Abstract
During the last decade, the intense study of adult hippocampal neurogenesis has led to several new lines of inquiry in the field of psychiatry. Although it is generally believed that adult mammalian neurogenesis is restricted to the hippocampus and olfactory bulb, a growing number of studies have described new neurons in the adult neocortex in both rodents and non-human primates. Interestingly, all of the new neurons observed in these studies have features of interneurons rather than pyramidal cells, the largest neuronal population of the neocortex. In this review, we discuss features of these interneurons that could help to explain why cortical neurogenesis has been so difficult to detect. In addition, these features suggest ways that production of even a small numbers of new neurons in the adult cortex could make a significant impact on neocortical function.
It is widely accepted that adult neurogenesis occurs in two mammalian brain regions: the dentate gyrus and olfactory bulb. Interestingly, the earliest reports of adult neurogenesis in these two regions also described new neurons in the adult neocortex (1; 2). These early neocortical neurogenesis findings have been replicated in recent studies (3–7), but the existence of adult neurogenesis in the neocortex remains controversial, largely due to the existence of negative reports. In non-human primates, new neurons have been reported in prefrontal, inferior temporal, and posterior parietal cortex (3; 6; 7), though other groups have found no new neurons in the neocortex of adult primates, including humans (8–11). In adult rodents, studies have reported finding new neurons in the anterior neocortex (5; 7; 12), but others found no new neocortical neurons in enriched, electroconvulsive seizure-treated, or control conditions (13; 14). Several additional studies have reported finding new neurons in the neocortex only after ischemia or targeted neuronal death (15–18). These contradictory findings are difficult to reconcile, because all of the studies of adult neurogenesis in the neocortex carried out within the past ten years have used essentially the same methods: injection and immunohistochemical detection of the S-phase marker bromodeoxyuridine (BrdU) to mark newly-born cells combined with immunohistochemistry for neuronal markers to determine whether the new cells are neurons. Virtually all positive and negative studies have used the neuron-specific marker NeuN (Neuronal Nuclei) to identify BrdU-labeled cells as mature neurons, and BrdU-labeled neurons in the adult rat neocortex have recently been characterized with several additional neuronal markers as well (5). The remainder of this review will describe features of new cortical neurons suggesting that they are interneurons rather than pyramidal neurons; discuss ways in which these features could explain why new neurons have been particularly difficult to detect; and suggest that their interneuron identity means that the birth of even small numbers of new neurons could have functional effects on the adult neocortex.
New Neurons in the Adult Brain Are Small and Sparsely Distributed
The number of cortical interneurons born during adulthood appears to be exceedingly small: only 1–2 cells per mm3 coexpressing BrdU and NeuN have been observed in the adult neocortex in macaques (7) and rats (5). In the macaque, this low density of new neurons represented 25% of all BrdU-labeled cells, while in the rat, only 33 strongly NeuN-expressing neurons were found among more than 7000 BrdU-labeled cells in the neocortex (5; 7). This disparity between density and proportion of BrdU-labeled cells suggests that the rate of neurogenesis is very similar in the two species but that adult rats have many more non-neuronal cells born and remaining labeled in the neocortex than adult monkeys. These low numbers suggest that new neurons are difficult to find in macaques and possibly even more difficult to detect in rodents because of the added “noise” of the large non-neuronal BrdU-labeled cell population. Two studies that found no adult neurogenesis in the neocortex examined only 40–50 BrdU-labeled cells (13; 19), suggesting that low sampling may indeed impede detection of new neurons in the rodent neocortex. In contrast, similar sampling methods are commonly used in the adult dentate gyrus, where a large proportion of BrdU-labeled cells in the granule cell layer are NeuN-expressing young granule neurons.
All of the new neurons observed in the adult neocortex have been relatively small, which may add to the difficulty in detection. Dayer et al. (5) found that all cells double-labeled with BrdU and neuronal markers had nuclear diameters between 5 and 10 μm. Cell size was not measured in other studies of neurogenesis in the adult neocortex, but photomicrographs (Figure 1) show similarly small new neurons in studies using different detection methods and in different species (2; 6; 7). These new neurons are outside the size range of neocortical pyramidal neurons, which typically have somata measuring 15–20 μm in diameter. They are much more similar in size to glial cells, potentially making identification more difficult. Interestingly, the neurons born in the hippocampus and olfactory bulb are also among the smallest in the brain. Dentate gyrus granule cell bodies measure approximately 10 μm in diameter, while olfactory bulb granule cells and periglomerular cell bodies are even smaller, approximately 7 μm (20; 21). In other controversial sites of adult neurogenesis, including the striatum (5; 22–24), hypothalamus (25), and spinal cord (26), new neurons also appear quite small, with cells in published examples having nuclear diameters between 5 and 10 μm. This size similarity found by different groups in multiple regions of the adult brain suggest that all neurons born in adulthood may be small, possibly reflecting a common lineage and/or constraints on growth of neurons born into a pre-existing structure.
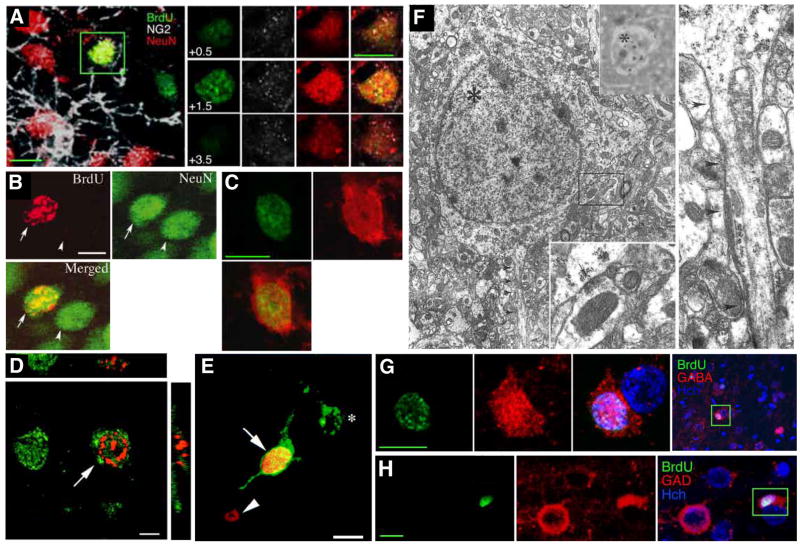
Figure 1.
New Neurons in the Adult Neocortex. (A) 4–5 week old neuron in the adult rat anterior neocortex labeled with BrdU (green) and the neuronal marker NeuN (red). The right panel shows colocalization of BrdU and NeuN in several confocal planes. (B) 4-week-old neuron in the adult mouse motor cortex after selective killing of mature motor neurons. (C) 4–5 week old neuron in the adult rat anterior neocortex labeled with BrdU (green) and the neuronal marker neuron-specific enolase (red). (D) 2-week-old neuron in the macaque prefrontal cortex labeled with BrdU (red) and NeuN (green). (E) A neuron in the macaque prefrontal cortex labeled with BrdU (red) and retrogradely labeled with Fluoro-Emerald (green), demonstrating the presence of an axon. (F) Electron micrographs of a 4-week-old 3H-thymidine-labeled neuron in the rat visual cortex. Top inset shows the autoradiographic silver grains over the nucleus; bottom inset shows a synapse onto the cell body at higher magnification; right panel shows the beginning of the axon (arrowheads) at higher magnification. (G, H) New neurons in the rat anterior cortex staining for BrdU (green) and GABA (G) or the GABA synthesizing enzyme GAD-67 (H), indicating that these are GABAergic interneurons. Scale bar, 5 μm (D), 10 μm (A, B, C, G, H), 20 μm (E). Photographs reprinted from Dayer et al., 2005 (A, C, G, H), Chen et al., 2004 (B), Gould et al., 2001 (D), Gould et al., 1999 (E), Kaplan, 1981 (F).
New Neurons in the Adult Brain Are Interneurons
Positive immunostaining of BrdU-labeled cells with GABA, GAD-67, calbindin, and calretinin suggests that most, if not all, of the new neurons in the adult neocortex are interneurons (5). The restriction of adult cortical neurogenesis to interneurons is perhaps not surprising, because adult neurogenesis in the dentate gyrus and olfactory bulb also occurs only within specific neuronal classes. In the olfactory bulb, both the granule cells and periglomerular cells are inhibitory interneurons. Interestingly, although the dentate gyrus granule cells are glutamatergic, there is strong evidence that they can be GABAergic as well (27). But because pyramidal neurons are very recognizable and make up 70–80% of neocortical neurons (28), it is easy to ignore or overlook the existence of neocortical interneurons. Using criteria or methods suitable for identifying BrdU-labeled pyramidal neurons could explain some of the difficulty in finding adult neocortical neurogenesis. One study that reported finding no adult neurogenesis in the macaque prefrontal cortex showed examples of small cells double-labeled with BrdU and NeuN (11). These cells were categorized as non-neuronal, because they were smaller than surrounding cortical neurons and had NeuN staining that was limited to the nucleus (11). Although these features clearly distinguish the cells from pyramidal neurons, both are typical of interneurons (29; 30), suggesting that new neurons actually were observed in this study.
It is generally agreed that neocortical interneurons belong to different subclasses whose developmental origins may be specified by particular transcription factors (31; 32). However, it has proven very difficult to achieve a consensus on how many subclasses exist or even which criteria (e.g., morphological, molecular, or electrophysiological) can be used to construct meaningful classes (32). Despite the difficulty in classifying interneurons, it seems likely that new neocortical neurons belong to only one or two interneuron subclasses according to any classification scheme. One well-quantified anatomical classification scheme uses a combination of dendritic morphology and calcium-binding protein expression to distinguish 23 subclasses of interneurons in the rat prefrontal cortex (33). New neurons expressing the calcium-binding proteins calbindin and calretinin have been observed in the adult rat frontal cortex, while none appear to express parvalbumin or somatostatin (5). The newborn calbindin-expressing interneurons observed by Dayer et al. (5) closely match the “class 7” calbindin-expressing cells of Gabbott et al. (33), which have somatic diameters of 8–14 μm and are found primarily in layers 5 and 6 in cingulate and prelimbic areas. The adult-born calretinin-expressing neurons (5) most closely match the “class 4” calretinin-expressing cells of Gabbott et al. (33). Interestingly, new granule cells in the adult dentate gyrus initially express calretinin before switching to calbindin expression (34), suggesting that new calretinin- and calbindin-expressing neocortical interneurons could potentially represent a single interneuron subtype at different maturational stages.
A different classification scheme for neocortical interneurons based primarily on axon morphology and electrophysiological properties distinguishes 10 different subtypes of cortical interneurons (28). The axons of the cortical neurons born in adulthood have not been examined, but based on the size of the cell bodies, the new neurons fit most closely into the neurogliaform category in this interneuron classification system. Interestingly, Gabbott et al. (33) also described their class 7 calbindin+ cells as resembling neurogliaform neurons, which have a small spider-like soma and dendritic tree on a dense, highly ramified axonal web (35; 36). The name neurogliaform was given to these neurons by Ramón y Cajal (36) because of their resemblance to glia:
“At first glance, these cells would be taken as small neuroglial cells with short radiations, but the lack of collateral appendages on the divergent dendritic processes and the undoubted presence of an axon promptly announce their nervous character” – S. Ramón y Cajal, 1911
The striking resemblance of these neurons to glia in standard staining preparations would undoubtedly hinder recognition of new neurogliaform neurons as neurons. However, establishing whether new cortical interneurons are indeed neurogliaform neurons must await further characterization. In addition to the morphological features of neurogliaform neurons described by Cajal many years ago, recent studies have begun to determine the electrophysiological features of neurogliaform neurons, providing further confirmation of their neuronal nature and describing some interesting properties of this interneuron class. Neurogliaform neurons appear to monitor the activity of several other classes of cortical interneurons through electrical coupling via gap junctions (37). Their possibly unique ability to activate G-protein-coupled GABAB receptors produces slow inhibition of pyramidal cell dendritic spines, which is likely to result in localized but long-lasting decreases in cortical activation (38; 39).
New neurons may originate from local precursors
The source of new cortical interneurons is still unknown, however there is some evidence that newborn neurons may be generated locally. It is widely accepted that the vast majority of dividing cells in the postnatal cortex are NG2-expressing cells known as oligodendrocyte precursor cells, or OPCs (5; 40; 41). During development oligodendroyctes and GABAergic interneurons arise from a common precursor (42; 43). Several recent studies have found that a fraction of NG2-expressing cells in the postnatal and adult brain express neuronal markers, including CRMP-4, βIII tubulin, PSA-NCAM and NeuN (5; 44; 45), and show electrophysiological properties of immature neurons (45; 46) – suggesting that OPCs retain the ability to generate interneurons postnatally. Interestingly, a recent study found that GFAP-expressing cells in the postnatal brain can generate neocortical interneurons, as well as oligodendrocytes and astrocytes (47). Taken together, these studies suggest that GFAP positive precursor/stem cells can generate NG2 positive multipotential progenitors that reside in the neocortex and differentiate into oligodendocytes as well as interneurons. Doublecortin, a microtubule associated protein strongly implicated in neuronal migration, has been widely used as a neuronal marker to label immature neurons in classical neurogenic regions such as the olfactory bulb and the dentate gyrus. In these two regions, immature neurons display strong levels of doublecortin immunoreactivity that have not been detected in the adult neocortex (5; 48). However, low levels of doublecortin expression have recently been observed throughout the neocortex in a subpopulation of newborn NG2 expressing cells (41). The comparatively low levels of doublecortin immunoreactivity in these newly-generated cortical cells may be related to the role of doublecortin in migration; the olfactory bulb granule neurons migrate long distances, while the new cortical neurons may be born very close to their final location.
Turnover Rate is Similar in Neocortex and Dentate Gyrus
If neurons are added to the neocortex, but in such small numbers that they are difficult to detect, does this suggest that the phenomenon is functionally irrelevant? It may be instructive to compare the number of neurons born in the adult neocortex to that in the dentate gyrus, where studies are beginning to demonstrate functional effects of adult neurogenesis (49; 50). The density of new neurons in the cortex is 100 times lower than in the dentate gyrus in macaques (7) and over 1000 times lower in rats (5; 51; 52). However, the neuronal composition and organization of the neocortex and the dentate gyrus are very different. Large numbers of granule cells are tightly packed into a highly homogeneous granule cell layer in the dentate gyrus. In contrast, interneurons are scattered throughout the large volume of the neocortex volume and make up only 15% of the neurons in this region (33), and fewer than 1% belong to the subclasses of small cortical interneurons that most closely match the neurogenic population (28; 33). Taking these differences in population size and the region volume into account, adult neurogenesis in the neocortex and dentate gyrus occur at “replacement rates” that are surprisingly similar despite the difference in density (see Table 1). This similarity in the proportional rate of adult neurogenesis in the dentate gyrus and the neocortex suggests that adult neurogenesis is more easily observed in the dentate gyrus and olfactory bulb due to properties of the neuronal population as a whole rather than differences in the neurogenetic process itself (Figure 2). In other words, new neurons are relatively easy to detect in the numerous, homogeneous, densely packed populations of the olfactory bulb and dentate gyrus, while finding turnover in small, scattered neuronal populations requires much more sensitive detection methods. One recent study used a novel technique, radioactive carbon dating, to determine the average age of neurons dissociated from adult postmortem human cortex and found no evidence for neocortical adult neurogenesis (9). This method can reportedly detect adult birth in as few as 1% of analyzed cells (9), which should be sensitive enough to detect adult neurogenesis in the dentate gyrus or to detect neurogenesis occurring at a significant rate in cortical pyramidal neurons. However, turnover of a subclass of interneurons comprising 1% of the neuronal population would not be detectable with this method even if the entire population turned over continuously.
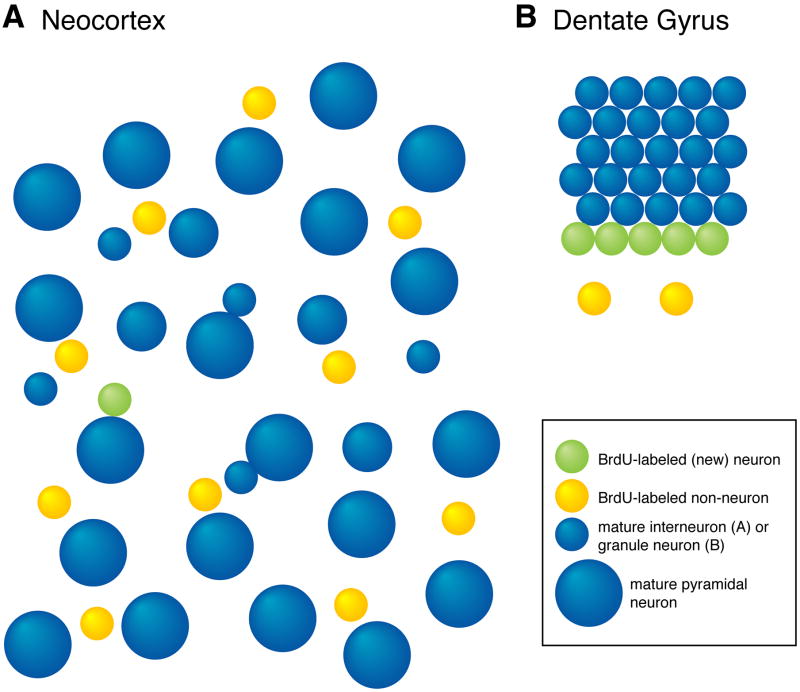
Figure 2.
Regional Differences Make New Neurons More Difficult to Find in Neocortex than in Dentate Gyrus. A. The large neocortical volume, large number of pyramidal neurons, and large number of BrdU-labeled non-neurons make new neocortical interneurons difficult to detect and recognize. B. The organization of newborn and mature granule cells in the dentate gyrus, small volume of the granule cell layer, and relatively small number of newborn non-neurons make it easier to detect the same relative number of new neurons (1 new neuron for every 5 mature neurons of the same type, in both A and B). This cartoon under-represents the differences between the two regions in at least two ways. First, the ratio of dentate gyrus new neuron density to neocortical new neuron density is 14:1 in the cartoon and greater than 1000:1 in the rodent brain (see Table 1). Second, the ratio of BrdU-labeled non-neurons to BrdU-labeled neurons is 10:1 in the cartoon (part A) and greater than 200:1 in the rodent neocortex.
Altering Small Numbers of Interneurons Could Have Functional Effects
Could the addition or replacement of neurons belonging to one or two small interneuron subclasses have a significant functional impact on information processing in the neocortex? Electrophysiological examination of hippocampal interneurons has revealed that different subclasses of interneurons provide distinct contributions to behaviorally relevant oscillations (53), suggesting that each interneuron subclass plays a unique role in shaping the firing activity of primary neurons, in part by targeting distinct regions of the primary neuron. Neurogliaform neurons target pyramidal cell dendrites and generate very long-lasting inhibitory currents that are likely to modulate overall excitability levels in localized areas of the cortex (39). Dampening of cortical activity such as that produced by neurogliaform neurons could play a role in preventing epileptiform activity. Interestingly, increased numbers of neurogliaform neurons have been reported in the deep layers of microdysgenetic cortex associated with temporal lobe epilepsy (54). The origin of these additional neurons has not been examined, but an increase in the normally-occurring production of calbindin-expressing interneurons could potentially lead to such a change, perhaps as a compensatory response to seizure activity.
Related to their ability to produce long-lasting inhibitory modulation, neurogliaform neurons appear to be the sole source of cortical inhibition mediated by GABAB, a receptor that has been implicated in depression and other psychiatric disorders. It is thus conceivable that changes in the numbers or networking of neurogliaform neurons could play a role in the changes in prefrontal cortex activation that have frequently been observed in mood disorders. Interestingly, a reduction in the density of small cells in the cortex has been observed in post-mortem brains from subjects with major depressive disorder and bipolar disorder (55). The affected cells in these mood disorder studies were identified as glial cells based on their appearance in Nissl stained sections as small round nuclei with scant perinuclear cytoplasm. However, these features are also characteristic of small interneurons, suggesting that the cell loss could possibly reflect decreased neurogenesis instead of, or in addition to, loss of glia. The loss of small cells in these human studies was observed in the subgenual, orbito-frontal, anterior cingulate, and dorsolateral prefrontal cortex. There is some evidence suggesting that neurogenesis may also be most pronounced in association areas. In non-human primates, new neurons have been observed in three cortical association areas (prefrontal, inferior temporal, and posterior parietal cortex), but not in the primary sensory striate cortex (6); in rodents new neurons have been found in cingulate cortex, somatosensory cortex, and secondary motor cortex, but not in primary motor cortex (5). These regional differences suggest that neurogenesis may have a function specific to association areas, and could also potentially explain the absence of constitutive adult neurogenesis in studies that focus on a particular region of the neocortex that may not be neurogenic (e.g., 15).
Further connections between adult neocortical neurogenesis and psychiatric diseases are suggested by studies of stress and therapeutic treatments. Social stress, chronic unpredictable stress, and corticosteroid administration all inhibit proliferation of cortical NG2-expressing cells (56–58), the likely source of new neurons in the adult neocortex. Conversely, electroconvulsive seizure treatment increases neocortical cell proliferation (19), and chronic administration of the anti-depressant fluoxetine prevents or reverses the stress-induced inhibition of cell division in the adult neocortex (56; 58). A recent study found that systemic administration of valproic acid (VPA), a classical mood stabilizer and a potent histone deacetylase inhibitor, increased the fraction of transplanted NG2 progenitors differentiating into neurons (41), presenting the possibility that therapeutic VPA administration could increase cortical neurogenesis by shifting endogenous NG2-expressing progenitors towards a neuronal fate. Although these parallels between clinical findings and adult neocortical neurogenesis are intriguing, any causative relationships remain highly speculative. Nevertheless, given all of the data supporting the existence of adult neurogenesis in rodents and non-human primates, and the features of new neurons that could potentially explain negative findings, it seems premature at this point to close the door on the search for adult neurogenesis in the human neocortex. On the contrary, the intriguing picture that is just beginning to emerge should encourage more researchers to look for evidence of new neocortical neurons and for clues to their specific identity and function.
Table I.
Comparison of Replacement Rates for Neurogenic Populations in Cortex and Dentate Gyrus1
| CB + CR small interneurons in Neocortex2 | Granule Cells in Dentate Gyrus | Notes/references | |
|---|---|---|---|
| (A) Number of Neurons (all cortical neurons or DG granule neurons) | 21,000,000 | 2,170,000 | (Korbo et al., 1990; West and Andersen, 1980) |
| (B) % of Neurons in A belonging to Neurogenic Class | 0.56% | 100% | (Gabbott et al., 1997)3 |
| (C) Number of Neurons in Neurogenic Class | 117,600 cells | 2,170,000 cells | = A*B |
| (D) Volume (Cortical Layer 5/6 or DG Granule Cell Layer) | 152 mm3 | 2.27 mm3 | (Korbo et al., 1990; Gabbott et al., 1997; West and Andersen, 1980)4 |
| (E) Density of Neurogenic Class | 774 cells/ mm3 | 956,000 cells/ mm3 | = C/D |
| (F) Density Ratio of Two Classes | 1 : | 1240 | = E /Edentate gyrus |
| (G) BrdU+ Neuron Density in DG | 850 cells/ mm3 | (Olariu et al., 2005; Dayer et al., 2003)5 | |
| (H) Expected Density for Replacement Rate Equivalent to DG | 0.69 cells/ mm3 | = G/F | |
| (I) Observed Density | 0.78 cells/ mm3 | (Dayer et al., 2005)6 |
The overall neuron density for two neurogenic populations, small calbindin- and calretinin-expressing interneurons in the cortex and granule cells in the dentate gyrus, are calculated from published estimates of neuron numbers and region volumes (rows A–E). The total neuron density in the two populations is compared (F). Finally, the density of new cortical neurons that would produce a replacement ratio equivalent to that of dentate gyrus granule neurons is calculated (H) using the observed density of new neurons in the granule cell layer (G) and compared to the observed density of new cortical neurons (I).
CB, calbindin-expressing; CR, calretinin-expressing
For cortex, % of CB class 7 and CR class 4 interneurons is calculated from % of neurons CB+ and CR+, % of CB+ and CR+ neurons in deep layers, and numbers of CB+ and CR+ classes in deep layers.
For cortex, volume calculated from total volume of 253 mm3 and 60% of cortical depth included in layers 5/6.
After a single BrdU injection, 1072 BrdU+ cells/mm3 x 79% of BrdU+ cells that are NeuN+
3.1 BrdU+/NeuN+ cells/ mm3 in cortex after four BrdU injections, divided by four to estimate the density after one injection.
Table modified from Dayer et al., 2005
Acknowledgments
HAC is supported by the Intramural Program of the NIH, NIMH (Z01-MH002784). AGD is supported by the Swiss National Foundation (Grant 3100A0-116496)
Footnotes
Financial Disclosures: HAC and AGD report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman J. Are new neurons formed in the brains of adult mammals ? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MS. Neurogenesis in the 3-month-old rat visual cortex. J Comp Neurol. 1981;195:323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 3.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Runyan CA, Shannon Weickert C, Saunders RC. Adult neurogenesis and immediate early gene response to working memory stimulation in the primate prefrontal cortex. Program No. 318.10. Abstract Viewer/Itinerary Planner: Society for Neuroscience 2006 [Google Scholar]
- 5.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 7.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 9.Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 11.Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Ehninger D, Kempermann G. Regional Effects of Wheel Running and Environmental Enrichment on Cell Genesis and Microglia Proliferation in the Adult Murine Neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 14.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- 18.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 19.Madsen TM, Yeh DD, Valentine GW, Duman RS. Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology. 2005;30:27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- 20.Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5. New York: Oxford University Press; 2004. pp. 455–498. [Google Scholar]
- 21.Shepherd GM, Chen WR, Greer CA. Olfactory Bulb. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5. New York: Oxford University Press; 2004. pp. 165–216. [Google Scholar]
- 22.Bedard A, Cossette M, Levesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- 23.Luzzati F, De Marchis S, Fasolo A, Peretto P. Neurogenesis in the caudate nucleus of the adult rabbit. J Neurosci. 2006;26:609–621. doi: 10.1523/JNEUROSCI.4371-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- 25.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 26.Shechter R, Ziv Y, Schwartz M. New GABAergic Interneurons Supported by Myelin-specific T Cells are Formed in Intact Adult Spinal Cord. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0705. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez R. The GABAergic phenotype of the “glutamatergic” granule cells of the dentate gyrus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 29.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 30.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 32.Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–527. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(sici)1096-9861(19970127)377:4<465::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 35.Jones EG. Neurogliaform or Spiderweb Cells. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. New York: Plenum Press; 1984. pp. 409–418. [Google Scholar]
- 36.Ramón y Cajal S. Histology of the nervous system of man and vertebrates. New York: Oxford University Press; 1995. [Google Scholar]
- 37.Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabadics J, Tamas G, Soltesz I. Spill-over of GABA after single action potential of neurogliaform cells results in slow postsynaptic GABAA responses. Program 318.10. Abstract Viewer/Itinerary Planner: Society for Neuroscience 2006 [Google Scholar]
- 39.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 40.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 41.Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 42.He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 Control Neuronal versus Oligodendroglial Cell Fate Acquisition in the Developing Forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 49.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 50.Cameron HA, Christie BR. Do new neurons have a functional role in the adult hippocampus? Debates in Neuroscience in press. [Google Scholar]
- 51.Olariu A, Cleaver KM, Shore LE, Brewer MD, Cameron HA. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–762. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- 52.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 53.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thom M, Holton JL, D'Arrigo C, Griffin B, Beckett A, Sisodiya S, et al. Microdysgenesis with abnormal cortical myelinated fibres in temporal lobe epilepsy: a histopathological study with calbindin D-28-K immunohistochemistry. Neuropathol Appl Neurobiol. 2000;26:251–257. doi: 10.1046/j.1365-2990.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 55.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 56.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic Unpredictable Stress Decreases Cell Proliferation in the Cerebral Cortex of the Adult Rat. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 57.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 58.Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic Social Stress Inhibits Cell Proliferation in the Adult Medial Prefrontal Cortex: Hemispheric Asymmetry and Reversal by Fluoxetine Treatment. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]