Abstract
A key problem in the treatment of numerous pathogenic eukaryotes centers on their development into latent forms during stress. For example, the opportunistic protist Toxoplasma gondii converts to latent cysts (bradyzoites) responsible for recrudescence of disease. We report that Toxoplasma eukaryotic initiation factor-2α (TgIF2α) is phosphorylated during stress and establish that protozoan parasites utilize translation control to modulate gene expression during development. Importantly, TgIF2α remains phosphorylated in bradyzoites, explaining how these cells maintain their quiescent state. Furthermore, we have characterized novel eIF2 kinases; one in the endoplasmic reticulum and a likely regulator of the unfolded protein response (TgIF2K-A) and another that is a probable responder to cytoplasmic stresses (TgIF2K-B). Significantly, our data suggest that 1) the regulation of protein translation through eIF2 kinases is associated with development, 2) eIF2α phosphorylation is employed by cells to maintain a latent state, and 3) endoplasmic reticulum and cytoplasmic stress responses evolved in eukaryotic cells before the early diverging Apicomplexa. Given its importance to pathogenesis, eIF2 kinase-mediated stress responses may provide opportunities for novel therapeutics.
A well characterized mechanism by which eukaryotic cells respond to
environmental stress involves phosphorylation of eukaryotic initiation
factor-2 (eIF2)3
(1–3).
The eIF2 combined with GTP delivers
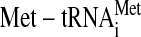 to the
translational machinery during initiation of protein synthesis. In mammalian
cells, four eIF2 kinases have been described that are each activated by unique
stress arrangements. For example, in response to accumulation of malfolded
protein in the lumen of the endoplasmic reticulum (so-called ER stress),
PEK/Perk (EIF2KA3) phosphorylates the α subunit of eIF2 at serine 51,
causing this translation factor to become an inhibitor of its own guanine
nucleotide exchange factor, eIF2B. The resulting repression in general
translation prevents further synthesis of secretory proteins that would
further overload the ER and allows cells sufficient time to trigger the
unfolded protein response (UPR)
(2). The UPR is a program of
mRNA expression involving genes that function in the assembly and transport of
secretory proteins (4). In
addition to ER stress, three other eIF2 kinases have been described that
recognize different forms of cytoplasmic stress in mammalian cells. These
include: GCN2 (EIF2KA4), which responds to nutrient deprivation and is well
conserved among eukaryotes (3,
5), HRI (EIF2KA1), which is
reported to be activated by heme deficiency, oxidative stress induced by
arsenite treatment, and heat shock
(6,
7), and PKR (EIF2KA2), which is
involved in the antiviral defenses
(8,
9).
to the
translational machinery during initiation of protein synthesis. In mammalian
cells, four eIF2 kinases have been described that are each activated by unique
stress arrangements. For example, in response to accumulation of malfolded
protein in the lumen of the endoplasmic reticulum (so-called ER stress),
PEK/Perk (EIF2KA3) phosphorylates the α subunit of eIF2 at serine 51,
causing this translation factor to become an inhibitor of its own guanine
nucleotide exchange factor, eIF2B. The resulting repression in general
translation prevents further synthesis of secretory proteins that would
further overload the ER and allows cells sufficient time to trigger the
unfolded protein response (UPR)
(2). The UPR is a program of
mRNA expression involving genes that function in the assembly and transport of
secretory proteins (4). In
addition to ER stress, three other eIF2 kinases have been described that
recognize different forms of cytoplasmic stress in mammalian cells. These
include: GCN2 (EIF2KA4), which responds to nutrient deprivation and is well
conserved among eukaryotes (3,
5), HRI (EIF2KA1), which is
reported to be activated by heme deficiency, oxidative stress induced by
arsenite treatment, and heat shock
(6,
7), and PKR (EIF2KA2), which is
involved in the antiviral defenses
(8,
9).
Very little research has been performed on eIF2 kinase and related stress response pathways in early-diverging eukaryotes, including pathogenic eukaryotes. However, viability, pathogenesis, and transmission of many parasites hinges on their ability to recognize and respond to cellular stress. Stress often serves as a signal for parasites to develop into a life cycle stage that is better suited to its current environment or into a dormant stage that allows microbial persistence. For example, in Toxoplasma gondii, a protozoan parasite in the phylum Apicomplexa, the stress response has clinical relevance as it underlies a development transition from the proliferating tachyzoites to encysted bradyzoites, which evade immunity and known drug treatments. T. gondii, transmissible through contaminated food or water, can cause congenital birth defects and serious complications in immunocompromised persons (10). The latent bradyzoites can reconvert into destructive tachyzoites if immunity wanes. Current drugs do not target cyst development or maintenance and have toxic side effects, hence creating an urgent need for new therapeutics. T. gondii differentiation is poorly understood, but it is established that a variety of cellular stresses can initiate this process in vitro, including alkaline pH, thermal stress, exposure to sodium arsenite, and arginine starvation (11–13). Thus, we have been investigating the role of the eIF2 kinase-mediated pathway in stress-induced differentiation of T. gondii.
We previously characterized eIF2α in T. gondii (TgIF2α) and showed that phosphorylation of this translation factor at the analogous serine 51 residue occurs in response to heat shock or alkaline pH stress (14). Interestingly, we reported a novel member of the eIF2 kinase family in T. gondii, named TgIF2K-A, that phosphorylated eIF2α and regulated translation control in the yeast model (14). These data suggested that key features of eIF2 kinase pathway are conserved in apicomplexan parasites and translational control may function in T. gondii.
In this study we show that TgIF2α phosphorylation and translational control occur in response to both ER and cytoplasmic stresses and persist during the quiescent bradyzoite stage of the life cycle. We have determined that TgIF2K-A localizes to the ER and shares regulatory features with PEK/Perk in mammalian cells. A novel T. gondii eIF2 kinase that we describe here, TgIF2K-B, is found in the cytosol. TgIF2K-A and –B, thus, allow T. gondii to respond to both ER and cytoplasmic stress arrangements, respectively. Importantly, our data suggest that translational control elicited by eIF2 kinase-mediated stress responses coincides with cyst development, microbial persistence, and latent infection. The conservation of these pathways in Apicomplexa suggests that eukaryotic cells developed these adaptation strategies very early in eukaryotic cell evolution.
EXPERIMENTAL PROCEDURES
Parasite Lines and Culture Methods—T. gondii tachyzoites (RH and ME49 strains) were cultivated in confluent monolayers of human foreskin fibroblast cells. Host cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen) and with no antibiotics. Host cells infected with RH strain were grown in Dulbecco's modified Eagle's medium supplemented with 1% fetal bovine serum (10% for ME49), free of antibiotics, in a humidified 37 °C incubator in 5% CO2. To induce differentiation using alkaline pH, ME49 parasites were incubated in RPMI 1640 media (pH 8.1) supplemented with 5% fetal bovine serum. Fresh medium was applied daily for 8 days, at which point the monolayer containing cysts was harvested. To induce differentiation using sodium nitroprusside (SNP), a final concentration of 50 μm SNP was added daily to Dulbecco's modified Eagle's medium; culture media was replaced at day 4, and cysts were harvested at day 8. Cultures with cysts were scraped and passed through a 25-gauge syringe needle on ice followed by centrifugation at 145 × g to remove debris. After 2 washes in ice-cold PBS, the bradyzoites were processed for immunoblotting as described below. In some experiments 1 μm salubrinal (Calbiochem) was added to parasite cultures daily for 4 days. The GFP-HDEL parasite clone (made in RH strain) was generously provided by Dr. Kristin Hager (Notre Dame) (15).
In experiments in which parasites were radiolabeled, equal numbers of extracellular tachyzoites were resuspended in labeling media, Dulbecco's modified Eagle's medium without l-methionine, l-cysteine, l-glutamine, or sodium pyruvate (Invitrogen #21013-024) supplemented with 5% fetal bovine serum, 1 mm l-glutamine, 0.5 mm sodium pyruvate. Thirty minutes into the treatment with 5 μm arsenite, 2.5 μm A23187, or vehicle, 0.145 mCi of Express Protein Label Mix containing [35S]methionine and [35S]cysteine (PerkinElmer Life Sciences) was added to the sample and incubated for the remainder of the stress period. Samples were washed twice in PBS, and a portion was counted to determine similar uptake of the radiolabel. Parasites were resuspended in lysis buffer and sonicated. Equal amounts of total protein from each lysate preparation were separated by SDS/PAGE, and radiolabeled proteins were visualized by autoradiography. Incorporation of [35S]Met/Cys radiolabel into proteins was determined by trichloroacetic acid precipitation followed by scintillation counting of the insoluble pellet.
Antibodies—Antibodies were generated against a phosphorylated polypeptide CMSDERLpSKRRFRS representing TgIF2α phosphorylated at the serine 71 regulatory site. To measure total levels of TgIF2α, polyclonal rabbit antisera was raised against purified recombinant TgIF2α as previously reported (14). Polyclonal antisera to TgIF2K-A was raised in rabbits using an antigen consisting of recombinant TgIF2K-A containing the carboxyl-terminal 194 amino acid residues. Recombinant TgIF2K-A was expressed in Escherichia coli and purified using an amino-terminal polyhistidine fusion tag in combination with nickel chromatography. Anti-TgIF2K-A was affinity-purified using purified recombinant antigen coupled to an Affi-gel 15 (Bio-Rad) column. TgIF2K-B antibodies were prepared against an amino-terminal polyhistidine-tagged recombinant protein containing the carboxyl-terminal 217 residues of TgIF2K-B and affinity-purified as described above. Antibody cross-reactive to the T. gondii homologue of BiP (15) was kindly provided by Dr. Jay Bangs (University of Wisconsin-Madison, WI).
Stress Tests—Filter-purified tachyzoites were treated for the designated times in various concentrations of sodium arsenite, calcium ionophore A23187, or tunicamycin (all from Sigma). To examine the effects of a specified stress on TgIF2α phosphorylation, equal numbers of filter-purified tachyzoites were incubated in media with the stress agent or subjected to a mock treatment. After treatment, parasites were collected by centrifugation and lysed in solution A (50 mm Tris (pH 7.9), 150 mm NaCl, 2 mm EDTA, 0.1% Nonidet P-40) supplemented with phosphatase inhibitor (50 mm NaF) and protease inhibitors (100 μm phenylmethylsulfonyl fluoride, 0.15 μm aprotinin, 1 μm pepstatin, and 1 μm leupeptin). Proteins were quantitated using the Bradford assay, and equal amounts were resolved by SDS-PAGE followed by protein transfer to nitrocellulose membranes and immunoblotting as described (14). Results are presented as means ± S.E. that were derived from three independent experiments. The Student's t test was used to determine the statistical significance.
Sucrose Gradient Centrifugation—Cycloheximide (50 μg/ml) was added to extracellular tachyzoites 10 min before harvest. Parasites were collected by centrifugation, washed twice in PBS containing 50 μg/ml cycloheximide, and resuspended in Breaking Solution (20 mm Tris-HCl (pH 7.9), 150 mm NaCl, 10 mm MgCl2, 0.1% Triton, 50 μg/ml cycloheximide, 0.04 units/μl RNase Out, and 250 μg/ml heparin) in the presence of protease inhibitors listed above. Lysis was achieved by briefly sonicating the resuspended parasites 3× on ice. Lysates were clarified by centrifugation at 16,000 × g for 10 min. Supernatant samples containing 3–5 A254 units were loaded onto a 15–45% sucrose gradient in Breaking Solution without RNase Out, heparin, or Triton as described previously (16). Ultracentrifugation was performed using Beckman rotor SW41 at 40,000 rpm for 2 h. Gradients were fractionated using an ISCO UA-6 absorbance monitor set at 254 nm, and 0.6-ml aliquots were collected.
Immunofluorescence Assays (IFA)—T. gondii tachyzoites engineered to express GFP-HDEL were allowed to infect confluent monolayers of human foreskin fibroblasts grown on glass coverslips. IFAs was carried out as described in Bhatti et al. (17) using a 1:100 dilution of affinity-purified anti-TgIF2K-A or 1:100 dilution of anti-GFP (Abcam, ab1218-100) as primary antibody. Secondary antibody consisted of anti-rabbit Alexa Fluor 488 or anti-mouse Alexa Fluor 594 (Invitrogen), respectively. Extracellular tachyzoites were examined after coating coverslips with 50 μl of 0.1 mg/ml poly-l-lysine. Parasites in 3% paraformaldehyde and PBS were inoculated onto the coated coverslips and allowed to sit at room temperature for 15 min before being processed for IFA as outlined above. For IFAs performed on both intracellular and extracellular parasites, 0.3 μm 4′,6-diamidino-2-phenylindole was applied for 5 min at room temperature in the dark as a co-stain. To visualize bradyzoite cyst wall antigens, a 1:100 dilution of fluorescein isothiocyanate-conjugated Dolichos biflorus agglutinin (Vector Laboratories) was applied.
In Vitro eIF2 Kinase Assays—TgIF2K-A or TgIF2K-B were immunoprecipitated from T. gondii protein lysates prepared in solution A (see above) supplemented with proteasome inhibitors using antibody specific to each eIF2 kinase. The antibody-bound eIF2 kinases were collected by using protein A-Sepharose and eIF2 kinase assays were carried out as described (14) using 10 μCi of [γ-32P]ATP in a final concentration of 50 μm and kinase buffer solution (20 mm Tris-HCl (pH 7.9), 50 mm KCl, 10 mm MgCl2, 2 mm MnCl2, and 5 mm 2-mercaptoethanol) supplemented with protease inhibitors. 1.3 μm purified recombinant yeast eIF2α was included in a 20-μl reaction mixture. Radiolabeled proteins were separated by SDS/PAGE and visualized by autoradiography.
Immunoelectron Microscopy—Preparations of Toxoplasma-infected fibroblasts were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in 0.25 m HEPES (pH 7.4) for 1 h at room temperature, then in 8% paraformaldehyde in the same buffer overnight at 4 °C. They were then infiltrated, frozen, and sectioned as previously described (18). The sections were immunolabeled with rabbit anti-TgIF2K-A or -B antibodies in PBS, 1% fish skin gelatin, then with mouse anti-rabbit IgG antibodies followed directly by 10 nm protein A-gold particles (Utrecht University, the Netherlands) before examination with a Philips CM120 Electron Microscope (Eindhoven, the Netherlands) under 80 kV.
Immunoprecipitation of eIF2 Kinases for BiP Interaction—RH tachyzoites were treated with 5 μm A23187 or DMSO control for 1 h at 37 °C. The parasites were lysed in 20 mm Tris (pH 7.6), 150 mm NaCl, and 0.5% Triton supplemented with protease inhibitors, and the suspension was briefly sonicated 3 times on ice. The lysates were precleared with protein-A-agarose (Invitrogen) followed by immunoprecipitation with affinity-purified TgIF2K-A or TgIF2K-B antibodies and protein-A-agarose for 16 h at 4 °C. The immunoprecipitates were collected by centrifugation and washed three times in lysis buffer. The immune complexes were heated to 95 °C for 10 min and separated on a 4–12% Tris-glycine gel (Invitrogen) and then transferred to a nitrocellulose membrane. The membrane was subsequently blocked in a 5% nonfat milk solution for 2 h at room temperature. The immunoblot was carried out using a BiP antibody (kindly provided by Dr. Jay Bangs, University of Wisconsin-Madison) (15) and anti-rabbit IgG-horseradish peroxidase conjugate (GE Healthcare). BiP was visualized using an ECL Western blotting substrate (Pierce).
Real-time PCR—Four hours post-infection, culture medium was replaced with media containing the designated concentrations of tunicamycin or the corresponding concentration of DMSO as the vehicle control. Culture media was replaced daily with fresh drug for 3 days. Parasites were released by needle passage, and total RNA was isolated using RNeasy kit (Qiagen). The cDNA was synthesized using Omniscript RT (Qiagen) and oligo(dT) primer (Ambion). The resulting cDNA was amplified using SYBR Green PCR Master Mix (Applied Biosystems) and 0.5 μm concentrations of each forward and reverse primer. Primers used were as follows: BAG1, sense, 5′-TGAGCGAGTGTCCGGTTATTT, and antisense, 3′-TAGAACGCCGTTGTCCATTG; LDH2, sense, 5′-ACAATGGCCCAGGCATTCT, and antisense, 3′-CAATAAACATATCGTGAAGCCCATA; β-tubulin (measured for normalization) sense, 5′-ATGTTCCGTGGTCGCATGT, and antisense, 3′-TGGGAATCCACTGAACGAAGT. PCR reactions were performed in triplicate using the 7500 Real-time PCR system and analyzed with relative quantification software (SDS software Version 1.2.1, Applied Biosystems).
Cloning, Sequence Data, and Analysis Tools—Sequences used include TgIF2K-A (GenBank™ accession number AAS48463), TgIF2K-B (GenBank™ accession number EU369393), human GCN2 (NP_001013725), human PEK (NP_004827), human PKR (NP_002750), and human HRI (GenBank™ accession number AAF70289). TgIF2K-B cDNA sequence was elucidated by designing multiple overlapping primers based on the ToxoDB gene prediction 583.m05439. cDNA fragments were amplified from T. gondii total RNA using the SuperScript™ III One-Step RT-PCR system (Invitrogen) and subcloned into a TA-TOPO vector (Invitrogen) for DNA sequencing. 3′-Rapid amplification of cDNA ends was performed using T. gondii mRNA and the GeneRacer™ kit (Invitrogen). Preliminary genomic sequence data were accessed via toxodb.org, Releases 1.0–2.2 (19). Genomic data were provided by The Institute for Genomic Research (supported by the National Institutes of Health Grant AI05093) and by the Sanger Center (Wellcome Trust). DNA and protein sequences were analyzed for homologues using BLAST programs at www.ncbi.nlm.nih.gov. Protein mapping and motif searches were performed using the Pfam HMM data base (pfam.wustl.edu/hmmsearch.shtml). Alignment of the sequences of multiple eIF2α kinase domains was performed using Vector NTI 10.0 (Informax/Invitrogen).
RESULTS
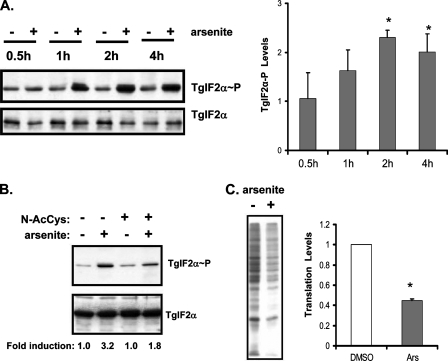
eIF2α Phosphorylation and Cytoplasmic Stress—We previously cloned the cDNA encoding the T. gondii eIF2α subunit orthologue (TgIF2α), which contains a conserved regulatory serine residue (amino acid 71) that is phosphorylated in response to alkaline pH or heat shock, stress conditions that induce parasite differentiation to bradyzoites in vitro (11, 14). Sodium arsenite is another cytoplasmic stress agent that induces T. gondii differentiation in vitro (12), and we found that this chemical treatment also induced a time-dependent increase of phosphorylation of TgIF2α (Fig. 1A). The importance of oxidative stress in the induction of TgIF2α phosphorylation in response to arsenite exposure was addressed by adding 20 mm N-acetylcysteine, which serves as an antioxidant, to the parasites 30 min before treatment with arsenite. As shown in Fig. 1B, the inclusion of N-acetylcysteine reduced phosphorylation of TgIF2α during arsenite exposure, supporting the idea that oxidative stress is a potent inducer of eIF2α phosphorylation in T. gondii.
FIGURE 1.
eIF2-mediated translational control in response to oxidative stress. A, tachyzoites exposed to 5 μm sodium arsenite or vehicle control over a time course were assayed for levels of TgIF2α phosphorylation by immunoblot (TgIF2α∼P). Immunoblots were also carried out with antibody recognizing total TgIF2α as a normalizing control. The right panel is a statistical analysis of three independent experiments, showing the mean ± S.E. of the -fold change in TgIF2α phosphorylation. An asterisk denotes a statistically significant difference between stressed and non-stressed parasites (p < 0.05). B, tachyzoites were exposed to 5 μm arsenite or vehicle for 1 h. Indicated samples were pretreated with 20 mm N-acetylcysteine (N-AcCys) for 30 min. Immunoblots were probed with antibodies to phosphorylated TgIF2α (upper panel) or total TgIF2α (lower panel). The -fold induction of TgIF2α phosphorylation was determined by densitometry of the autoradiogram, which was in the linear range of exposure. C, tachyzoites were subjected to 5 μm arsenite (Ars) or vehicle (no stress) for a total of 1 h. 30 min after the start of the stress treatment, [35S]Met/Cys was added to radiolabel the proteins in each sample. Samples were then resolved on SDS-PAGE for autoradiography. Changes in the level of translation were assayed in three independent experiments and analyzed for statistical significance (graph) as the mean ± S.E. An asterisk denotes a statistically significant difference (p < 0.05).
In other species, phosphorylation of eIF2α has been reported to repress general translation. Although we have shown that TgIF2α is phosphorylated in response to stress, it has not been determined what effect this has on protein production in the parasite. We investigated this by radiolabeling newly synthesized proteins in tachyzoites with [35S]Met/Cys during the period of stress treatment (or mock treatment). Treatment of T. gondii with arsenite led to a ∼60% reduction in protein synthesis compared with the mock-treated cells (Fig. 1C). These results show that a range of different cytoplasmic stresses in T. gondii induce eIF2α phosphorylation, coincident with a dramatic lowering of global translation.
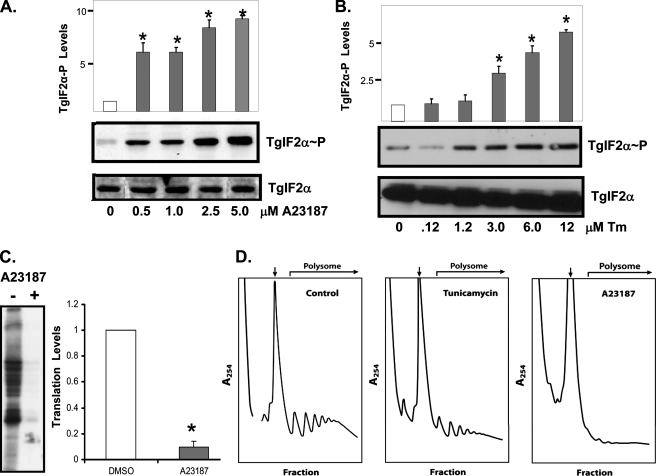
ER Stress and Translation Control in T. gondii—In addition to responding to cytoplasmic stress, phosphorylation of eIF2α is a central part of the UPR that remedies ER stress in mammalian cells. To determine whether stress inflicted upon the parasite ER elicits TgIF2α phosphorylation, we treated extracellular parasites with the calcium ionophore A23187. Calcium ionophore increases intracellular Ca2+ levels by releasing it from ER stores and is a well established inducer of ER stress in higher eukaryotes (20). Subjecting extracellular tachyzoites to increasing concentrations of A23187 for 1 h showed a marked and dose-dependent increase of TgIF2α phosphorylation (Fig. 2A). Tunicamycin is another well characterized ER stress agent that inhibits N-linked glycosylation of secreted proteins (2, 21, 22). Phosphorylation of TgIF2α was also sharply induced in response to treatment with as little as a 1.2 μm concentrations of this ER stress agent (Fig. 2B). These results support the idea that T. gondii activates the eIF2 kinase pathway in response to both ER and cytoplasmic stress arrangements.
FIGURE 2.
eIF2-mediated translational control in response to ER stress. Tachyzoites were incubated in the presence of increasing concentrations of A23187 (A) or tunicamycin (Tm; B) for 1 h. Levels of phosphorylated TgIF2α (upper panel) and total TgIF2α (lower panel) were monitored by immunoblotting. C, tachyzoites were subjected to 2.5 μm A23187 or vehicle for a total of 1.5 h after the start of the stress treatment, and [35S]Met/Cys was added to radiolabel the proteins in each sample. Samples were then resolved on SDS-PAGE for autoradiography. D, polysome profiles of extracellular tachyzoites treated with 5 μm A23187 or 12 μm tunicamycin or vehicle (no stress) for 1 h. Polysomes are indicated in the profiles, with an arrow indicating the 80 S monosomes. Statistical analysis was performed for A, B, and C as detailed in Fig. 1.
We next addressed whether ER stress agents regulated global translation in T. gondii. Protein synthesis, as measured by incorporation with [35S]Met/Cys, was significantly reduced in parasites treated with A23187 for 1 h (Fig. 2C). It is noteworthy that the higher level of TgIF2α phosphorylation seen with A23187 relative to arsenite corresponded to a greater reduction in protein synthesis. To directly address whether TgIF2α phosphorylation is accompanied by a decrease in translation initiation, we generated polyribosome profiles from tachyzoites subjected to A23187 or tunicamycin. Samples stressed with tunicamycin showed a 45% reduction in polysomes, whereas those stressed with A23187 showed a 93% reduction (Fig. 2D). The diminished polysome fractions and corresponding enhanced free ribosome and monosome fractions in the ER-stressed parasites indicate that these ER stresses trigger a translation initiation defect. Together, these studies demonstrate that protozoan parasites, such as T. gondii, are capable of directing an ER stress response. Moreover, similar to mammalian cells, TgIF2 kinase-mediated translation control is induced in response to a broad range of stress conditions, including those eliciting cytoplasmic and ER perturbations.
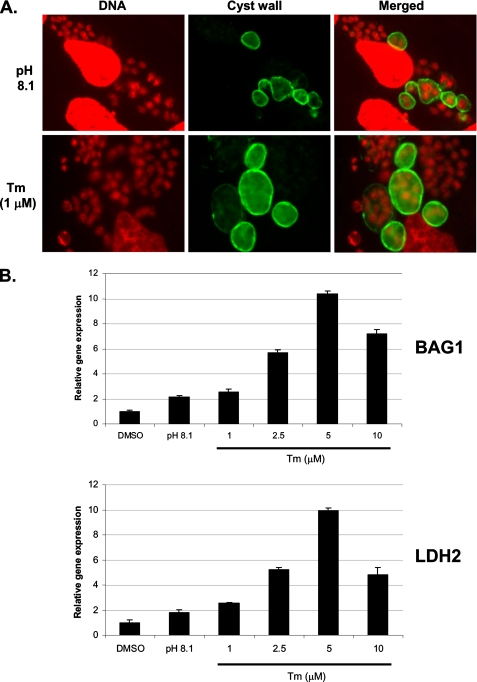
ER Stress Induced by Tunicamycin Triggers Expression of Bradyzoite Genes—Our findings that ER stress agents induce TgIF2α phosphorylation prompted us to examine if disruption of this organelle can trigger differentiation to bradyzoites. We focused on tunicamycin in this assay because A23187 has been reported to induce rapid egress of parasites from their host cells (23). Treatment of intracellular tachyzoites with 1.0 μm tunicamycin triggered the expression of bradyzoite cyst wall antigens within 3 days, as detected by staining with D. biflorus lectin (Fig. 3A). We also assessed mRNA levels for two bradyzoite-specific genes using real-time PCR in parasites subjected to mock treatment, alkaline pH, or increasing concentrations of tunicamycin for 3 days. Treatment with tunicamycin significantly increased BAG1 and LDH2 message levels in a dose-dependent fashion, with concentrations >1.0 μm producing more dramatic responses than the standard method of differentiation using alkaline pH (Fig. 3B). 10 μm tunicamycin appeared to exert stress on the host cell monolayer, which likely accounts for the decrease in BAG1 and LDH2 message levels compared with 5 μm tunicamycin. We conclude that TgIF2α phosphorylation and translation control due to ER stress induces the expression of bradyzoite-specific genes and cyst development.
FIGURE 3.
Tunicamycin induces bradyzoite gene expression. A, immunofluorescence assay to detect bradyzoite cyst wall antigens using Dolichos lectin (green). 4′,6-Diamidino-2-phenylindole was applied as a nuclear (DNA) co-stain (red). Intracellular parasites appear as smaller red dots; larger red structures are host cell nuclei. B, real-time PCR was used to monitor mRNA levels for BAG1 (upper graph) and LDH2 (lower graph) during alkaline pH stress and with increasing concentrations of tunicamycin (Tm) for 3 days. Statistical tests used include one-way an analysis of variance test and Bonferroni test. The mean difference for BAG1 and LDH2 transcripts measured between the control (DMSO) and each of the tunicamycin-treated parasites is significant at p < 0.01.
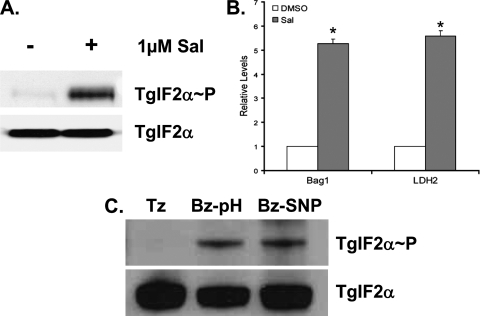
Inhibition of eIF2α Dephosphorylation Induces Cyst Development—Salubrinal (Sal) is a selective inhibitor of cellular complexes that dephosphorylate eIF2α (24). We tested if Sal had the same effect on Toxoplasma eIF2α phosphorylation. Tachyzoites subjected to tunicamycin stress were allowed to recover for 1 h either in the presence of 1 μm Sal or a vehicle control. Parasites that are allowed to recover from tunicamycin stress exhibit very low levels of TgIF2α phosphorylation; however, those treated with Sal continue to show high levels of TgIF2α phosphorylation (Fig. 4A). This experiment validates that Sal functions as an inhibitor of TgIF2α dephosphorylation. We then tested if cyst development could be induced through inhibition of TgIF2α dephosphorylation by adding 1 μm Sal to tachyzoite cultures daily for 4 days. As assessed by real-time PCR, the Sal-treated cultures exhibit significantly higher levels of transcripts encoding bradyzoite-specific genes BAG1 and LDH2 (Fig. 4B). Cyst formation in the Sal-treated cultures was confirmed by IFA analysis with Dolichos lectin (data not shown). These data strongly support the idea that TgIF2α phosphorylation is associated with bradyzoite cyst development.
FIGURE 4.
TgIF2α phosphorylation and bradyzoite cyst development. A, extracellular ME49 parasites were exposed to 12 μm tunicamycin for 1 h. After the stress treatment, the parasites were washed and cultured in the presence (+) or absence (-) of 1 μm Sal for an additional hour. Levels of total TgIF2α and TgIF2α-P were evaluated by immunoblot. B, BAG1 and LDH2 mRNA levels were evaluated using real-time PCR from intracellular ME49 parasites exposed to 1 μm Sal or the vehicle (DMSO) for 4 days. C, sustained TgIF2α phosphorylation in bradyzoites. Tachyzoites (Tz) and tachyzoites induced to bradyzoites in vitro using alkaline (pH 8.1) (Bz-pH) or 50 μm sodium nitroprusside (Bz-SNP) for 8 days were harvested to determine the levels of TgIF2α phosphorylation by immunoblot (upper panel). Samples were also probed with antibody recognizing total TgIF2α (lower panel).
TgIF2α Phosphorylation Is Maintained in Latent Bradyzoites— The results above strongly support the idea that the phosphorylation of TgIF2α is a contributor to the genesis of bradyzoite development. Mature bradyzoites are virtually quiescent and would be expected to have lowered translation rates compared with actively replicating tachyzoites. Therefore, we examined if mature bradyzoites exhibited more phosphorylated TgIF2α relative to tachyzoites. Immunoblots of tachyzoites and day 8 bradyzoites were probed with antibodies specific to total TgIF2α or the phosphorylated form of TgIF2α. Bradyzoites exhibited significant levels of TgIF2α phosphorylation, whereas none was detected in unstressed tachyzoites (Fig. 4C). This is consistent with the idea that TgIF2α phosphorylation reduces protein translation in bradyzoites, which would contribute to the dormant nature of this stage of the life cycle.
TgIF2K-B, a Novel eIF2 Kinase in T. gondii—Given that both ER and cytoplasmic stresses elicit TgIF2α phosphorylation, we wished to determine whether there are additional T. gondii eIF2 kinases that function in conjunction with the previously identified TgIF2K-A to remedy various stresses. A second predicted eIF2 kinase, which we designated TgIF2K-B, was identified that shared the signature features diagnostic of this protein kinase family (ToxoDB.org accession number 583.m05439). We used multiple reverse transcription-PCRs to clone and sequence cDNA fragments representing TgIF2K-B, which revealed an open reading frame of 7665 bp (supplemental Fig. S1). An mRNA of ∼10 kilobases was detected by Northern blotting (supplemental Fig. S1). As judged by 3′-rapid amplification of cDNA ends, TgIF2K-B mRNA includes a 3′-untranslated region that is nearly 1.0 kilobases, suggesting a 5′-UTR that is at least 1.0 kilobases. The predicted start AUG fits Kozak consensus rules and is preceded by an in-frame stop codon 207 bp upstream (25).
The deduced TgIF2K-B polypeptide is 2554 amino acid residues in length, with a very large centralized kinase domain that is 1890 residues long (supplemental Figs. S1 and S2). Each eIF2 kinase domain has an insert of variable length and sequence between subdomains IV and V, and the deduced TgIF2K-B polypeptide sequence contains a very large insert, 866 residues in length. This insert flanks the V subdomain sequence LYIQMEYC, which is similar among the eIF2 kinase family members. As judged by the x-ray crystal structures of human PKR and yeast GCN2 (26, 27), this segment is in proximity to the hinge regions connecting the amino- and carboxyl-terminal lobes of the kinase domain (supplemental Fig. S2). A second insert, 700 residues in length, is found in the TgIF2K-B activation loop between subdomains VII and VIII. The VIII subdomain region contains a threonine residue in the different eIF2 kinase family members that is autophosphorylated, contributing to activation during stress (28, 29). TgIF2K-B contains a threonine at this position, consistent with the idea that this regulatory feature may be conserved in this predicted eIF2 kinase (supplemental Fig. S2). Interestingly, there are no discernable regions of sequence homology outside of the described kinase domain similarity.
Given the unusual inserts in the kinase domain, we wished to determine whether TgIF2K-B functioned as an eIF2 kinase in T. gondii. Polyclonal antiserum was developed against TgIF2K-B as well as against TgIF2K-A. Both antibodies were able to immunoprecipitate kinase activity specific for the serine-51 residue of eIF2α, supporting the idea that these proteins are functional eIF2 kinases (Fig. 5).
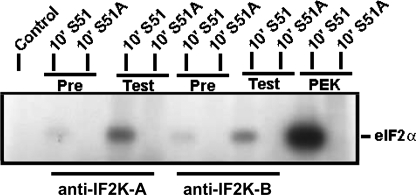
FIGURE 5.
Immunoprecipitation of TgIF2K-A and -B verify eIF2 kinase activities. Parasite lysates were incubated with antiserum raised against TgIF2K-A or TgIF2K-B (Test) or pre-immune sera (Pre) as a control. The resulting immunocomplex was used in an in vitro kinase assay with wild-type eIF2α (S51) or mutant non-phosphorylatable eIF2α (S51A) as substrate (14). Reactions were separated by SDS-PAGE and processed for autoradiography. Recombinant PEK protein was used as a positive control in the eIF2 kinase reaction.
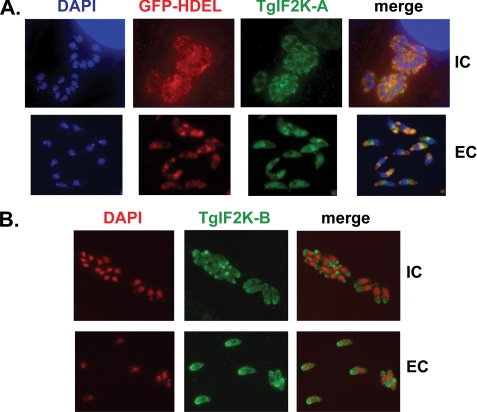
Differential Localization of TgIF2K-A and TgIF2K-B—Because of the fact that ER stress agents are potent inducers of TgIF2α phosphorylation and that TgIF2K-A has predicted transmembrane domains preceding its kinase domain (14), we hypothesized that it may be functionally related to the mammalian eIF2 kinase PEK. PEK is a type 1 transmembrane protein whose amino-terminal region is located in the lumen of the ER. Upon accumulation of malfolded protein in the ER, this regulatory portion of PEK facilitates activation of the carboxyl-terminal kinase domain, which resides in the cytoplasm and has access to eIF2α. Consequently, PEK is unique among the mammalian eIF2 kinases in that it harbors a transmembrane domain that localizes it to the ER where it plays this central role in the UPR (2). IFA using our anti-TgIF2K-A antiserum revealed an enriched staining around the parasite nucleus in wild-type tachyzoites, which is indicative of parasite ER (data not shown). Additional IFAs were performed using a transgenic parasite clone engineered to express GFP fused to the ER retention signal HDEL (15). There was co-localization between native TgIF2K-A and the GFP-HDEL fusion protein, consistent with residence in the parasite ER compartment (Fig. 6A). The co-localization pattern was similar in both extracellular (free) tachyzoites and intracellular (invaded) tachyzoites. By comparison, IFA images using antibody specific to TgIF2K-B revealed that this eIF2 kinase is diffuse throughout the parasite cytoplasm (Fig. 6B).
FIGURE 6.
Localization of TgIF2K-A and TgIF2K-B. A, IFA were performed on a transgenic parasite clone expressing GFP fused to the ER retention signal, HDEL (15). Intracellular (IC) or extracellular (EC) GFP-HDEL parasites were co-stained with affinity-purified anti-TgIF2K-A (green), anti-GFP (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue). B, IFA performed on wild-type parasites using anti-TgIF2K-B (green) and 4′,6-diamidino-2-phenylindole (red) shows diffuse staining of parasite cytoplasm in intracellular (IC) and extracellular (EC) parasites.
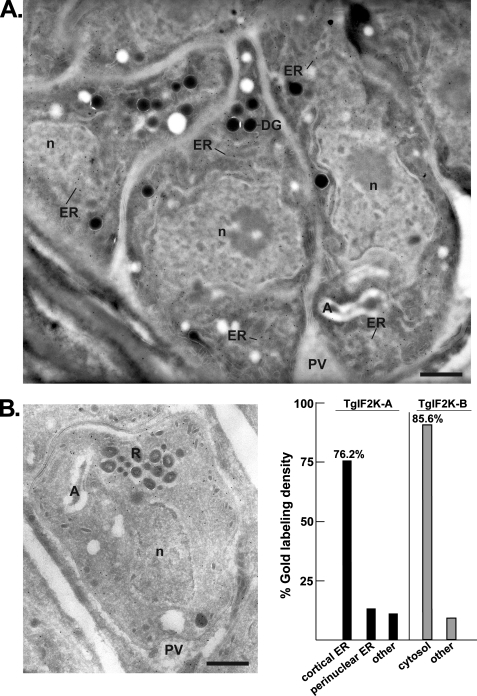
To better define the localization of TgIF2K-A and TgIF2K-B in tachyzoites, we performed immunoelectron microscopy using our affinity-purified antibodies. Results confirm an enrichment of TgIF2K-A in the parasite ER surrounding the nucleus (Fig. 7A). Anti-TgIF2K-A intensely stains multiple regions of the ER, both cortical and perinuclear. These results are consistent with the idea that TgIF2K-A is localized to the ER, analogous to mammalian PEK, to monitor and respond to ER stress. Also consistent with IFA results, the immunoelectron microscopy data show primarily a cytosolic localization for TgIF2K-B (Fig. 7B). TgIF2K-B does not appear to be associated with any particular structure but, rather, is observed interspersed between parasite organelles. Stereologic analysis was performed to demonstrate the specificity of gold labeling for TgIF2K-A and TgIF2K-B (Fig. 7C). About 76 and 13% of gold labeling for TgIF2K-A were specifically localized to cortical ER and the nuclear envelope, respectively. By comparison, about 86% of TgIF2K-B labeling was detected in the parasite cytoplasm.
FIGURE 7.
Ultrastructural detection of TgIF2K-A and TgIF2K-B in Toxoplasma. A and B, immunogold localization of TgIF2K-A (panel A) or TgIF2K-B (panel B) on intracellular tachyzoites. Cryosections were labeled with the anti-TgIF2K-A (dilution, 1/4) or anti-TgIF2K-B antibodies (dilution, 1/20) and revealed with protein A-gold particles (10 nm). n, nucleus; DG, dense granule; R, rhoptry; A, apicoplast; PV, parasitophorous vacuole. Scale bars are 0.5 μm. C, stereological analysis of gold labeling demonstrating the specificity in localization of the TgIF2K-A and TgIF2K-B compartments in intracellular T. gondii. Density (gold particles per μm2) of labeled structures was determined from 20 to 25 cellular cryosections. The percentage of individual intracellular compartment density was determined from the sum of gold density normalized for the variation in expression of TgIF2K-A or TgIF2K-B.
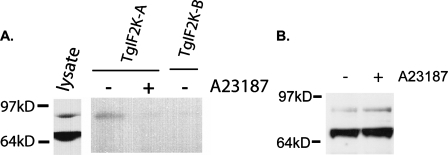
TgIF2K-A Associates with BiP in a Stress-dependent Manner—In the lumen of the ER, the amino-terminal regulatory portion of PEK has been shown to associate with the chaperone BiP/GRP78, which is proposed to be a repressor of PEK (21, 30). Upon accumulation of malfolded protein in the ER, BiP is released from PEK. This facilitates dimerization between PEK polypeptides, consequently activating PEK phosphorylation of cytosolic eIF2α (21). A BiP homologue has been identified in T. gondii that exists in two forms, a predominant 70-kDa protein and a minor form of 86 kDa (see parasite lysate control in Fig. 8 and Refs. 15 and 31). Given that ER stress conditions elicit TgIF2α phosphorylation in T. gondii and TgIF2K-A is a transmembrane protein localized to the ER, we hypothesized that it may be regulated analogously to PEK. TgIF2K-A may bind BiP specifically during non-stressed conditions, and an ER stress condition would signal release of BiP from TgIF2K-A, leading to its activation. To address this model, we immunoprecipitated TgIF2K-A from lysates prepared from tachyzoites treated for 1 h with ionophore or no ER stress agent. We then performed an immunoblot for T. gondii BiP using these immunocomplexes. As a control we also carried out the following immunoprecipitation of TgIF2K-B, which is located in the cytoplasm and, therefore, would not be in proximity to BiP. We found that only the 86-kDa form of BiP was associated with TgIF2K-A (Fig. 8A). Binding of TgIF2K-A with the 86-kDa BiP was substantially reduced in immunoprecipitates of parasites that were subjected to ER stress. By comparison, neither form of BiP was detectable in the TgIF2K-B immunocomplex (Fig. 8A). Levels of both forms of BiP were similar between unstressed parasites and those treated for 1 h with A23187 (Fig. 8B). These results support the idea that release of BiP from TgIF2K-A during ER stress directs translational control in T. gondii, a regulatory mechanism shared with mammalian PEK. By comparison, the cytoplasmic TgIF2K-B is subject to a different form of control.
FIGURE 8.
Interaction between TgIF2K-A and T. gondii BiP. A, equal numbers of extracellular tachyzoites exposed to 5 μm A23187 for 1 h (+) or mock treatment (-) were processed for immunoprecipitation with TgIF2K-A. Proteins in the immunoprecipitates were resolved by SDS-PAGE, and the presence of BiP in the immunocomplex was visualized by immunoblot analysis. As a control, a similar immunoprecipitation was carried out using anti-TgIF2K-B antibody, and after separation of immunocomplexed proteins by SDS-PAGE, a BiP immunoblot was performed. Parasite lysate probed for T. gondii BiP shows the protein migrating as 70- and 86-kDa products. B, protein lysates (10 μg) were generated from extracellular tachyzoites exposed to 5 μm A23187 for 1 h (+) or mock treatment (-) and processed for BiP immunoblot.
DISCUSSION
eIF2 Kinases Mediate Responses to ER and Cytosolic Stresses in T. gondii—A variety of environmental stresses induce T. gondii differentiation in vitro, and this study characterized a key component of the parasitic stress response pathway, the phosphorylation of TgIF2α. In this study we show stress impacting either the cytosol or ER induces TgIF2α phosphorylation in T. gondii. In the case of ER stress, the calcium ionophore A23187 or tunicamycin block translation initiation coincident with eIF2α phosphorylation (Fig. 2). Stresses known to elicit cytosolic stress, including heat shock and oxidative stress induced by arsenite, are also potent activators of TgIF2α phosphorylation (Fig. 1) (14). We identified an eIF2 kinase in each compartment, TgIF2K-A in the ER and TgIF2K-B in the cytoplasm. Although neither TgIF2K-A nor TgIF2K-B shares sequence homology outside the kinase domains with the four different mammalian eIF2 kinases, there are many functional similarities between TgIF2K-A and PEK/Perk. Both are suggested to be ER transmembrane proteins, and TgIF2K-A and PEK each associate with BiP-related ER chaperones during non-stressed conditions (21, 30, 32) (Fig. 8). TgIF2K-A appears to bind an 86-kDa version of BiP, suggesting that different versions of this ER chaperone serve to regulate TgIF2K-A. In the case of PEK, accumulation of misfolded protein in the lumen of the ER is proposed to bind BiP, maintaining PEK in a repressed conformation. The ensuing release of BiP from PEK allows for PEK to dimerize and autophosphorylate, steps that are thought to be integral for activation of the eIF2 kinase (21, 30, 33). Like PEK, there is substantially reduced BiP binding to TgIF2K-A during ER stress conditions. This suggests that the eIF2 kinase activity of TgIF2K-A is regulated analogously to PEK, supporting the idea that an ER stress response akin to portions of the UPR is conserved in T. gondii. To our knowledge, the observation that ER stress induces eIF2α phosphorylation and translational control is the first report that a UPR functions in Apicomplexa parasites. This is of significance because studies have suggested that Giardia lamblia, another early-branching eukaryotic cell, may have diverged before the appearance of this type of UPR (34). A transmembrane-containing eIF2 kinase has recently been identified in trypanosomes, but it localized to the flagellar pocket and not the ER (35). Our studies argue that the Apicomplexa are the earliest reported eukaryote likely to possess a functional UPR.
Development and Differentiation Involves eIF2α Phosphorylation— Appropriate regulation of gene expression is critical for the survival of cells subjected to environmental stress. In many species of protozoa and fungi, the response to stress involves conversion into a latent form that is protected by a cyst. One of the qualities that has made T. gondii such a successful pathogen is its ability to develop into a bradyzoite cyst in the tissues of its infected host. The cyst form allows the parasite to evade immunity and provides a mechanism of transmission to a new host (in the event the original host is consumed). Studies have shown that the host immune response involving production of interferon gamma is a key contributor to the establishment of chronic toxoplasmosis (36). If host immunity becomes impaired through disease or chemotherapy, then the T. gondii cysts will re-emerge to produce acute disease. Blocking the ability of the parasite to interconvert between tachyzoite and bradyzoites stages would be a significant advancement in the treatment of this pathogen, but the process is poorly understood.
Our results establish that eIF2 kinases and translation control exist in T. gondii. Moreover, eIF2 kinase-mediated pathways participate in the parasite stress response, including stress-induced developmental changes that underlie pathogenesis and facilitate transmission. In addition to induced TgIF2α phosphorylation and a reduction in global protein synthesis, ER stress elicited by tunicamycin is a potent inducer of bradyzoite differentiation, as measured by real-time PCR and IFA for bradyzoite-specific genes (Fig. 3). Moreover, cyst formation can be induced by inhibiting TgIF2α dephosphorylation (Fig. 4, A and B). In addition to triggering bradyzoite differentiation, TgIF2α phosphorylation and reduced global translation is likely to be crucial for maintaining the quiescent state of bradyzoites. Consistent with this idea, we found that TgIF2α phosphorylation was significantly elevated in bradyzoites compared with non-stressed tachyzoites (Fig. 4C).
The idea that the eIF2 kinase stress response is important for induction of morphogenesis that provides for resistance to environmental stresses is also suggested for other eukaryotic organisms. For example, when the fungi Candida albicans is subjected to nutrient limitation, the target of GCN2 eIF2 kinase, GCN4, is required to trigger filamentous growth (37). Furthermore, deletion of eIF2 kinases related to GCN2 in the slime mold Dictyostelium results in significant morphological and patterning defects triggered during stressed-induced differentiation from growing amoebae to fruiting bodies (38, 39). Together with our results, these studies point to eIF2 kinase-mediated translation control as having a key role in development and progression through the life cycle in response to changes in the environment of the organism.
New Members of the eIF2 Kinase Family Facilitate Translational Control—A central theme developed in studies of eIF2 kinases is that each family member has a unique regulatory domain juxtaposed to the kinase domain that allows for induction of eIF2α phosphorylation by a discrete set of environmental stresses. This modular domain arrangement appears to have been extended to unicellular eukaryotic parasites. T. gondii has two validated eIF2 kinases, the ER-based TgIF2K-A and a new cytoplasmic eIF2 kinase, TgIF2K-B. Two additional eIF2 kinases most closely related to GCN2 are predicted in the T. gondii genome data base, 20.m03847 and 641.m01524. Given the important role of GCN2 in directing translational control in response to nutrient deprivation, these two additional predicted eIF2 kinases may function to protect the parasite during starvation conditions.
Bioinformatic analyses were carried out with the completed genomes of T. gondii and other apicomplexans, including Plasmodium falciparum, Cryptosporidium parvum, and Theileria annulata. Each of the apicomplexans has a predicted eIF2 kinase harboring at least one transmembrane domain like TgIF2K-A, including the previously identified P. falciparum protein kinase PfPK4 (supplemental Fig. S3) (40). Each apicomplexan parasite examined also contains a putative orthologue of the eIF2 kinase GCN2. Interestingly, there is no clear orthologue of TgIF2K-B among these other apicomplexan species, suggesting that this eIF2 kinase responds to an environmental stress that is distinctive to T. gondii. Three eIF2 kinases are also suggested to be central to stress responses in the kinetoplastid parasite, Trypanosoma brucei (35). This includes a GCN2-related eIF2 kinase, TbeIF2K1, and a transmembrane containing kinase, TbeIF2K2, suggested to have a role in sensing protein or nutrient transport in T. brucei. The identification of these new eIF2 kinases suggests important roles for translational control in stress responses among numerous unicellular parasites, which may offer new targets for drug therapies.
Supplementary Material
Acknowledgments
We thank Dr. Kristin Hager (Notre Dame) for providing the GFP-HDEL parasites and Dr. Jay Bangs (University of Wisconsin-Madison) for providing anti-BiP antiserum. We thank Marc Pypaert (Yale Center for Cell and Molecular Imaging) for excellent assistance and scientific comments for electron microscopy, Dr. Jeremy Sanford (Indiana University School of Medicine) for technical assistance with polyribosome profiling, and Dr. Anthony Sinai (University of Kentucky) for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01GM49164 and R01GM64350 (to R. C. W.). This study was also supported in part by American Heart Association Grant 0750201Z (to W. J. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: eIF2, eukaryotic initiation factor-2; ER, endoplasmic reticulum; UPR, unfolded protein response; TgIF2α, Toxoplasma eukaryotic initiation factor-2α; PBS, phosphate-buffered saline; GFP, green fluorescent protein; IFA, immunofluorescence assays; Sal, salubrinal.
References
- 1.Dever, T. E. (2002) Cell 108545 -556 [DOI] [PubMed] [Google Scholar]
- 2.Wek, R. C., and Cavener, D. R. (2007) Antioxid. Redox Signal. 92357 -2371 [DOI] [PubMed] [Google Scholar]
- 3.Wek, R. C., Jiang, H. Y., and Anthony, T. G. (2006) Biochem. Soc. Trans. 347 -11 [DOI] [PubMed] [Google Scholar]
- 4.Travers, K. J., Patil, C. K., Wodicka, L., Lockhart, D. J., Weissman, J. S., and Walter, P. (2000) Cell 101249 -258 [DOI] [PubMed] [Google Scholar]
- 5.Hinnebusch, A. G. (2005) Annu. Rev. Microbiol. 59407 -450 [DOI] [PubMed] [Google Scholar]
- 6.Chen, J. J. (2007) Blood 1092693 -2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu, L., Han, A. P., and Chen, J.-J. (2001) Mol. Cell. Biol. 217971 -7980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadler, A. J., and Williams, B. R. (2007) Curr. Top. Microbiol. Immunol. 316253 -292 [DOI] [PubMed] [Google Scholar]
- 9.Barber, G. N. (2005) Cell Death Differ. 12563 -570 [DOI] [PubMed] [Google Scholar]
- 10.Black, M. W., and Boothroyd, J. C. (2000) Microbiol. Mol. Biol. Rev. 64 607-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss, L. M., and Kim, K. (2000) Front. Biosci. 5391 -405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soete, M., Camus, D., and Dubremetz, J. F. (1994) Exp. Parasitol. 78361 -370 [DOI] [PubMed] [Google Scholar]
- 13.Fox, B. A., and Bzik, D. J. (2002) Nature 415926 -929 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan, W. J., Jr., Narasimhan, J., Bhatti, M. M., and Wek, R. C. (2004) Biochem. J. 380523 -531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hager, K. M., Striepen, B., Tilney, L. G., and Roos, D. S. (1999) J. Cell Sci. 1122631 -2638 [DOI] [PubMed] [Google Scholar]
- 16.Ramirez, M., Wek, R. C., and Hinnebusch, A. G. (1991) Mol. Cell. Biol. 113027 -3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatti, M. M., and Sullivan, W. J., Jr. (2005) J. Biol. Chem. 2805902 -5908 [DOI] [PubMed] [Google Scholar]
- 18.Folsch, H., Pypaert, M., Schu, P., and Mellman, I. (2001) J. Cell Biol. 152595 -606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kissinger, J. C., Gajria, B., Li, L., Paulsen, I. T., and Roos, D. S. (2003) Nucleic Acids Res. 31 234-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prostko, C. R., Brostrom, M. A., and Brostrom, C. O. (1993) Mol. Cell. Biochem. 128255 -265 [DOI] [PubMed] [Google Scholar]
- 21.Ma, K., Vattem, K. M., and Wek, R. C. (2002) J. Biol. Chem. 27718728 -18735 [DOI] [PubMed] [Google Scholar]
- 22.Luk, F. C., Johnson, T. M., and Beckers, C. J. (2007) Mol. Biochem. Parasitol. 157169 -178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endo, T., Sethi, K. K., and Piekarski, G. (1982) Exp. Parasitol. 53179 -188 [DOI] [PubMed] [Google Scholar]
- 24.Boyce, M., Bryant, K. F., Jousse, C., Long, K., Harding, H. P., Scheuner, D., Kaufman, R. J., Ma, D., Coen, D. M., Ron, D., and Yuan, J. (2005) Science 307935 -939 [DOI] [PubMed] [Google Scholar]
- 25.Kozak, M. (1991) J. Biol. Chem. 26319867 -19870 [PubMed] [Google Scholar]
- 26.Dar, A. C., Dever, T. E., and Sicheri, F. (2005) Cell 122887 -900 [DOI] [PubMed] [Google Scholar]
- 27.Padyana, A. K., Qiu, H., Roll-Mecak, A., Hinnebusch, A. G., and Burley, S. K. (2005) J. Biol. Chem. 28029289 -29299 [DOI] [PubMed] [Google Scholar]
- 28.Romano, P. R., Garcia-Barrio, M. T., Zhang, X., Wang, Q., Taylor, D. R., Zhang, F., Herring, C., Mathews, M. B., Qin, J., and Hinnebusch, A. G. (1998) Mol. Cell. Biol. 182282 -2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, F., Romano, P. R., Nagamura-Inoue, T., Tian, B., Dever, T. E., Mathews, M. B., Ozato, K., and Hinnebusch, A. G. (2001) J. Biol. Chem. 27624946 -24958 [DOI] [PubMed] [Google Scholar]
- 30.Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P., and Ron, D. (2000) Nat. Cell Biol. 2 326-332 [DOI] [PubMed] [Google Scholar]
- 31.Yung, S. C., Unnasch, T. R., and Lang-Unnasch, N. (2003) J. Parasitol. 89 767-776 [DOI] [PubMed] [Google Scholar]
- 32.Harding, H. P., Zhang, Y., and Ron, D. (1999) Nature 397271 -274 [DOI] [PubMed] [Google Scholar]
- 33.Marciniak, S. J., and Ron, D. (2006) Physiol. Rev. 861133 -1149 [DOI] [PubMed] [Google Scholar]
- 34.Reiner, D. S., McCaffery, J. M., and Gillin, F. D. (2001) Cell Microbiol 3 459-472 [DOI] [PubMed] [Google Scholar]
- 35.Moraes, M. C., Jesus, T. C., Hashimoto, N. N., Dey, M., Schwartz, K. J., Alves, V. S., Avila, C. C., Bangs, J. D., Dever, T. E., Schenkman, S., and Castilho, B. A. (2007) Eukaryot. Cell 61979 -1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman, L. A., and Hunter, C. A. (2002) Int. Rev. Immunol. 21373 -403 [DOI] [PubMed] [Google Scholar]
- 37.Tripathi, G., Wiltshire, C., Macaskill, S., Tournu, H., Budge, S., and Brown, A. J. (2002) EMBO J. 215448 -5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rai, M., Xiong, Y., and Singleton, C. K. (2006) Differentiation 74583 -595 [DOI] [PubMed] [Google Scholar]
- 39.Fang, R., Xiong, Y., and Singleton, C. K. (2003) BMC Dev. Biol. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohrle, J. J., Zhao, Y., Wernli, B., Franklin, R. M., and Kappes, B. (1997) Biochem. J. 328677 -687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.