Abstract
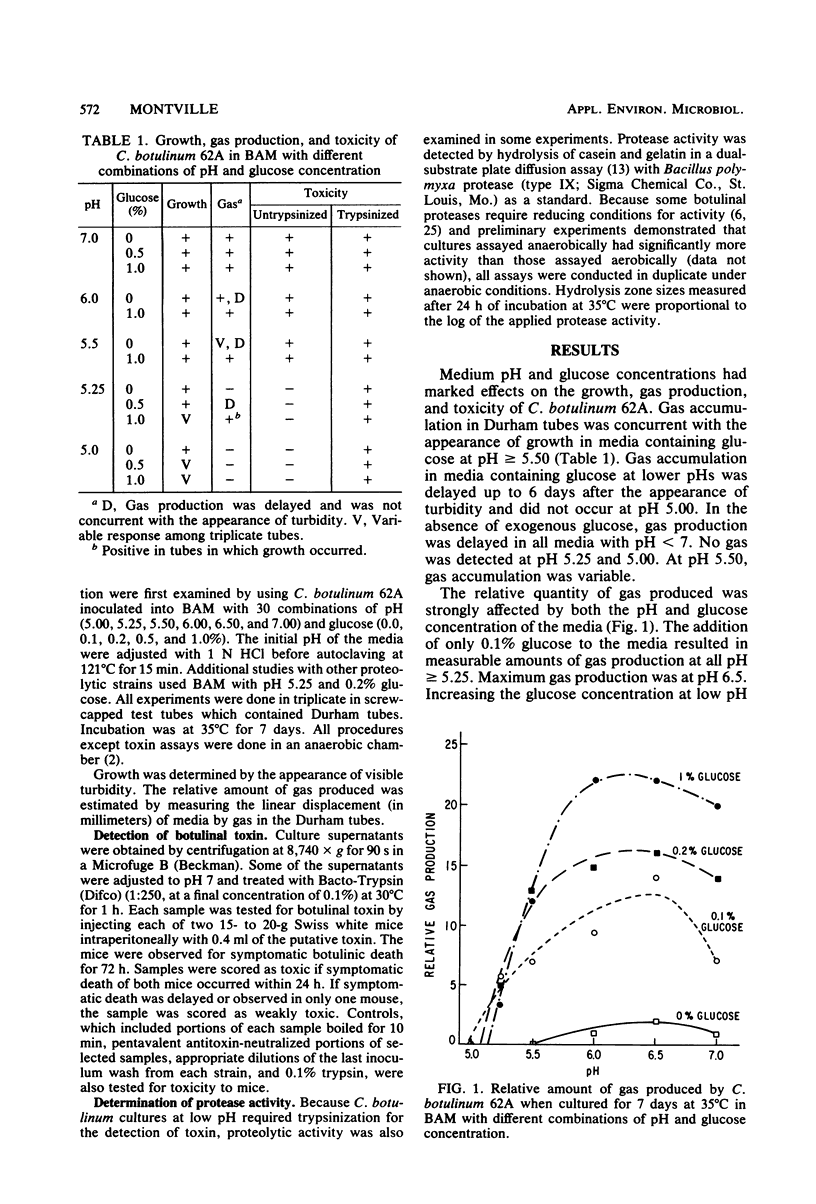
Reports that Clostridium botulinum toxin can sometimes be detected in the absence of indicators of overt spoilage led to a systematic study of this phenomenon in a model system. Media with various combinations of pH (5.0 to 7.0) and glucose (0.0 to 1.0%) were inoculated with vegetative cells of C. botulinum 62A and incubated anaerobically at 35 degrees C. Although growth and toxin production occurred at all pH and glucose combinations, accumulation of gas was delayed or absent in media with low pH, low glucose levels, or both. Other proteolytic C. botulinum strains gave similar results. Trypsin activation was required to detect toxin in some low pH cultures. The trypsinization requirement correlated with low proteolytic activity in the cultures. Proteolytic activity of the strains examined was 5- to 500-fold lower in botulinal assay medium than in cooked meat medium. The results indicate that the absence of gas accumulation does not preclude the presence of botulinal toxin and that proteolytic cultures grown under adverse conditions may require trypsinization for the detection of toxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONVENTRE P. F., KEMPE L. L. Physiology of toxin production by Clostridium botulinum types A and B. II. Effect of carbohydrate source on growth, autolysis, and toxin production. Appl Microbiol. 1959 Nov;7:372–374. doi: 10.1128/am.7.6.372-374.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONVENTRE P. F., KEMPE L. L. Physiology of toxin production by Clostridium botulinum types A and B. IV. Activation of the toxin. J Bacteriol. 1960 Jan;79:24–32. doi: 10.1128/jb.79.1.24-32.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. The Utilization of Amino Acids and of Glucose by Clostridium botulinum. J Bacteriol. 1940 May;39(5):485–497. doi: 10.1128/jb.39.5.485-497.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Sugiyama H. Role of arginine residues in the structure and biological activity of botulinum neurotoxin types A and E. Biochem Biophys Res Commun. 1980 Mar 28;93(2):369–375. doi: 10.1016/0006-291x(80)91086-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta B. R., Sugiyama H. Isolation and characterization of a protease from Clostridium botulinum type B. Biochim Biophys Acta. 1972 Jun 16;268(3):719–729. doi: 10.1016/0005-2744(72)90276-8. [DOI] [PubMed] [Google Scholar]
- Elberg S. S., Meyer K. F. The Extracellular Proteolytic System of Clostridium parabotulinum. J Bacteriol. 1939 May;37(5):541–565. doi: 10.1128/jb.37.5.541-565.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondalen T. Osteochondrosis and arthrosis in pigs. I. Incidence in animals up to 120 kg live weight. Acta Vet Scand. 1974;15(1):1–25. doi: 10.1186/BF03547490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Dual-substrate plate diffusion assay for proteases. Appl Environ Microbiol. 1983 Jan;45(1):200–204. doi: 10.1128/aem.45.1.200-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J. Effect of plating medium on heat activation requirement of Clostridium botulinum spores. Appl Environ Microbiol. 1981 Oct;42(4):734–736. doi: 10.1128/aem.42.4.734-736.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatjes G. J., Smelt J. P. Clostridium botulinum can grow and form toxin at pH values lower than 4.6. Nature. 1979 Oct 4;281(5730):398–399. doi: 10.1038/281398a0. [DOI] [PubMed] [Google Scholar]
- SIMMONS R. J., COSTILOW R. N. Enzymes of glucose and pyruvate catabolism in cells, spores, and germinated spores of Clostridium botulinum. J Bacteriol. 1962 Dec;84:1274–1281. doi: 10.1128/jb.84.6.1274-1281.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin L. C. The action of batulinum toxin at the neuromuscular junction. Med Biol. 1981 Feb;59(1):11–20. [PubMed] [Google Scholar]
- Siegel L. S., Metzger J. F. Effect of fermentation conditions on toxin production by Clostridium botulinum type B. Appl Environ Microbiol. 1980 Dec;40(6):1023–1026. doi: 10.1128/aem.40.6.1023-1026.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. S., Metzger J. F. Toxin production by Clostridium botulinum type A under various fermentation conditions. Appl Environ Microbiol. 1979 Oct;38(4):606–611. doi: 10.1128/aem.38.4.606-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt J. P., Raatjes G. J., Crowther J. S., Verrips C. T. Growth and toxin formation by Clostridium botulinum at low pH values. J Appl Bacteriol. 1982 Feb;52(1):75–82. doi: 10.1111/j.1365-2672.1982.tb04375.x. [DOI] [PubMed] [Google Scholar]
- Tjaberg T. B., Fossum K. Proteases of Clostridium botulinum. IV. Inhibitors against proteases from Clostridium botulinum. Acta Vet Scand. 1973;14(4):560–569. [PubMed] [Google Scholar]
- Tjaberg T. B., Fossum K. Proteases of Clostridium botulinum. V. Studies on the serological relationship between proteases from Clostridium botulinum and other spore-forming bacteria. Acta Vet Scand. 1973 May;14(5):700–711. doi: 10.1186/BF03547399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaberg T. B. Proteases of Clostridium botulinum. 3. Isolation and characterization of proteases from Clostridium botulinum types A,B,C,D and F. Acta Vet Scand. 1973;14(4):538–559. [PubMed] [Google Scholar]
- Tjaberg T. B. Proteases of Clostridium botulinum. I. Classification of proteases and literature survey. Acta Vet Scand. 1973;14(1):184–192. doi: 10.1186/BF03547421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaberg T. B. Proteases of Clostridium botulinum. II. The relationship between growth medium and the production of proteases by Clostridium botulinum types A, B, C, D, E and F. Acta Vet Scand. 1973;14(1):193–200. doi: 10.1186/BF03547422. [DOI] [PMC free article] [PubMed] [Google Scholar]


