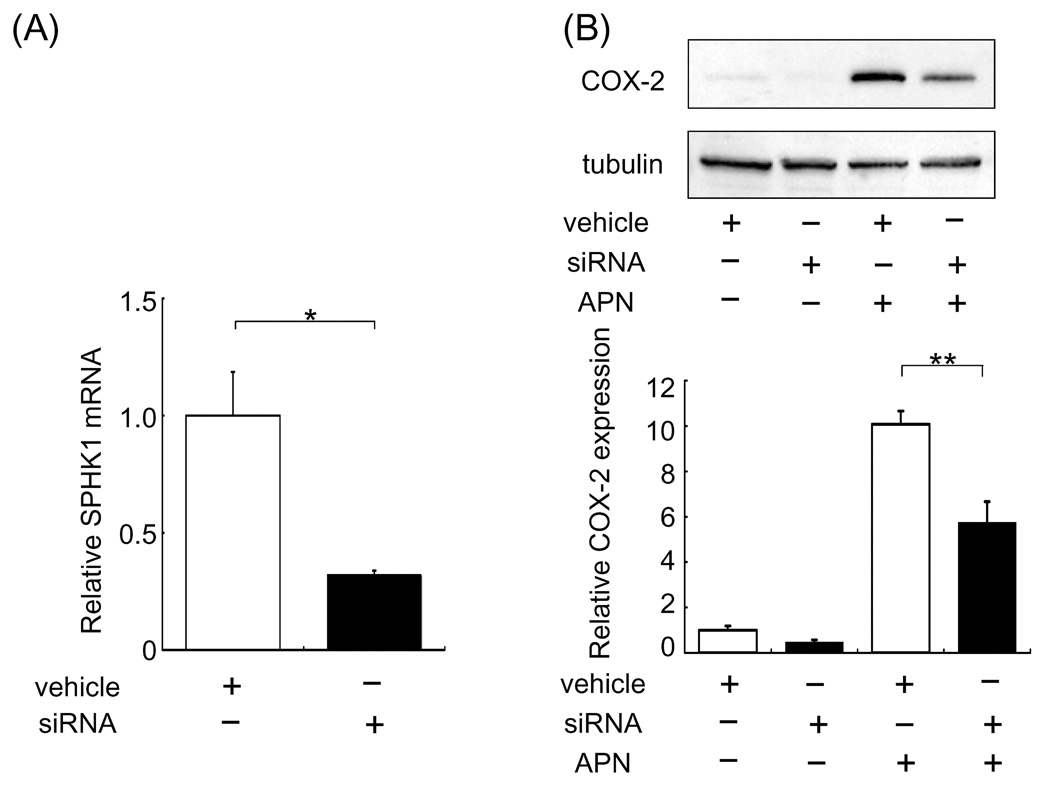
Knockdown of SphK-1 reduces COX-2 induction by APN in cardiac myocytes. Cardiomyocytes were transfected with 100nM siRNA of SphK-1. (A) SphK-1 mRNA levels were reduced following treatment with siRNA directed toward SphK-1. (B) Treatment with siRNA against SphK-1 diminished APN-induced COX-2 upregulation on cardiac myocytes. Forty-eight hours after siRNA transfection, cardiac cells were treated with APN (30 µg/ml) or vehicle for 18 hours. Values are expressed relative to vehicle-treated cultures and reported as means ± SEM. *P< 0.05, **P<0.01, (A) n=4 in each group, (B) n=6 in each group.
3.3 APN-induced COX-2 expression is diminished by S1P receptor antagonist
S1P acts as a ligand for S1P receptors, a family of specific-G-protein-coupled receptors [18], and S1P receptors are expressed by cardiomyocytes [19]. Indeed, S1P upregulated COX-2 expression in cardiac myocytes, and S1P-induced COX-2 expression was reduced in a dose-dependent manner by the addition of VPC23019, which antagonizes S1P receptor 1 and 3 (Fig. 3A). Thus, we assessed whether APN-induced COX-2 upregulation is mediated by S1P receptors in cardiac myocytes using VPC23019. Pretreatment with 0.1 and 1.0µM, but not 0.01µM, VPC23019 diminished APN-induced COX-2 expression (Fig. 3B).
Fig. 3.
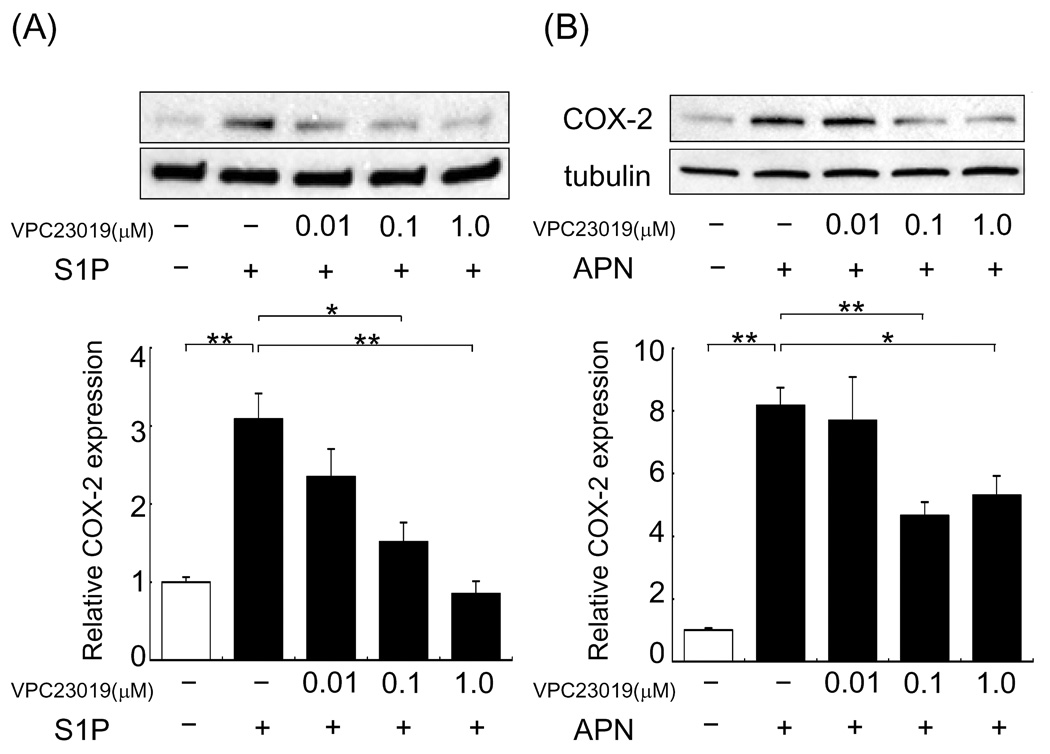
S1P receptor inhibition reduces COX-2 upregulation by APN or S1P in cardiac myocytes. (A) S1P receptor-inhibition reduces COX-2 upregulation by S1P. Cardiac myocytes were pretreated with VPC23019 or vehicle for 1 hour prior to incubation with S1P or vehicle for 6 hours. Values are expressed relative to vehicle-treated cultures and reported as means ± SEM. *P<0.05, **P<0.01 (A) n=3 in each group. (B) S1P receptor inhibition reduces COX-2 upregulation by APN in cardiac myocytes. Cardiac myocytes were pretreated with 0.01, 0.1 and 1.0µM VPC23019 or vehicle for 1 hour prior to incubation with APN (30 µg/ml) or vehicle for 18 hours. Values are expressed relative to vehicle-treated cultures and reported as means ± SEM. *P<0.05, **P<0.01, n= 3–8 in each group.
4. Discussion
The present study demonstrates the existence of an APN – COX-2 regulatory axis in vivo. Previously we reported that the cardioprotective actions of APN overexpression could be partially blocked by a COX-2 inhibitor [8]. To extend these observations, we tested whether adiponectin-deficiency would reduce the induction of cardiac COX-2 by ischemia-reperfusion injury in vivo. APN-KO hearts showed reduced COX-2 induction by ischemia-reperfusion compared to WT hearts, indicating APN is necessary for full COX-2 expression in the heart. These findings are of importance because they demonstrate that endogenous adiponectin participates in the myocardial COX-2 response to injury.
This study also elucidates aspects of the mechanism by which APN regulates COX-2 in neonatal rat cardiac myocytes. APN-induced COX-2 expression was considerably suppressed by incubation with SKI or siRNA of SphK-1. A S1P receptor antagonist also repressed COX-2 upregulation by APN. These data suggest that APN increases COX-2 expression through a SphK-1-S1P receptor pathway. S1P is a sphingolipid metabolite that regulates diverse biological processes in a variety of cell types [20]. Whereas S1P levels are controlled by activities of SphK-1 and SphK-2, SphK-1 is the dominant isoform in heart tissue [21]. Deficiency of SphK-1 has been shown to exaggerate cardiac cell apoptosis in oxidative stress [22] or hypoxia and glucose deprivation [23].
In contrast to these protective actions of SphK-1, Kase and colleagues have recently reported that globular APN promotes an inflammatory state in endothelial cells through SphK-1- dependent NF-κB activation [24]. In contrast, others have reported that APN suppresses TNF-α induced NF-κB activation and subsequent endothelial adhesion molecules in endothelial cells [25], and it is well established that adiponectin has anti-inflammatory and anti-atherogenic actions [2, 6].
S1P functions both as an intracellular second messenger and an extracellular ligand of a family of five S1P receptors [26]. S1P receptor 1, 2 and 3 are expressed in adult mammalian cardiomyocytes [27, 28]. It has been reported that VPC23019, a S1P receptor 1 and 3 antagonist, reduces the pro-survival effects of SphK-1 in cardiac cells [23]. It has also been shown that S1P exerts protective effects on cardiomyocytes survival during hypoxia through phosphatidylinositol 3-kinase-Akt signaling via S1P receptor 1 [23, 28]. In this study we showed that VPC23019 inhibited both S1P-induced and APN-induced COX-2 upregulation in cardiac myocytes. Thus, APN may stimulate S1P receptors via S1P production, in an autocrine or paracrine manner, resulting in stimulation of COX-2 expression.
In summary, it is shown that APN-induced COX-2 expression in the heart is partially dependent on SphK-1. Because COX-2 is cardioprotective, the ability of APN to stimulate SphK-1-COX-2 signaling in cardiac myocytes may contribute to its ability to protect the heart from injury.
Acknowledgements
This work was supported by NIH National Heart, Lung, and Blood Institute grant NO1-HV- 28178 and by other grants from the NIH (HL77774, HL86785, and HL81587, AG15052) to Kenneth Walsh. Yasumasa Ikeda was supported by Japan Health Sciences Foundation. Koji Ohashi was supported by Manpei Suzuki Diabetes Foundation. Noriyuki Ouchi was supported by an American Heart Association, Northeast Affiliate, Scientist Development Grant.
Abbreviations
- APN
Adiponectin
- SKI
sphingosine kinase inhibitor
- APN-KO
APN-deficient
- WT
wild-type
- SphK-1
sphingosine kinase-1
- S1P
sphingosine-1-phosphate
- COX-2
cyclooxygenase-2
- AMPK
AMP-activated protein kinase
- TNF-α
tumor necrosis factor-α
- PG
prostaglandin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 3.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–1116. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 6.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci W, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J. Molec. Cell. Cardiol. 2007 doi: 10.1016/j.yjmcc.2007.03.808. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. Jama. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 11.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc. Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine-1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine-1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nodai A, Machida T, Izumi S, Hamaya Y, Kohno T, Igarashi Y, Iizuka K, Minami M, Hirafuji M. Sphingosine-1-phosphate induces cyclooxygenase-2 via Ca2+- dependent, but MAPK-independent mechanism in rat vascular smooth muscle cells. Life Sci. 2007;80:1768–1776. doi: 10.1016/j.lfs.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel DR, Adachi T, Ido Y, Heibeck T, Jiang B, Lee Y, Melendez JA, Cohen RA, Colucci WS. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J. Mol. Cell. Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J. Biol. Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine-1-phosphate can regulate cellular processes by divergent pathways. Biochem. Soc. Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 19.Bunemann M, Brandts B, zu Heringdorf DM, van Koppen CJ, Jakobs KH, Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1- phosphate. J. Physiol. 1995;489(Pt 3):701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalfant CE, Spiegel S. Sphingosine-1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J. Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda Y, Kihara A, Igarashi Y. Distribution of sphingosine kinase activity in mouse tissues: contribution of SPHK1. Biochem. Biophys. Res. Commun. 2003;309:155–160. doi: 10.1016/s0006-291x(03)01551-1. [DOI] [PubMed] [Google Scholar]
- 22.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ. Res. 2007;100:41–49. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 23.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc. Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Kase H, Hattori Y, Jojima T, Okayasu T, Tomizawa A, Suzuki K, Banba N, Monden T, Satoh H, Akimoto K, Kasai K. Globular adiponectin induces adhesion molecule expression through the sphingosine kinase pathway in vascular endothelial cells. Life Sci. 2007;81:939–943. doi: 10.1016/j.lfs.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 27.Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac S1P receptors. J. Histochem. Cytochem. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine-1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]


