Abstract
Cell number in the spinal nucleus of the bulbocavernosus (SNB) of rats was the first neural sex difference shown to differentiate under the control of androgens, acting via classical intracellular androgen receptors. SNB motoneurons reside in the lumbar spinal cord and innervate striated muscles involved in copulation, including the bulbocavernosus (BC) and levator ani (LA). SNB cells are much larger and more numerous in males than in females, and the BC/LA target muscles are reduced or absent in females. The relative simplicity of this neuromuscular system has allowed for considerable progress in pinpointing sites of hormone action, and identifying the cellular bases for androgenic effects. It is now clear that androgens act at virtually every level of the SNB system, in development and throughout adult life. In this review we focus on effects of androgens on developmental cell death of SNB motoneurons and BC/LA muscles; the establishment and maintenance of SNB motoneuron soma size and dendritic length; BC/LA muscle morphology and physiology; and behaviors controlled by the SNB system. We also describe new data on neurotherapeutic effects of androgens on SNB motoneurons after injury in adulthood.
Keywords: Motoneuron, Bulbocavernosus, Levator ani, Androgen, Neuromuscular, Dendrite, Sexual differentiation, Cell death, Testosterone, Dihydrotestosterone
Introduction
In 1980, Marc Breedlove and Art Arnold described a sexually dimorphic group of motoneurons in the lumbar spinal cord of rats (Breedlove and Arnold, 1980). As these motoneurons innervated the striated perineal muscles bulbocavernosus (BC) and levator ani (LA), they named the cell group the spinal nucleus of the bulbocavernosus (SNB). In that first report, SNB motoneurons were shown to be much more numerous in adult males than in females, and to accumulate radiolabeled testosterone or dihydrotestosterone, but not estradiol (Breedlove and Arnold, 1980). In rapid succession the authors demonstrated that the sex difference in cell number could be reversed by neonatal, but not adult treatment with gonadal steroids (Breedlove and Arnold, 1981; Breedlove et al., 1982), and that the SNB nucleus is completely female-like in genetically male rats bearing an inactivating mutation of the androgen receptor (Breedlove and Arnold, 1981). They concluded: “the sexually dimorphic nature of the SNB depends on neither the adult hormone state nor the presence of a Y chromosome, but on the interaction of androgens with their receptors early in development” (Breedlove and Arnold, 1981).
The SNB therefore became the first neural sex difference shown to be due to androgens, acting via classical, intracellular androgen receptors (AR). In the twenty-five-plus years since, the importance of androgens and AR in this system has been repeatedly confirmed, albeit with some interesting twists and turns. The AR so abundantly expressed by SNB cells during adulthood, for example, turn out to be something of a red herring for understanding the sex difference in SNB cell number. Androgens apparently do not act directly at SNB cells to control their number, as is discussed below, although they may act on AR expressed in the motoneurons to control motoneuron soma size.
The last 25 years has also seen the mushrooming of the field of sexual differentiation of the nervous system in general, and it soon became clear that many sex differences in the brain were primarily dependent on estrogenic metabolites of testosterone (reviewed in Hutchinson, 1997). For some time, the SNB seemed to be unique in its androgen dependence, which led some writers to conclude that testosterone acts through androgenic metabolites and AR to masculinize the spinal cord and periphery, but through estrogen receptors to masculinize the brain. This has turned out to be an oversimplification. Although the SNB remains the premier example of an androgen-dependent neural sex difference, the articles in the current issue attest to the growing awareness of the roles of androgens and AR in other sex differences and neural systems.
At this point, however, more is probably known about the roles of androgens in sexual differentiation of the SNB system than for any other sex difference in the nervous system. The reasons for this are twofold: the androgen-dependence of the SNB has been known for such a long time, and students of the SNB enjoy the advantages offered by any neuromuscular system --very large size of neurons, accessibility of nerve terminals, easy identification of target cells, and relative simplicity of connections. As a result, substantial progress has been made in identifying effects of androgens on multiple processes both in development and in adulthood; in some cases, the cellular and molecular bases for androgenic effects have also been revealed. Here, we first review the evidence for the role of androgens and AR in the determination of SNB cell number and soma size. We next turn to hormone effects on the SNB target muscles, and to the behaviors controlled by this neuromuscular system. Finally, we consider the development and maintenance of SNB dendritic arbors, the study of which has uncovered a novel role for androgens, as well as an unexpected requirement for estrogens in the establishment of SNB motoneuron morphology and function.
Motoneuron Death and the Establishment of SNB Cell Number
In male rats, the SNB [also known as the dorsomedial nucleus or DM (Schrøder, 1980)] consists of approximately 200 motoneurons that innervate the perineal muscle complex consisting of the bulbocavernosus (BC) and levator ani (LA; collectively, BC/LA), as well as the external anal sphincter (Breedlove and Arnold, 1980; Schr¸der, 1980; McKenna and Nadelhaft, 1986). The SNB in mature females, who lack or have greatly reduced perineal musculature (Hayes, 1965; Čihák et al., 1970; Tobin and Joubert, 1991), is comprised of only approximately 60 motoneurons, innervating primarily the external anal sphincter (McKenna and Nadelhaft, 1986; Ueyama et al., 1987).
This sex difference in SNB motoneuron number develops perinatally. Before birth, the number of motoneurons in the SNB increases in both male and female rats, the result of a migration of developing SNB cells into their characteristic medial location (Sengelaub and Arnold, 1986). SNB motoneuron numbers reach their maxima in both sexes just before birth, and this peak is followed by a decline through postnatal day (P) 10, when cell number reaches its adult range (Figure 1). The decline is due to sexually dimorphic cell death, as revealed by counts of degenerating cells in the SNB (Nordeen et al., 1985). Females typically lose up to 70% of their SNB motoneurons, whereas males lose about 25%.
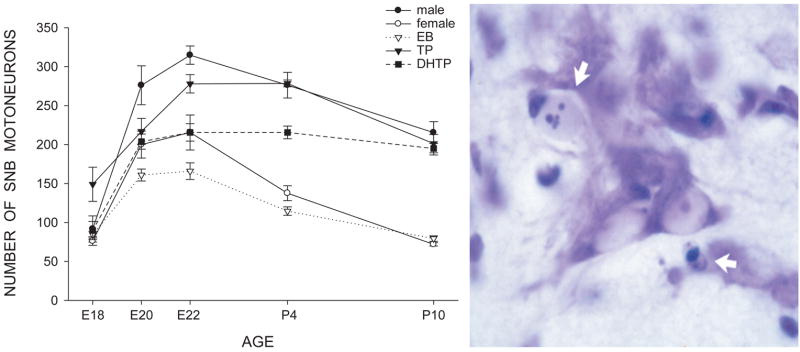
Figure 1.
Development of SNB motoneuron number is regulated through an androgen-mediated normally occurring cell death. (Left) Counts of motoneurons in the SNB from embryonic day (E)18 through postnatal day (P)10 for normal males and females, as well as females treated both pre- and postnatally with either estradiol benzoate- (EB), testosterone propionate- (TP), and dihydrotestosterone propionate- (DHTP). Females treated with TP or DHTP have a masculine number of SNB cells on P10, whereas females treated with EB do not differ from controls. Points represent means ± SEM. (Right) Photomicrograph of a transverse, cresylecht violet-stained section through the SNB at P2 showing both normal motoneurons and degenerating cells (arrows). (Compiled from data originally published in Nordeen et al., 1985, Goldstein and Sengelaub, 1990, 1992.)
Gonadal hormones act in the establishment of sex differences in SNB motoneuron number by regulating this normally-occurring motoneuron death. Females treated perinatally with testosterone propionate (TP) have reduced cell death during development and significantly more SNB motoneurons in adulthood than do normal females (Breedlove and Arnold, 1983b; Nordeen et al., 1985; Sengelaub and Arnold, 1986). Conversely, male rats with a mutation of the androgen receptor gene, as in the testicular feminization mutation (Tfm), experience a feminine pattern of cell degeneration in the developing SNB (Sengelaub et al., 1989a), and exhibit a feminine number of SNB cells in adulthood (Breedlove and Arnold, 1981). These genetic males develop functional testes that secrete normal levels of testosterone yet remain unresponsive to the steroid because of greatly reduced androgen binding relative to normal males (Naess et al., 1976). Prenatal treatment with the antiandrogen flutamide, a drug known to block androgen receptor activation, also feminizes SNB motoneuron number of males (Breedlove and Arnold, 1983a).
Androgens are thought to regulate the death of SNB motoneurons indirectly through their action at the target musculature. SNB motoneurons lack AR during the time in which exposure to androgens spare them (Fishman et al., 1990; Jordan et al., 1991, 1997). In addition, the normal androgen-induced masculinization of the SNB can be prevented by local application of the anti-androgen flutamide to the target muscles (Fishman and Breedlove, 1992). Studies implicating the SNB target muscles as the site of action have demonstrated that these muscles can be maintained by androgen treatment during development even if they have been denervated by removal of the lumbar spinal cord at birth (Fishman and Breedlove, 1988), and moreover, the muscles possess androgen receptors at birth (Fishman et al., 1990). In a study by Freeman et al. (1996), Tfm females who were genetically mosaic for androgen insensitivity were treated with testosterone during the period of SNB motoneuron death. These researchers capitalized on the fact that the AR gene resides on the X chromosome (Yarbrough et al., 1990). As a result of the random X-chromosome inactivation that occurs in every cell of XX embryos during development, female carriers of the Tfm mutation have some SNB cells that express the wild-type AR, and some that express the Tfm AR. Despite lacking functional androgen receptors, SNB motoneurons expressing the Tfm allele were spared by early testosterone treatment (Freeman et al., 1991). Because the SNB target musculature was also spared in these females, and these muscles contained functional androgen receptors, it seems plausible that androgen action in the neuromuscular periphery is responsible for the indirect support of SNB motoneuron survival.
Many of the actions of testosterone are mediated after its conversion to dihydrotestosterone (DHT) or estrogenic metabolites (reviewed by Hutchinson, 1997). For example, in rats, the sexually dimorphic nucleus of the preoptic area of the hypothalamus is larger in adult males than in females. This sex difference is the result of estrogenic action during development. Females treated with estradiol benzoate (EB) perinatally develop masculine sexually dimorphic nuclei (Dohler et al., 1984a), while treatment of males with estrogen antagonists results in feminine sexually dimorphic nuclei (Dohler et al., 1984b). Furthermore, DHT treatment is ineffective in masculinizing the sexually dimorphic nucleus in females, and males given androgen antagonists perinatally develop sexually dimorphic nuclei that are no different from those of normal males (Dohler et al., 1986). By contrast, in the SNB, treatment of females with EB completely fails to alter cell number from the normal feminine pattern (Figure 1) (Breedlove et al., 1982; Goldstein and Sengelaub, 1990; Breedlove, 1997). Similarly, SNB motoneuron number is not affected in males treated postnatally with the aromatase inhibitor 4-OH-androstenedione (Currie et al., 1990). The findings in Tfm rats, and the failure of estrogens to prevent SNB motoneuron death, suggests that the sparing of both the SNB and its target musculature specifically requires activation of the androgen receptor.
Observations of animals treated with the non-aromatizable androgen DHT provide some surprises, however. If females are treated throughout the pre- and postnatal critical period with DHT, the SNB system is completely masculine in terms of motoneuron number (Figure 1) and presence of the target muscles (Goldstein and Sengelaub, 1992). Early postnatal treatment of females with DHT results in only a small increase in SNB motoneuron number (Breedlove and Arnold, 1983b), presumably a consequence of the lack of androgens during the prenatal phase of motoneuron death. Interestingly, treatment of females with DHT only prenatally does not spare SNB motoneurons (Breedlove and Arnold, 1983b; Sengelaub et al., 1989b) but the perineal musculature is retained (albeit at a reduced size relative to normal males; Breedlove and Arnold, 1983b). The BC muscles in these females are innervated by motoneurons anomalously located in the dorsolateral nucleus (DLN) (Breedlove, 1985; Kurz et al., 1990).
This curious pattern is the result of a failure of prenatal DHT treatment to support the postnatal survival of SNB motoneurons, leaving the BC without adequate innervation. DHTP-treated females undergo a decline in the number of Nissl-stained motoneurons in the SNB through P10 (Sengelaub et al., 1989), as well as a decrease in the number of SNB motoneurons that can be retrogradely labeled via the BC muscle (Kalkbrenner and Sengelaub, 1992). Following these declines, the number of motoneurons in the DLN projecting to the BC muscle increases over six-fold by P28 (Figure 2). Thus, it appears that because of the anatomical proximity of the BC to the ischiocavernosus, a target muscle of DLN motoneurons, DLN axons opportunistically invade the denervated SNB targets in the late postnatal period (Kalkbrenner and Sengelaub, 1992). This result indicates that retention of the target musculature through androgenic action is not sufficient to spare SNB motoneurons from normally-occurring death (Sengelaub et al., 1989b). Androgenic hormones may be required to stimulate the production of trophic factors from the neuromuscular periphery that then mediate the rescue of SNB motoneurons. Although these factors have not been identified, injection of the trophic factor, ciliary neurotrophic factor, into the perineum of newborn females spares SNB motoneurons (Forger et al., 1993), and the ability of testosterone to masculinize SNB motoneuron number is blocked by perineal injections of antagonists to trophic factors (Xu et al., 2001).
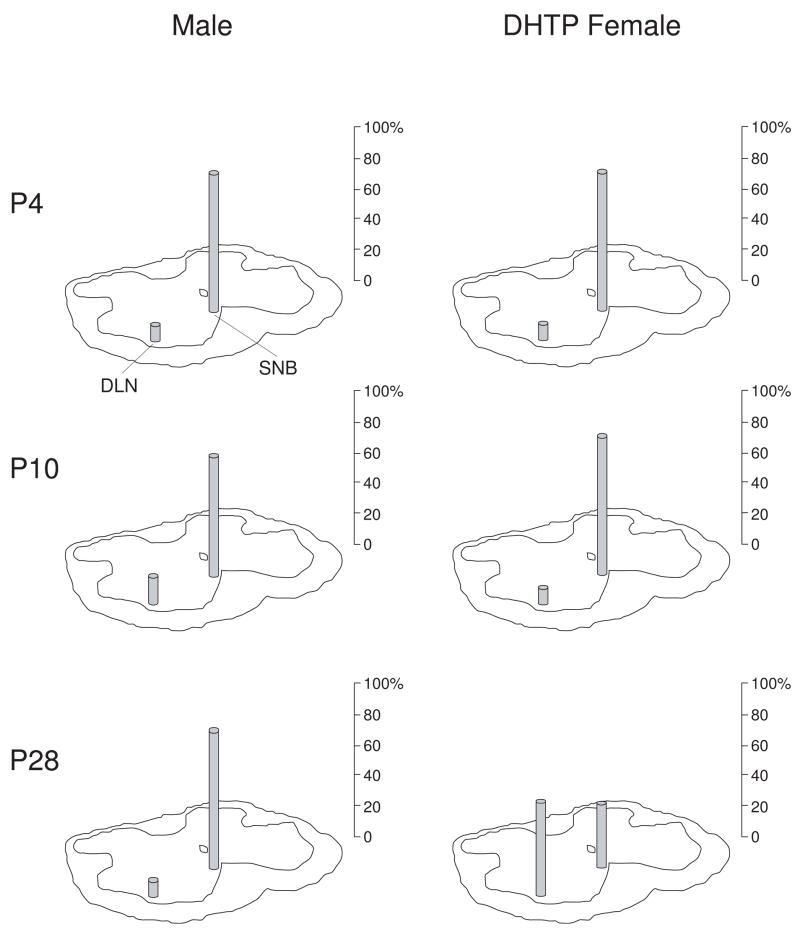
Figure 2.
Relative proportions of motoneurons in the SNB and DLN retrogradely labeled via the BC muscle at postnatal (P) days 4, 10, and 28 in both normal males (left) and females treated prenatally with dihydrotestosterone propionate (DHTP; right). Motoneurons in normal males, and females cross-fostered from pregnant dams treated on prenatal days 17–22 with DHTP (4 mg/day) were labeled following unilateral injections of horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP; 0.05 μL, 0.2%) into the BC muscle on embryonic (E) day 22, and days P4, P10, and P28 (n=5–9 per group); post-injection survival times varied by age (6 hours at E22, 24 hours at P4 and P10, 48 hours at P28). Counts of labeled motoneurons in the DLN and SNB were made under bright-field illumination at 500X and corrected by the method of Konigsmark (1970). On P4 and P10, the majority of the retrogradely labeled motoneurons are found in the SNB in both males and DHTP-treated females. By P28, however, the number of motoneurons in the DLN projecting to the BC muscle in DHTP-treated females has substantially increased. (Data from Kalkbrenner and Sengelaub, 1992.)
Although the development of the SNB has been most intensively studied in rats, a similar sex difference (male > female) in the number of perineal motoneurons is seen in many mammals, including mice, gerbils, dogs, hyenas, monkeys, and humans (Forger and Breedlove, 1986; Wee et al., 1987; Ueyama et al., 1987; Ulibarri et al., 1995; Forger et al., 1996). In those cases in which it has been examined, these sex differences in other mammals also depend on differential exposure to androgens in males and females during development.
SNB Soma Size
Effects of androgens during development and in adulthood on SNB cell size
The earliest reports on the SNB noted a sex difference in cell size: both the cell bodies (somata) and nuclei of SNB motoneurons are significantly larger in adult male rats than in females (Breedlove and Arnold, 1980; 1981). Similar observations have been made in white-footed mice (Forger and Breedlove, 1987), gerbils (Ulibarri et al., 1995), and several strains of Mus musculus (Wee and Clemens, 1987; Park et al., 2002; Monks et al., 2003; Zuloago et al., 2007). Larger somata likely reflect differences in electrophysiological properties, protein synthesis, and more robust dendritic organization, all of which are androgen sensitive in SNB motoneurons (see below). In rats, the larger soma size in males is due to both organizational and activational effects of gonadal steroids. SNB cell size is completely feminine in Tfm males lacking functional AR (Breedlove and Arnold, 1981), and SNB somata are reduced in wild-type males deprived of androgen stimulation during development, even if adult levels of T are made equivalent (Breedlove and Arnold, 1983a). Conversely, treatment of females with TP or DHTP during perinatal life increases SNB soma size in adulthood (Breedlove and Arnold, 1983b; Ward et al., 1996).
It is possible that developmental exposure to androgens causes an increase in average SNB soma size by shifting in the population of cells comprising the SNB. Most SNB motoneurons of animals exposed to androgens perinatally innervate the BC and LA muscles, whereas the majority of SNB cells in females or males lacking androgen stimulation innervate the external anal sphincter (McKenna and Nadelhaft, 1986). Because anal sphincter motoneurons may be smaller and less androgen sensitive than those innervating the BC/LA (Collins et al., 1992), effects of perinatal androgens on SNB soma size could simply be a byproduct of altered survival. Several observations argue for organizational effects of gonadal steroids on SNB cell size, per se, however. The critical period for androgen effects on soma size extends to P12, whereas administration of TP or DHTP after the first week of life does not change the survival of SNB cells (Breedlove and Arnold, 1983b; Lee et al., 1989). In addition, perinatal treatment of female rats with EB, alone or in combination with DHTP, increased SNB soma size in adulthood, even though EB does not prevent the death of SNB motoneurons (Breedlove, 1997; but see Breedlove and Arnold, 1981). This suggests two separate mechanisms for effects of hormones on SNB cell number and cell size.
Manipulating androgens in adulthood also has marked effects on SNB soma size (Figure 3A–C). Castration of adult males leads to the shrinkage of SNB somata (Breedlove and Arnold, 1981), which can be prevented by treating castrates with T. Treatment of female rats with T in adulthood also increases SNB cell size, although not to the level seen in males (Breedlove and Arnold, 1981). DHT partially protects SNB cells from shrinkage after castration of adult male rats, but is not as effective as T when given in the same dose (Forger et al., 1992). Estrogenic metabolites of T do not seem to explain the difference in efficacy between T and DHT, however, as EB alone or in conjunction with DHT has no effect on soma size (Forger et al., 1992). Similarly, blockade of estradiol synthesis in intact adult male rats with the aromatase inhibitor, fadrozole, does not affect SNB soma size (Burke et al., 1999).
Figure 3.
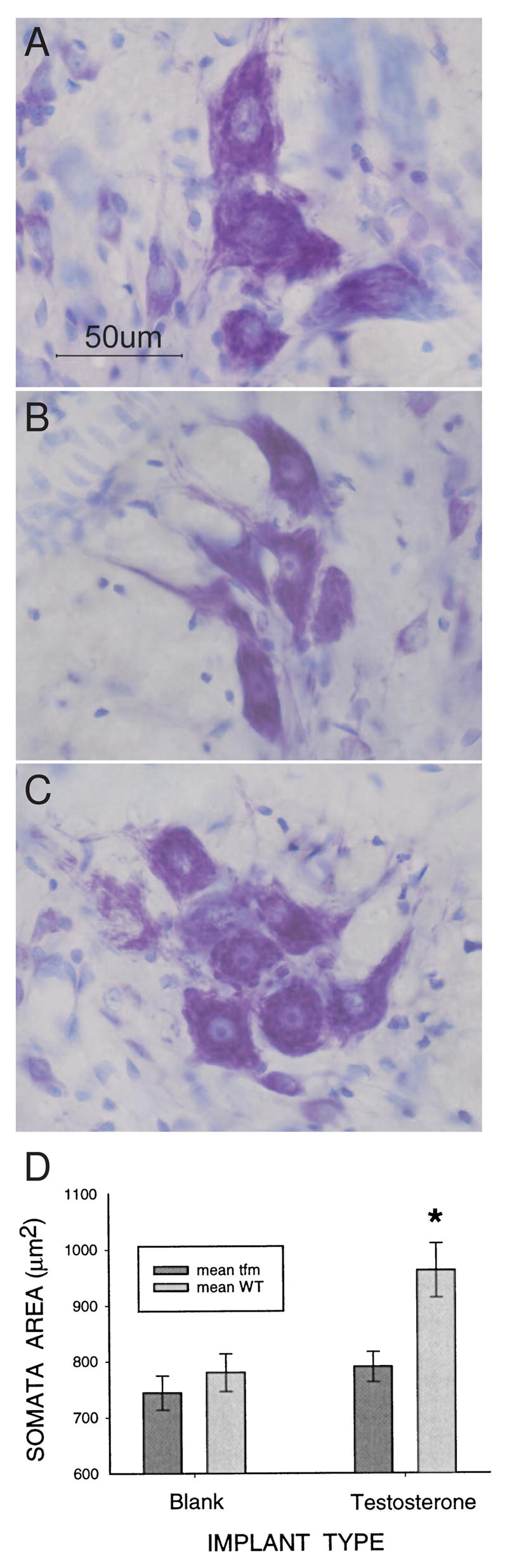
Digital micrographs of thionin-stained SNB somata in a normal adult male (A), a castrate (B), and a castrate treated with testosterone (C), demonstrating the androgen sensitivity of soma size in adulthood. (D) Testosterone increases SNB soma size only in cells expressing the wild-type (WT) androgen receptor (AR). Adult female carriers of the Tfm mutation were implanted with blank capsules or capsules filled with testosterone. SNB soma size was assessed 4–6 weeks later. Some SNB motoneurons in these females express the WT AR, whereas others express the non-functional, Tfm AR. Only SNB motoneurons expressing the WT AR responded to testosterone with an increase in soma size. (Panels A–C, D.R. Sengelaub; Panel D reprinted from Watson et al., (2001) with permission from the Society for Neuroscience.)
A similar pattern of results is seen in gerbils, where long-term castration of adult males leads to a reduction in SNB soma size that can be prevented by treatment with T, but not EB (Fraley and Ulibarri, 2002). As in rats, DHT is partially effective in preventing the castration-induced reduction in SNB cell size in gerbils (Fraley and Ulibarri, 2002). This suggests that testosterone increases SNB soma size of adult rodents by acting via AR, and may do so without conversion to either estradiol or DHT.
The expansion and shrinkage of SNB somata seen in laboratory rodents in response to androgen manipulations may be a natural part of the physiology of seasonally-breeding animals. In white-footed mice (Peromyscus leucopus), SNB somata and nuclei, and the BC/LA muscles all shrink in response to short daylengths mimicking late fall, and regrow when mice are transferred to long daylengths characteristic of spring (Forger and Breedlove, 1987). These changes in the SNB system parallel the regression and growth of the testes, and likely result from photoperiod-dependent changes in circulating testosterone (Forger and Breedlove, 1987). Similarly, transfer to short daylengths leads to testicular regression and a reduction in SNB soma size in seasonally-breeding Siberian hamsters (Phodopus sungorus; Hegstrom and Breedlove, 1999). Thus, for rodents in the wild, the size of SNB motoneurons may vary according to the reproductive state of the animal, and regression of the SNB system may contribute to seasonal infertility.
Site of action for effects of androgens on SNB soma size
The site(s) of hormone action for organizational effects of gonadal steroids on SNB cell size are not known. SNB motoneurons do not accumulate radiolabeled testosterone or express AR perinatally (Fishman et al., 1990; Jordan et al., 1991; Jordan et al., 1997), and do not express estrogen receptors at any age (Breedlove and Arnold, 1980; Taylor et al., 1995). Thus, hormones present during perinatal life presumably act indirectly to influence later SNB cell size.
In adulthood, the motoneurons themselves, the BC/LA muscle complex, and supraspinal afferents of SNB cells all express AR and are potential direct sites of androgen action (Dube et al., 1976; Breedlove and Arnold, 1980; Shen et al., 1990; Wagner et al., 1993). A clever experiment by Watson and colleagues (2001) made use of female carriers of the Tfm mutation (described above) that are mosaic for the mutated and wild-type AR (Figure 3D). Administration of androgen in adulthood induced an increase in soma size only in those SNB cells expressing the wild-type, functional AR (Watson et al., 2001). This both confirms the necessity for the AR in this response and identifies the motoneurons themselves as a necessary site of androgen action.
Other evidence, however, implicates the target muscles in the androgenic control of SNB soma size. SNB motoneurons of adult male rats that were axotomized and forced to reinnervate a non-androgen sensitive muscle did not respond to testosterone with an increase in soma size (Araki et al., 1991). In addition, if SNB cells are separated from their target muscles by axotomy at two weeks of age, they do not respond to changes in testosterone levels with changes in soma size later in life (Lubischer and Arnold, 1995). On the other hand, testosterone increased SNB somata after axotomy in adulthood (Yang and Arnold, 2000). This suggests that if there has been no perturbation during development, the target muscles are not a required site of action for effects of androgens on SNB soma size. Finally, Rand and Breedlove (1995) sutured small, hormone-containing capsules to the BC muscles of castrated male rats to determine whether androgen stimulation localized to the muscles could alter SNB cell size. They found effects on dendritic arbors (see below), but no effect of hormone treatment at the muscle on SNB soma size. Taken together, SNB motoneurons themselves are a necessary site of hormone action for the androgenic maintenance of soma size, and may be the only site of action.
Molecular Mechanisms
The molecular mechanism(s) whereby androgens control SNB soma size are not known. Castration of adult male rats reduces the expression of trophic factor receptors in SNB motoneurons (Forger et al., 1998; Osborn et al., 2007; Ottem et al., 2007), suggesting that SNB cells of castrates may be less responsive to trophic support. Other studies have explored the role of AR and steroid receptor cofactors in effects of androgens on SNB cell size. SNB motoneurons express the AR at higher levels than do other lumbar motoneurons, but this alone does not appear to explain their unusual androgen responsiveness (Collins et al., 1992; Jordan, 1997). The binding of steroid receptors to hormonal ligands recruits a complex of coregulatory proteins to the promoter region of a target gene (McKenna and O’Malley, 2002) and these proteins play an essential role in steroid-regulated gene transcription. Although SNB motoneurons express several steroid receptor coactivators (SRC-1, SCR-2, CBP, p300, cJUN), most other lumbar motoneurons, including those that do not respond morphologically to changes in androgen, also do (O’Bryant and Jordan, 2005). In addition, soma size of SNB motoneurons in SRC-1 knockout mice does not differ from that of wild-type controls (Monks et al., 2003). Thus, at this point, the molecular basis of the unusual androgen responsiveness of SNB motoneurons is not clear.
Androgen Effects on the BC/LA Muscles
Androgen-dependence of the BC/LA in development
Early investigators reported that the BC and LA muscles are present in males and females at birth, but later involute in females during the first postnatal week (Čihák et al., 1970). The muscles could be maintained in females treated with testosterone during the late prenatal or early postnatal period (Čihák et al., 1970; Breedlove and Arnold, 1983b). Later, however, Tobin and Joubert challenged the interpretation that the LA “involutes” in female rats. They found an LA muscle in untreated adult females (consisting of about 500 fibers, compared to approximately 5,000 fibers in the LA of males), which was both innervated and androgen responsive (Tobin and Joubert, 1988, 1989). From a detailed study of the development of LA fiber number and size, they concluded that the sex difference in the LA was due not to degeneration of the muscles in females, but to a dramatic increase in fiber number in male rats during early postnatal life (Tobin and Joubert, 1991).
The discrepancies between the interpretations of Tobin and Joubert and earlier investigators may be explained in part by the species and strains examined. Although an LA muscle was present in every adult female Great Wistar rat examined (Tobin and Joubert, 1988), no LA fibers were found in the majority of Sprague Dawley rats examined, based on microscopic analysis of serial sections through the perineum (Bengston et al, 1996). In mice, the LA of adult females is miniscule, averaging only about 50 fibers (Jacob et al., 2004). Moreover, although cell addition and growth undoubtedly contribute to the sex difference in LA size, several observations suggest that cell death also plays an important role in sexual differentiation of both the LA and BC muscles. Signs of degeneration have been noted in the LA of postnatal females based on simple observation of histological sections (Čihák et al., 1970). More recently, we used TUNEL (terminal deoxynucleotidyl nick end labeling) to examine dying cells in the developing perineums of mice. We find TUNEL-positive cells in the BC and LA muscles of both sexes perinatally, but the density of apoptotic cells is significantly greater in females (Figure 4; Jacob and Forger, unpublished). Moreover, LA muscle fiber number is increased in female mice lacking the pro-death gene, Bax (Jacob et al., 2004), and is massively increased in double knockout mice lacking Bax and Bak, two genes critical for cell death in many tissues (Jacob, Ray, Lindsten and Forger, unpublished). Thus, cell death contributes to sexual differentiation of the LA, at least in mice.
Figure 4.
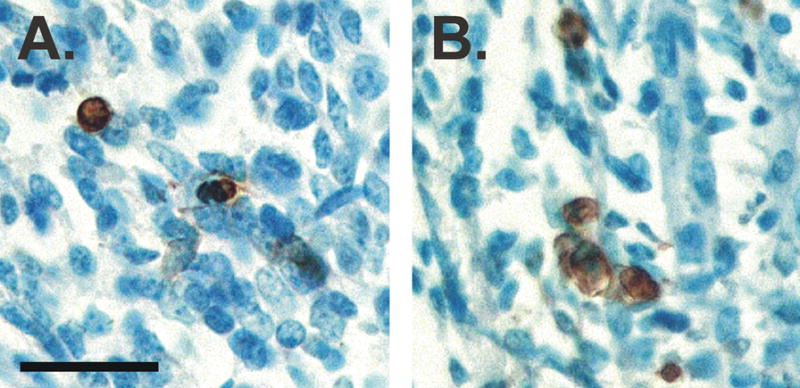
Photomicrographs of sections through the LA muscle of a male (A) and female (B) mouse on embryonic day 18. Tissue was stained with TUNEL to label dying cells. The density of TUNEL-positive cells (brown) is about two-fold higher in females than in males. Scale bar = 25 μm. (Panels A and B, N. Forger.)
As is seen for effects of T on BC/LA size in adulthood (see below), androgenic metabolites are responsible for masculinization of the perineal muscles during development. Female rats injected with TP or DHTP around the time of birth have easily identified BC/LA muscles in adulthood, whereas those treated with EB do not (Breedlove et al., 1982; Breedlove, 1997). EB also does not augment effects of DHT on muscle size (Breedlove, 1997). In addition, prenatal treatment of male rats with flutamide feminizes the BC/LA (Breedlove and Arnold, 1983a), and male rats without functional AR lacked identifiable BC/LA muscles (Breedlove and Arnold, 1981). The AR is expressed abundantly in the BC/LA of newborn male rats (Fishman et al., 1990), and removing SNB input to the BC/LA did not prevent the androgenic sparing of these muscles (Fishman and Breedlove, 1988). Thus, androgens may act directly at the developing BC/LA muscles to masculinize muscle size.
Neuromuscular synapse elimination in juveniles
Androgenic metabolites of testosterone also are responsible for establishing the normal innervation pattern of the perineal muscles during the juvenile period. Mammalian muscle fibers are initially innervated by multiple motor nerve terminals, and the number of inputs per fiber is subsequently pruned. This process of neuromuscular synapse elimination takes place primarily during the third and fourth week of life in the SNB target musculature (Jordan et al., 1988), and is regulated by androgens. Castration accelerates synapse elimination in the LA muscle, while androgen treatment after castration can delay or prevent this process (Jordan et al., 1989a,b). Unlike androgens, treatment with E does not maintain multiple innervation in the LA (Jordan et al., 1995). Similarly, androgen treatment during the period of synapse elimination significantly increases the size of LA muscle fibers, whereas E does not (Jordan et al., 1995).
Hormone dependence of the BC/LA muscles in adulthood
The BC and LA muscles of adult rodents have long been considered the most androgen-sensitive muscles in the body. The muscles shrink markedly after adult castration and enlarge with androgen treatment in male rats and mice (Wainman and Shipounoff, 1941; Dorfman and Kincle, 1963; Vyskocil and Gutmann, 1977). These anabolic effects of androgens involve changes in perineal muscle fiber size, without a change in fiber number (Venable, 1966). Androgens in adulthood also maintain BC/LA neuromuscular junction size (Bleisch and Harrelson, 1989; Balice-Gordon et al., 1990; Lubischer and Bebinger, 1999), acetylcholine receptor number (Bleisch et al., 1982; Bleisch and Harrelson, 1989), and peripheral nerve activity (Fargo et al., 2003).
The androgen-responsiveness of the BC/LA is much greater than for other striated muscles, presumably because the BC/LA complex is enriched for androgen binding sites and AR protein compared to other striated muscles (Dube et al., 1976; Tremblay et al., 1977; Monks et al., 2006). Effects of T on muscle size in adulthood are primarily or exclusively androgen-mediated, as BC/LA mass in male rats castrated in adulthood is increased significantly by treatment with T or DHT, but not by EB (Forger et al., 1992). Moreover, the aromatase inhibitor, fadrozole, does not affect BC/LA size of intact, adult males (Verhovshek et al., 2000), suggesting that endogenously produced estrogens are not required for the maintenance of muscle mass.
Although both T and DHT increase BC/LA mass of castrates, T appears to be more effective in this regard (Forger et al., 1992). This may be because exogenously supplied DHT is quickly converted to inactive metabolites at the BC/LA muscle, whereas T itself is relatively stable (Dionne et al., 1977; Tremblay et al., 1977; Krieg et al., 1974). In addition, 5 α-reductase activity is negligible in striated muscles, including the BC/LA (Tramblay et al., 1977), suggesting that conversion of T to DHT does not normally occur there. Indeed, blocking conversion of T to DHT in prepubertal rats with finasteride does not block growth of the BC/LA to normal adult size (George et al., 1989). Thus, despite the widespread notion that DHT is a more “active” androgen than is T, the relative potency of the two hormones is cell-type specific and depends on the steroidogenic enzymes and/or AR co-factors that are expressed.
Unlike SNB motoneurons, the BC/LA complex also expresses estrogen receptors (Dube et al., 1976). The role of the estrogen receptor in the BC/LA is not clear, but two observations suggest possible direct effects of estrogens on muscle function. The LA responds to T and EB, but not DHT, by increasing the activity of glucose-6-phosphate dehydrogenase (Knudsen and Max, 1980), and in a reduced preparation consisting of the pudendal nerve attached to the BC muscle, both DHT and E could prevent a reduction in muscle excitability following castration (Foster and Sengelaub, 2004b). Roles of estrogens in behavioral responses involving the SNB neuromuscular system are discussed further below.
Site of action for effects of androgens on BC/LA muscle mass
Muscle tissue is composed of multiple cell types, including mature fibers, satellite cells, fibroblasts, and Schwann cells associated with motor nerve terminals. Interestingly, although the anabolic effects of androgens on striated muscle have long been known, the cellular/molecular basis for this is poorly understood. In particular, it was established only quite recently which cells in muscle tissue express AR and are therefore potential direct sites of androgen action. A study of the distribution of receptor expression shows that about 75% of nuclei in muscle fibers of the LA are AR-positive (AR+), compared to 5–10% for a relatively non-androgen sensitive limb muscle (Figure 5; Monks et al., 2004). The LA and limb muscle contained equivalent proportions of AR+ fibroblasts, and terminal Schwann cells were not AR+ in either muscle (Monks et al., 2004). Thus, both muscle fibers and fibroblasts are potential direct sites of androgen action in striated muscle, but the effect of androgens on muscle size correlates with AR expression by myofiber nuclei. Evidence that androgens may act locally, presumably via the AR expressed within BC/LA myofibers, to maintain muscle size in adult rats comes from a study by Rand and Breedlove. When small capsules were sutured onto each side of the BC muscle, one capsule containing T and the other containing the anti-androgen flutamide, the BC/LA muscles on the T-treated side were larger in mass and exhibited greater fiber size (Rand and Breedlove, 1992). This suggests a direct site of hormone action for effects of androgens on BC muscle size.
Figure 5.
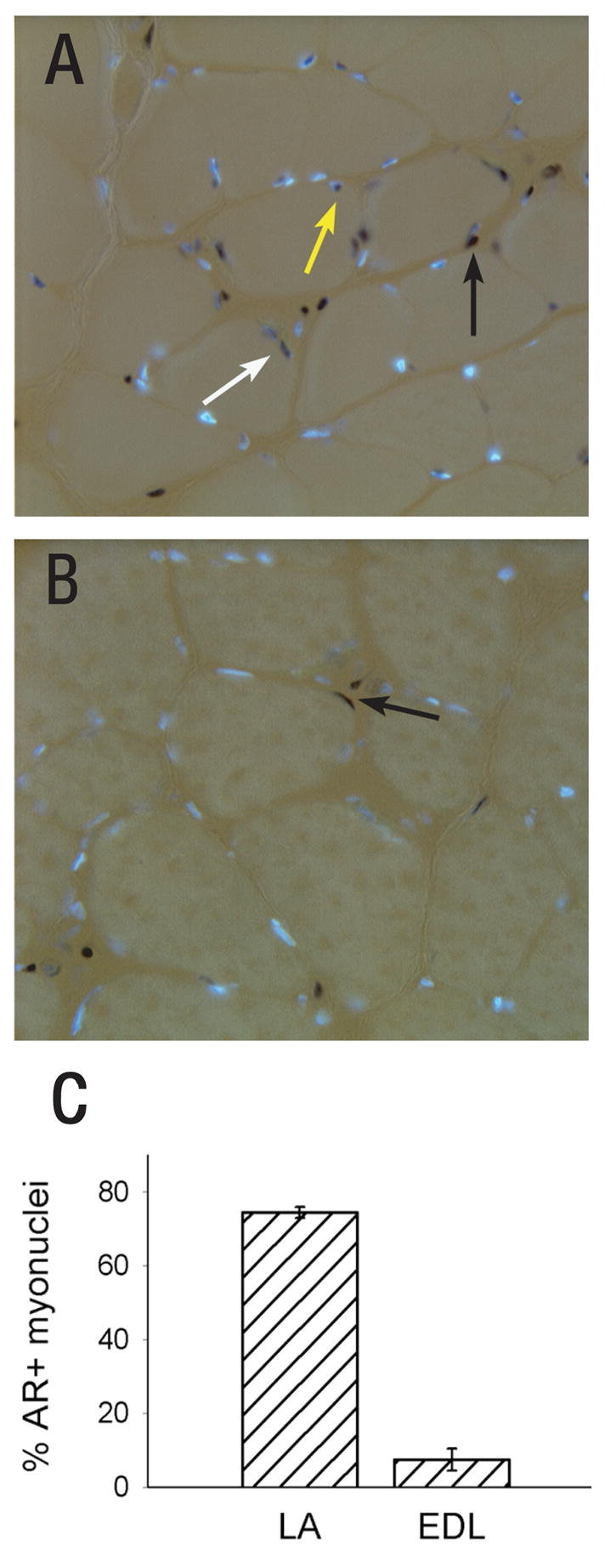
Androgen receptor (AR) expression is unusually high in myofibers of the LA. In the photomicrographs shown in (A) and (B), DAPI (blue) labels AR negative nuclei and black peroxidase labels AR+ nuclei. A fluorescent basal lamina stain (not shown) was used to discriminate between AR in muscle fiber nuclei (white and yellow arrows) or in fibroblasts, which are found in the interstitial space between fibers (black arrows). (A) Cross section through the LA reveals many AR+ myonuclei; (B) Cross section through the extensor digitorum longus (EDL) limb muscle shows few AR+ myonuclei. (C) The percentage of AR+ muscle fibers in the LA and EDL. (Reprinted from Monks et al. (2004) with permission from John Wiley and Sons.)
Behaviors Mediated by the SNB Neuromuscular System
The LA and BC muscles attach exclusively to the base of the penis in male rats (Sachs, 1982; Hart and Melese-d’Hospital, 1983) and, perhaps not surprisingly, play a role in copulation. The BC contracts rhythmically during erection and ejaculation in rats and several other species, including humans (Hart, 1972; Beckett et al., 1975; Sachs, 1982; Karacan et al., 1983). Contraction of the LA in rats is tightly coupled with that of the BC and augments penile erection (Holmes and Sachs, 1994). The actions of the perineal muscles and their hormone dependence have been studied in some detail in penile reflex tests in which a male rat or mouse is held in a supine position and the penile sheath retracted; a series of erections, flips of the penis, and ejaculations follows. Following adult castration, these reflexive penile erections quickly disappear, and T or DHT, but not estradiol, prevent this decrement (Hart, 1979; Meisel et al., 1984). T and DHT also restore erections of long-term castrates (Gray et al., 1980; Hart, 1979), and flutamide inhibits the T-induced restoration of penile reflexes, confirming that the actions of T are primarily via AR (Gray et al., 1980). The site of action for this androgen effect is not known, although direct implantation of T into the spinal cord increased reflexes in castrated, spinally-transected males (Hart and Haugen, 1968).
Because the same hormone metabolites that support SNB somata and BC/LA muscles following castration also maintain the penile reflexes, morphological regression of the SNB neuromuscular system might be responsible for the decrements in behavior. Several observations suggest that caution is warranted before accepting this interpretation, however. Effects of hormones on the restoration of penile reflexes in long-term castrates are very rapid: administration of T or DHT increase reflexive erections within 6 hours and restore reflexes to normal levels after only 24 to 48 h (Gray et al., 1980; Sachs, 1982; Hart et al., 1983; Meisel et al., 1984; Leedy et al., 1987). Although increases in synapse number onto SNB cells can be seen within just 48 hours of T treatment in long-term castrates (Leedy et al., 1987), this time frame is probably too short for regrowth of the BC/LA muscles or SNB somata and dendrites in castrates.
In addition, investigators have repeatedly noted dissociations between morphology of the SNB system and performance on tests of sexual behavior. Prenatal flutamide treatment did not affect mounts or intromissive-like behaviors in adult male rats, despite the fact that the SNB system was severely reduced (Breedlove and Arnold, 1983a). Similarly, Tfm males, in which the SNB system is completely feminine, sometimes show intromissions and ejaculations (Olsen, 1979). Dissociations between penile reflex tests and sexual behavior tests are also noted. For example, castrated male rats maintained on long-term, high-dose EB treatment quickly lose the capacity for reflexive erections and show a marked decrease in mass of the BC, but exhibit sexual behavior in tests with females (O’Hanlon et al., 1981). Estrogen-maintained castrates also exhibit erections during and between intromissions, and electromyographic recordings of the BC during copulatory tests indicate that the pattern of EMG activity during copulation is similar in estrogen-maintained and control males (Holmes and Sachs, 1992). This may be related to the observation that EB is as effective as DHT in maintaining excitability of the BC following castration (Fargo et al., 2003; Foster and Sengelaub, 2004b).
Thus, an intact SNB system is not strictly required for copulation. Nonetheless, activity of SNB motoneurons and their target muscles may contribute importantly to fertility. Following surgical removal or denervation of the BC, intense, cup-like erections are eliminated, although weaker, strictly vascular erections are maintained (Sachs, 1982; Hart and Melese-d’Hospital, 1983; Monaghan and Breedlove, 1989). These cup-like erections and other actions of the penis are important for formation and adequate placement of copulatory plugs (Wallach and Hart, 1983), and may provide some of the vaginocervical stimulation essential for the maintenance of pregnancy. Indeed, in rats and mice in which the BC is surgically removed, mating rarely results in successful pregnancy (Sachs, 1982; Elmore and Sachs, 1988).
Dendritic development
Quantitative dendritic reconstruction following retrograde labeling with horseradish peroxidase has demonstrated that the development of SNB motoneuron dendrites in male rats is protracted, extending over the first seven weeks of postnatal life (Goldstein et al., 1990). Over this period, SNB dendrites undergo an initial period of exuberant growth which is followed by a retraction to adult length and distribution. In the first postnatal week, SNB dendrites have established their general distribution, and the arbor extends to the limits of the gray matter laterally and dorsally and is sparse in the lateral aspects of lamina VII. Over the next three weeks, SNB dendrites increase approximately five times in total length, and by P28 they are nearly twice the length of normal adults.
After P28 there is a period of gradual dendritic retraction (Hebbeler and Sengelaub, 2003), and by P49 dendritic length and topology are mature and indistinguishable from those of much older males (Goldstein et al., 1990). While the length of the SNB motoneuron dendrites decreases over this period, their distribution in the gray matter also changes. Dendritic retraction is greatest in areas where the arbors from motoneurons in the two halves of the nucleus overlap (Goldstein et al., 1993), and interactions facilitated by this arbor overlap appear to be involved in regulating the normal retraction of SNB dendrites. Elimination of motoneurons from one side of the SNB nucleus on the day of birth results in a prevention of the normal decline in SNB dendritic length from the exuberant P28 levels. At adulthood, dendritic length in the remaining SNB is over 50% greater than that of normal adults, and increases are predominately in those regions where the arbors from missing SNB motoneurons normally project (Goldstein et al., 1993).
The maturation of SNB dendritic morphology is coincident with the onset of sexual functioning of the SNB system. Intromission, ejaculation, and the penile reflexes mediated by SNB motoneurons, first appear at approximately 7 weeks of age (Sodersten et al., 1977; Sachs and Meisel, 1979). The temporal correlation between SNB motoneuron maturation and reflex onset suggests that structural changes associated with the morphology of SNB somata or dendrites may underlie reflex expression. Electrophysiological maturation is also coincident with the mature morphology that follows the period of retraction (Foster and Sengelaub, 2004a). Following unilateral stimulation of the L6 dorsal root, bilateral recordings at the level of the pudendal motor nerves show that resultant activation of SNB motoneurons is of low amplitude at P28, both ipsilaterally and contralaterally. Activation was measured as peak-to-peak amplitudes or area under the activity curves through a range of stimulus intensities. Between P28 and P49, the amplitude of ipsilateral activation further decreases to adult levels, while contralateral activation dramatically increases. Similarly, latency between stimulus and response decreases from lengthy P28 values to faster adult values by P49. Thus, the function of the SNB neuromuscular system matures during the dendritic reorganization from P28 and P49 during which time juvenile function is replaced by a mature phenotype.
Alterations in the development of SNB dendritic morphology appear to have functional consequences as well. For example, reductions in maternal care (specifically maternal licking of male pups) results in reductions in SNB dendritic length, soma size, and number, as well as reductions in the weight of the BC/LA musculature (Lenz and Sengelaub, 2006; Moore et al., 1992). These changes in the SNB system are associated with deficits in male sexual behavior in adulthood, including increases in latency to ejaculation, post-ejaculatory intromission, and inter-intromission intervals (Moore, 1984). The tactile component of the maternal licking appears to be a critical factor. Using an artificial rearing paradigm, where the amount of stimulation could be directly varied, Lenz et al., 2006 have shown that low levels of postnatal tactile stimulation result in reductions in SNB dendritic length in adulthood. This alteration in SNB dendritic arbor is significantly correlated with several SNB-specific copulatory behaviors, including latency to ejaculation, percent erections with cups, and the number of erection clusters.
Like other aspects of SNB development, dendritic development is also sensitive to androgens (Figure 6). SNB dendrites in males castrated at one week of age fail to grow beyond precastration lengths. SNB dendrites in castrates that receive testosterone replacement grow normally, attaining the typical exuberant length by P28 (Goldstein et al., 1990). Interestingly, treatment of castrated males with either of testosterone’s metabolites, DHT or estradiol, supports SNB dendritic growth through the first 4 postnatal weeks (Goldstein and Sengelaub, 1994), but typically not to the level of testosterone-treated or intact males. Similarly, blocking estrogen receptors by systemic treatment with tamoxifen (Taylor et al., 1995), or preventing estradiol synthesis with fadrozole (Burke et al., 1999), during the period of dendritic growth in gonadally intact males results in dendritic lengths that are significantly below those of normal males (and comparable to those of DHT-treated castrates). Indeed, combined treatment with both metabolites is as effective as testosterone in fully supporting dendritic growth to normal P28 lengths (Burke et al., 1997). Thus, the development of dendritic extent is the first feature of the SNB system with a required role for estrogens. Examination of SNB dendritic growth in perinatally masculinized females further supports a role for estrogens. As described above, females masculinized by perinatal DHT treatment retain their SNB motoneurons and target musculature. Ovarian hormones support SNB dendritic growth in these females, because at P28, SNB dendritic length in intact females is significantly greater than that of ovariectomized females (Hebbeler et al., 2001).
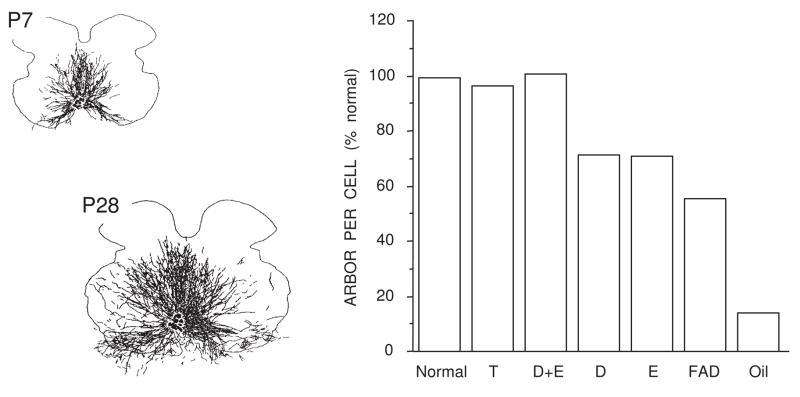
Figure 6.
SNB dendritic growth requires both androgens and estrogens for normal masculine development. (Left) Computer-generated composites of retrogradely-labeled somata and processes drawn at 320 μm intervals through the entire rostrocaudal extent of the SNB of normal males at P7 (top) and P28 (bottom), illustrating the exuberant growth of SNB dendrites. (Right) SNB dendritic lengths at P28 in intact males, postnatally castrated males treated with testosterone propionate (T), dihydrotestosterone propionate combined with estradiol benzoate (D+E), D or E alone, gonadally intact males treated with fadrozole (FAD), and oil-treated castrates. Data are expressed as a percentage of dendritic length in intact males. (Compiled from data originally published in Goldstein et al., 1990, Goldstein and Sengelaub, 1994, Burke et al., 1997, 1999.)
While estrogens appear to be an important factor for support of SNB dendritic growth, SNB motoneurons do not accumulate estradiol during development (Taylor et al., 1995) or in adulthood (Breedlove and Arnold, 1980), suggesting that estrogens exert their effect on SNB dendritic growth indirectly. SNB motoneurons receive afferents from dorsal root ganglia (Sohrabji et al., 1994) and hypothalamic nuclei (Shen et al., 1990; Wagner et al., 1993) that contain estrogen-concentrating neurons. However, the elimination of dorsal root ganglion afferents by dorsal rhizotomy does not prevent SNB dendritic growth in otherwise normal males (Goldstein et al., 1996). Similarly, removal of supraspinal afferents by spinal transection does not prevent SNB dendritic growth in normal males, nor does it block the ability of estrogens to support dendritic growth in castrates (Hebbeler and Sengelaub, 2003). As mentioned previously, the SNB target muscles bind both androgens and estrogens in adults (Dube et al., 1976). In adulthood, SNB dendritic morphology is influenced by androgens acting at the target musculature (see below). Similarly, it appears that estrogens act in the neuromuscular periphery to support postnatal SNB dendritic growth (Nowacek and Sengelaub, 2006). Local blockade of estrogen receptors at the BC muscle of gonadally intact males results in severely reduced dendritic lengths similar to those seen in castrates, whereas local administration of estradiol to the BC muscle of castrated males supports dendritic growth.
This estrogenic influence is limited to the early postnatal period: the morphology of SNB motoneurons is insensitive to estrogens after 4 weeks of age (Goldstein and Sengelaub, 1994; Hebbeler et al., 2001; Warren and Sengelaub, 2002) or in adulthood (Forger et al., 1992; Fargo and Sengelaub, 2007). The transient influence of estrogens on SNB dendritic growth is coincident with a period in which high levels of NMDA receptors are expressed in the spinal cord, and locally in the SNB nucleus (Verhovshek et al., 2005). NMDA receptors are present throughout the spinal gray matter at P7, but are greatly reduced over the next few weeks except in the substantia gelatinosa (Kalb et al., 1992; Stegenga and Kalb, 2001). Given that gonadal hormones have been shown to modulate the expression of NMDA receptors in the brain (Kus et al., 1995; Gazzaley et al., 1996; Cyr et al., 2001), the temporally restricted effects of estrogens in the SNB may be explained through a modulation of this transient NMDA receptor population. Injections of the NMDA receptor antagonist MK-801 into intact males or estradiol-treated castrates attenuates dendritic growth through P28. These results suggest that dendritic development in the SNB involves NMDA receptors, and furthermore that the component of SNB dendritic development sensitive to estrogens requires their activation (Hebbeler et al., 2002).
As mentioned above, after P28 SNB dendrites normally retract to mature lengths. The increase in androgen levels that occur with the onset of puberty may be what halts this dendritic retraction at around P49. If males are castrated at P28 and treated with adult levels of T, retraction is prevented and they retain the early exuberant SNB dendritic length into adulthood (Goldstein et al., 1990). Unlike the initial, exuberant dendritic outgrowth, however, SNB dendritic retraction is specifically sensitive to T. SNB dendritic retraction is not affected in gonadally intact males treated with fadrozole, or castrates treated with EB, DHTP, or both EB and DHTP combined, and at P49 SNB lengths in animals receiving any of these treatments do not differ from those of normal males (Hebbeler et al., 1999).
Adult Dendritic Plasticity
As is seen for SNB somata, nerve terminals, and the BC/LA muscles, SBN dendritic morphology is androgen-dependent throughout life. In adulthood, castration results in a substantial reduction in SNB dendritic length which can be completely reversed with androgen replacement (Figure 7; Kurz et al., 1986). This kind of androgen-dependent, reversible plasticity had not been reported previously in an adult mammalian system, and was particularly exciting because changes in androgen levels occur normally and correlate with changes in the frequency of copulatory behaviors. For example, a naturally occurring analog of hormone depletion and replacement can be seen in seasonally breeding mammals such as white-footed mice. As in rats, castration results in decreased dendritic arbors in these mice (Forger and Breedlove, 1987). Similarly, circulating testosterone levels decline with advanced aging, and these declines are accompanied by reductions in sexual behavior. Fargo et al. (2006) found that the BC/LA muscles and their innervating SNB motoneurons underwent profound atrophy with advancing age, with muscle weight and motoneuron dendritic length declining to less than 50% of young adult levels. Treatment of aged animals with testosterone completely reverses the age-related declines in muscle weight and SNB motoneuron morphology, demonstrating that the SNB system retains its androgen-mediated plasticity throughout life.
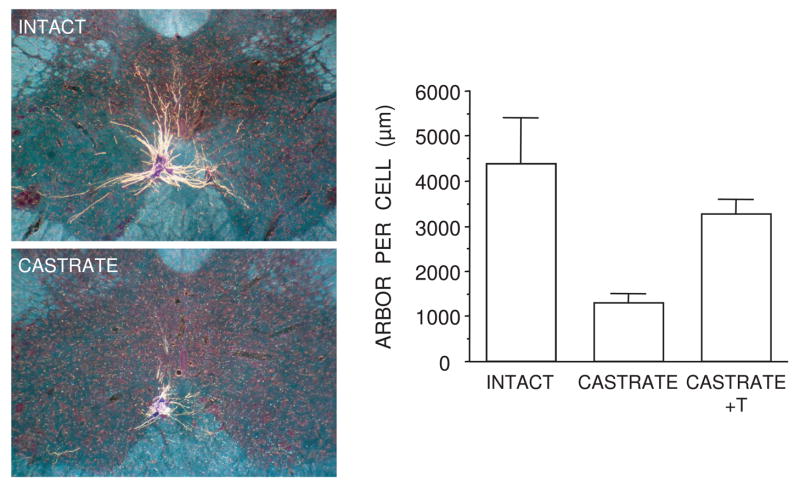
Figure 7.
SNB dendritic maintenance in adulthood requires androgens. (Left) Darkfield digital micrographs of transverse sections through the lumbar spinal cord of an adult normal male (top) and a castrated male (bottom) after injection of horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP), into the left BC muscle. (Right) SNB dendritic lengths expressed as length of arbor per labeled motoneuron for gonadally intact males, castrated males, and castrates treated with testosterone (T). Bar heights represent means ± SEM. (Data after Kurz et al., 1986.)
Concomitant with changes in dendritic extent, androgens control the number and size of gap junctions and synapses on SNB motoneurons (Leedy et al., 1987; Matsumoto et al., 1988a,b). Although the molecular bases for androgenic effects on dendrites, gap junctions or synapses are not fully understood, androgen manipulation directly or indirectly affects the expression of a number of candidate genes and proteins in SNB motoneurons, including those for the gap junction protein connexin 32 (Matsumoto et al., 1992), N-cadherin (Monks et al., 2001), calcitonin gene-related peptide (Popper and Micevych, 1990), AR (Matsumoto et al., 1998), the ciliary neurotrophic factor receptor α (Forger et al., 1998), and the major cytoskeletal elements, β-actin and β-tubulin (Matsumoto et al., 1992; 1993).
Rand and Breedlove (1995) showed that testosterone can regulate SNB dendrites by acting at the target musculature. In castrated males, SNB motoneurons projecting to testosterone-implanted BC/LA muscles have significantly longer dendritic lengths than those projecting to muscles on the contralateral side treated with the anti-androgen flutamide. This result suggests that, similar to the effects described earlier for hormonal support of dendritic growth during postnatal development (Nowacek and Sengelaub, 2006), androgens regulate a neurotrophic signal from the muscle that is critical in the maintenance of dendritic organization in adulthood.
Given the estrogenic support of SNB dendritic growth during the early postnatal period it was possible that both androgens and estrogens could underlie adult dendritic plasticity. However, examination of motoneuron and muscle morphology in intact or castrated males, testosterone-, dihydrotestosterone-, or estradiol-treated castrates, and males treated with either the 5-α-reductase inhibitor finasteride or the aromatase inhibitor fadrozole revealed that testosterone is the most powerful mediator of adult SNB system morphology. EB treatment in castrates fails to maintain masculine adult SNB dendritic or target muscle morphology (Verhovshek et al., 2000), despite effects on neuromuscular physiology (Fargo et al., 2003; Foster and Sengelaub, 2004b). Similarly, blockade of estrogen synthesis with fadrozole reduced sex behavior but did not alter SNB dendritic morphology.
Recent work examining androgen effects on SNB dendrites in strains of Mus musculus have produced mixed results. Consistent with results from rats, BC/LA muscle mass and SNB soma size are reduced in castrated B6D2F1 mice compared to either testosterone-treated castrates or sham-operated animals. However, there are no effects of androgen deprivation on SNB dendritic arbors in this strain (Park et al., 2002). In contrast, using C57BL6J mice, Zuloaga et al. (2007) found reductions in SNB dendritic length after castration that were comparable to those discussed above in rats and white footed mice. The source of these differences in dendritic response to androgen is unknown, but strain differences in androgen response are a strong possibility. B6D2F1 mice are particularly notable in that a significant minority (~30%) of males of this strain continues to exhibit sexual behavior many weeks after castration (Clemens et al., 1988). This raises the possibility that the B6D2F1 strain may be less sensitive to changes in androgen levels than other mouse strains, resulting in retentions of SNB dendritic morphology after castration. On the positive side, given that C57BL6 mice are commonly used for creating transgenic mouse lines, the androgen-sensitivity of the SNB system in this strain suggests they will provide a powerful tool for understanding the mechanisms underlying androgen-regulated plasticity (Zuloaga et al., 2007).
Neurotherapeutic Effects
Gonadal steroids exhibit a wide array of neuroprotective and neurotherapeutic effects (Jones et al., 2001; Henderson and Reynolds, 2002; Woolley and Cohen, 2002). For example, testosterone protects against cell death in cultured hippocampal neurons (Pike, 2001) and injury-induced dendritic atrophy in cortical pyramidal cells (Forgie and Kolb, 2003). The application of androgens stimulates motoneuron axonal growth after peripheral nerve injury, and demonstrates a therapeutic role for gonadal steroids in axon regeneration (Kujawa et al., 1989; Jones et al., 2001).
Partial depletion of SNB motoneurons, induced by injections of the neurotoxin saporin into the target musculature, results in substantial somal and dendritic atrophy in surviving motoneurons (Fargo and Sengelaub, 2004a,b). While motoneuron depletion occurs within a few days after saporin injection, dendritic atrophy in remaining SNB motoneurons progresses linearly over several weeks, and by four weeks is decreased to 66% of intact lengths. Evidence of recovery in dendritic lengths is present at 6 weeks post depletion, and by 10 weeks, SNB dendritic lengths recover to those of normal, intact males (Ferguson and Sengelaub, 2007).
Testosterone treatment is neurotherapeutic in SNB motoneurons, attenuating the induced somal and dendritic atrophy, and concomitant reductions in excitability, resulting from the death of nearby motoneurons (Fargo and Sengelaub, 2004a,b). While initial dendritic atrophy (2 weeks after motoneuron depletion) is similar to that of untreated saporin-injected males, T treatment prevents further decrements in length (Ferguson and Sengelaub, 2007). This attenuation of dendritic atrophy by T is dose-dependent (Figure 8), and four weeks after motoneuron depletion, SNB dendritic lengths in males treated with physiological levels of T are 102% longer than those of untreated, saporin-injected males, and not different from those of intact control males (Coons et al., 2007).
Figure 8.
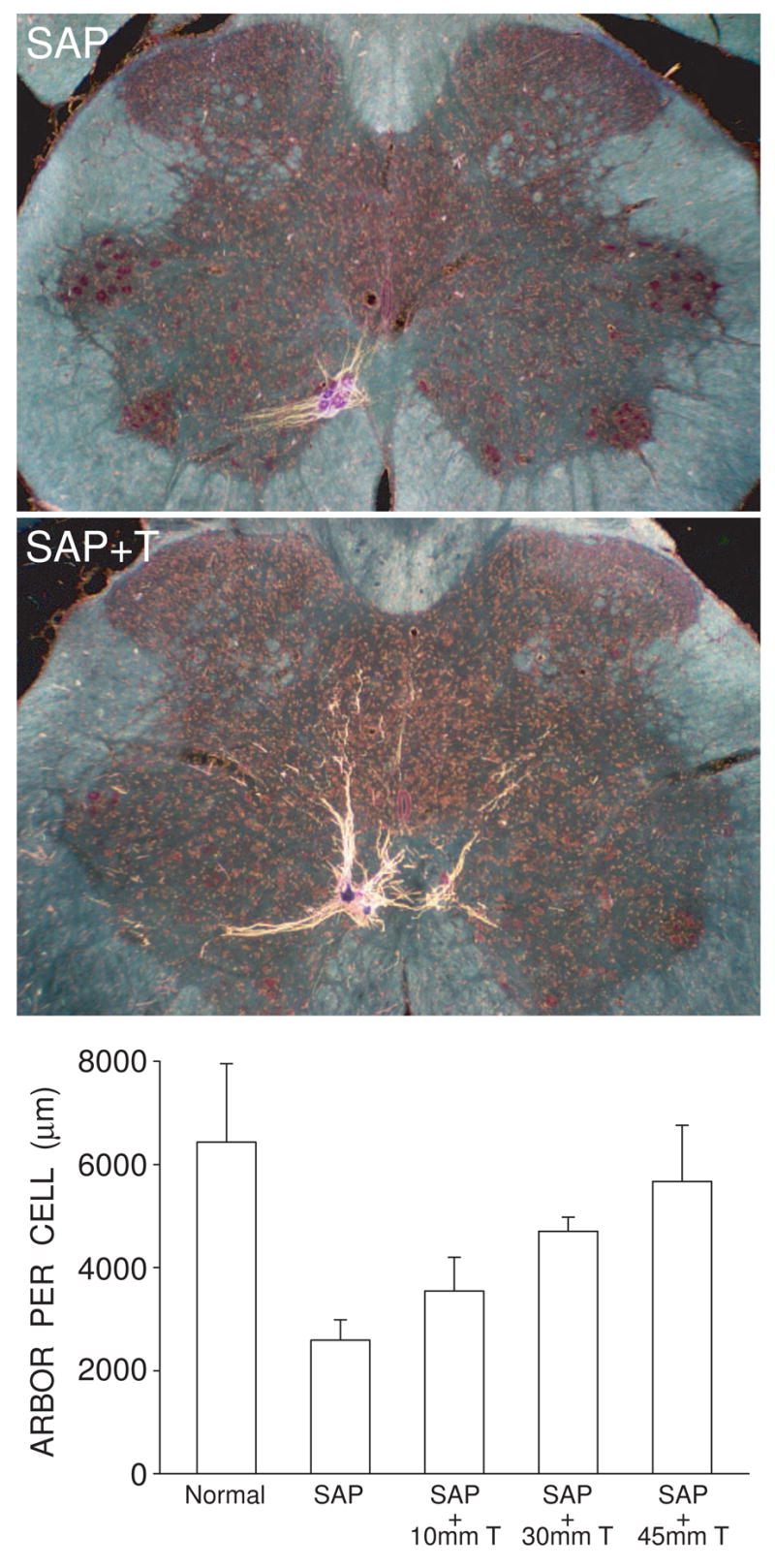
Testosterone treatment is neurotherapeutic in SNB motoneurons, attenuating induced dendritic atrophy resulting from the death of nearby motoneurons. (Left) Darkfield digital micrographs of transverse sections through the lumbar spinal cords of males whose SNB motoneurons have been partially depleted with the toxin saporin, and then left untreated (SAP) or given supplemental testosterone (SAP + T), after BHRP injection into the left BC muscle. (Right) SNB dendritic lengths expressed as length of arbor per labeled motoneuron for normal males and saporin-treated males with or without supplemental testosterone. Partial motoneuron depletion reduces the dendritic arbor of remaining motoneurons. However, in rats whose androgen levels had been supplemented with varying levels of T, this atrophy is attenuated in a dose-dependent manner. Bar heights represent means ± SEM. (Data from Coons et al., 2007.)
Consistent with most other morphological effects in adult SNB motoneurons, the neuroprotective effects of steroid treatment appear to be strictly androgenic, as treatment with either T or DHT, but not EB, attenuates induced somal and dendritic atrophy following saporin injections (Fargo and Sengelaub, 2007). Thus, the SNB stands in contrast to some other areas in the nervous system, where estradiol has powerful neuroprotective effects (Henderson and Reynolds, 2002; Hoffman et al., 2006).
The neuroprotective effects of androgens after saporin-induced motoneuron depletion in this system could be mediated by the muscle (Fargo and Sengelaub, 2004a,b). As described above, Rand and Breedlove (1995) showed that testosterone can regulate dendrites in adulthood by acting at the target musculature, suggesting that androgens regulate a target-derived neurotrophic signal critical for the maintenance of dendritic organization. The BC/LA muscles produce brain-derived neurotrophic factor (BDNF) (Yang and Arnold, 1999). By applying BDNF peripherally to cut BC/LA nerves, Yang et al. (2004) showed that SNB dendritic morphology can be regulated by trophic substances from the neuromuscular periphery as long as testosterone is also present. The requirement for testosterone may be at least partially due to the fact that the expression of the BDNF receptor, trkB, in SNB motoneurons is regulated by T: motoneurons of castrated animals deprived of T show reduced expression of trkB receptors compared to motoneurons of intact animals or castrated animals given T replacement (Osborn et al., 2007; Ottem et al., 2007).
Conclusions
In summary, it is clear that directly or indirectly, androgenic action dominates the SNB system throughout the lifespan, regulating structural and functional features at every level. Although the early hypothesis that masculinization of the spinal cord is mediated by androgens, while estrogens are responsible for the establishment of sex differences in the brain must be abandoned, androgens and AR are undeniably of central importance for the SNB neuromuscular system.
Motoneuron number in the SNB was the first neural sex difference shown to be due to androgenic action, and this system has gone on to demonstrate many additional and equally important “firsts”, only some of which we list here. The sex difference in SNB motoneuron number was the first shown to result from androgen-regulated neuronal cell death, and thus also the first sexually dimorphic neural system where the cellular basis of a critical period was understood. Similarly, the SNB system was the first to demonstrate steroid effects on neuromuscular synapse elimination during development. The SNB was the first sexually dimorphic neural system demonstrated to respond to neurotrophic factors (both in development and adulthood). SNB motoneurons provided the first example of androgen-dependent, reversible dendritic plasticity in an adult mammal, and the first documentation of an important role for androgens in attenuating dendritic atrophy following adult neuronal injury. Work on the SNB was also the first to demonstrate that hormones can act indirectly (e.g., via target cells) to affect a population of neurons, as well the first neural system to show a cell-autonomous response to steroids in vivo.
This modest population of motoneurons has generated over 200 papers, and provided insights that have generalized to questions, processes, and structures throughout the nervous system. With the addition of new tools, such as transgenic models and molecular approaches, the SNB system will no doubt continue to be a rich source of information. Not bad for 200 motoneurons.
Acknowledgments
The authors wish to thank their anonymous reviewers for their helpful comments on the manuscript. This review was prepared with support from NIH grants NS24877, HD35315, AG09308, and NS047264 (to D.R.S.), and MH072825, MH068482 (to N.G.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki I, Harada Y, Kuna M. Target-dependent hormonal control of neuron size in the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1991;11:3025–3033. doi: 10.1523/JNEUROSCI.11-10-03025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Breedlove SM, Bernstein S, Lichtman JW. Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci. 1990;10:2660–2671. doi: 10.1523/JNEUROSCI.10-08-02660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengston L, Lopez V, Watamura S, Forger NG. Short- and long-term effects of ciliary neurotrophic factor on androgen-sensitive motoneurons in the lumbar spinal cord. J Neurobiol. 1996;31:263–273. doi: 10.1002/(SICI)1097-4695(199610)31:2<263::AID-NEU10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson A. Androgens modulate endplate size and ACh receptor density at synapses in rat levator ani muscle. J Neurobiol. 1989;20:189–202. doi: 10.1002/neu.480200403. [DOI] [PubMed] [Google Scholar]
- Bleisch WV, Harrelson AL, Luine VN. Testosterone increases acetylcholine receptor number in the “levator ani” muscle of the rat. J Neurobiol. 1982;13:153–161. doi: 10.1002/neu.480130207. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Hormonal control of the anatomical specificity of motoneuron-to-muscle innervation in rats. Science. 1985;227:1357–1359. doi: 10.1126/science.3975621. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Neonatal androgen and estrogen treatments masculinize the size of motoneurons in the rat spinal nucleus of the bulbocavernosus. Cell Mol Neurobiol. 1997;17:687–697. doi: 10.1023/a:1022590104697. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237:173–181. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983a;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983b;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KA, Kuwajima M, Sengelaub DR. Aromatase inhibition reduces dendritic growth in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1999;38:301–312. [PubMed] [Google Scholar]
- Burke KA, Widows MR, Sengelaub DR. Synergistic effects of testosterone metabolites on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1997;33:1–10. doi: 10.1002/(sici)1097-4695(199707)33:1<1::aid-neu1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Čihák R, Gutmann E, Hanzlikova V. Involution and hormone-induced persistence of the muscle sphincter (levator ani) in female rats. J Anat (Lond) 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Clemens LG, Wee BEF, Weaver DR, Roy EJ, Goldman BD, Rakerds B. Retention of masculine sexual behavior following castration in male B6D2F1 mice. Physiol Behav. 1988;42:69–76. doi: 10.1016/0031-9384(88)90262-4. [DOI] [PubMed] [Google Scholar]
- Collins WF, III, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992;577:326–330. doi: 10.1016/0006-8993(92)90292-h. [DOI] [PubMed] [Google Scholar]
- Coons KD, Wilson RE, Sengelaub DR. Protection from dendritic atrophy with testosterone following partial motoneuron depletion: dose-dependence in males and efficacy in females. Soc Neurosci Abstr Viewer/Itinerary Planner. 2007 Program No. 56.11. [Google Scholar]
- Currie JC, Houston PA, Henderson A, Payne AP. Neonatal hormone manipulations and the maintenance of perineal muscles and their motor neurones in Albino Swiss rats. J Reprod Fertil. 1990;89:597–603. doi: 10.1530/jrf.0.0890597. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morisette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Pre- and postnatal influence of testosterone propionate and diethylstilbesterol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984a;302:291–295. doi: 10.1016/0006-8993(84)90242-7. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Sickmoller PM, Jarzab B, Gorski RA. Pre- and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Endocrinology. 1986;42:443–448. doi: 10.1159/000124484. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen anatagonist. Neuroendocrinology. 1984b;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- Dube JY, Lesage R, Tremblay RR. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Elmore LA, Sachs BD. Role of the bulbospongiosus muscles in sexual behavior and fertility in the house mouse. Physiol Behav. 1988;44:125–129. doi: 10.1016/0031-9384(88)90355-1. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Foster AM, Harty MW, Sengelaub DR. Estrogen alters excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. J Neurobiol. 2003;56:66–77. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J Neurobiol. 2004a;60:348–359. doi: 10.1002/neu.20027. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol. 2004b;469:96–106. doi: 10.1002/cne.10991. [DOI] [PubMed] [Google Scholar]
- Fargo KW, Iwema CL, Clark-Phelps MC, Sengelaub DR. Androgen-mediated plasticity in an aging sexually dimorphic motor system. Horm Behav. 2006;51:20–30. [Google Scholar]
- Fargo KN, Sengelaub DR. Androgenic, but not estrogenic, protection of motoneurons from somal and dendritic atrophy induced by the death of neighboring motoneurons. Dev Neurobiol. 2007;67:1094–1106. doi: 10.1002/dneu.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AS, Sengelaub DR. Dendritic atrophy following partial motoneuron depletion: time course of recovery and protection with testosterone. Soc Neurosci Abstr Viewer/Itinerary Planner. 2007 Program No. 56.24. [Google Scholar]
- Fishman RB, Breedlove SM. Neonatal androgen maintains sexually dimorphic muscles in the absence of innervation. Muscle Nerve. 1988;11:553–560. doi: 10.1002/mus.880110606. [DOI] [PubMed] [Google Scholar]
- Fishman RB, Chism L, Firestone GL, Breedlove SM. Evidence for androgen receptors in sexually dimorphic perineal muscles of neonatal male rats. Absence of androgen accumulation by the perineal motoneurons. J Neurobiol. 1990;21:694–704. doi: 10.1002/neu.480210504. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Sexual dimorphism in human and canine spinal cord: Role of early androgen. Proc Natl Acad Sci USA. 1986;83:7527–7531. doi: 10.1073/pnas.83.19.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Seasonal variation in mammalian striated muscle mass and motoneuron morphology. J Neurobiol. 1987;18:155–165. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- Forger NG, Fishman RB, Breedlove SM. Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav. 1992;26:204–213. doi: 10.1016/0018-506x(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Forger NG, Roberts LS, Wong V, Breedlove SM. Ciliary neurotrophic factor maintains motoneurons and their target muscles in developing rats. J Neurosci. 1993;13:4720–4726. doi: 10.1523/JNEUROSCI.13-11-04720.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol. 1996;375:333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Forger NG, Wagner CK, Contois M, Bengston L, MacLennan AJ. Ciliary neurotrophic factor receptor α in spinal motoneurons is regulated by gonadal hormones. J Neurosci. 1998;18:8720–8729. doi: 10.1523/JNEUROSCI.18-21-08720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgie ML, Kolb B. Manipulation of gonadal hormones in neonatal rats alters the morphological response of cortical neurons to brain injury in adulthood. Behav Neurosci. 2003;117:257–262. doi: 10.1037/0735-7044.117.2.257. [DOI] [PubMed] [Google Scholar]
- Foster AM, Sengelaub DR. Development of penile reflexes and electrophysiological activity in a sexually dimorphic spinal rat spinal neuromuscular system. Soc Neurosci Abstr Viewer/Itinerary Planner. 2004a Program No. 946.15. [Google Scholar]
- Foster AM, Sengelaub DR. Hormone sensitivity of muscle activation in the sexually dimorphic SNB/BC neuromuscular system of the rat. Neurosci Lett. 2004b;359:41–44. doi: 10.1016/j.neulet.2004.01.065. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Ulibarri CM. Long term castration affects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res. 2002;953:265–271. doi: 10.1016/s0006-8993(02)02949-9. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Watson NV, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Horm Behav. 1996;30:424–433. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George FW, Johnson L, Wilson JD. The effect of a 5 alpha-reductase inhibitor on androgen physiology in the immature male rat. Endocrinol. 1989;125:2434–2438. doi: 10.1210/endo-125-5-2434. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Kalkbrenner A, Sengelaub DR. Changes in dendritic morphology of rat spinal motoneurons during development and after unilateral target deletion. Dev Brain Res. 1993;73:151–163. doi: 10.1016/0165-3806(93)90133-u. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–946. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Mills AC, Sengelaub DR. Motoneuron development after deafferentation. I. Dorsal rhizotomy does not alter growth in the spinal nucleus of the bulbocavernosus. Dev Brain Res. 1996;91:11–19. doi: 10.1016/0165-3806(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. IV. Masculinization of the spinal nucleus of the bulbocavernosus with testosterone metabolites. J Neurobiol. 1990;21:719–730. doi: 10.1002/neu.480210506. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Timing and duration of dihydrotestosterone treatment affect the development of motoneuron number and morphology in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 1992;326:147–157. doi: 10.1002/cne.903260113. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J Neurobiol. 1994;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Gray GD, Smith ER, Davidson JM. Hormonal regulation of penile erection in castrated male rats. Physiol Behav. 1980;24:463–468. doi: 10.1016/0031-9384(80)90237-1. [DOI] [PubMed] [Google Scholar]
- Hart BL. The action of extrinsic penile muscles during copulation in the male dog. Anat Rec. 1972;173:1–6. doi: 10.1002/ar.1091730101. [DOI] [PubMed] [Google Scholar]
- Hart BL. Activation of sexual reflexes of male rats by dihydrotestosterone but not estrogen. Physiol Behav. 1979;23:107–109. doi: 10.1016/0031-9384(79)90129-x. [DOI] [PubMed] [Google Scholar]
- Hart BL, Haugen CM. Activation of sexual reflexes in male rats by spinal implantation of testosterone. Physiol Behav. 1968;3:735–738. [Google Scholar]
- Hart BL, Melese-D’Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–813. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- Hart BL, Wallach SJR, Melese-d’Hospital PY. Differences in responsiveness to testosterone of penile reflexes and copulatory behavior of male rats. Horm Behav. 1983;17:274–283. doi: 10.1016/0018-506x(83)90026-0. [DOI] [PubMed] [Google Scholar]
- Hayes KJ. The so-called ‘levator ani’ of the rat. Acta Endocrinol. 1965;48:337–347. doi: 10.1530/acta.0.0480337. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. Estrogen regulates growth but not retraction of dendrites in a sexually dimorphic rat spinal nucleus. Soc Neurosci Abstr. 1999;25:1269. [Google Scholar]
- Hebbeler SL, Sengelaub DR. Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: morphologic changes and implications for estrogen sites of action. J Comp Neurol. 2003;467:80–96. doi: 10.1002/cne.10911. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. Ovariectomy attenuates dendritic growth in hormone-sensitive spinal motoneurons. J Neurobiol. 2001;48:301–314. doi: 10.1002/neu.1059. [DOI] [PubMed] [Google Scholar]
- Hebbeler SL, Verhovshek T, Sengelaub DR. N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J Comp Neurol. 2002;451:142–152. doi: 10.1002/cne.10347. [DOI] [PubMed] [Google Scholar]
- Hegstrom CD, Breedlove SM. Seasonal plasticity of neuromuscular junctions in adult male Siberian hamsters (Phodopus sungorus) Brain Res. 1999;819:83–88. doi: 10.1016/s0006-8993(98)01315-8. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Reynolds DW. Protective effects of estrogen on aging and damaged neural systems. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 821–837. [Google Scholar]
- Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Sachs BD. Erectile function and bulbospongiosus EMG activity in estrogen-maintained castrated rats vary with behavioral context. Horm Behav. 1991;26:406–419. doi: 10.1016/0018-506x(92)90010-s. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Sachs BD. Physiology and mechanics of rat levator ani muscle: evidence for a sexual function. Physiol Behav. 1994;55:255–266. doi: 10.1016/0031-9384(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB. Gender-specific steroid metabolism in neural differentiation. Cell Molec Neurobiol. 1997;17:603–626. doi: 10.1023/a:1022581902880. [DOI] [PubMed] [Google Scholar]
- Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2004;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Horm Behav. 1997;32:1–10. doi: 10.1006/hbeh.1997.1397. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Breedlove SM, Arnold AP. Ontogeny of steroid accumulation in spinal lumbar motoneurons of the rat: implications for androgen’s site of action during synapse elimination. J Comp Neurol. 1991;313:441–448. doi: 10.1002/cne.903130304. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. Synapse elimination occurs late in the hormone-sensitive levator ani muscle of the rat. J Neurobiol. 1988;19:335–356. doi: 10.1002/neu.480190403. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. I. Androgen delays the loss of multiple innervation in the levator ani muscle. J Neurosci. 1989a;9:229–238. doi: 10.1523/JNEUROSCI.09-01-00229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Letinsky MS, Arnold AP. The role of gonadal hormones in neuromuscular synapse elimination in rats. II. Multiple innervation persists in the adult levator ani muscle after juvenile androgen treatment. J Neurosci. 1989b;9:239–247. doi: 10.1523/JNEUROSCI.09-01-00239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997;379:88–98. [PubMed] [Google Scholar]
- Jordan CL, Watamura S, Arnold AP. Androgenic, not estrogenic, steroids alter neuromuscular synapse elimination in the rat levator ani. Dev Brain Res. 1995;84:225–232. doi: 10.1016/0165-3806(94)00175-y. [DOI] [PubMed] [Google Scholar]
- Kalb RG, Lidow MS, Halsted MJ, Hockfield S. N-Methyl-d-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc Natl Acad Sci USA. 1992;89:8502–8506. doi: 10.1073/pnas.89.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Sengelaub DR. Development of altered motoneuron-to-muscle specificity in androgen-sensitive rat spinal nuclei. Soc Neurosci Abstr. 1992;18:233. [Google Scholar]
- Karacan I, Aslan C, Hirshkowitz M. Erectile mechanisms in man. Science. 1983;220:1080–1082. doi: 10.1126/science.6844930. [DOI] [PubMed] [Google Scholar]
- Konigsmark BW. Methods for the counting of neurons. In: Nauta WJH, Ebbeson S0E, editors. Contemporary Research Methods in Neuroanatomy. Springer-Verlag; New York: 1970. pp. 315–340. [Google Scholar]
- Knudsen JF, Max SR. Aromatization of androgens to estrogens mediates increased activity of glucose 6-phosphate dehydrogenase in rat levator ani muscle. Endocrinol. 1980;106:440–443. doi: 10.1210/endo-106-2-440. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Bowers CA, Sengelaub DR. Morphology of rat spinal motoneurons with normal and hormonally altered specificity. J Comp Neurol. 1990;292:638–656. doi: 10.1002/cne.902920412. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Kus L, Handa RJ, Sanderson JJ, Kerr JE, Beitz AJ. Distribution of NMDAR1 receptor subunit mRNA and [125I]MK-801 binding in the hypothalamus of intact, castrate and castrate-DHTP treated male rats. Mol Brain Res. 1995;28:55–60. doi: 10.1016/0169-328x(94)00179-i. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jordan CL, Arnold AP. Critical period for androgenic regulation of soma size of sexually dimorphic motoneurons in rat lumbar spinal cord. Neurosci Letts. 1989;98:79–84. doi: 10.1016/0304-3940(89)90377-7. [DOI] [PubMed] [Google Scholar]
- Leedy MG, Beattie MS, Bresnahan JC. Testosterone-induced plasticity of synaptic inputs to adult mammalian motoneurons. Brain Res. 1987;424:386–390. doi: 10.1016/0006-8993(87)91484-3. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Graham MD, Fleming A, Sengelaub DR, Monks DA. Penile reflexes and SNB motoneuron morphology in artificially reared male rats under various conditions of tactile stimulation. Soc Neurosci Abstr Viewer/Itinerary Planner. 2006 Program No. 150.10. [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Res. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Arnold AP. Evidence for target regulation of the development of androgen sensitivity in rat spinal motoneurons. Dev Neurosci. 1995;17:106–117. doi: 10.1159/000111279. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Bebinger DM. Regulation of terminal Schwann cell number at the adult neuromusculr junction. J Neurosci. 1999;19(1–5):RC46. doi: 10.1523/JNEUROSCI.19-24-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expression of gap junction and b-actin mRNAs in adult rat motoneurons. Neurosci Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Hyodo S. Androgenic regulation of expression of β-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J Neuroendocrinol. 1993;5:357–363. doi: 10.1111/j.1365-2826.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi GA, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci. 1988a;8:4177–4183. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Micevych PE, Arnold AP. Androgen regulates synaptic input to motoneurons of the adult rat spinal cord. J Neurosci. 1988b;8:4168–4176. doi: 10.1523/JNEUROSCI.08-11-04168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248:532–549. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. Minireview: nuclear receptor coactivators – an update. Endocrinol. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- Meisel RL, O’Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm Behav. 1984;18:56–64. doi: 10.1016/0018-506x(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Breedlove SM. The role of the bulbocavernosus in penile reflex behavior in rats. Brain Res. 1992;587:178–180. doi: 10.1016/0006-8993(92)91443-i. [DOI] [PubMed] [Google Scholar]
- Monks DA, Getsios S, MacCalman CD, Watson NV. N-cadherin is regulated by gonadal steroids in adult sexually dimorphic spinal motoneurons. J Neurobiol. 2001;47:255–264. doi: 10.1002/neu.1033. [DOI] [PubMed] [Google Scholar]
- Monks DA, Kopachik W, Breedlove SM, Jordan CL. Anabolic responsiveness of skeletal muscles correlates with androgen receptor protein but not mRNA. Can J Physiol Pharmacol. 2006;84:272–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- Monks DA, O’Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: Enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks DA, Xu J, O’Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen-mediated sexual differentiation of spinal motoneurons. Neuroendocrinol. 2003;78:45–51. doi: 10.1159/000071705. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL, Dou H, Juraska JM. Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Res. 1992;572:52–56. doi: 10.1016/0006-8993(92)90449-j. [DOI] [PubMed] [Google Scholar]
- Naess O, Haug E, Ahramadal A, Aakvvaag A, Hansson V, French F. Androgen receptors in the anterior pituitary and central nervous system of the androgen “insensitive” (Tfm) rat: Correlation between receptor binding and effects of androgens on gonadotropin secretion. Endocrinology. 1976;99:1295–1303. doi: 10.1210/endo-99-5-1295. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nowacek AS, Sengelaub DR. Estrogenic support of motoneuron dendritic growth via the neuromuscular periphery in a sexually dimorphic motor system. J Neurobiol. 2006;66:962–976. doi: 10.1002/neu.20274. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and –unresponsive motoneurons. Horm Behav. 2005;47:29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- O’Hanlon JK, Meisel RL, Sachs BD. Estradiol maintains castrated male rats’ sexual reflexes in copula, but not ex copula. Behav Neural Biol. 1981;32:269–273. doi: 10.1016/s0163-1047(81)90645-2. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Induction of male mating behavior in androgen-insensitive (tfm) and normal (King-Holzman) male rats: effect of testosterone propionate, estradiol benzoate, and dihydrotestosterone. Horm Behav. 1979;13:66–84. doi: 10.1016/0018-506x(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB expression in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (trkB) in the sexually dimorphic spinal nucleus of the bulbocavernosus (SNB) Endocrinol. 2007 April 26, 2007; doi: 10.1210/en.2007-0308. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Park JJ, Zup SL, Verhovshek T, Sengelaub DR, Forger NG. Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and Bcl-2 overexpressing mice. J Neurobiol. 2002;53:403–412. doi: 10.1002/neu.10103. [DOI] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Popper P, Micevych PE. Steroid regulation of calcitonin gene-related peptide mRNA expression in motoneurons of the spinal nucleus of the bulbocavernosus. Mol Brain Res. 1990;8:159–166. doi: 10.1016/0169-328x(90)90060-q. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen locally regulates rat bulbocavernosus and levator ani size. J Neurobiol. 1992;23:17–30. doi: 10.1002/neu.480230104. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66:433–443. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Meisel RL. Pubertal development of penile reflexes and copulation in male rats. Psychoneuroendocrinology. 1979;4:287–296. doi: 10.1016/0306-4530(79)90013-1. [DOI] [PubMed] [Google Scholar]
- Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Arnold AP. Development and loss of early projections in a sexually dimorphic rat spinal nucleus. J Neurosci. 1986;6:1613–1620. doi: 10.1523/JNEUROSCI.06-06-01613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengelaub DR, Jordan CL, Kurz EM, Arnold AP. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. II. Development of the spinal nucleus of the bulbocavernosus in androgen insensitive (Tfm) rats. J Comp Neurol. 1989a;280:630–636. doi: 10.1002/cne.902800412. [DOI] [PubMed] [Google Scholar]
- Sengelaub DR, Nordeen EJ, Nordeen KW, Arnold AP. Hormonal control of neuron number in sexually dimorphic nuclei in the rat spinal cord. III. Differential effects of the androgen dihydrotestosterone. J Comp Neurol. 1989b;280:637–644. doi: 10.1002/cne.902800413. [DOI] [PubMed] [Google Scholar]
- Shen P, Arnold AP, Micevych PE. Supraspinal projections to the ventromedial lumbar spinal cord in adult male rats. J Comp Neurol. 1990;300:263–272. doi: 10.1002/cne.903000209. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Damassa DA, Smith ER. Sexual behavior in developing male rats. Horm Behav. 1977;8:320–341. doi: 10.1016/0018-506x(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Ovarian hormones differentially regulate estrogen and nerve growth factor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegenga SL, Kalb RG. Developmental regulation of N-methyl-D-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience. 2001;105:499–507. doi: 10.1016/s0306-4522(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Taylor SR, Widows MR, Sengelaub DR. Estrogenic influence and possible site of action in development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. Soc Neurosci Abstr. 1995;21:40. [Google Scholar]
- Tobin C, Joubert Y. The levator ani of the female rat: a suitable model for studying the effects of testosterone on the development of mammalian muscles. Biol Struct Morphog. 1988;1:28–33. [PubMed] [Google Scholar]
- Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY, Ho-Kim MA, Lesage R. Determination of rat muscles androgen-receptor complexes with methyltrienolone. Steroids. 1977;29:185–195. doi: 10.1016/0039-128x(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Mizuno N, Takahashi O, Nomura S, Arakawa H, Matsushima R. Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol. 1985;232:548–556. doi: 10.1002/cne.902320411. [DOI] [PubMed] [Google Scholar]
- Ulibarri C, Popper P, Micevych PE. Motoneurons dorsolateral to the central canal innervate perineal muscles in the Mongolian gerbil. J Comp Neurol. 1995;356:225–237. doi: 10.1002/cne.903560207. [DOI] [PubMed] [Google Scholar]
- Venable JH. Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat. 1966;119:271–301. doi: 10.1002/aja.1001190206. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Harty M, Sergent M, Sengelaub DR. Estrogen alters excitability but not morphology of a sexually dimorphic neuromuscular system in adult rats. Soc Neurosci Abstr. 2000;26:593. doi: 10.1002/neu.10224. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Wellman CL, Sengelaub DR. NMDA receptor binding declines differentially in three spinal motor nuclei during postnatal development. Neurosci Lett. 2005;384:122–126. doi: 10.1016/j.neulet.2005.04.080. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Sisk CL, Clemens LG. Neurons in the paraventricular nucleus of the hypothalamus that project to the sexually dimorphic lower lumbar spinal cord concentrate 3H-estradiol in the male rat. J Neuroendocrinol. 1993;5:545–551. doi: 10.1111/j.1365-2826.1993.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff GC. The effects of castration and testosterone propionate on the striated perineal musculature in the rat. Endocrinology. 1941;29:975–978. [Google Scholar]
- Wallach SJ, Hart BL. The role of the stiated penile muscles of the male rat in seminal plug dislodgement and deposition. Physiol Behav. 1983;31:815–821. doi: 10.1016/0031-9384(83)90278-0. [DOI] [PubMed] [Google Scholar]
- Ward OB, Wexler AM, Carlucci JR, Eckert MA, Ward I. Critical periods of sensitivity of sexually dimorphic spinal nuclei to prenatal testosterone exposure in female rats. Horm Behav. 1996;30:407–415. doi: 10.1006/hbeh.1996.0045. [DOI] [PubMed] [Google Scholar]
- Warren MA, Sengelaub DR. Treatment with testosterone, but not its metabolites, prevents dendritic retraction during development in a sexually dimorphic rat spinal nucleus. Soc Neurosci Abstr Viewer/Itinerary Planner. 2002 Program No. 33.19. [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: Direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21:1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Cohen RS. Sex steroids and neuronal growth in adulthood. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 717–777. [Google Scholar]
- Xu J, Gingras KM, Bengston L, Di Marco A, Forger NG. Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons. J Neurosci. 2001;21:4366–4372. doi: 10.1523/JNEUROSCI.21-12-04366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Arnold AP. The bulbocavernosus and levator ani (BC/LA) muscle complex express BDNF protein. Soc Neurosci Abstr. 1999;25:1269. [Google Scholar]
- Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Morris JA, Monks DA, Breedlove SM, Jordan CL. Androgen-sensitivity of somata and dendrites of spinal nucleus of the bulbocavernosus (SNB) motoneurons in male C57BL6J mice. Horm Behav. 2007;51:207–212. doi: 10.1016/j.yhbeh.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]