Abstract
VprR77Q has been associated with long-term nonprogressive (LTNP) HIV infection. We wished to investigate the prevalence, clinical correlates, and effect on treatment response of VprR77Q in a cohort of antiretroviral-naïve individuals initiating Highly Active Antiretroviral Therapy (HAART). Baseline plasma samples from 728 subjects were genotyped using RT-PCR and direct DNA sequencing. Cox proportional hazards regression was used to model the effects of VprR77Q on virologic and immunologic responses, and survival following initiation of HAART, over a median 4.5 years follow-up. We found that 308 subjects (42.3%) harbored VprR77Q alone or in combination with another amino acid, while 420 (57.7%) harbored an amino acid other than Q. A cross-sectional analysis found no correlation between R77Q and baseline plasma viral load (pVL), CD4 count, diagnosis of AIDS, or sociodemographic characteristics including age, gender and history of injection drug use (p > 0.1). In multivariate analyses, no significant associations between VprR77Q and initial pVL and CD4 responses to HAART (p > 0.1) or survival following initiation of treatment were observed. The high prevalence and the lack of association with pre-therapy clinical parameters in this cohort argue against an association of R77Q with LTNP status. These results do not support an association between R77Q and HAART response.
Keywords: HIV, Vpr, R77Q, antiretroviral therapy response, HOMER cohort
Introduction
Viral protein R (Vpr) is a highly conserved, 96-kDa accessory protein involved in the pathogenesis of HIV-1. Although its role in HIV-1 infection remains somewhat unclear, Vpr has been shown to affect multiple functions in vitro, including increasing the fidelity of the reverse transcription process, targeting the preintegration complex to the host cell nucleus, inducing host cell cycle arrest at the G2/M phase, and inducing apoptosis in infected as well as uninfected cells (for a review see [1]).
The apoptosis-inducing function of Vpr maps to a tightly-conserved H(S/F)RIG amino acid motif at its C-terminal end [2,3]. Earlier studies indicated that HIV gene defects mapping to the C-terminus of HIV Vpr are associated with long-term nonprogressive infection [4], although other studies have reported no obvious correlation between Vpr genetics and rate of HIV disease progression [5]. Recently, a study by Lum et al. [6] indicated that an R77Q substitution, located within the C-terminal H(S/F)RIG amino acid motif, appeared to significantly impair the ability of Vpr to induce apoptosis in vitro [6]. Furthermore, this R77Q mutation was associated with long-term nonprogressive HIV-infection [6].
In the present study we wished to investigate the prevalence, clinical correlates, and effect on treatment response of VprR77Q in a large population-based cohort of antiretroviral-naïve individuals initiating their first Highly Active Antiretroviral Therapy (HAART) regimen.
Study subjects: the HOMER cohort
In the province of British Columbia, Canada, antiretrovirals are distributed free of charge to HIV-infected individuals through a centralized drug treatment program, in accordance with provincial and international HIV treatment guidelines [7]. The present study focuses on the HAART Observational Medical Evaluation and Research (HOMER) cohort, which includes all HIV-positive, antiretroviral-naïve adults who initiated triple antiretroviral therapy (consisting of two nucleoside reverse transcriptase inhibitors [nRTIs] and either a protease inhibitor [PI] or a non-nucleoside reverse transcriptase inhibitor [NNRTI]) through the British Columbia Drug Treatment Program between Aug. 1996 and Sept. 1999 (N = 1191). This cohort has been the focus of a number of studies and has been described in detail previously [7,8,9]. Permission to conduct this research was granted by the institutional Research Ethics Board (Providence Health Care/University of British Columbia).
Baseline clinical correlates of HIV VprR77Q in this antiretroviral-naïve population
The present study focused on 728 of 1191 (61.1%) HOMER cohort subjects for whom a baseline (pre-therapy) sample, collected ≤ 6 months prior to initiation of therapy, was available. Analysis of the baseline sociodemographic and clinical characteristics of cohort subjects revealed that the subset of subjects for whom Vpr genotype data were available (N = 728 of 1191) in general represented the cohort participants, with the exception that those with Vpr genotypes had slightly higher baseline pVL and were more likely to initiate NNRTI-containing HAART than those without Vpr genotypes (Table 1). Vpr genotyping was performed by extracting viral RNA from plasma using guanidinium isothiocyanate, followed by nested RT-PCR amplification of a portion of the HIV-1 genome spanning vif, vpr and vpu, using first round primers 5′-ATCTTAAGACAGCAGTACAA-3′ (bases 4741–4760 of HIV-1 HXB2 Genbank Acc# K03455) and 5′-TCTACCATGTTATTTTTCCACAT-3′ (6529–6507) and second round primers 5′-TCCTCTGGAAAGGTGAAGGGG-3′ (4951–4971) and 5′-GGTCTGTGGGTACACAGGCATGT-3′ (6459–6437). If no product was obtained, a shorter 2-round RT-PCR protocol targeting only the C-terminus of Vpr was used. PCR products were sequenced in both 5′ and 3′ directions using the BigDye dye terminator cycle sequencing kit (Applied Biosystems) and run on an ABI 3700 automated sequencer. Sequences from full-length PCR products have been deposited into GenBank (Accession #s DQ203856 to DQ204405). The same baseline plasma samples were used to determine baseline HIV co-receptor usage, using the Virologic Phenosense assay, as described previously [9]. The Phenosense assay classifies isolates as R5, X4 or R5/X4 (indicating dual and/or mixed-tropic virus).
Table 1. Cross-sectional analysis of the association of Vpr codon 77 genotype with baseline (pre-therapy) parameters.
Associations between baseline VprR77Q mutation and baseline parameters including age, gender, baseline AIDS diagnosis, history of injection drug use, HIV co-receptor usage (R5 vs. R5/X4) [9], plasma viral load (pVL) and CD4 count were determined using the Chisquared test (for dichotomous variables) or the Wilcoxon Rank-sum test (for continuous variables).
| Stratified by availability of Vpr genotype data | Stratified by Vpr genotype (N = 728 successfully genotyped samples) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Total study group (N = 1191) | Vpr genotype data not available (N = 463) | Vpr Genotyping successful (N = 728) | p | Vpr R77 non-Q(n = 420) | Vpr R77Q (n = 308) | p |
| Male Gender (%) | 1004 (84.3%) | 390 (84.2%) | 614 (84.3%) | 0.960 | 350 (83.3%) | 264 (85.7%) | 0.382 |
| Median Age, years [IQR] | 37 [32–44] | 36.6 [31.5–43.4] | 37.2 [32.2–43.7] | 0.278 | 36.9 [32.2–43.4] | 37.8 [32.2–44.1] | 0.602 |
| Median HIV pVL (log10 copies/ml) [IQR] | 5.08 [4.60–5.49] | 5.00 [4.46–5.45] | 5.11 [4.69–5.52] | 0.003 | 5.11 [4.65–5.53] | 5.13 [4.78–5.51] | 0.214 |
| Median CD4 count (cells/mm3) [IQR] | 280 [130–420] | 290 [120–440] | 270 [130–415] | 0.216 | 260 [130–400] | 280 [130–420] | 0.827 |
| Baseline AIDS diagnosis (%) | 154 (12.9%) | 60 (13.0%) | 95 (13.1%) | 0.964 | 56 (13.3%) | 39 (12.7%) | 0.791 |
| NNRTI-containing therapy (%) | 316 (25.7%) | 103 (22.3%) | 203 (27.9%) | 0.030 | 117 (27.9%) | 86 (27.9%) | 0.984 |
| History of injection drug use (%) | 321 (27.0%) | 130 (28.1%) | 205 (28.2%) | 0.976 | 120 (28.6%) | 85 (27.6%) | 0.773 |
[IQR]: Interquartile Range
Of the 728 genotyped subjects, 308 (42.3%) harbored the VprR77Q alone (n = 276) or as a mixed viral population with another amino acid (R/Q, n = 19; H/Q, n = 9; other, n = 4). The remaining 420 subjects (57.7%) harbored the wild-type Vpr77R amino acid alone (n = 364), as a mixture with other non-Q amino acids (n = 4), or harbored a non- R-non-Q amino acid (R77H, n = 45; other amino acid, n = 7). For the present study, we divided the cohort into subjects harboring the VprR77Q (pure or mixed virus population, n = 308) and subjects harboring an amino acid other than Q at codon 77 (n = 420).
In a cross-sectional analysis of baseline sociodemographic and clinical parameters, no significant correlations were observed between variation at Vpr codon 77 and baseline pVL, CD4 count, diagnosis of AIDS, or sociodemographic characteristics including age, gender, and history of injection drug use (p > 0.1; Table 1). In addition, the VprR77Q mutation was not significantly correlated with baseline HIV co-receptor usage as determined by the Virologic Phenosense assay, though a trend was observed (21.5% of VprR77Q isolates and 16.1% of non-Q isolates were characterized by R5/X4 tropism, p = 0.08). Interestingly, VprR77Q was more frequently observed among non-B subtypes (76% of non-B subtypes harbored the R77Q, as compared to 42% of B-subtypes, p = 0.005). It is necessary to note, however, that the HOMER cohort consists of approximately 97% subtype B infections, and therefore the number of non-B clades is low (N = 17).
Association of baseline VprR77Q mutation with survival and other clinical outcomes following initiation of HAART
Subjects were followed for a median of 4.5 years (Interquartile Range [IQR] 3.8–6.0 years) after initiation of HAART. Associations between VprR77Q and survival as well as pVL and CD4 response following initiation of therapy were assessed by Kaplan-Meier methods. Cox proportional hazards regression was used to calculate univariate and multivariate Hazard Ratios (HR) and 95% confidence intervals (CI). Baseline variables included in the model were gender (male vs. female [reference group]), age (per 10 year increment), AIDS-defining illness (yes vs. no), plasma HIV RNA (per log10 increment), CD4 count (per 100 cell decrement), proportion of time spent on antiretroviral therapy in the first year of follow-up (per 10% increment), a surrogate of adherence [10], type of therapy at initiation (PI-containing vs. NNRTI-containing), and history of injection drug use (yes vs. no). All factors significant in univariate analyses were included in multivariate analyses. All tests of significance were two-sided, with a p-value < 0.05 indicating statistical significance.
The primary outcome investigated was time to non-accidental death (occurring on or before June 30, 2003, the date of latest linkage with mortality statistics from the British Columbia Vital Statistics Agency). Deaths were classified according to the International Classification of Diseases, Tenth revision (ICD-10). Accidental deaths were censored at date of death. Additional clinical outcomes included time to plasma HIV RNA suppression (time from therapy initiation to first of two consecutive pVL < 500 copies/ml), time to plasma HIV RNA rebound (subsequent time to the first of two consecutive pVL ≥500 copies/ml), and time to first CD4 decline below baseline. Eventfree subjects were censored on the date of the last tested sample up to and including June 30, 2003.
In univariate analyses, as well as multivariate analyses adjusting for baseline sociodemographic and clinical parameters, we observed no significant association between VprR77Q and time to non-accidental death following initiation of therapy (Figure 1). A total of 49 (41%) and 71 (59%) non-accidental deaths occurred among the R77Q and non-R77Q study subjects, respectively, during study follow-up. In addition, neither univariate nor multivariate analyses indicated significant associations between VprR77Q and shorter-term clinical therapy responses including time to pVL suppression < 500 copies/ml, subsequent pVL rebound ≥500 copies/ml, or time to CD4 decline below baseline. Consistent with previous analyses [7], the strongest determinants of survival and clinical response following initiation of treatment were baseline CD4 count and continuous use of antiretroviral therapy, as estimated by prescription refill records [10] (data not shown).
Figure 1. Association between VprR77Q mutation at baseline on survival following initiation of first HAART regimen.
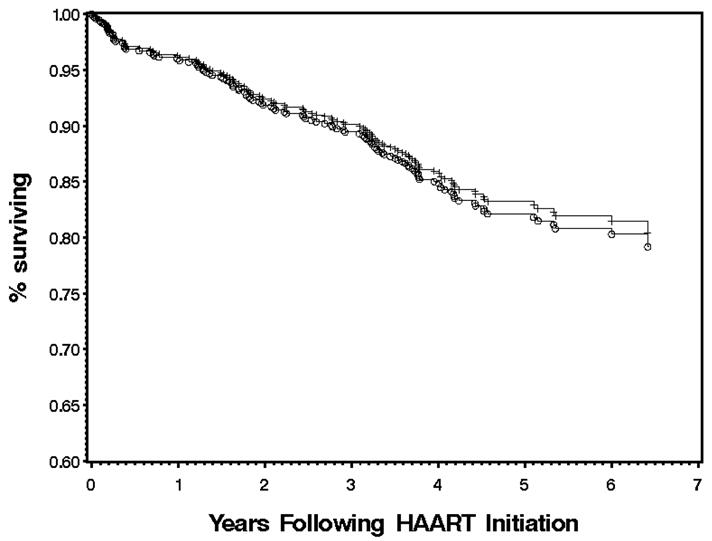
Kaplan-Meier analyses of the influence of baseline VprR77Q on time to non-accidental death. Individuals harboring HIV with the VprR77Q mutation are represented by plus (+) signs, while individuals with an amino acid other than Q at Vpr codon 77 are represented by circles (○). Event-free subjects were censored on the date of the last tested sample up to and including June 30, 2003. Subjects lost to follow-up were censored at their last contact date with the Drug Treatment Program.
It has recently been demonstrated that the VprR77Q mutation is associated with impaired induction of apoptosis in vitro [6], and data suggested an association with long-term nonprogressive HIV infection in vivo [6]. While these in vitro observations have not been challenged, the results from the present study are consistent with evidence indicating that HIV VprR77Q is not a major determinant of HIV disease progression [11] and does not affect clinical response or survival over the first six years following initiation of HIV therapy [12].
The relatively high prevalence (42%) of VprR77Q in this cohort, which represents the majority of HIV-infected British Columbians requiring treatment for their HIV infection over a three-year period in the late 1990s, strongly argues against an association between VprR77Q and long-term nonprogression. The prevalence of VprR77Q observed here is consistent with a prevalence of 40% reported in a study of 96 minimally pre-treated patients initiating nucleoside therapy [12]. While it is not clear why the nonprogressor group obtained from the Los Alamos database [6] included a large proportion of individuals with R77Q, it is possible that unobserved epidemiological linkage may account for these observations.
The ability to draw conclusions from cross-sectional data is somewhat limited, particularly since the duration of infection prior to the subject’s enrollment in the HIV Drug Treatment Program is generally not known. Nonetheless, the observed lack of association of VprR77Q with pre-therapy clinical parameters, including pVL, CD4 count, and AIDS diagnosis, provides evidence against an association of VprR77Q and HIV disease intensity during untreated infection, an observation also consistent with recent results from a small study of HIV subtype A- and B-infected individuals [11]. Consistent with the previous study, which observed a correlation between VprR77Q and HIV subtype A [11], we also observed a significant association between R77Q and non-B subtypes. We are unable to investigate individual viral subtype associations due to the small number of individuals in the cohort who harbor non-B subtypes. In addition, we observed no correlation between VprR77Q and HIV co-receptor usage, a hypothesis we investigated due to the known association of syncytium-inducing, CXCR4-using isolates with more rapid HIV disease progression.
Finally, we observed no association between the VprR77Q mutation and survival following initiation of HAART, as well as initial virologic and immunologic response to HAART. Taken together, these data do not support an association between R77Q and antiretroviral treatment response, and argue against a clinically-relevant role for HIV VprR77Q in vivo.
Acknowledgments
Z.L.B. is supported by doctoral research awards from the Michael Smith Foundation for Health Research (MSFHR) and the Canadian Institutes of Health Research (CIHR). A.D.B. is supported by grants from the National Institutes of Health (R01 AI62261-01-1 and R01 AI40384) and the Burroughs Wellcome Award for Translational Research (ID#1005160).
References
- 1.Le Rouzic E, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2005;2:11. doi: 10.1186/1742-4690-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macreadie IG, Arunagiri CK, Hewish DR, et al. Extracellular addition of a domain of HIV-1 Vpr containing the amino acid sequence motif H(S/F)RIG causes cell membrane permeabilization and death. Mol Microbiol. 1996;19:1185–92. doi: 10.1111/j.1365-2958.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 3.Arunagiri C, Macreadie I, Hewish D, et al. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2:69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Ge YC, Palasanthiran P, et al. Gene defects clustered at the C-terminus of the vpr gene of HIV-1 in long-term nonprogressing mother and child pair: in vivo evolution of vpr quasispecies in blood and plasma. Virology. 1996;223:224–32. doi: 10.1006/viro.1996.0471. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Huang Y, Yuan H, et al. Genetic characterization of vif, vpr, and vpu sequences from long-term survivors of human immunodeficiency virus type 1 infection. Virology. 1997;228:340–49. doi: 10.1006/viro.1996.8378. [DOI] [PubMed] [Google Scholar]
- 6.Lum JJ, Cohen OJ, Nie Z, et al. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J Clin Invest. 2003;111:1547–54. doi: 10.1172/JCI16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 8.Brumme ZL, Dong WWY, Yip B, et al. Clinical and immunological impact of HIV envelope V3 sequence variation after starting initial triple antiretroviral therapy. AIDS. 2004;18:F1–F9. doi: 10.1097/00002030-200403050-00001. [DOI] [PubMed] [Google Scholar]
- 9.Brumme ZL, Goodrich J, Mayer HB, et al. Molecular and Clinical Epidemiology of CXCR4-using HIV-1 in a large population of therapy-naïve individuals. J Infect Dis. 2005;192:466–74. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- 10.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–58. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fischer A, Lejczak C, Lambert C, et al. Is the Vpr R77Q mutation associated with long-term non-progression of HIV infection? AIDS. 2004;18:1346–47. doi: 10.1097/00002030-200406180-00018. [DOI] [PubMed] [Google Scholar]
- 12.Cavert W, Webb CH, Balfour HH., Jr Alterations in the C-terminal region of the HIV-1 accessory gene vpr do not confer clinical advantage to subjects receiving nucleoside antiretroviral therapy. J Infect Dis. 2004;189:2181–84. doi: 10.1086/420788. [DOI] [PubMed] [Google Scholar]


