Abstract
In this report, we demonstrate that H-NS is essential for establishing the Mg2+-responsive transcriptional regulation of the PhoP regulon in Salmonella. Deletion of this regulatory gene abolished the transcriptional repression of PhoP-activated genes when bacteria were grown in high environmental Mg2+, thus stimulating expression of phoP and other PhoP regulon genes. In the absence of H-NS, transcriptional activation was PhoP-dependent for those genes only activated by PhoP, but was PhoP-independent for those genes activated by both PhoP and SlyA. The H-NS protein footprints the phoP promoter in a sequence located upstream of the PhoP box; mutation of this cis-acting factor abolished transcriptional repression of the phoP gene equivalent to the phenotype exhibited in the hns mutant. Further results showed that H-NS gel shifts other PhoP regulon promoters, indicating that a PhoP-activated gene would be transcriptionally repressed via direct H-NS binding and inhibition of its activator PhoP. Furthermore, H-NS footprints a newly identified SlyA box and the reverse PhoP box in the pagC promoter, suggesting that both SlyA and PhoP compete with this regulatory protein. Therefore, H-NS should pair with SlyA and PhoP to establish a forward regulatory loop to regulate expression of pagC, and perhaps other PhoP- and SlyA-dependent genes.
The PhoP/PhoQ two-component system controls several cellular functions essential for bacterial virulence in Salmonella typhimurium by responding to environmental Mg2+, pH, and host-secreted antimicrobial peptides (1–4). The sensor protein PhoQ interacts with these signals and induces phosphorylation of the response regulator PhoP (5, 6), which then binds to its target promoters with higher affinity in vivo (7) and stimulates transcription of PhoP-activated genes (8). A conserved hexanucleotide repeat separated by 5 nucleotides, termed PhoP box, present in the promoter region of many PhoP-regulated genes (7, 9) is recognized by the PhoP protein. In addition, other regulators are also involved in activation of these PhoP-dependent loci. We and other researchers have demonstrated that MarR-type regulator SlyA is required for the transcriptional activation of two chromosomal loci, ugtL and pagC, through a feed-forward loop (10, 11). The ugtL and pagC genes are Salmonella-specific loci that are activated by PhoP (12, 13) and expressed in response to low Mg2+ conditions that activate the PhoP/PhoQ system (1). The PhoP protein binds to the ugtL promoter by interacting with a reverse PhoP box, and substitutions of this sequence abolished its transcription (10). Meanwhile, SlyA, whose transcription appears to be activated by PhoP (14), footprints a DNA sequence located downstream of the transcription start (+1) of the ugtL promoter (10), implying that a direct protein-DNA interaction is essential for its function (10).
Contrary to the results from the in vivo expression (7), a protein-DNA interaction assay showed that the unphosphorylated PhoP protein exhibits a similar affinity to PhoP-dependent promoters in vitro when compared with the phosphorylated form (15). This raised the possibility that an unknown cellular factor is involved in establishing an in vivo transcriptional repression of PhoP-activated genes in response to high Mg2+. Indeed, another study suggested that the unphosphorylated form of the PhoP protein could mediate transcriptional activation when it was overexpressed (16), probably because PhoP could compete in the promoter region with the unknown repressor when the concentration reached a high level.
DNA-binding protein H-NS is a global transcriptional regulator that specifically silences horizontally acquired virulence genes in Salmonella (17). This process is fulfilled through interactions between H-NS and AT-rich DNA regions with low specificity (for review see Ref. 18). Interestingly, a recent report claimed that a mutation at the hns locus derived from Salmonella wild-type 14028s was lethal, but was able to be suppressed when additional deletions occurred at the phoP or rpoS locus (17). It remains unknown whether bacterial viability was affected by simultaneously deleting the 90-bp upstream and a partial coding region in the hns gene or by a cryptic mutation existing in that particular resulting strain. Nevertheless, their results implied that H-NS might interact with various PhoP-dependent promoters throughout the Salmonella genome (17).
Here we report that H-NS controls transcriptional repression of horizontally acquired PhoP-activated genes when Salmonella cells experience high Mg2+ conditions. We show that a strain derived from wild-type ATCC 14028s harboring the deleted hns coding region was viable, and transcription of these PhoP-regulated genes was kept activated under both low and high Mg2+ conditions. This is because the unphosphorylated PhoP is able to bind to their promoters when H-NS is absent. H-NS undergoes a two-step interaction, i.e. binding to the regulator phoP promoter and the individual target promoter, which establishes the PhoP-directed, Mg2+-responsive regulation. The H-NS protein footprints an adjacent sequence located upstream of the PhoP box in the phoP promoter, and also footprints the SlyA box and PhoP box in the PhoP- and SlyA-dependent pagC promoter. We demonstrate that PhoP functions as a transcriptional activator for PhoP-activated transcription, whereas it functions as a transcriptional anti-repressor for PhoP- and SlyA-dependent transcription.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions—Strains used in this study are described in Table 1. All Salmonella enterica serovar typhimurium strains are derived from the wild-type strain 14028s. Phage P22-mediated transductions in Salmonella are performed as described previously (19). Bacteria are grown at 37 °C in Luria-Bertani broth or in N minimal medium, pH 7.4, supplemented with 0.1% casamino acids and 38 mm glycerol (20). MgCl2 is added to the required concentrations. When necessary, antibiotics were added at final concentrations of 50 μg/ml for ampicillin, 20 μg/ml for chloramphenicol, and 50 μg/ml for kanamycin. Escherichia coli DH5α is used as a host for the preparation of plasmid DNA.
TABLE 1.
Bacterial strains used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| S. enterica serovar typhimurium | ||
| 14028s | Wild-type | ATCC |
| YS11708 | Δhns | This work |
| YS11590 | ΔphoP | This work |
| YS11068 | ΔslyA | This work |
| YS11945 | Δhns ΔphoP | This work |
| YS10382 | pcgL-lacZY::KmR | This work |
| YS11743 | pcgL-lacZY::KmR ΔphoP | This work |
| YS11744 | pcgL-lacZY::KmR Δhns | This work |
| YS11745 | pcgL-lacZY::KmR ΔhnsΔphoP | This work |
| YS11644 | pagC-lacZY::KmR | This work |
| YS11664 | pagC-lacZY::KmR ΔslyA | This work |
| YS11782 | pagC-lacZY::KmR ΔphoP | This work |
| YS11780 | pagC-lacZY::KmR Δhns | This work |
| YS11935 | pagC-lacZY::KmR Δhns ΔslyA | This work |
| YS11932 | pagC-lacZY::KmR Δhns ΔphoP | This work |
| YS11938 | pagC-lacZY::KmR Δhns ΔphoP ΔslyA | This work |
| YS11535 | Δup-phoP::CmR | This work |
| YS11536 | Δup-phoP::CmRup-6 | This work |
| YS11368 | hns-HA | This work |
| YS11370 | hns-HA ΔphoP::CmR | This work |
| YS11369 | hns-HA ΔslyA::CmR | This work |
|
YS11398
|
hns-HA ΔslyA ΔphoP::CmR |
This work
|
| E. coli | ||
|
DH5α
|
F–supE44 ΔlacU169 (φ80
lacZ ΔM15) hsdR17 recA1
endA1 gyrA96 thi-1 relA1
|
30 |
| Plasmids | ||
| pACYC184 | repp15A CmRTcR | 31 |
| pKD3 | repR6K γApR FRT CmR FRT | 21 |
| pKD46 | reppSC101ts ApR ParaBAD γ β exo | 21 |
| pCP20 | reppSC101ts ApR CmRcI857 λPR | 32 |
| pCE37 | repR6Kγ KmR FRT lacZY this | 22 |
| pUHE21-2lacq | reppMB1 ApRlacIq | 33 |
| pYS1000 | repp15A CmR Plac1–6 lacZ this | This work |
| pYS1100 | repp15A CmR PphoP1 lacZ this | This work |
| pYS1115 | repp15A CmR PphoP1 up-6 lacZ this | This work |
| pYS1244 | repp15A CmR PphoP1 up-far lacZ this | This work |
| pYS1118 | reppMB1 AprlacIq hns | This work |
| pYS1119 | reppMB1 AprlacIq hns-HA | This work |
DNA and Oligonucleotides—Chromosomal DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega). DNA primers used as probes, and for the construction of plasmids and strains, are described in Table 2.
TABLE 2.
Primers used in this study (classified) All oligonucleotides were purchased from IDT (Integrated DNA Technologies).
| Primer No. | Sequence |
|---|---|
| Construction of deletion mutants | |
| ΔphoP | |
| 399 | tgt tct tat tgt taa cac aag gga gaa gag cat atg aat atc ctc ctt ag |
| 400 | gcg gca gaa aat ggc gag caa att tat tca gtg tag gct gga gct gct tc |
| ΔslyA | |
| 11 | ata ata act tag caa gctaat tat aag gag cat atg aat atc ctc ctt ag |
| 12 | gtc aca tgg cca cac gta tgc ccc tgc acc gtg tag gct gga gct gct tc |
| Δhns | |
| 350 | cgg cgg gat ttt aag cat cca gga agt aaa gtg tag gct gga gct gct tc |
| 351 | cca ccc caa tat aag ttt gag att act aca cat atg aat atc ctc ctt ag |
| Δup-phoP up-6 | |
| 395 | tcg acg aac tta aat aat gcc tgc ctc acc cat atg aat atc ctc ctt ag |
| 396 | aaa tag tca ccc tct ttc tga aga aaa gag gtg tag gct gga gct gct tc |
|
575
|
ttt ggg gat aaa cag tta ata aac cag aca tta tca cac cctt ctt tct gaa gaa aag
|
| Construction of chromosomal HA epitope in hns locus | |
| 350 | cgg cgg gat ttt aag cat cca gga agt aaa gtg tag gct gga gct gct tc |
|
352
|
aag caa ctg gaa gat ttc ctg atc aag gaa tat ccg tat gat gtt cct gat tat gct
taa cat atg aat atc ctc att ag
|
| Construction of chromosomal lacZ fusion | |
| pcgL | |
| 38 | ttc ccg tta agc aga gac cct gaa acg gcg cat atg aat atc ctc ctt ag |
| 39 | cag gcg taa tga tta gat ttc gca aca aaa gtg tag gct gga gct gct tc |
| pagC | |
| 130 | ggc ttc aac gtc ggg gtt gga tac cgt ttc gac tac aag gac gac gat gac aag taa cat atg aat atc ctc ctt ag |
|
131
|
aag gcg aac ctt ccg cat agc tta tgc ttt gtg tag gct gga gct gct tc
|
| Construction of plasmids | |
| pYS1000 | |
| 1 | gcg tcg act tta cac ttt aag ctt ttt atg ttt atg ttg tgt gga act gca gcc gct cga gca gga aac agc tat gac cat gat tac g |
| 2 | ccc aag ctt aaa aaa aac cgg gca ttg ccc ggt ttt ttt aat gga ttt cct tac gcg aaa tac ggg c |
| pYS1100 | |
| 409 | gcg tcg acg aac tta aat aat gcc |
| 410 | ccg ctc gag tag cgt tga tta tgg tgc |
| pYS1115 | |
| 538 | ctc ttt tct tca gaa aga ggg tgt gat aat gtc tgg ttt att aac |
| 539 | cac cct ctt tct gaa gaa aag agg gtg agg cag |
| pYS1118 | |
| 595 | cgg gat cca gtt tga gat tac tac aat gag cg |
| 594 | ccc aag ctt aat att cct tga tca gga aat c |
| pYS1119 | |
| 595 | cgg gat cca gtt tga gat tac tac aat gag cg |
| 596 | ccc aag ctt aag cat aat cag gaa cat cat acg gat att cct tga tca gga aat c |
| pYS1244 | |
| 775 | aat aat gcc tgc ctc acc ctc ata tga aca gaa aga ggg tga cta t |
|
776
|
gag ggt gag gca ggc att att taa gtt cgt cga
|
| Reverse transcription-PCR | |
| phoP | |
| 223 | atg atg cgc gta ctg gtt g |
| 226 | gta gta atc agc ttc cct g |
| pcgL | |
| 580 | atg gta aaa tgc gta tct tc |
| 581 | caa agt ctt ttg gct ggt g |
| pagK | |
| 582 | atg aaa cac gtc aag agc |
| 583 | tta tga tct tga gag tct g |
| phoN | |
| 578 | atg aaa agt cgt tat tta g |
| 579 | tca ctg att ctt cag gag |
| pagC | |
| 584 | atg aaa aat att att tta tcc |
| 585 | ctt tgt gca tac ccc acg g |
| ugtL | |
| 586 | atg aag aaa tca gat ggt g |
| 587 | gta aca gca ata ata gcc |
| rpoD | |
| 346 | cca tct gcc gga aga tat cg |
|
347
|
cgg cat cag gcg ctt ctt cc
|
| ChIP assay | |
| phoP promoter | |
| 409 | gcg tcg acg aac tta aat aat gcc |
| 410 | ccg ctc gag tag cgt tga tta tgg tgc |
| pcgL promoter | |
| 675 | aca cat cgt tat ctg tgc |
| 676 | tgt tac acc tcg cga gag |
| pagK promoter | |
| 673 | tgt taa ctg gcc tgt tcc |
| 674 | tac ctg tta aat tgt ggc |
| phoN promoter | |
| 677 | aga ttt taa cct aat gcg |
| 678 | aga ctc act ccg gat cag |
| pagC promoter | |
| 505 | tgg aac gtc att gac |
|
506
|
ttt att ccc gct ccg
|
| EMSA assay and footprinting assay | |
| phoP promoter | |
| 469 | cgc cgg caa att ata tcg gtc |
| 470 | ggt gtt cat taa ggt agt aat c |
| pcgL promoter | |
| 675 | aca cat cgt tat ctg tgc |
| 676 | tgt tac acc tcg cga gag |
| pagK promoter | |
| 673 | tgt taa ctg gcc tgt tcc |
| 674 | tac ctg tta aat tgt ggc |
| phoN promoter | |
| 677 | aga ttt taa cct aat gcg |
| 678 | aga ctc act ccg gat cag |
| pagC promoter | |
| 513 | ata atg act tgt gaa gtt c |
| 514 | cta tca gtt aca aca ttt c |
Construction of Chromosomal Mutations, lac Fusions, and Epitope-tagged Proteins—PCR products were used to generate a deletion in the coding region of a gene or introduce the HA2 epitope sequence in bacterial chromosome as described previously (21). Primers are listed as pairs for individual genes in Table 2 and plasmid pKD3 was used as the template (21). All resulting strains were confirmed using colony PCR and DNA sequencing. A lac gene was integrated behind a coding region in chromosome using plasmid pCE37 (22) into the FLP recombination target sequence generated after the CmR cassette, which was derived from a one-step gene disruption using primers in Table 2, was removed using plasmid pCP20 (21). Δup-phoP::CmR up-6 (YS11536) was constructed as follows: the CmR cassette was introduced 36-bp upstream of the PhoP box in the phoP promoter using PCR fragments synthesized with primers 395 and 396 from pKD3 to generate Δup-phoP::CmR (YS11535). Then PCR amplification was carried out using the chromosomal DNA of this strain and primers 395 and 575. This DNA product was electroporated into the wild-type strain harboring pKD46 and chloramphenicol-resistant colonies were selected. The hexamer substitution (up-6) located 12 to 6 bp upstream of the PhoP box was confirmed using colony PCR and DNA sequencing. The CmR cassette was removed using plasmid pCP20.
Construction of Plasmids—Plasmid pYS1000 was constructed as following: plasmid pACYC184 was digested with HindIII and SalI, and the resulting 3.6-kb fragment was purified with the Gel Extraction Kit (Qiagen). This fragment was ligated with the HindIII and SalI digests of a DNA fragment amplified from the E. coli lacZ gene using primers 1 and 2 (Table 2). Plasmid pYS1100 was constructed by using a PCR fragment containing 108 bp of the phoP promoter region, covering one promoter (PphoP-1) generated with primers 409 and 410, and strain 14028s chromosomal DNA, used as the template, which were digested with SalI and XhoI, and then ligated between the SalI and XhoI sites of pYS1000. Plasmids pYS1115 and pYS1244 were constructed with primers 538 and 539, and primers 775 and 776 using the Gene Tailor Site-directed Mutagenesis System (Invitrogen) with Platinum Taq Polymerase High Fidelity (Invitrogen). Plasmids pYS1118 and pYS1119 were constructed by using PCR fragments containing the 430-bp wild-type hns gene generated with primers 594 and 595, or primers 595 and 596, respectively, and strain 14028s chromosomal DNA, used as the template, which were digested with BamHI and HindIII, and then ligated between the BamHI and HindIII sites of pUHE21. All plasmids were confirmed by DNA sequencing.
Reverse Transcription-PCR (RT-PCR)—Bacterial cells were grown for 4 h in N medium supplemented with 0.01 (low) or 10 mm (high) MgCl2. Expression of the hns gene was induced from strains harboring pYS1118 by adding 0.2 mm IPTG under the same growth conditions. Total RNA was isolated from bacterial culture using the SV Total RNA Isolation System (Promega) according to the manufacturer's instructions. RNA concentration was determined by spectrophotometry at 260 nm. RNA quality was confirmed by agarose gel electrophoresis. cDNA was synthesized using murine leukemia virus reverse transcriptase (BioLabs). DNA was amplified with the primers listed in Table 2 using Taq polymerase (BioLabs) and performed in a thermocycler (Bio-Rad).
Isolation of C-terminal HA-tagged H-NS (H-NS-HA) Protein— Salmonella hns-HA strain (YS11368) harboring plasmid pUHE-hns-HA (pYS1119) was grown at 37 °C with shaking to A600 nm 0.5 in 500 ml of LB medium; then IPTG (final concentration, 0.5 mm) was added, and bacteria were incubated for another 2 h. Cells were harvested, washed with PBS once, suspended in 10 ml of PBS, and opened by sonication. The whole cell lysate was used for H-NS-HA purification by mixing with 0.3 ml of EZview Red Anti-HA affinity gel (Sigma), washed with PBS three times, and H-NS-HA was eluted with 0.25 ml of 0.1 mg/ml HA peptide. Pure protein sample was confirmed using the Color Silver Stain Kit (Pierce) following the instructions from the manufacturer, then dialyzed in a Slide-A-Lyzer cassette (Pierce) overnight against PBS. Protein concentration was determined using the BCA Protein Assay Kit (Pierce).
Electrophoretic Mobility Shift Assay (EMSA)—1 pmol of 32P-labeled DNA fragments amplified with primers in Table 2 were incubated at room temperature for 30 min with various amounts of H-NS-HA in 15 μl of an EMSA buffer consisting of 10 mm Tris-HCl, pH 8.0, 50 mm NaCl, 50 mm KCl, and 10 mm MgCl2. 100 pmol of unlabeled DNA was added to 32P-labeled DNA when required. 1.8 μg of anti-HA antibody (Sigma) was added to the H-NS-HA and DNA mixture when required. After addition of the DNA dye solution (40% glycerol, 0.05% bromphenol blue, 0.05% xylene cyanol), the mixture was directly subjected to 5% PAGE. Signals were detected by autoradiography.
DNase I Protection Assay—DNase I protection assays were carried out using DNA fragments amplified by PCR using Salmonella chromosomal DNA as template. Prior to the PCR, primers 470 and 514 (Table 2) were labeled with T4 polynucleotide kinase and [γ-32P]ATP (GE Healthcare). The phoP promoter region was amplified with primers 469 and 32P-470 and the pagC promoter region was amplified with primers 513 and 32P-514. Approximately 25 pmol of labeled DNA and 0, 50, 100, or 200 pmol of the H-NS-HA protein were used in a 100-μl reaction. DNase I digestion was carried out as described previously (23). DNase I was purchased from Invitrogen, and 0.05 units were used per reaction. Samples (3 μl) were analyzed by 6% denaturing polyacrylamide electrophoresis by comparison with a DNA sequence ladder generated with the appropriate primer by using a Maxam and Gilbert A+G reaction. The positions of radioactive DNA fragments in the gels were detected by autoradiography.
Chromatin Immunoprecipitation (ChIP) Assay—Strains harboring chromosomally encoded H-NS protein with a C-terminal HA epitope were grown in 25 ml of N medium as described above for 4 h, washed with PBS once to remove Tris (amino group), and resuspended in 25 ml of PBS. H-NS was cross-linked to promoter DNA by adding formaldehyde to 1% final concentration. ChIP assays were performed as described previously (24). Enriched promoter DNA fragments were detected by PCR using the primers listed in Table 2.
Immunoblot Analysis of HA-tagged H-NS Proteins—Bacterial suspensions for Western blot analysis were ultrasonicated. Total protein of whole cell lysates were determined using the BCA Protein Assay Kit (Pierce), and normalized to the same amount before being treated with SDS loading buffer. Expression of the hns gene was induced from strains harboring pYS1119 by adding IPTG to the required concentrations. H-NS protein was separated in 15% SDS-PAGE, transferred to nitrocellular membrane (Bio-Rad), and incubated with monoclonal anti-HA antibody. Protein signals were detected using ECL Western blotting substrate (Pierce).
β-Galactosidase Assay—β-Galactosidase assays were carried out in triplicate, and the activity was determined as described previously (25). Data corresponds to at least three independent assays conducted in duplicate.
RESULTS
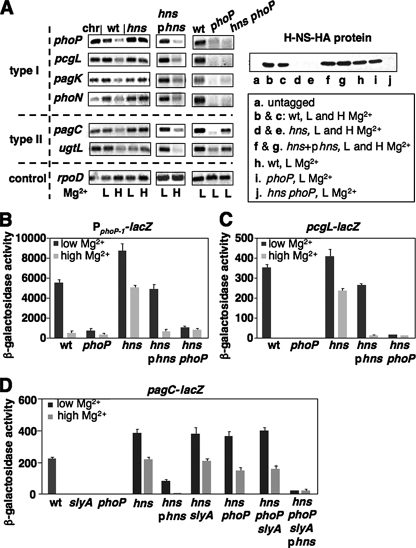
The Regulatory Gene hns Controls Signal-responsive Repression in Horizontally Acquired PhoP-activated Loci—The unphosphorylated PhoP protein binds to target promoter DNA with a similar affinity to the phosphorylated PhoP protein in vitro (15); when overexpressed, it induces PhoP-activated genes in vivo (16). This raised the possibility that repression of PhoP-activated transcription may require other regulatory proteins in high Mg2+ conditions, in which dephosphorylation of the PhoP protein is facilitated (5, 6). To see if this repression process could be mediated by a universal transcriptional regulator, we constructed strains harboring a deletion mutation in chromosomal loci that were identified as global transcriptional regulators, including hns (26). We conducted RT-PCR to determine mRNA levels of the phoP transcripts in wild-type and isogenic strains carrying a mutation in those genes. When wild-type bacteria were grown in high (10 mm) and low (0.01 mm) Mg2+, expression of a PhoP-activated gene is repressed and activated, respectively (1). Therefore, the wild-type mRNA level was lower when bacteria were grown in high Mg2+ than in low Mg2+ (Fig. 1A). Different from the wild-type and other mutant strains we tested, the phoP mRNA level in the hns mutant grown in high Mg2+ was as high as that grown in low Mg2+ (Fig. 1A and data not shown). This suggests that H-NS should be the cellular factor that mediates transcriptional repression of the phoP gene in high Mg2+ conditions, thus, deletion of this locus results in derepressing phoP expression in the signal depleting condition (i.e. high Mg2+) for the PhoP/PhoQ system. We compared transcription of those PhoP-activated genes, identified specifically from Salmonella, in wild-type and the isogenic hns mutant. The rationale for choosing these loci was because a previous report suggested that H-NS specifically silences horizontally acquired genes (17). We classified these genes as type I, which is activated by PhoP, including phoP, pcgL, pagK, and phoN (12, 27, 28) and type II, which is activated by PhoP and SlyA, including ugtL and pagC (10, 11). Consistent with an established model (1), expression of these loci were repressed under the high Mg2+ condition because the mRNA level from wild-type was reduced when bacteria were grown in high Mg2+ (Fig. 1A). Mutation of the hns gene, however, abolished the transcriptional repression of both type I and II genes in this signal-depleting condition because their mRNA levels in the hns mutant grown in high Mg2+ were as high as that grown in low Mg2+ (Fig. 1A). The phenotype of the transcriptional activation in the hns mutant strain is solely the result of a lack of hns gene function because transcription of these genes including phoP could be restored to wild-type levels, i.e. the mRNA level was higher in low Mg2+ than in high Mg2+, by a plasmid (pYS1118) containing a wild-type copy of the hns gene (Fig. 1A). Deletion of the phoP gene in hns mutant strains inhibited transcription of the type I genes under all tested conditions (Fig. 1A), suggesting that removing transcriptional repressor H-NS is not sufficient to activate their transcription. Different from the type I genes, transcriptional derepression by deleting the hns locus allows activation of the type II genes even in the absence of PhoP (Fig. 1A). PhoP appears to be unrelated to hns expression because the protein level of H-NS in a phoP mutant was similar to that in wild-type (Fig. 1A). Thus, our results demonstrate that PhoP functions as a transcriptional anti-repressor and activator for the type I genes, and as a transcriptional anti-repressor for the type II genes.
FIGURE 1.
H-NS is required for repression of PhoP-dependent transcription in high Mg2+ conditions. A, RT-PCR analysis of mRNA level of PhoP-activated genes: type I, phoP, pcgL, pagK, and phoN; type II, pagC and ugtL; synthesized from bacteria was determined in wild-type (wt, 14028s), hns mutant (YS11708), hns mutant (YS11708) harboring pUHE21–2lacq with a wild-type copy of the hns gene (phns, pYS1118), phoP mutant (YS11590), and hns phoP mutant (YS11945). Wild-type chromosomal DNA (chr) was used as control. The constitutively transcribed rpoD gene indicated that similar amounts of total RNA were used. The protein levels of H-NS were determined in the same growth conditions from strains harboring hns-HA fusion in the chromosome or in plasmid pYS1119, whose sequence is identical to pYS1118 except a HA tag sequence at the C terminus of the hns gene (Table 1). B, PphoP1-lacZ transcriptional fusion expressed by bacteria harboring pYS1100 was determined in wild-type (14028s), phoP mutant (YS11590), hns mutant (YS11708), hns mutant (YS11708) harboring pYS1118, and hns phoP mutant (YS11945) strains. C, pcgL-lacZ transcriptional fusion expressed by bacteria was determined in wild-type (YS10382), phoP mutant (YS11743), hns mutant (YS11744), hns mutant (YS11744) harboring pYS1118, and hns phoP mutant (YS11745) strains. D, pagC-lacZ transcriptional fusion expressed by bacteria was determined in wild-type (YS11644), slyA mutant (YS11664), phoP mutant (YS11782), hns mutant (YS11780), hns mutant (YS11780) harboring pYS1118, hns slyA mutant (YS11935), hns phoP mutant (YS11932), hns phoP slyA mutant (YS11938), and hns phoP slyA mutant (YS11938) harboring pYS1118 strains. Bacteria were grown in N minimal medium, pH 7.4, with 0.01 mm (low or L) and 10 mm (high or H) Mg2+. Complementation required the addition of 0.2 mm IPTG. All graphed values (Miller unit) are mean ± S.D., and data correspond to three independent assays conducted in duplicate.
Further evidence came from β-galactosidase assays using strains harboring lacZ fusions under control of the phoP promoter, type I promoter, and type II promoter. To compare the in vivo phoP transcription without interfering with the regulatory function of the PhoP/PhoQ system, we constructed a plasmid (pYS1100) carrying lacZ fused to a 108-bp DNA fragment including a phoP promoter (PphoP-1 fragment from -123 to -34, Fig. 2C). This DNA could initiate a PhoP-dependent transcription from -34 (or P1 in Fig. 2C, also see Ref. 33). Meanwhile, we constructed strains harboring a chromosomal pcgL-lacZ fusion and a chromosomal pagC-lacZ fusion in addition to a set of isogenic strains with mutations in the regulatory genes. Expression of all three lacZ fusions were Mg2+-responsive and PhoP-dependent because β-galactosidase activity from wild-type strains was higher in low Mg2+ than in high Mg2+, and because this activity from wild-type strains was higher than in phoP mutants in low Mg2+ (Fig. 1, B–D). In addition, expression of pagC-lacZ was SlyA-dependent because β-galactosidase activity from wild-type was higher than in a slyA mutant (Fig. 1D). Consistent with RT-PCR results (Fig. 1A), activation of the phoP, pcgL, and pagC genes in the high Mg2+ condition occurred when the hns gene was deleted because β-galactosidase activity from hns mutants grown in high Mg2+ was greatly induced (Fig. 1, B–D). The lacZ induction in these hns mutants is solely the result of a lack of hns gene function because transcription of lacZ could be restored to wild-type levels by pYS1118, i.e. β-galactosidase activity was greatly reduced in high Mg2+ (Fig. 1, B–D). Mutation of the phoP locus resulted in inhibition of lacZ transcription from promoters of phoP and pcgL no matter whether H-NS was present or absent (Fig. 1, B and C). Thus, H-NS binding to a PhoP-activated promoter is to prevent unphosphorylated PhoP, which is produced in high Mg2+ conditions, from initiating transcription of this gene.
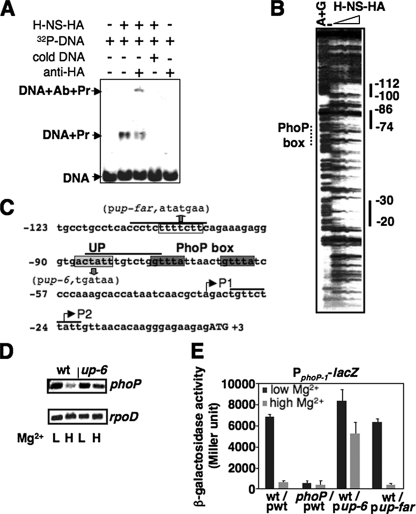
FIGURE 2.
H-NS binds to DNA regions located upstream of the PhoP box in the phoP promoter. A, EMSA. 32P-Labeled phoP DNA fragment was incubated with 100 pmol of H-NS-HA (shown as Pr) in the absence or presence of “cold” phoP DNA fragment, or in the absence or presence of anti-HA antibodies (shown as Ab). H-NS-DNA mixtures were subjected to 5% polyacrylamide electrophoresis. Location of DNA migration was detected by autoradiography. B, DNase I footprinting analysis of the phoP promoter performed with probes for the noncoding strand (see “Experimental Procedures”) and increasing amounts of C-terminal HA-tagged H-NS (0, 50, and 100 pmol) proteins. Solid lines correspond to regions protected by the H-NS protein. Lane AG corresponds to the Maxam and Gilbert A+G reaction ladder. Numbering is from the start codon of the phoP open reading frame. Dotted line corresponds to the region of the PhoP box. C, sequence of the chromosomal purB-phoP intergenic region in S. typhimurium. Black lines indicate the DNA region protected by the H-NS-HA protein from the result in B. The dark gray boxes correspond to the PhoP box. The light gray box and open box correspond to the sequences for substitutions in plasmids pup-6 and pup-far. The up-6 substitution was also introduced in the chromosome to construct the up-6 strain (YS11536). The substituted sequences are shown in parentheses. P1 and P2 are two transcription start sites. Numbering is from the A (as +1) in the predicted phoP start codon (shown as uppercase letters). D, RT-PCR analysis of the mRNA level of phoP and rpoD synthesized from bacteria grown in N minimal medium, pH 7.4, with 0.01 mm (L) and 10 mm (H) Mg2+ was determined in wild-type (wt, YS11535) and up-6 (YS11536) strains. E, PphoP1-lacZ transcriptional fusion expressed by bacteria harboring pYS1100 (pwt, with wild-type sequence), pYS1115 (pup-6, with substituted UP sequence), and pYS1244 (pup-far, with substituted farther upstream sequence in C) was determined in wild-type (14028s) or phoP mutant (YS11590). Data correspond to three independent assays conducted in duplicate, and values were mean ± S.D.
On the other hand, PhoP is not essential for activation of the type II gene pagC when H-NS is absent because lacZ expression from the pagC promoter was similar in a hns mutant and a hns phoP double mutant (Fig. 1D). This indicates that PhoP functions differently in the type I and II genes. Because expression of the pagC gene is also SlyA-dependent (11), we compared the transcriptional level of the pagC-lacZ fusion from wild-type and isogenic slyA mutant strains carrying a deletion at the hns locus. lacZ expression was inhibited in a slyA mutant, but remained activated in a hns slyA mutant under all tested conditions (Fig. 1D). This result, and the above result from a hns phoP mutant, suggested that PhoP and SlyA are not required for pagC transcription when H-NS is absent. Indeed, we found that the transcriptional level of the pagC-lacZ fusion in a hns phoP slyA mutant was as high as that in the hns mutant (Fig. 1D). These experiments demonstrate that PhoP and SlyA are designed to antagonize H-NS, which represses transcription of the type II PhoP-dependent loci. Thus, the pagC promoter, perhaps ugtL as well, could be regarded as a “constitutively activated” promoter in the absence of H-NS.
The H-NS Protein Binds to a DNA Region Located Upstream of the PhoP Box in the Promoter of the phoP Gene—We conducted an EMSA using C-terminal HA-tagged H-NS protein (H-NS-HA) and a 235-bp DNA fragment including the purB-phoP intergenic region (see “Experimental Procedures”). We found that this H-NS protein could gel shift the DNA fragment (Fig. 2A), indicating the presence of a binding site for H-NS in this DNA region. Specific H-NS binding was further identified by a supershift reaction, in which a monoclonal anti-HA antibody (Sigma) could bind to the H-NS protein and further decrease the mobility of the protein-DNA complex in a polyacrylamide gel (Fig. 2A). Thus, we conducted DNase I footprinting assays and found that the H-NS protein protects three DNA regions in the purB-phoP intergenic region (Fig. 2B): the -112 to -100 sequence, 11 bp downstream of the purB coding region; the -86 to -74 sequence, 6 bp upstream of the PhoP box; and the -30 to -21 sequence, 4 bp downstream of the PhoP-dependent transcription start P1 (the first nucleotide of the phoP start codon as +1, Fig. 2C). The -30 to -20 sequence was not present in the DNA fragment cloned in pYS1100 (see “Experimental Procedures”). This ruled out the possibility that this region is required for H-NS repression because the upstream region of P1 is sufficient to confer a Mg2+-responsive lacZ expression (Fig. 1B). To examine the -86 to -74 sequence adjacent to the PhoP box, we constructed a strain (the up-6 mutant) harboring a chromosomal substitution from actatt to tgataa (Fig. 2C) at the H-NS binding site (UP) present in the phoP promoter, and a pYS1100-derived plasmid (pup-6) carrying this hexamer substitution. Determination of phoP transcripts from the up-6 mutant indicated that mRNA levels of the phoP gene were similar in this mutant grown in low and high Mg2+ conditions (Fig. 2D). Furthermore, the wild-type strain harboring plasmid pup-6 exhibited similar β-galactosidase activities in low and high Mg2+ conditions (Fig. 2E), which was the same phenotype displayed by the hns mutant carrying the wild-type plasmid (Fig. 1B). On the other hand, lacZ expression from wild-type harboring another pYS1100-derived plasmid (pYS1244) with a heptamer substitution from ttttctt to atatgaa within the -112 to -100 sequence was similar to that from wild-type harboring a wild-type plasmid (Fig. 2E). These results indicate that binding of the H-NS protein to the UP region adjacent to the PhoP box in the phoP promoter is required for its transcriptional repression in high Mg2+.
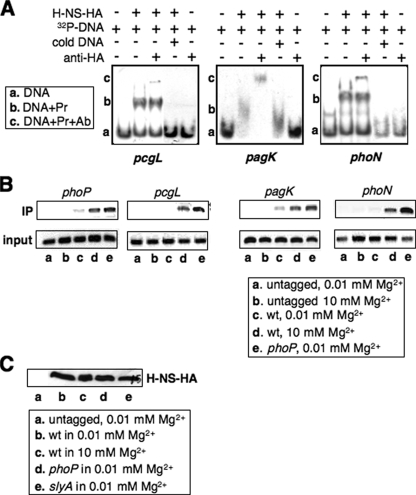
The H-NS Protein Represses Type I PhoP-activated Genes by Binding to Their Promoters—To examine whether transcriptional repression of type I genes results from H-NS binding to their promoters, we conducted EMSA using H-NS-HA protein and PCR-generated DNA fragments carrying regulatory sequences present upstream of pcgL, pagK, and phoN coding regions (see “Experimental Procedures”). We found that the H-NS protein could gel shift all tested DNA fragments, which were supershifted by anti-HA antibodies (Fig. 3A). This result indicates that a binding site for H-NS is present in these type I promoter regions. Thus, we compared the H-NS binding ability in these promoters in a strain harboring a chromosomal hns-HA fusion with that expressed by the isogenic strain with a mutation in the regulatory gene phoP, and carried out a ChIP experiment (29) using bacteria grown in low and high Mg2+. A stronger enrichment of DNA fragments present in the phoP, pcgL, pagK, and phoN promoters was observed for the HA-tagged wild-type strain grown in high Mg2+ (Fig. 3B, lane d) than in low Mg2+ (Fig. 3B, lane c). Furthermore, a dramatic enrichment was observed for the phoP mutant strain grown in low Mg2+, which was stronger than the wild-type strain grown in high Mg2+ (Fig. 3B, lanes e and d). The ChIP assay was specific because there was no significant enrichment of H-NS-bound DNA to the examined promoters when a control (i.e. wild-type strain lacking a HA-tag) strain was used (Fig. 3B, lanes a and b). We ruled out the possibility that strong binding of the H-NS protein to these type I promoters in wild-type cells grown in high Mg2+ (Fig. 3B, lane d) or in the phoP mutant strain (Fig. 3B, lane e) could be caused by high expression of the H-NS protein under these conditions (as opposed to a low level of the PhoP protein), because immunoblot analysis in cell lysates from ChIP experiments revealed that the H-NS protein level was constant in wild-type grown in both Mg2+ conditions and in a phoP or slyA mutant grown in low Mg2+ (Fig. 3C). This indicates that expression of H-NS is independent of PhoP and SlyA, as well as Mg2+ concentration. Taken together, our observations demonstrate that the H-NS protein competes with PhoP to repress transcription directed by type I PhoP-dependent promoters.
FIGURE 3.
H-NS binds to promoter regions of PhoP-activated loci by competing with PhoP protein. A, EMSA. 32P-Labeled promoter fragments from pcgL, pagK, and phoN were incubated with 100 pmol of H-NS-HA (shown as Pr) in the absence or presence of cold pcgL, pagK, and phoN DNA fragments, respectively, or in the absence or presence of anti-HA antibodies (shown as Ab). H-NS-DNA mixtures were subjected to 5% polyacrylamide electrophoresis. Location of the DNA migration was detected by autoradiography. B, ChIP assay. In vivo H-NS binding to promoters of phoP, pcgL, pagK, and phoN were detected in untagged (14028s), wild-type (YS11368), and phoP mutant (YS11370) strains grown for 4 h in N medium, pH 7.4, containing 0.01 and 10 mm Mg2+. Input is total DNA and IP is immunoprecipitated DNA. PCR amplification was performed for 27 cycles, and DNA fragments were separated on 1% agarose gel and visualized by ethidium bromide. C, immunoblot analysis of cell extracts used in B. Protein samples were separated in SDS-PAGE (15% acrylamide). Monoclonal anti-HA antibodies (Sigma) were used for detection of HA-tagged H-NS.
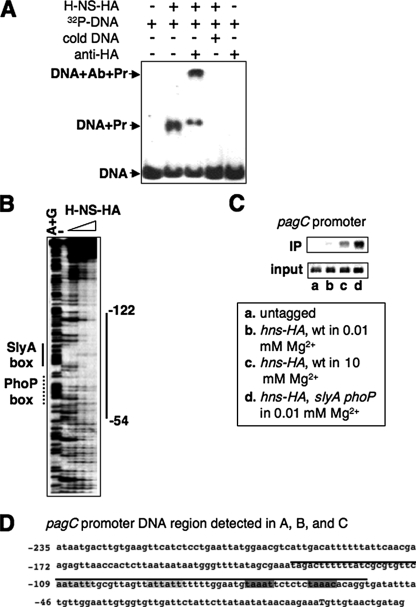
H-NS Represses Transcription of the Type II Promoters by Binding to the PhoP Box and SlyA Box—We conducted EMSA using H-NS-HA protein and the 250-bp DNA fragment containing regulatory motifs of the pagC promoter.3 We found that H-NS could gel shift the pagC promoter DNA (Fig. 4A), which indicates the presence of a possible binding site for H-NS in this type II promoter region. Gel shift resulted specifically from H-NS binding because a monoclonal anti-HA antibody (Sigma) was able to further decrease the mobility of the H-NS protein and pagC DNA complex in a polyacrylamide gel (Fig. 4A). We then conducted DNase I footprinting assays and found that the H-NS protein binds to the pagC promoter throughout the DNA region from -122 to -54 (Fig. 4, B and D). We identified the pagC sequence aatatt-(N10)-attatt from -109 to -88 as the SlyA-binding site (the SlyA box) and the sequence taaatt-(N5)-taaaca from -76 to -61 as the PhoP-binding site (the PhoP box, Fig. 4D) because substitutions in these sequences resulted in a complete inhibition in pagC transcription.3 The region protected by the H-NS protein extends through the whole SlyA box and PhoP box (Fig. 4D), suggesting that binding of the H-NS protein to this promoter should compete with PhoP and the PhoP-activated SlyA (14). We carried out a ChIP experiment to compare the H-NS binding ability in this type II promoter using the strain harboring the chromosomal hns-HA fusion and an isogenic strain with mutations in both phoP and slyA genes grown in low and high Mg2+. A dramatic enrichment of the DNA fragment corresponding to the pagC promoter was observed for the HA-tagged wild-type strain grown in high Mg2+ (Fig. 4C, lane c), but very weak H-NS binding was observed in this strain grown in low Mg2+ (Fig. 4C, lane b). Furthermore, DNA enrichment was even stronger for the phoP slyA mutant strain grown in low Mg2+ (Fig. 3B, lane d). The ChIP assay was specific because there was no significant enrichment of H-NS-bound DNA to the pagC promoter when the control (i.e. wild-type strain lacking a HA-tag) was used (Fig. 4C, lane a). Thus, these results suggest that the H-NS protein establishes Mg2+-dependent transcription of the type II pagC promoter by competing with PhoP and SlyA.
FIGURE 4.
H-NS binds to the PhoP box and SlyA box in the pagC promoter. A, EMSA. 32P-Labeled promoter fragment from pagC was incubated with 100 pmol of H-NS-HA (shown as Pr) in the absence or presence of cold pagC DNA fragment or in the absence or presence of anti-HA antibodies (shown as Ab). H-NS-DNA mixtures were subjected to 5% polyacrylamide electrophoresis. Location of DNA migration was detected by autoradiography. B, DNase I footprinting analysis of the pagC promoter performed with probes for the noncoding strand (see “Experimental Procedures”) and increasing amounts of C-terminal HA-tagged H-NS (0, 50, 100, and 150 pmol) proteins. The thick solid line corresponds to regions protected by the H-NS protein. The thin solid line and dotted line correspond to the SlyA box and PhoP box, respectively. Lane AG corresponds to the Maxam and Gilbert A+G reaction ladder. Numbering is from the transcription start (as +1) of the pagC gene. C, ChIP assay. In vivo H-NS binding to pagC promoters was detected in untagged (14028s), wild-type (YS11368), and slyA phoP mutant (YS11398) strains grown for 4 h in N medium, pH 7.4, containing 0.01 and 10 mm Mg2+. Input is total DNA and IP is immunoprecipitated DNA. PCR amplification was performed for 28 cycles, and DNA fragments were separated on 1% agarose gel and visualized by ethidium bromide. D, sequence of the regulatory region in the pagC promoter. Black line indicates the DNA region protected by the H-NS-HA protein from the result in B. The light gray boxes correspond to the SlyA box. The dark gray boxes correspond to the PhoP box. Numbering is from the transcription start (as +1 and shown as a capital letter).
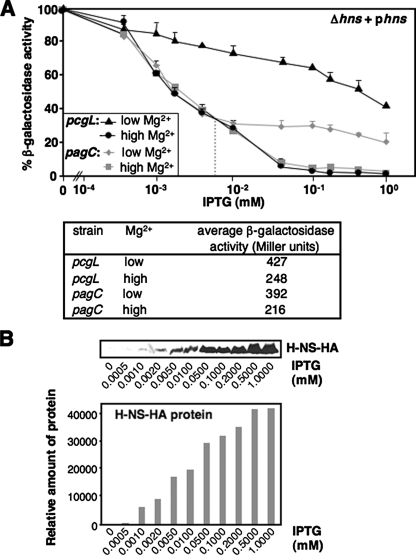
The Molecular Mechanism of Mg2+-dependent Regulation Mediated by the PhoP/PhoQ System and H-NS—We titrated the H-NS protein that could mediate repression of the type I gene pcgL and the type II gene pagC using hns mutants harboring a chromosomal lacZ fusion and a plasmid (pYS1118) with an inducible hns gene. When bacteria were grown in medium without IPTG, β-galactosidase activity from each strain grown in low (0.01 mm) and high (10 mm) Mg2+ was determined (see the actual values in lower panel of Fig. 5A) and set as 100% (Fig. 5A). In the signal-depleting condition (i.e. high Mg2+), lacZ expression controlled by the pcgL promoter was fully repressed, like a wild-type strain, when supplemented IPTG concentrations reached 0.05 mm or higher (Fig. 5A). On the contrary, in the signal-stimulating condition (i.e. low Mg2+), transcription remained at 78% maximum in the same IPTG concentration (Fig. 5A). This result, and a similar result in Fig. 1C, indicates that only the phosphorylated PhoP could antagonize H-NS at the pcgL promoter (Fig. 6). We determined the H-NS protein levels induced by IPTG using the hns mutant harboring a plasmid (pYS1119), which has an identical sequence to pYS1118 with a C-terminal HA tag added to the hns gene. As revealed by an immunoblot analysis, induction of the H-NS protein is proportional to the concentration of supplemented IPTG (Fig. 5B).
FIGURE 5.
H-NS controls PhoP-activated transcription and establishes response of the PhoP/PhoQ system to Mg2+. A, pcgL-lacZ (black lines) and pagC-lacZ (gray lines) transcriptional fusions expressed by bacteria was determined in hns mutant (YS11744 and YS11780, respectively) strains harboring pYS1118 grown for 4 h in N medium, pH 7.4, containing Mg2+ concentrations of 0.01 mm (or low, triangle for pcgL and diamond for pagC) or 10 mm (or high, circle for pcgL and square for pagC), and supplemented with different concentrations (0, 0.0005, 0.001, 0.002, 0.005, 0.01, 0.05, 0.1, 0.2, 0.5, and 1 mm) of IPTG. Percentage of β-galactosidase activity = (β-galactosidase activity in y mm IPTG ÷ β-galactosidase activity in 0 mm IPTG) × 100. “y mm” means a given IPTG concentration tested. The table in the lower panel shows β-galactosidase activity from each strain grown in either low and high Mg2+ without supplementing IPTG. The gray dashed vertical line divides stages I and II in pagC repression by low and high concentrations of H-NS. B, immunoblot analysis of cell extracts prepared from the hns mutant (YS11708) harboring pYS1119. Bacteria were grown for 4 h in N medium, pH 7.4, containing 0.01 mm Mg2+ and supplemented with different IPTG concentrations used in A. Protein samples were separated in SDS-PAGE (15% acrylamide). Monoclonal anti-HA antibodies (Sigma) were used for HA-tagged H-NS. Relative protein amount (shown in the lower panel) was measured by using software Quantity One and calculated by using the density from 0 mm IPTG sample (=2,992) as the baseline.
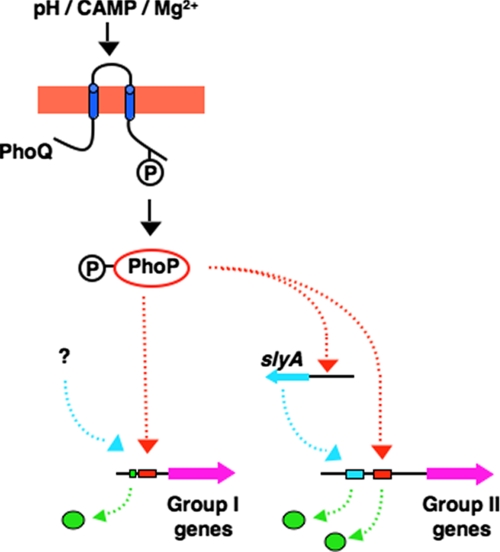
FIGURE 6.
Model illustrating the H-NS-dependent Mg2+ response of the PhoP/PhoQ system. In wild-type, the PhoP protein is phosphorylated during growth in low Mg2+. The type I PhoP-activated genes (left) are transcriptionally activated via competition of the phosphorylated PhoP, which binds with high affinity (probably facilitated by other factors) to the PhoP box (the red box) over H-NS near the UP motif (the green box), whereas type II PhoP-activated genes (green circles) are activated during growth in low Mg2+ when PhoP-activated SlyA, along with the phosphorylated PhoP, bind to the promoters replacing H-NS in the SlyA box and PhoP box, and stimulating their transcription in S. typhimurium. Competition over the unphosphorylated PhoP allows the H-NS protein to occupy these chromosomal regions and repress transcription of the type I and II genes when wild-type bacteria are grown in high Mg2+ conditions. In the absence of H-NS (Hns- strain), the unphosphorylated PhoP, regardless of its low DNA affinity, is able to bind to type I promoters, which facilitates transcriptional activation of these genes even in high Mg2+ conditions. Meanwhile, type II promoters become independent of SlyA and PhoP in Hns- bacteria.
The SlyA amount was maintained at similar levels in low and high Mg2+ (11), thus, Mg2+-responsive transcription of the pagC gene should depend primarily on the phosphorylation of PhoP. Indeed, this pagC expression was inhibited as rapidly as the pcgL gene in high Mg2+ (Fig. 5A). Unexpectedly, low IPTG concentrations resulted in transcriptional repression in low Mg2+ equal to high Mg2+, which was followed by a slight reduction of pagC transcription even when the IPTG concentration became higher (Fig. 5A). We therefore divided H-NS-mediated transcriptional repression of the pagC gene into two stages, the first stage different from that of the pcgL gene when H-NS concentrations are low, and the second stage the same as the pcgL gene when H-NS concentrations are high. Because the H-NS protein induced in the first stage is lower than the actual amount in bacterial cytoplasm (data not shown), this Mg2+-independent process implies the possibility that SlyA may facilitate binding of the unphosphorylated PhoP to the pagC promoter exhibited at the first stage. Our results demonstrate that the phosphorylated PhoP is much more resistant to H-NS repression exhibited in the second stage of pagC expression equivalent to expression of type I genes in low Mg2+ (Fig. 5A).
DISCUSSION
We have identified the regulatory mechanism governing transcriptional repression of the PhoP/PhoQ two-component signaling network of S. enterica. We have established that transcriptional regulation of the PhoP regulon is controlled by the H-NS protein, which competes with PhoP protein, as well as SlyA in particular cases. This model is supported by the following data. (i) The transcriptional repression of phoP and all tested PhoP-activated genes was abolished in hns mutants when bacteria were grown under PhoP-dephosphorylated conditions (Fig. 1, A–E). (ii) The H-NS protein gel shifted all tested PhoP-dependent promoter DNA (Figs. 2A, 3A, and 4A) and footprinted the phoP promoter (Fig. 2B). (iii) The H-NS protein footprinted both the SlyA binding site and the PhoP binding site in the pagC promoter (Fig. 4B). (iv) Mutation of a H-NS binding site in the phoP promoter abolished phoP transcriptional repression to levels observed in a hns mutant (Fig. 2, D and E). (v) H-NS binding to all tested PhoP-dependent promoters was enhanced in a phoP mutant grown under PhoP-phosphorylated conditions and in wild-type bacteria grown under PhoP-dephosphorylated conditions (Fig. 3B). (vi) H-NS binding to the PhoP-, SlyA-dependent pagC promoter was enhanced in a phoP slyA mutant grown under PhoP-phosphorylated conditions and in wild-type bacteria grown under PhoP-dephosphorylated conditions (Fig. 4C). (vii) Inducing H-NS selectively repressed pcgL and pagC transcription in hns mutants grown under PhoP-dephosphorylated conditions (Fig. 5A).
We ruled out the possibility that the H-NS protein level could be influenced in different mutants or when bacteria were grown under all tested conditions in this study (Fig. 3C), therefore, H-NS-mediated transcriptional repression solely depends on its selective binding to the target promoters. H-NS not only binds to the promoter of the master regulator PhoP (Fig. 2, A and B), but also binds to promoters of individual PhoP-activated genes (Figs. 3A and 4A), suggesting that H-NS and PhoP should form a counteracting regulatory pair in these horizontally acquired PhoP-activated genes. Most PhoP-activated promoters (i.e. type I promoters in this study) described to date harbor a conserved hexanucleotide repeat separated by 5 nucleotides, termed the PhoP box, located 12 bp upstream of the -10 region (9). We found one H-NS binding site (termed the UP element here) located in one helical turn upstream of the PhoP box in the phoP promoter; this spatial adjacency could explain competition between H-NS and phosphorylated PhoP when they approach these binding sites as demonstrated in this study (Fig. 3B) and illustrated in Fig. 6. This competition appears to be specific in vivo. Although H-NS could bind several DNA regions in phoP promoter in vitro (Fig. 2, B and C), only the hexamer mutation in the UP site could abolish repression of the phoP transcription in high Mg2+ (Fig. 2, D and E). This feature of the phoP promoter should be applicable for other type I promoters that also possess the AT-rich sequences located upstream from the identified PhoP box (7). Although no UP consensus sequence could be found, H-NS is able to bind to these promoter DNA regions (Fig. 3, A and B).
Regulatory activity of PhoP is modulated by changing its phosphorylation level, which likely changes its conformation, and therefore, changes its in vivo affinity to target promoters when the PhoP/PhoQ system responds to environmental Mg2+ (5, 6). On the other hand, both unphosphorylated and phosphorylated forms of PhoP could bind to promoters with similar affinities in vitro (15). Taken together, it is likely that additional cellular factor(s) could be required for preferential binding of the phosphorylated PhoP in vivo. One example is SlyA, which mediates a feedback activation of the PhoP/PhoQ system3 by interacting with the H-NS binding site, i.e. the UP element in the phoP promoter. Two functions of PhoP were suggested based on results in this study. (i) As a transcriptional anti-repressor. In wild-type, Mg2+-responsive transcription could be because the phosphorylated PhoP, but not unphosphorylated PhoP, was able to counteract H-NS in these promoters (Fig. 3B). (ii) As a transcriptional activator. Under high Mg2+ conditions, removing H-NS in the phoP mutant could activate type II promoters (Fig. 1, A and D), but not activate type I promoters probably because an unphosphorylated PhoP is still required to turn on their expression (Fig. 1, A and C–E). Because the PhoP box is located within the -35 region of type I promoters (7), it is possible that PhoP is required to directly interact with RNA polymerase for their transcription initiation. Transcriptional activation of phoP and other PhoP-activated genes in the hns mutants can be turned on under PhoP-dephosphorylated conditions (high Mg2+), possibly because unphosphorylated PhoP protein is able to bind to their promoter regions and function as a transcriptional activator, like the phosphorylated PhoP protein, in the absence of the competitor H-NS.
Type II promoters (ugtL and pagC in this study) are atypical in that they contain a PhoP box located on the opposite strand (Fig. 4D),3 and that they require SlyA for their activation (10, 11). A reverse PhoP box and SlyA box are all located farther upstream from the putative -35 region of the ugtL and pagC promoters. Contrary to the type I promoters, PhoP is not essential for transcription of these type II genes in the absence of H-NS (Fig. 1, A and D), indicating that this regulator functions solely to antagonize H-NS, i.e. as an anti-repressor. This difference is possibly derived from the involvement of the additional regulator SlyA. Although SlyA was transcriptionally activated by PhoP (10, 14), protein levels of SlyA appeared to be kept at similar levels when bacteria were grown under both low and high Mg2+ conditions (11). However, SlyA could only bind weakly to the pagC promoter in high Mg2+ (data not shown), indicating a coordinate binding of the PhoP and SlyA proteins onto type II promoters with an unknown mechanism. H-NS binds to both the PhoP box and SlyA box present in the type II promoters although their nucleotide sequences are different (Fig. 4D).3 Our results, therefore, provide a molecular mechanism for the feed-forward loop designed for the transcriptional activation of type II promoters, i.e. to counteract the inhibitory effect of H-NS in the upstream elements, PhoP box and SlyA box, PhoP and SlyA proteins should simultaneously bind to DNA and remove this repressor (Fig. 6). It is unknown how these distal regulatory elements enhance transcriptional initiation of the RNA polymerase complex. One possibility is that RNA polymerase requires an “entrance passage” located upstream of the -35 sequence, where, in high Mg2+, H-NS functions as a “gate-keeper” by occupying these regions and preventing polymerase complex from approaching.
In summary, we provide a molecular mechanism for the signal-dependent gene regulation controlled by the PhoP/PhoQ system, and perhaps by other two-component regulatory systems. Results in this study show that a type I PhoP-dependent promoter interacts with H-NS and PhoP in the following order: phosphorylated PhoP > H-NS > unphosphorylated PhoP. Therefore, H-NS antagonizes low DNA-affinity PhoP when it is unphosphorylated in high Mg2+, but not high DNA-affinity PhoP when it is phosphorylated in low Mg2+. Results in this study also show that PhoP and SlyA antagonize H-NS in type II PhoP-dependent promoters, in which the Mg2+ concentration obviously determines PhoP binding to these promoters. However, it remains to be investigated which cellular signal molecule could influence SlyA binding to these promoters.
Acknowledgments
We thank Roy Curtiss III and Josephine Clark-Curtiss for discussions and Guozheng Qin for technical support. We also thank one anonymous reviewer for thoughtful comments.
This work was supported, in whole or in part, by National Institutes of Health Grant AI24533 (to W. K.). This work was also supported by start-up funds from Arizona State University (for Y. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HA, hemagglutinin; IPTG, isopropyl 1-thio-β-d-galactoside; PBS, phosphate-buffered saline; EMSA, electrophoretic mobility shift assay; ChIP, chromatin immunoprecipitation; RT, reverse transcription.
W. Kong, N. Weatherspoon, and Y. Shi, submitted for publication.
References
- 1.Garcia Vescovi, E., Soncini, F. C., and Groisman, E. A. (1996) Cell 84 165-174 [DOI] [PubMed] [Google Scholar]
- 2.Prost, L. R., Daley, M. E., Le Sage, V., Bader, M. W., Le Moual, H., Klevit, R. E., and Miller, S. I. (2007) Mol. Cell 26 165-174 [DOI] [PubMed] [Google Scholar]
- 3.Bader, M. W., Sanowar, S., Daley, M. E., Schneider, A. R., Cho, U., Xu, W., Klevit, R. E., Le Moual, H., and Miller, S. I. (2005) Cell 122 461-472 [DOI] [PubMed] [Google Scholar]
- 4.Prost, L. R., and Miller, S. I. (2008) Cell Microbiol. 10 576-582 [DOI] [PubMed] [Google Scholar]
- 5.Castelli, M. E., Cauerhff, A., Amongero, M., Soncini, F. C., and Vescovi, E. G. (2003) J. Biol. Chem. 278 23579-23585 [DOI] [PubMed] [Google Scholar]
- 6.Montagne, M., Martel, A., and Le Moual, H. (2001) J. Bacteriol. 183 1787-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lejona, S., Aguirre, A., Cabeza, M. L., Garcia Vescovi, E., and Soncini, F. C. (2003) J. Bacteriol. 185 6287-6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soncini, F. C., Garcia Vescovi, E., Solomon, F., and Groisman, E. A. (1996) J. Bacteriol. 178 5092-5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minagawa, S., Ogasawara, H., Kato, A., Yamamoto, K., Eguchi, Y., Oshima, T., Mori, H., Ishihama, A., and Utsumi, R. (2003) J. Bacteriol. 185 3696-3702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi, Y., Latifi, T., Cromie, M. J., and Groisman, E. A. (2004) J. Biol. Chem. 279 38618-38625 [DOI] [PubMed] [Google Scholar]
- 11.Navarre, W. W., Halsey, T. A., Walthers, D., Frye, J., McClelland, M., Potter, J. L., Kenney, L. J., Gunn, J. S., Fang, F. C., and Libby, S. J. (2005) Mol. Microbiol. 56 492-508 [DOI] [PubMed] [Google Scholar]
- 12.Hilbert, F., Garcia-del Portillo, F., and Groisman, E. A. (1999) J. Bacteriol. 181 2158-2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, S. I., Kukral, A. M., and Mekalanos, J. J. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 5054-5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norte, V. A., Stapleton, M. R., and Green, J. (2003) J. Bacteriol. 185 3508-3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perron-Savard, P., De Crescenzo, G., and Le Moual, H. (2005) Microbiology 151 3979-3987 [DOI] [PubMed] [Google Scholar]
- 16.Lejona, S., Castelli, M. E., Cabeza, M. L., Kenney, L. J., Garcia Vescovi, E., and Soncini, F. C. (2004) J. Bacteriol. 186 2476-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarre, W. W., Porwollik, S., Wang, Y., McClelland, M., Rosen, H., Libby, S. J., and Fang, F. C. (2006) Science 313 236-238 [DOI] [PubMed] [Google Scholar]
- 18.Dorman, C. J. (2007) Nat. Rev. Microbiol. 5 157-161 [DOI] [PubMed] [Google Scholar]
- 19.Davis, R. W., Bolstein, D., and Roth, J. R. (1980) Advanced Bacterial Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 20.Snavely, M. D., Miller, C. G., and Maguire, M. E. (1991) J. Biol. Chem. 266 815-823 [PubMed] [Google Scholar]
- 21.Datsenko, K. A., and Wanner, B. L. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6640-6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellermeier, C. D., Janakiraman, A., and Slauch, J. M. (2002) Gene (Amst.) 290 153-161 [DOI] [PubMed] [Google Scholar]
- 23.Kato, A., Tanabe, H., and Utsumi, R. (1999) J. Bacteriol. 181 5516-5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin, D., and Groisman, E. A. (2005) J. Biol. Chem. 280 4089-4094 [DOI] [PubMed] [Google Scholar]
- 25.Miller, J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 26.Hengge-Aronis, R. (1999) Curr. Opin. Microbiol. 2 148-152 [DOI] [PubMed] [Google Scholar]
- 27.Kier, L. D., Weppelman, R. M., and Ames, B. N. (1979) J. Bacteriol. 138 155-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belden, W. J., and Miller, S. I. (1994) Infect. Immun. 62 5095-5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuras, L., and Struhl, K. (1999) Nature 399 609-613 [DOI] [PubMed] [Google Scholar]
- 30.Hanahan, D. (1983) J. Mol. Biol. 166 557-580 [DOI] [PubMed] [Google Scholar]
- 31.Chang, A. C., and Cohen, S. N. (1978) J. Bacteriol. 134 1141-1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherepanov, P. P., and Wackernagel, W. (1995) Gene (Amst.) 158 9-14 [DOI] [PubMed] [Google Scholar]
- 33.Soncini, F. C., Vescovi, E. G., and Groisman, E. A. (1995) J. Bacteriol. 177 4364-4371 [DOI] [PMC free article] [PubMed] [Google Scholar]