Abstract
AMP-activated protein kinase (AMPK) is activated in adipocytes during exercise and other states in which lipolysis is stimulated. However, the mechanism(s) responsible for this effect and its physiological relevance are unclear. To examine these questions, 3T3-L1 adipocytes were treated with cAMP-inducing agents (isoproterenol, forskolin, and isobutylmethylxanthine), which stimulate lipolysis and activate AMPK. When lipolysis was partially inhibited with the general lipase inhibitor orlistat, AMPK activation by these agents was also partially reduced, but the increases in cAMP levels and cAMP-dependent protein kinase (PKA) activity were unaffected. Likewise, small hairpin RNA-mediated silencing of adipose tissue triglyceride lipase inhibited both forskolin-stimulated lipolysis and AMPK activation but not that of PKA. Forskolin treatment increased the AMP:ATP ratio, and this too was reduced by orlistat. When acyl-CoA synthetase, which catalyzes the conversion of fatty acids to fatty acyl-CoA, was inhibited with triacsin C, the increases in both AMPK activity and AMP:ATP ratio were blunted. Isoproterenol-stimulated lipolysis was accompanied by an increase in oxidative stress, an effect that was quintupled in cells incubated with the AMPK inhibitor compound C. The isoproterenol-induced increase in the AMP:ATP ratio was also much greater in these cells. In conclusion, the results indicate that activation of AMPK in adipocytes by cAMP-inducing agents is a consequence of lipolysis and not of PKA activation. They suggest that AMPK activation in this setting is caused by an increase in the AMP:ATP ratio that appears to be due, at least in part, to the acylation of fatty acids. Finally, this AMPK activation appears to restrain the energy depletion and oxidative stress caused by lipolysis.
AMP-activated protein kinase (AMPK)2 is a sensor of cellular energy state that responds to metabolic stresses and other regulatory signals. The mechanism of activation of AMPK is a complex phenomenon and is still not fully understood, although it is recognized that it involves phosphorylation of the critical Thr-172 residue of its α catalytic subunit by upstream kinases such as LKB1, Ca2+-calmodulin-dependent protein kinase kinase, and possibly Tak1, a member of the mitogen-activated protein kinase kinase kinase family (1–5). AMPK is also regulated by AMP allosterically and the current view is that this both increases AMPK activity directly and makes it a poorer substrate for phosphatases (6).
The role and regulation of AMPK in muscle, liver, and various cultured cells have been extensively studied. It is now well established that energy depletion because of starvation, hypoxia, and exercise increases the intracellular AMP:ATP ratio and secondarily AMPK activity (7). Upon its activation, a major role of AMPK is to replete cellular energy stores by stimulating processes that generate ATP, such as fatty acid oxidation, and inhibiting ATP-consuming pathways (e.g. lipogenesis, triglyceride synthesis, and gluconeogenesis) that are not acutely needed for cell survival (7). In addition, changes in the levels of various hormones and fuels have been demonstrated to activate or inhibit AMPK suggesting that its regulation and physiological relevance are more complex than initially appreciated. In keeping with this notion, AMPK has also been shown to protect cells by reducing or even preventing a variety of stress responses, including the generation of free radicals and inflammation (8–12).
Adipose tissue is a key player in whole-body energy regulation. One of its major roles is to provide free fatty acids (FFA) as a fuel for other tissues in times of need. In these circumstances, catecholamines, and possibly other hormones and neurotransmitters, rapidly activate β-adrenergic receptors and the cAMP-dependent protein kinase (PKA) axis. This results in the stimulation of lipolysis by a complex regulatory mechanism that appears to involve hormone-sensitive lipase, adipose-tissue triglyceride lipase (ATGL), and perilipin, a lipid droplet-associated phosphoprotein that has proven to be essential for β-adrenergic stimulation of lipolysis (13–17). In recent years, ATGL has been identified as the predominant lipase responsible for the initial step in hydrolyzing triglycerides, at least in rodents (18–20).
Despite its prominent role in energy metabolism, few investigations have addressed the regulation of AMPK in adipose tissue. Previous studies conducted in our laboratory first showed that in rodents exercise increases AMPK activity in intra-abdominal adipose tissue (21, 22). Acute exercise is known to increase the release of catecholamines, which participate in the lipolytic response of adipose tissue to exercise. In keeping with this notion, β-adrenergic agonists and agents that increase cAMP have been found to stimulate both lipolysis and AMPK activation in cultured adipocytes (23, 24), and to cause a decrease in their cellular energy state in vitro (25–28) and in rats in vivo (29). Despite this, the mechanism(s) by which these agents activate AMPK, and whether it is dependent on their ability to stimulate lipolysis is unknown, as is the physiological significance of AMPK activation in this setting. To address these questions, we first examined activation of AMPK by agents that increase cAMP in cultured adipocytes in situations in which their ability to stimulate lipolysis was inhibited downstream of PKA. We then investigated the consequences of inhibiting AMPK activation on reactive oxygen species (ROS) generation and energy depletion in these cells.
EXPERIMENTAL PROCEDURES
Culture of 3T3-L1 Adipocytes—3T3-L1 preadipocytes were purchased from American Type Culture Collection (Manassas, VA). 3T3-L1 preadipocytes placed in 12-well plates were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% bovine calf serum and differentiated to adipocytes using standard protocols (30). 3T3-L1 attained a fully differentiated state on day 10 after the initiation of the differentiation protocol, and experiments were done on days 12–16. DMEM, bovine calf serum, and fetal bovine serum were purchased from Invitrogen.
Generation and Differentiation of Stable Lines of Peri-/- Mouse Embryonic Fibroblast Adipocytes—Stable lines of MEFs were generated from embryos of perilipin null mice (Peri-/-) as described previously (13, 14, 31). MEF adipocytes were generated by retroviral expression of peroxisome proliferator-activated receptor-γ (31) followed by selection, expansion, and differentiation using a standard differentiation medium (13, 14). MEFs attained a differentiated adipocyte phenotype within 7 days of culturing in differentiation medium (13, 14).
Adenoviral Expression of GFP and Peri A Constructs in Peri-/- MEF Adipocytes—Adenoviruses expressing Aequoria victoria green fluorescent protein (Ad-GFP) or wild-type perilipin A (Ad-WT Peri A) were generated and verified as described previously (13, 14, 32). Recombinant adenovirus was transduced into Peri-/- MEFs with Lipofectamine Plus™ (Invitrogen) on day 3 after induction of differentiation. The amount of each adenovirus used was selected to ensure comparable levels of expression of the different Peri A constructs, which was confirmed by Western blots and densitometry (13, 14, 32). GFP was transduced in Peri-/- MEFs as a negative control.
Recombinant Adenoviruses Expressing Small Hairpin RNA (shRNA) Directed Against Murine ATGL—ATGL shRNA design was based on GenBank™ accession number NM025802 (sequence GGAGAGAACGTCATCATAT) as described (13). A “scrambled” version of these shRNA (CGCGCTTTGTAGGATTCA) was generated as a control for nonspecific effects of shRNA. All shRNAs were cloned into the pQuiet vector to generate recombinant adenoviruses. Recombinant adenovirus expressing shRNAs was transduced into MEFs cells with Lipofectamine Plus™ on day 2 after induction of differentiation.
Lipolysis Assays—Lipolysis was stimulated by addition of the β-adrenergic agonist isoproterenol (10 μm), the adenylate cyclase activator forskolin (20 μm), or the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) (0.5 mm) into serum-free culture medium containing 0.5% fatty acid-free bovine serum albumin (Celliance, Toronto, Canada) in the presence or absence of the general lipase inhibitor orlistat (100 μm), the ACS inhibitor triacsin C (6 μm), or the AMPK inhibitor compound C (50 μm). Lipolysis was assessed from the release of glycerol and FFA in the culture medium as described previously (14) using free glycerol reagent (Sigma) and nonesterified fatty acids measurement kit (Waco Diagnostics, Richmond, VA). Triacsin C was purchased from Biomol (Plymouth Meeting, PA), compound C from Calbiochem, and other compounds from Sigma.
Immunoblot Analysis—Cultured cells were scraped on ice in cell lysis buffer (plus 1 mm phenylmethylsulfonyl fluoride), sonicated, and centrifuged (14,000 × g for 15 min at 4 °C). Protein concentrations of cell supernatants were determined using the bicinchoninic acid (BCA) reagents (Pierce) using bovine serum albumin as the standard. Proteins (20 μg) were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham Biosciences). Membranes were blocked in Tris-buffered saline (pH 7.5) containing 0.05% Tween 20 (TBST) and 5% milk for 1 h at room temperature and then probed with antibodies specific to β-actin, perilipin, or ATGL for 1 h at room temperature, or specific to P-AMPK Thr-172, pan-αAMPK, P-ACC Ser-79, P-LKB1 Ser-431, P-CREB Ser-133, phospho-(Ser/Thr)-PKA substrate overnight at 4 °C. Bound antibodies were detected with the appropriate horseradish peroxidase-linked whole secondary antibodies. Protein immunoblots were visualized by enhanced chemiluminescence, and bands were quantified with scanning densitometry. Antibodies for pan-α-AMPK, P-AMPK Thr-172, and P-CREB Ser-133, phospho-(Ser/Thr)-PKA substrate, and cell lysis buffer were purchased from Cell Signaling Technology (Beverly, MA); P-ACC Ser-79 was from Upstate Biotechnology, Inc. (Lake Placid, NY); β-actin was from Sigma; P-LKB1 Ser-431 and mouse and rabbit antibodies conjugated to horseradish peroxidase were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Specific polyclonal ant-rabbit ATGL and perilipin antibody were generated and affinity-purified as described (13–15).
Determination of AMPK Activity—3T3-L1 express mainly the α1 isoform of AMPK (data not shown and see Ref. 33). AMPK α1 activity was measured after 1 h of treatment with or without isoproterenol (10 μm) in 3T3-L1 adipocytes. In addition, a possible nonspecific direct effect of orlistat or triacsin C on AMPK activity was ruled out by measuring AMPK activity in a cell-free system with/without the presence of orlistat (100 μm) or triacsin C (6 μm). To do so, untreated 3T3-L1 lysates containing 100 μg of protein were immunoprecipitated with AMPKα1-specific polyclonal antibody (Bethyl Laboratories Inc., Montgomery, TX) and protein A/G-agarose beads. Beads were washed, and the immobilized enzyme was assayed based on the phosphorylation of SAMS peptide (HMRSAMSGLHLVKRR) (0.2 mmol/liter) by 0.2 mmol/liter ATP (containing 2 μCi of [γ-32P]ATP) in the presence and absence of 0.2 mmol/liter AMP and the presence or absence of orlistat (100 μm) or triacsin C (6 μm). Label incorporation into the SAMS peptide was measured using a scintillation counter.
Nucleotide Studies in 3T3-L1 Adipocytes—Cellular levels of ATP, ADP, and AMP were measured spectrophotometrically as described previously (34, 35). In brief, 3T3-L1 adipocytes were treated as described under “Lipolysis Assays.” After the incubation period, the media were kept for glycerol measurements, and the cells were washed once with cold phosphate-buffered saline, and proteins were precipitated with 1% trichloroacetic acid. The acid was then removed from the aqueous phase of the trichloroacetic acid extracts with repeated washes with ether. The samples were then dried to powder and kept at -80 °C until the assay.
Determination of Intracellular cAMP Content in 3T3-L1 Adipocytes—3T3-L1 adipocytes were treated as described under “Lipolysis Assays.” After the incubation period, cAMP was extracted and measured using a commercially available cAMP EIA kit according to the instructions provided by the manufacturer (Biomedical Technologies Inc., Stoughton, MA).
Detection of Reactive Oxygen Species—3T3-L1 adipocytes were incubated overnight with compound C or vehicle (DMSO) and thereafter treated for 1 h with isoproterenol and loaded with the redox-sensitive dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF, Invitrogen) during the last 30 min of this incubation period. After the loading period, the media were kept for glycerol assay, and cells were washed with and incubated in pre-warmed Hanks' buffered saline solution at 37 °C. Reactive oxygen species (H2O2 and OH) formation was measured for 1 h in a multiwell fluorescence plate reader (Fluoroskan Ascent, Labsystems) set at 37 °C using excitation and emission filters of 485 and 538 nm, respectively. At the end of the DCF experiment, tert-butyl hydroperoxide (1 mm) (Sigma) was added to the incubation media to verify that the measurement maximal capacity of the system had not been reached during the experiment.
Statistical Analysis—Results were analyzed using Statview version 5.0.1 and are presented as means ± S.E. of at least three independent experiments. Statistical significance was determined by a one-way or two-way analysis of variance for nonrepeated or repeated measures, as appropriate. Fisher's protected least significant difference post hoc test was used in the event of a significant (p < 0.05) ratio.
RESULTS
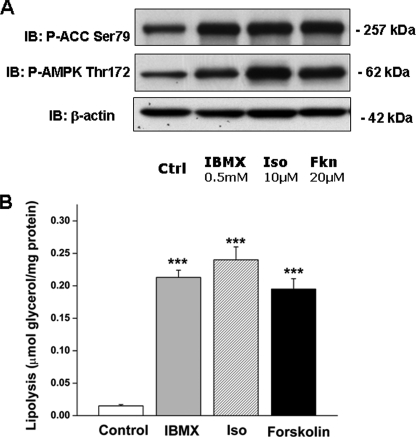
Agents That Increase cAMP Stimulate Both Lipolysis and AMPK Activation in Adipocytes—Incubation of 3T3-L1 adipocytes with a β-adrenergic agonist (isoproterenol, 10 μm) and an adenylyl cyclase activator (forskolin, 20 μm) caused a 20-fold increase in lipolysis (Fig. 1B). In confirmation of previous reports (23, 24), these agents also activated AMPK as reflected by increases in the phosphorylation of its α-subunit at Thr-172 and of its downstream target ACC at Ser-79 (Fig. 1A). That isoproterenol activates AMPK was confirmed by measurements of the activity of AMPK α1, the main isoform expressed in 3T3-L1 adipocytes (data not shown). Incubation of the cells with the phosphodiesterase inhibitor IBMX (0.5 mm) also stimulated lipolysis and increased AMPK and ACC phosphorylation (Fig. 1, A and B). Because the phosphodiesterase catalyzes the conversion of cAMP to AMP, this essentially rules out this source of AMP as a major cause of AMPK activation during lipolysis.
FIGURE 1.
Agonists that increase cAMP stimulate both lipolysis and AMPK activation. L1 adipocytes were treated with the phosphodiesterase inhibitor IBMX (0.5 mm), the β-agonist isoproterenol (Iso, 10 μm), or the adenylyl cyclase activator forskolin (FKN, 20 μm) for 1 h, following a 4-h preincubation period in serum-free DMEM containing 0.5% fatty acid-free BSA. Ctrl, control. A, activation of AMPK as assessed from immunoblots of phospho-AMPK Thr-172 and P-ACC Ser-79. IB, immunoblot. B, lipolysis was quantified on the basis of glycerol release into the incubation media. Results for glycerol release are means ± S.E. (n = 9) and were obtained in three independent experiments. Immunoblots shown are representative of those obtained in a total of nine lysates. Significantly different from control group: ***, p < 0.001.
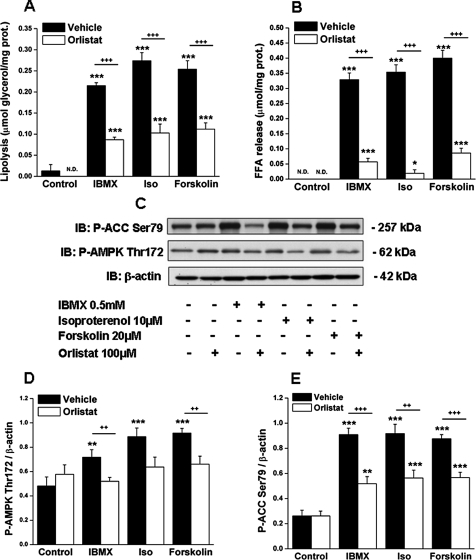
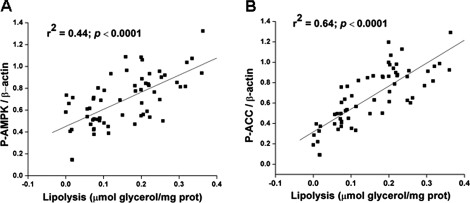
Iinhibition of Lipolysis by Orlistat Reduces AMPK Activation by Agents That Increase cAMP—We next sought to determine whether AMPK activation by isoproterenol, forskolin, and IBMX is related to their ability to stimulate lipolysis. To test this possibility, we incubated adipocytes with orlistat, a general lipase inhibitor (36). As shown in Fig. 2, orlistat inhibited the ability of all three lipolytic agents to stimulate lipolysis by ∼50% (Fig. 2, A and B), and it had a similar inhibitory effect on AMPK activation (Fig. 2, C–E). The possibility that orlistat could have a direct inhibitory action on AMPK was ruled out by its lack of effect on AMPK activity in a cell-free system (data not shown, see under “Experimental Procedures”). Regression analyses revealed a strong positive correlation between both P-AMPK Thr-172 and lipolysis (r2 = 0.44; p < 0.0001) and between P-ACC Ser-79 and lipolysis (r2 = 0.64; p < 0.0001) (Fig. 3, A and B).
FIGURE 2.
Inhibition of lipolysis with the general lipase inhibitor orlistat blunts the activation of AMPK by agents that increase cAMP. 3T3-L1 adipocytes were treated with IBMX (0.5 mm), isoproterenol (Iso, 10 μm), or forskolin (20 μm) for 1 h, following a 4-h preincubation period in serum-free DMEM containing 0.5% fatty acid-free BSA with/without orlistat (100 μm). Lipolysis was assessed by measuring the release of glycerol (A) or FFA (B) into the incubation media and the activation of AMPK by immunoblots (IB) of phospho-AMPK and phospho-ACC (C) as described in the legend of Fig. 1. Densitometric analyses of P-AMPK (D) and P-ACC (E) are shown. N.D., nondetectable. Immunoblots shown are representative of nine samples obtained in three independent experiments. Results in A, B, D, and E are expressed as means ± S.E. (n = 9) and were obtained from three independent experiments. Significantly different from control group: *, p < 0.05; **, p < 0.01; ***, p < 0.001; significantly different from orlistat-treated cells: ++, p < 0.01; +++, p < 0.001.
FIGURE 3.
Correlation of measures of AMPK activation and lipolysis in adipocytes stimulated by agents that increase cAMP. Lipolysis was assessed on the basis of glycerol release and was correlated with the abundance of P-AMPK Thr-172 (A) and P-ACC Ser-79 (B). Individual values were obtained in the experiments described in Fig. 2.
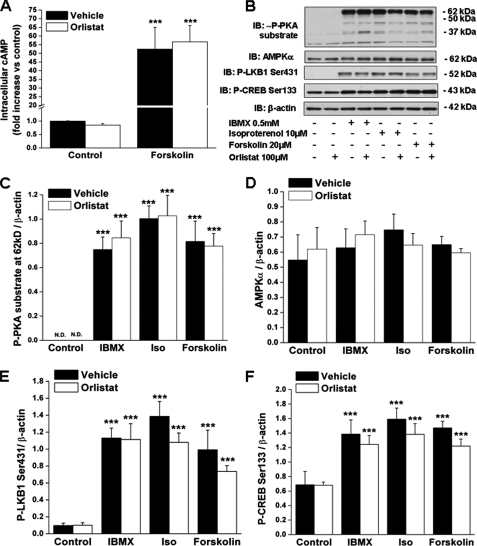
Inhibition of Stimulated Lipolysis by Orlistat Does Not Alter cAMP or PKA Signaling—Forskolin, the only agent tested, caused a 50-fold increase in intracellular cAMP levels (Fig. 4A). Likewise, the three lipolytic agents augmented PKA activity as reflected by their ability to increase the abundance of phosphorylated serine and threonine residues of PKA substrates (∼P-PKA substrate) (Fig. 4B). The most abundant P-PKA substrate was found at ∼62 kDa (Fig. 4B), which corresponds to the molecular weight of perilipin, the predominant PKA substrate in adipocytes (17, 37). These agents also increased the phosphorylation of Ser-133 on the cAMP-response element-binding protein (CREB) and Ser-431 on LKB1, two downstream targets of cAMP and PKA (38–40) (Fig. 4, B, E and F). Treatment with orlistat did not affect any of these events (Fig. 4, A–C, E and F), indicating that it inhibited lipolysis downstream of cAMP and PKA. Collectively, the data suggest that the activation of AMPK by agents that increase cAMP in adipocytes is the result of increased lipolysis and that it is not a direct effect of cAMP or PKA activation.
FIGURE 4.
Orlistat inhibits AMPK activation without affecting cAMP levels and PKA signaling. A, intracellular levels of cAMP in 3T3-L1 adipocytes treated with forskolin with/without orlistat as described in Fig. 2. B, cell lysates obtained in the experiment described in Fig. 2 were immunoblotted (IB) with antibodies recognizing phosphorylated serine and threonine residues of PKA substrates (∼P-PKA), totalα-AMPK, the PKA downstream targets P-LKB1 Ser-431, P-CREB Ser-133, and β-actin. Densitometric analyses are as follows: C, P-PKA substrate ∼62 kDa; D, α-AMPK; E, P-LKB1 Ser-431; and F, P-CREB Ser-133. Immunoblots shown are representative of nine samples obtained in three independent experiments. Results in A, C, D, E, and F are expressed as means ± S.E. (n = 9). Significantly different from control group: ***, p < 0.001. Iso, isoproterenol.
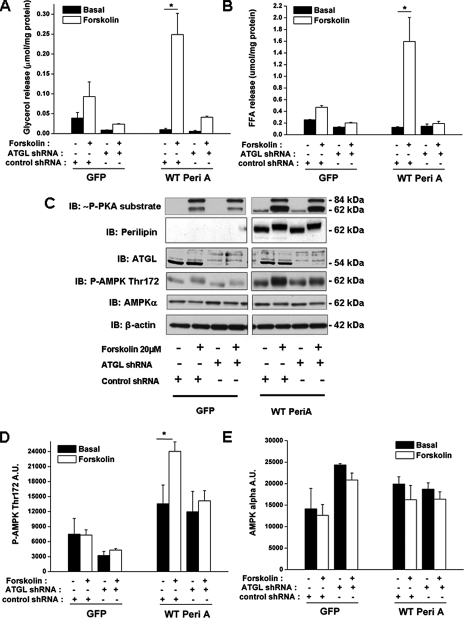
Inhibition of Stimulated Lipolysis by Genetic Means Abrogates Forskolin-induced AMPK Activation—To examine further the relationship between lipolysis and AMPK activation, we next used genetic approaches to inhibit or restore forskolin-stimulated lipolysis downstream of PKA. For this purpose cultured adipocytes derived from MEFs of Peri-/- mice were utilized because such cells exhibit a robust reduction in forskolin-stimulated lipolysis despite normal PKA activation (13, 14), in keeping with the observation that perilipin is essential for PKA-mediated lipolysis (15–17). In addition, we have found that the adenoviral expression of wild-type perilipin A restores forskolin-stimulated lipolysis in these adipocytes and that shRNA-mediated silencing of ATGL reverses this effect (13, 14). Finally, these cells exhibit the biochemical and physiological hallmarks of true adipocytes and that they be more easily infected with adenoviruses than 3T3-L1 adipocytes (13, 14).
In agreement with our previous reports (13, 14), the stimulation of lipolysis (glycerol release) by forskolin was only 3-fold in the Peri-/- MEF adipocytes infected with Ad-GFP as a negative control, whereas in adipocytes infected with wild-type perilipin A glycerol release was increased by 26-fold (Fig. 5A). Similar changes were found for the release of FFA (Fig. 5B). Transduction of the cells with an ATGL-directed shRNA led to the near total abrogation of forskolin-stimulated glycerol and FFA release both in the peri-null and in the MEF adipocytes expressing WT perilipin A (Fig. 5, A and B). In keeping with the results obtained with orlistat in 3T3-L1 adipocytes, the very weak forskolin-induced stimulation of lipolysis in the peri-null adipocytes was not accompanied by AMPK phosphorylation (Fig. 5, A–D). In contrast, the adenoviral expression of WT Peri A led to the robust restoration of both forskolin-induced lipolysis and AMPK phosphorylation (Fig. 5, A–D). Conversely, shRNA-mediated silencing of ATGL in these cells reversed both forskolin-induced lipolysis and AMPK phosphorylation (Fig. 5, A–D).
FIGURE 5.
The absence of perilipin or the shRNA-mediated silencing of ATGL inhibit both forskolin-stimulated lipolysis and AMPK activation in Peri-/- MEF adipocytes. Adipocytes retrovirally engineered from murine embryonic fibroblasts of perilipin null mice (Peri-/- MEFs) were transduced with an adenovirus expressing GFP (negative control) or wild-type perilipin A (WT Peri A) and with either an ATGL-directed shRNA or a scrambled shRNA. After differentiation to adipocytes, cells were serum-depleted in DMEM containing 2% fatty acid-free BSA and treated for 2 h with/without forskolin. A, medium glycerol; B, medium FFA. Representative immunoblots of phosphorylated serine and threonine residues of PKA substrates (∼P-PKA), perilipin, ATGL, P-AMPK Thr-172, α-AMPK, and β-actin (C). Densitometric analysis of P-AMPK Thr-172 (D) and α-AMPK (E) immunoblots. Results in A and B are expressed as means ± S.E. (n = 9) obtained in three independent experiments. Significantly different from basal control group: *, p < 0.05, ***, p < 0.001. IB, immunoblot.
Immunoblots demonstrated that the adenoviral expression of Peri A was successful (Fig. 5C). Accordingly, Peri-/- MEF adipocytes (GFP) were characterized by the absence of perilipin (Fig. 5C, lanes 1–4), whereas the adipocytes transduced with wild-type Peri A (WT Peri A; Fig. 5C, lanes 5–8) expressed perilipin. ATGL was present in both of these cells, and its expression was reduced by ∼100% as a result of transduction of an shRNA directed against ATGL (Fig. 5C). None of the genetic maneuvers significantly affected the abundance of AMPKα (Fig. 5, C and E). For reasons unknown, in experiments carried out with the MEF adipocytes, the phosphorylation of ACC Ser-79 was not changed by forskolin treatment as it was in the 3T3-L1 adipocytes (data not shown). Notwithstanding this discrepancy, these results support the notion that in adipocytes the activation of AMPK by agents that induce cAMP is a consequence of the stimulation of lipolysis.
Inhibition of Stimulated Lipolysis by shRNA-mediated Silencing of ATGL Does Not Alter PKA Signaling—Forskolin treatment increased the abundance of phosphorylated serine and threonine residues of PKA substrates (∼P-PKA substrate) both in the perilipin null and in the MEF adipocytes expressing perilipin A (Fig. 5C). This phosphorylation event was not affected by the ATGL-directed shRNA suggesting PKA activity was not altered (Fig. 5C). The major P-PKA substrates were found at ∼84 and ∼62 kDa, which corresponds, respectively, to the molecular weight of hormone-sensitive lipase, a known PKA substrate, and perilipin, the predominant PKA substrate in adipocytes (17, 37). The light P-PKA substrate band found at ∼62 kDa in the perilipin null adipocytes suggests the presence of another PKA substrate whose identity has yet to be determined. Forskolin treatment of the MEF adipocytes expressing WT Peri A led to the hyperphosphorylation of perilipin (17), as suggested by its higher migration in the immunoblotted samples (Fig. 5C). That hyperphosphorylation of perilipin was not affected by transduction of the ATGL shRNA indicates that PKA activity was not altered by this genetic manipulation.
Effect of Stimulating and Inhibiting Lipolysis on the Concentration of Adenine Nucleotides—Because AMPK activity is known to be modulated by changes in the energy state of cells, we next evaluated the effect of stimulating and inhibiting lipolysis on cellular levels of ATP, ADP, and AMP in 3T3-L1 adipocytes. As shown in Table 1, forskolin caused an ∼25% decrease in ATP levels, no significant change in ADP, and an ∼2.8-fold increase in AMP, resulting in an ∼4-fold increase in the AMP: ATP ratio compared with control cells. Orlistat treatment, which partially inhibited lipolysis and AMPK activation (Fig. 2), also markedly reduced the increase in the AMP:ATP ratio caused by forskolin, although not to control values. Neither the lipolytic agents, nor orlistat, nor the genetic manipulations affected the abundance of the α-AMPK subunit (Fig. 4, B and D, and Fig. 5, C and E).
TABLE 1.
Cellular adenine nucleotide levels in 3T3-L1 adipocytes Data are expressed as means ± S.E. (n = 5) and were obtained in four independent experiments.
| ATP | ADP | AMP | AMP/ATP | |
|---|---|---|---|---|
| nmol/mg protein | nmol/mg protein | nmol/mg protein | ||
| Control | 11.39 ± 0.83 | 3.72 ± 0.81 | 0.38 ± 0.04 | 0.034 ± 0.004 |
| Control + orlistat | 12.91 ± 0.73 | 4.23 ± 0.82 | 0.4 ± 0.03 | 0.031 ± 0.001 |
| Forskolin | 8.39 ± 0.59a | 5.19 ± 0.89 | 1.06 ± 0.14b | 0.130 ± 0.023b |
| Forskolin + orlistat | 9.15 ± 1.14a | 4.64 ± 1.03 | 0.68 ± 0.11a,c | 0.074 ± 0.009a,c |
Significantly different from control counterpart, p < 0.05
Significantly different from control counterpart, p < 0.001
Significantly different from the group treated with forskolin alone, p < 0.01
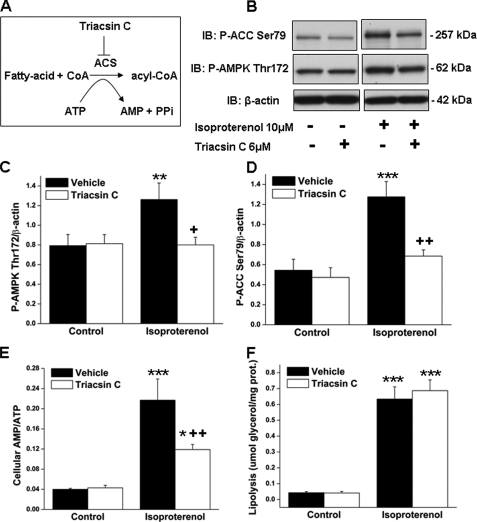
Effect of the Inhibition of Acyl-CoA Synthetase on Cellular Energy State and AMPK Activation—A portion of the FFA released during lipolysis in the adipocyte can be converted to fatty acyl-CoA by an energy-consuming reaction catalyzed by acyl-CoA synthetase (ACS, EC 6.2.1.3) in which ATP is consumed and AMP is generated (Fig. 6A). To test whether this reaction is relevant for the energy depletion that occurs when lipolysis is stimulated, differentiated 3T3-L1 adipocytes were incubated with isoproterenol in the presence or absence of the ACS inhibitor triacsin C (41–43). Isoproterenol, like forskolin (Table 1), caused a ∼5-fold increase in the cellular AMP:ATP ratio (Fig. 6E) as a result of both a decrease in ATP and an increase in AMP (data not shown). Co-incubation with triacsin C, a concentration (6 μm) previously shown to inhibit ACS in 3T3-L1 adipocytes (41), blunted the isoproterenol-induced increases in both the AMP:ATP ratio and the abundance of P-AMPK and P-ACC (Fig. 6, B–E). In contrast, triacsin C treatment had no effect on lipolysis in intact cells (Fig. 6F). The possibility that triacsin C could have a direct inhibitory action on AMPK was ruled out by its lack of effect on AMPK activity in a cell-free system (data not shown, see “Experimental Procedures”). Collectively, these data suggest that the acylation of FFAs by ACS plays a role in lipolysis-induced decrease in energy state and AMPK activation.
FIGURE 6.
Treatment with the acyl-CoA synthetase inhibitor triacsin C blunts the increases in both the cellular AMP:ATP ratio and AMPK activity when lipolysis is stimulated by isoproterenol. Stimulation of lipolysis releases intracellular fatty acids that can be either released into the media or, as shown in A, activated by ACS to form fatty acyl-CoA in a reaction that consumes ATP and produces AMP; triacsin C is an ACS inhibitor. 3T3-L1 adipocytes were preincubated for 4 h in serum-free DMEM containing 2% fatty acid-free BSA and then incubated with triacsin C (6 μm) or vehicle (DMSO) for 30 min prior to the addition of isoproterenol for 1 h. B, cell extracts were immunoblotted (IB) for P-ACC Ser-79, P-AMPK Thr-172, and β-actin. Densitometric analyses of immunoblots for P-AMPK (C) and P-ACC (D) are shown. E, cellular AMP to ATP ratio. F, lipolysis was quantified on the basis of glycerol release into the medium. Immunoblots shown are representative of three separate experiments performed in duplicate. Results are expressed as mean ± S.E. (n = 6) and were obtained in three independent experiments. Significantly different from control group, *, p < 0.05; **, p < 0.01;***, p < 0.001; significantly different from vehicle group, +, p < 0.05; ++ p < 0.01.
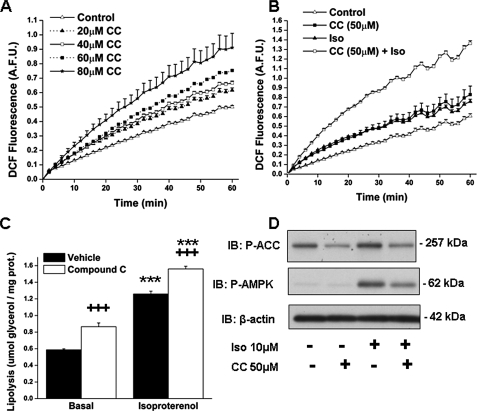
Effect of the Inhibition of AMPK by Compound C on ROS Generation in the Presence and Absence of Isoproterenol—Exogenous FFAs have been shown to increase ROS generation in 3T3-L1 adipocytes (44, 45). We observed a similar phenomenon when cellular FFAs were increased by stimulating lipolysis. Thus, isoproterenol treatment of 3T3-L1 adipocytes for 1 h led to a 25% increase in ROS generation, as measured by DCF fluorescence (0.757 ± 0.24 versus 0.606 ± 0.24 arbitrary fluorescence units in control cells; p < 0.05). AMPK activation has been found to reduce ROS generation in endothelial cells (10, 46). To assess if decreased AMPK activity has the opposite effect, 3T3-L1 adipocytes were incubated with the competitive inhibitor of AMPK compound C (29, 47). As shown in Fig. 7A, a 20-h incubation with 20–80 μm CC increased ROS production in 3T3-L1 adipocytes in a dose-dependent manner (p < 0.05 between each concentration and the one that precedes it). Lower concentrations of CC had no effect (data not shown). Most impressively, 1 h of isoproterenol stimulation in the presence of 50 μm CC resulted in a further 5-fold increase in ROS production (p < 0.001) (Fig. 7B). In these experiments, preincubation of the cells with CC for 20 h increased their basal rate of lipolysis; however, it did not impair the ability of isoproterenol to stimulate glycerol release (Fig. 7C). Treatment of the cells with 50 μm compound C for 20 h decreased both basal and isoproterenol-stimulated AMPK activity as reflected by the phosphorylation of AMPK Thr-172 and ACC Ser-79 (Fig. 7D).
FIGURE 7.
Inhibition of AMPK activity with compound C increases ROS production and lipolysis. 3T3-L1 adipocytes were treated with the AMPK inhibitor CC or vehicle for 20 h. A, dose-response of increases in DCF fluorescence, a measure of cellular H2O2 and OH content, in response to compound C. B, DCF fluorescence of adipocytes incubated with compound C (50 μm) or vehicle for 20 h prior to 1 h stimulation ± isoproterenol (Iso, 10 μm); C, glycerol released into the media; and D, representative immunoblots of P-ACC Ser-79, P-AMPK Thr-172 and β-actin. A.F.U., arbitrary fluorescent units. For A and B, values are means ± S.E. of 2–3 samples in a representative experiment that was repeated at least three times. For C, values are means ± S.E. (n = 9) and were obtained in three independent experiments. Significantly different from basal control group, ***, p < 0.001; significant effect of compound C: +++, p < 0.001.
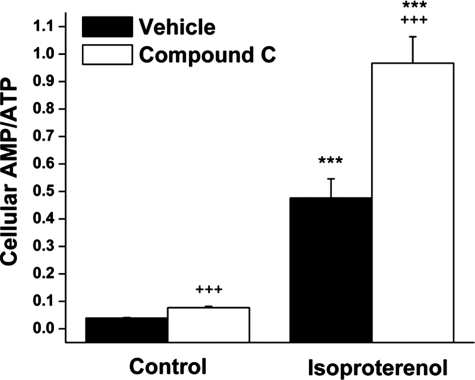
Effect of the Inhibition of AMPK by Compound C on Adenine Nucleotides Levels—As shown in Fig. 8, incubation of the cells with compound C (50 μm) for 20 h led to a 2-fold increase in their AMP:ATP ratio. This was the result of a significant decrease in ATP as well as increase in AMP levels (data not shown). These effects were substantially magnified when the adipocytes were subsequently incubated for an additional hour with isoproterenol (Fig. 8).
FIGURE 8.
Inhibition of AMPK activity with compound C increases both base-line and isoproterenol-induced changes in AMP:ATP ratio. 3T3-L1 adipocytes were treated with compound C (50 μm) or vehicle for 20 h prior to 1 h stimulation ± isoproterenol (10 μm). Cellular adenine nucleotides content was measured as described under the “Experimental Procedures.” Values are expressed as means ± S.E. (n = 9) and were obtained in three independent experiments. Significantly different from control group, ***, p < 0.001; significant effect of compound C, +++, p < 0.001.
DISCUSSION
We investigated the mechanism and the physiological relevance of AMPK activation by agents that increase cAMP in cultured adipocytes. The major findings are as follows. 1) AMPK activation by these agents appears to be secondary to an increase in the AMP:ATP ratio that accompanies lipolysis. 2) It is not a direct effect of increases in cAMP levels and PKA activity. 3) This decrease in energy state appears to be due, at least in part, to the acylation of the fatty acids released during lipolysis. 4) When AMPK activation is prevented, the stimulation of lipolysis by isoproterenol is associated with an even greater decrease in energy state and an increase in oxidative stress.
The observation that agents that raise cAMP levels (isoproterenol, forskolin, and IBMX) activate AMPK in cultured adipocytes is in agreement with previous reports (23, 24, 29, 33). A novel finding was that activation of AMPK by these agents was the result of enhanced lipolysis. Thus, AMPK activation, like lipolysis, was blunted by 50% by the general lipase inhibitor orlistat in 3T3-L1 adipocytes (Fig. 2) and completely in MEF adipocytes in which lipolysis was inhibited by genetic modifications (Peri-/- or ATGL shRNA) (Fig. 5). Furthermore, the ability of forskolin to stimulate lipolysis and activate AMPK was restored in perilipin-null MEF adipocytes in which wild-type perilipin A was expressed (Fig. 5). In contrast, inhibition of lipolysis did not affect cAMP levels or the cAMP-induced increase in PKA activity, as evidenced by the finding that phosphorylation of the downstream targets of PKA, LKB1 Ser-431, CREB Ser-133, and perilipin were all unchanged. In addition, neither orlistat nor the ATGL-directed shRNA altered the abundance of phosphorylated serine and threonine residues of PKA substrates (Fig. 4, B and C, and Fig. 5C). Thus, increases in cAMP and PKA activation, in the absence of lipolysis, are insufficient to cause the observed activation of AMPK.
LKB1 is an AMPK kinase (1, 2). Whether the phosphorylation of its Ser-431 residue observed in this study contributed to the increase in AMPK activity is unclear. Others have found that the phosphorylation of LKB1 at Ser-431 is essential for the inhibition of Rat-2 cell growth (39), the regulation of cell polarity in Drosophila (48), and the induction of AMPK phosphorylation in response to ONOO- in HeLa-S3 cells in which LKB1 was knocked in (49). In our system, this phosphorylation event was insufficient by itself to induce AMPK activation, as evidenced by the ability of orlistat to reduce the activation of AMPK without inhibiting LKB1 Ser-431 phosphorylation (Fig. 4, B and E). Whether the phosphorylation of LKB1 Ser-431 is necessary for lipolysis-induced AMPK activation remains to be determined.
The results also suggest that the increase in AMPK caused by lipolysis is related to a decrease in energy state. In keeping with this conclusion, AMPK is activated by an increase in the AMP: ATP ratio (6), and it has long been known that β-adrenergic stimulation of adipocytes is associated with a decrease in ATP levels (25–28, 50). More recently, this association has been demonstrated by Goodyear and co-workers (29) who found that both the AMP:ATP ratio and AMPK activity were increased in adipose tissue of rats that had been exercised or injected with epinephrine (29). They also found that these effects of exercise and epinephrine were prevented by the β-adrenergic blocker propanolol. The present data suggest that all of these changes are secondary to the stimulation of lipolysis. Thus, forskolin-induced increases in both the AMP:ATP ratio and AMPK activation were comparably diminished when lipolysis was inhibited by orlistat (Table 1).
Lipolysis by itself is not an energy-consuming metabolic process. On the other hand, it has been found that about ∼30% of the FFAs released during fasting is reesterified into triglycerides in rat adipose tissue (51, 52) and as much as 40% in healthy humans (52, 53). Moreover, in rat adipose tissue in vitro, the absolute rates of reesterification of FFAs are reported to increase proportionally with the rate of lipolysis (51, 54, 55). The reesterification of FFAs requires their acylation, a reaction catalyzed by ACS (EC 6.2.1.3). This reaction both uses ATP and generates AMP (Fig. 6A); thus two molecules of ATP are required for every molecule of FFA that is acylated, and a total of 7–9 ATPs are required for the synthesis of a triglyceride molecule, depending on the origin of glycerol 3-phosphate. The energy required for this acylation reaction has been reported to be the largest single drain of ATP in adipocytes stimulated by lipolytic hormones (56). The finding in this study that triacsin C, an ACS inhibitor (41, 43), blunted both the decrease in energy state and activation of AMPK caused by isoproterenol supports this conclusion (Fig. 6, B–E). It also suggests that the acylation of intracellular fatty acids is, at least in part, responsible for both the increase in the AMP:ATP ratio and the activation of AMPK that occurs during lipolysis. A similar hypothesis has been proposed, but not tested, by others, based on studies in which intracellular fatty acids were increased in adipocytes during lipolysis (57), in muscle treated with isoprenaline (58), and in hepatocytes incubated with exogenous FFAs (59).
These results do not exclude the possibility that other events could contribute to the decrease in energy state that accompanies lipolysis. One of these could be the uncoupling of oxidative phosphorylation, a phenomenon that has been observed in isolated rat adipocytes stimulated with lipolytic hormones (50, 56) and is in keeping with the intrinsic uncoupling effects of FFAs on mitochondria (60). Another is the energy consumed by the formation of glycerol 3-phosphate used for reesterification of FFAs. In keeping with this possibility, glycerol 3-phosphate formation from glucose (56, 61, 62) and from pyruvate and lactate (glyceroneogenesis) (55) has been shown to increase in adipocytes during lipolysis. Hypothetically, glucose or FFA catabolism could be impaired during lipolysis. However, previous studies have shown that various lipolytic hormones increase glucose oxidation and lactate production and, if anything, that would increase cellular ATP levels (61–64). Also, fatty acid oxidation has been reported to account for only a very small portion of oxygen consumption and ATP production in adipocytes (51). It is noteworthy that in the experiments presented here, triacsin C did not completely eliminate the isoproterenol-induced decrease in cellular energy state. Apart from the above-mentioned possibilities, this could also be due to a lack of effect of triacsin C on certain ACS isoforms (65).
Another novel finding was that inhibition of AMPK activity by compound C under base-line conditions led to increases in both ROS production and lipolysis (Fig. 7, A and C). In addition, we observed a 25% increase in ROS generation when lipolysis was stimulated with isoproterenol (no compound C) (Fig. 7B) or other lipolytic agents.3 Most impressively, the effect of isoproterenol was magnified 5-fold in the presence of compound C. Enhanced ROS production has been found previously in 3T3-L1 adipocytes incubated with exogenous saturated or unsaturated FFAs (45), an effect attributed to an up-regulation of NAD(P)H oxidase activity (44). Likewise, increased ROS production has been demonstrated in white adipose tissue of an obese insulin-resistant rodent, the KKAy mice (44). Our data suggest the following: 1) that an increase in endogenous FFA availability augments ROS generation, and 2) that AMPK activation during lipolysis probably limits this oxidative stress. Studies in which AMPK activity in the adipocyte is down-regulated by genetic means are needed to confirm this conclusion; however, it is noteworthy that a similar effect of AMPK activation on ROS generation has been observed in human endothelial cells incubated with linoleate or palmitate (10, 46) and in neutrophils treated with phorbol myristate acetate (12). In both situations, AMPK activation was shown to diminish NAD(P)H oxidase activity. Enhanced ROS production has been found to have several detrimental effects on adipocyte function, including insulin resistance (66, 67), enhanced expression and release of pro-inflammatory cytokines (44, 45, 66–68), and increased apoptosis (69). Whether a failure to activate AMPK during lipolysis contributes to such changes remains to be determined.
A second effect of AMPK inhibition by compound C was to decrease cellular energy state under both basal and isoproterenol-stimulated conditions (Fig. 8). To our knowledge, this study provides the first evidence that in the adipocyte AMPK preserves ATP under base-line conditions and, perhaps more importantly, when the stimulation of lipolysis causes energy depletion. As already noted, AMPK acts acutely in energy-stressed cells to stimulate processes that generate ATP (glucose transport and fatty acid oxidation) and to down-regulate ATP-consuming pathways that are not acutely needed for cell survival (fatty acid, triglyceride and protein synthesis, as well as gluconeogenesis) (7). This concept has been well illustrated in muscle and liver; however, its applicability to the adipocyte has been less explored (70). Some reports suggest that AMPK inhibits lipid (71–73) and protein synthesis (74) in adipocytes as it does in other cells. Its effects on glucose transport and fatty acid oxidation in the adipocyte are less clear (72, 75–77). Another means by which AMPK could restrain energy depletion in adipocytes is by reducing lipolysis. In keeping with this notion, most studies suggest that AMPK acts as an anti-lipolytic signal (33, 70, 71, 74, 78, 79), possibly by phosphorylating and inhibiting hormone-sensitive lipase (70), although others have found it is prolipolytic (24, 29) or may not alter lipolysis (73, 79). In this study, the inhibition of AMPK by compound C induced a 30% increase in lipolysis over 20 h under base-line conditions but did not affect its stimulation by isoproterenol (Fig. 7C). The reason for this difference is unclear. The possibility that compound C might exert effects that are independent of AMPK inhibition needs to be considered, and for this, studies in which AMPK activity is down-regulated by genetic means will be required.
Although, if anything, AMPK appears to inhibit lipolysis in the adipocyte, during exercise and stimulation by agents that increase cAMP, both AMPK activity and lipolysis are concurrently enhanced. We would propose that, in these settings, rather than preventing lipolysis, AMPK acts to restrain it to protect the fat cell from further energy depletion. If correct, AMPK would not be acting as a simple on and off switch but as a rheostat that controls the lipolytic rate. An analogous situation also occurs during exercise in the liver when both AMPK activity and gluconeogenesis, an energy-requiring process that can occur at a high rate, are simultaneously enhanced despite the fact that AMPK has been shown to inhibit both key gluconeogenic enzymes and hepatic glucose production (80).
In conclusion, the activation of AMPK in adipocytes by agents that increase cAMP appears to be secondary to an increase in the AMP:ATP ratio that accompanies lipolysis and not the direct result of increases in cAMP levels and PKA activity. This decrease in energy state appears to be due, at least in part, to the acylation of the fatty acids released during lipolysis. The data also suggest that AMPK activation may restrain energy depletion and ROS production that occur during the stimulation of lipolysis. Studies in which AMPK is genetically knocked down are needed to confirm this role.
Acknowledgments
We thank Drs. Vera Schultz, Gordon Yaney, Keith Tornheim, Jude Deeney, Eva Tomas, and Meghan Kelly for technical assistance and valuable advice.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1DK19514, RO1DK067509, PO1HL68758 (to N. B. R.), and RO1DK50647 (to A. S. G.). This work was also supported by United States Department of Agriculture Agriculture Research Service Co-operative Agreement Contract 58-1950-7-707 and a grant from the Robert C. and Veronica Atkins Foundation (to A. S. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Theabbreviationsusedare:AMPK,AMP-activatedproteinkinase; PKA, cAMP-dependent protein kinase; shRNA, small hairpin RNA; DMEM, Dulbecco's modified Eagle's medium; IBMX, isobutylmethylxanthine; BSA, bovine serum albumin; FFA, free fatty acid; GFP, green fluorescent protein; MEF, mouse embryonic fibroblast; WT, wild type; ACS, acyl-CoA synthetase; DCF, 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; ROS, reactive oxygen species; CREB, cAMP-response element-binding protein; P-CREB, phospho-CREB; CC, compound C; ATGL, adipose-tissue triglyceride lipase; Peri A, perilipin A; ACC, acetyl-CoA carboxylase.
M.-S. Gauthier and N. B. Ruderman, unpublished data.
References
- 1.Hawley, S. A., Boudeau, J., Reid, J. L., Mustard, K. J., Udd, L., Makela, T. P., Alessi, D. R., and Hardie, D. G. (2003) J. Biol. 2 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods, A., Johnstone, S. R., Dickerson, K., Leiper, F. C., Fryer, L. G., Neumann, D., Schlattner, U., Wallimann, T., Carlson, M., and Carling, D. (2003) Curr. Biol. 13 2004-2008 [DOI] [PubMed] [Google Scholar]
- 3.Woods, A., Dickerson, K., Heath, R., Hong, S. P., Momcilovic, M., Johnstone, S. R., Carlson, M., and Carling, D. (2005) Cell Metab. 2 21-33 [DOI] [PubMed] [Google Scholar]
- 4.Hawley, S. A., Pan, D. A., Mustard, K. J., Ross, L., Bain, J., Edelman, A. M., Frenguelli, B. G., and Hardie, D. G. (2005) Cell Metab. 2 9-19 [DOI] [PubMed] [Google Scholar]
- 5.Momcilovic, M., Hong, S.-P., and Carlson, M. (2006) J. Biol. Chem. 281 25336-25343 [DOI] [PubMed] [Google Scholar]
- 6.Hardie, D. G. (2007) Annu. Rev. Pharmacol. Toxicol. 47 185-210 [DOI] [PubMed] [Google Scholar]
- 7.Hardie, D. G., Hawley, S. A., and Scott, J. W. (2006) J. Physiol. (Lond.) 574 7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cacicedo, J. M., Gauthier, M.-S., Ruderman, N. B., and Ido, Y. (2007) Diabetes 56 A515 (Abstr. 2039) [Google Scholar]
- 9.Cacicedo, J. M., Yagihashi, N., Keaney, J. F., Jr., Ruderman, N. B., and Ido, Y. (2004) Biochem. Biophys. Res. Commun. 324 1204-1209 [DOI] [PubMed] [Google Scholar]
- 10.Lee, W. J., Lee, I. K., Kim, H. S., Kim, Y. M., Koh, E. H., Won, J. C., Han, S. M., Kim, M.-S., Jo, I., Oh, G. T., Park, I.-S., Youn, J. H., Park, S.-W., Lee, K.-U., and Park, J.-Y. (2005) Arterioscler. Thromb. Vasc. Biol. 25 2488-2494 [DOI] [PubMed] [Google Scholar]
- 11.Pilon, G., Dallaire, P., and Marette, A. (2004) J. Biol. Chem. 279 20767-20774 [DOI] [PubMed] [Google Scholar]
- 12.Alba, G., El Bekay, R., Alvarez-Maqueda, M., Chacon, P., Vega, A., Monteseirin, J., Santa Maria, C., Pintado, E., Bedoya, F. J., Bartrons, R., and Sobrino, F. (2004) FEBS Lett. 573 219-225 [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi, H., Perfield, J. W., II, Souza, S. C., Shen, W.-J., Zhang, H.-H., Stancheva, Z. S., Kraemer, F. B., Obin, M. S., and Greenberg, A. S. (2007) J. Biol. Chem. 282 996-1002 [DOI] [PubMed] [Google Scholar]
- 14.Miyoshi, H., Souza, S. C., Zhang, H.-H., Strissel, K. J., Christoffolete, M. A., Kovsan, J., Rudich, A., Kraemer, F. B., Bianco, A. C., Obin, M. S., and Greenberg, A. S. (2006) J. Biol. Chem. 281 15837-15844 [DOI] [PubMed] [Google Scholar]
- 15.Souza, S. C., Muliro, K. V., Liscum, L., Lien, P., Yamamoto, M. T., Schaffer, J. E., Dallal, G. E., Wang, X., Kraemer, F. B., Obin, M., and Greenberg, A. S. (2002) J. Biol. Chem. 277 8267-8272 [DOI] [PubMed] [Google Scholar]
- 16.Souza, S. C., de Vargas, L. M., Yamamoto, M. T., Lien, P., Franciosa, M. D., Moss, L. G., and Greenberg, A. S. (1998) J. Biol. Chem. 273 24665-24669 [DOI] [PubMed] [Google Scholar]
- 17.Greenberg, A., Egan, J., Wek, S., Garty, N., Blanchette-Mackie, E., and Londos, C. (1991) J. Biol. Chem. 266 11341-11346 [PubMed] [Google Scholar]
- 18.Zimmermann, R., Strauss, J. G., Haemmerle, G., Schoiswohl, G., Birner-Gruenberger, R., Riederer, M., Lass, A., Neuberger, G., Eisenhaber, F., Hermetter, A., and Zechner, R. (2004) Science 306 1383-1386 [DOI] [PubMed] [Google Scholar]
- 19.Zechner, R., Strauss, J. G., Haemmerle, G., Lass, A., and Zimmermann, R. (2005) Curr. Opin. Lipidol. 16 333-340 [DOI] [PubMed] [Google Scholar]
- 20.Haemmerle, G., Lass, A., Zimmermann, R., Gorkiewicz, G., Meyer, C., Rozman, J., Heldmaier, G., Maier, R., Theussl, C., Eder, S., Kratky, D., Wagner, E. F., Klingenspor, M., Hoefler, G., and Zechner, R. (2006) Science 312 734-737 [DOI] [PubMed] [Google Scholar]
- 21.Park, H., Kaushik, V. K., Constant, S., Prentki, M., Przybytkowski, E., Ruderman, N. B., and Saha, A. K. (2002) J. Biol. Chem. 277 32571-32577 [DOI] [PubMed] [Google Scholar]
- 22.Kelly, M., Keller, C., Avilucea, P. R., Keller, P., Luo, Z., Xiang, X., Giralt, M., Hidalgo, J., Saha, A. K., Pedersen, B. K., and Ruderman, N. B. (2004) Biochem. Biophys. Res. Commun. 320 449-454 [DOI] [PubMed] [Google Scholar]
- 23.Moule, S. K., and Denton, R. M. (1998) FEBS Lett. 439 287-290 [DOI] [PubMed] [Google Scholar]
- 24.Yin, W., Mu, J., and Birnbaum, M. J. (2003) J. Biol. Chem. 278 43074-43080 [DOI] [PubMed] [Google Scholar]
- 25.Angel, A., Desai, K. S., and Halperin, M. L. (1971) J. Lipid Res. 12 203-213 [PubMed] [Google Scholar]
- 26.Angel, A., Desai, K., and Halperin, M. L. (1971) Metabolism 20 87-99 [DOI] [PubMed] [Google Scholar]
- 27.Vallano, M. L., Lee, M. Y., and Sonenberg, M. (1983) Am. J. Physiol. 245 E266-E272 [DOI] [PubMed] [Google Scholar]
- 28.Kather, H. (1990) J. Clin. Investig. 85 106-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh, H.-J., Hirshman, M. F., He, H., Li, Y., Manabe, Y., Balschi, J. A., and Goodyear, L. J. (2007) Biochem. J. 403 473-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohanski, R., Frost, S., and Lane, M. (1986) J. Biol. Chem. 261 12272-12281 [PubMed] [Google Scholar]
- 31.Rosen, E. D., Hsu, C.-H., Wang, X., Sakai, S., Freeman, M. W., Gonzalez, F. J., and Spiegelman, B. M. (2002) Genes Dev. 16 22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, H. H., Souza, S. C., Muliro, K. V., Kraemer, F. B., Obin, M. S., and Greenberg, A. S. (2003) J. Biol. Chem. 278 51535-51542 [DOI] [PubMed] [Google Scholar]
- 33.Daval, M., Diot-Dupuy, F., Bazin, R., Hainault, I., Viollet, B., Vaulont, S., Hajduch, E., Ferre, P., and Foufelle, F. (2005) J. Biol. Chem. 280 25250-25257 [DOI] [PubMed] [Google Scholar]
- 34.Maizels, E. Z., Ruderman, N. B., Goodman, M. N., and Lau, D. (1977) Biochem. J. 162 557-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry, O. H., and Passoneau, J. V. (1972) A Flexible System of Enzymatic Analysis, pp. 147-156, Academic Press, New York
- 36.Nolan, C. J., Leahy, J. L., Delghingaro-Augusto, V., Moibi, J., Soni, K., Peyot, M. L., Fortier, M., Guay, C., Lamontagne, J., Barbeau, A., Przybytkowski, E., Joly, E., Masiello, P., Wang, S., Mitchell, G. A., and Prentki, M. (2006) Diabetologia 49 2120-2130 [DOI] [PubMed] [Google Scholar]
- 37.Brasaemle, D. L. (2007) J. Lipid Res. 48 2547-2559 [DOI] [PubMed] [Google Scholar]
- 38.Collins, S. P., Reoma, J. L., Gamm, D. M., and Uhler, M. D. (2000) Biochem. J. 345 673-680 [PMC free article] [PubMed] [Google Scholar]
- 39.Sapkota, G. P., Kieloch, A., Lizcano, J. M., Lain, S., Arthur, J. S. C., Williams, M. R., Morrice, N., Deak, M., and Alessi, D. R. (2001) J. Biol. Chem. 276 19469-19482 [DOI] [PubMed] [Google Scholar]
- 40.Self, D. W., Genova, L. M., Hope, B. T., Barnhart, W. J., Spencer, J. J., and Nestler, E. J. (1998) J. Neurosci. 18 1848-1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brasaemle, D. L., Rubin, B., Harten, I. A., Gruia-Gray, J., Kimmel, A. R., and Londos, C. (2000) J. Biol. Chem. 275 38486-38493 [DOI] [PubMed] [Google Scholar]
- 42.Ajuwon, K. M., and Spurlock, M. E. (2005) J. Nutr. 135 1841-1846 [DOI] [PubMed] [Google Scholar]
- 43.Mashek, D. G., McKenzie, M. A., Van Horn, C. G., and Coleman, R. A. (2006) J. Biol. Chem. 281 945-950 [DOI] [PubMed] [Google Scholar]
- 44.Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., Nakayama, O., Makishima, M., Matsuda, M., and Shimomura, I. (2004) J. Clin. Investig. 114 1752-1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subauste, A. R., and Burant, C. F. (2007) Am. J. Physiol. 293 E159-E164 [DOI] [PubMed] [Google Scholar]
- 46.Ruderman, N. B., Cacicedo, J. M., Itani, S., Yagihashi, N., Saha, A. K., Ye, J. M., Chen, K., Zou, M., Carling, D., Boden, G., Cohen, R. A., Keaney, J., Kraegen, E. W., and Ido, Y. (2003) Biochem. Soc. Trans. 31 202-206 [DOI] [PubMed] [Google Scholar]
- 47.Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., Musi, N., Hirshman, M. F., Goodyear, L. J., and Moller, D. E. (2001) J. Clin. Investig. 108 1167-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin, S. G., and St Johnston, D. (2003) 421 379-384 [DOI] [PubMed]
- 49.Xie, Z., Dong, Y., Zhang, M., Cui, M.-Z., Cohen, R. A., Riek, U., Neumann, D., Schlattner, U., and Zou, M.-H. (2006) J. Biol. Chem. 281 6366-6375 [DOI] [PubMed] [Google Scholar]
- 50.Hepp, D., Challoner, D. R., and Williams, R. H. (1968) J. Biol. Chem. 243 2321-2327 [PubMed] [Google Scholar]
- 51.Vaughan, M. (1962) J. Biol. Chem. 237 3354-3358 [PubMed] [Google Scholar]
- 52.Reshef, L., Olswang, Y., Cassuto, H., Blum, B., Croniger, C. M., Kalhan, S. C., Tilghman, S. M., and Hanson, R. W. (2003) J. Biol. Chem. 278 30413-30416 [DOI] [PubMed] [Google Scholar]
- 53.Beale, E. G., Hammer, R. E., Antoine, B., and Forest, C. (2002) FASEB J. 16 1695-1696 [DOI] [PubMed] [Google Scholar]
- 54.Brooks, B., Arch, J. R. S., and Newsholme, E. A. (1982) FEBS Lett. 146 327-330 [DOI] [PubMed] [Google Scholar]
- 55.Reshef, L., Hanson, R. W., and Ballard, F. J. (1970) J. Biol. Chem. 245 5979-5984 [PubMed] [Google Scholar]
- 56.Rognstad, R., and Katz, J. (1966) Proc. Natl. Acad. Sci. U. S. A. 55 1148-1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hardie, D., and Carling, D. (1997) Eur. J. Biochem. 246 259-273 [DOI] [PubMed] [Google Scholar]
- 58.Alam, N., and Saggerson, E. D. (1998) Biochem. J. 334 233-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawaguchi, T., Osatomi, K., Yamashita, H., Kabashima, T., and Uyeda, K. (2002) J. Biol. Chem. 277 3829-3835 [DOI] [PubMed] [Google Scholar]
- 60.Wojtczak, L., and Schonfeld, P. (1993) Biochim. Biophys. Acta 1183 41-57 [DOI] [PubMed] [Google Scholar]
- 61.Cahill, G. F., Jr., Leboeuf, B., and Flinn, R. B. (1960) J. Biol. Chem. 235 1246-1250 [PubMed] [Google Scholar]
- 62.Rodbell, M. (1964) J. Biol. Chem. 239 375-380 [PubMed] [Google Scholar]
- 63.Vaughan, M. (1961) J. Biol. Chem. 236 2196-2199 [PubMed] [Google Scholar]
- 64.Hagen, J. H., and Ball, E. G. (1960) J. Biol. Chem. 235 1545-1549 [PubMed] [Google Scholar]
- 65.Kim, J.-H., Lewin, T. M., and Coleman, R. A. (2001) J. Biol. Chem. 276 24667-24673 [DOI] [PubMed] [Google Scholar]
- 66.Lin, Y., Berg, A. H., Iyengar, P., Lam, T. K. T., Giacca, A., Combs, T. P., Rajala, M. W., Du, X., Rollman, B., Li, W., Hawkins, M., Barzilai, N., Rhodes, C. J., Fantus, I. G., Brownlee, M., and Scherer, P. E. (2005) J. Biol. Chem. 280 4617-4626 [DOI] [PubMed] [Google Scholar]
- 67.Unoki, H., Bujo, H., Yamagishi, S.-I., Takeuchi, M., Imaizumi, T., and Saito, Y. (2007) Diabetes Res. Clin. Pract. 76 236-244 [DOI] [PubMed] [Google Scholar]
- 68.Kamigaki, M., Sakaue, S., Tsujino, I., Ohira, H., Ikeda, D., Itoh, N., Ishimaru, S., Ohtsuka, Y., and Nishimura, M. (2006) Biochem. Biophys. Res. Commun. 339 624-632 [DOI] [PubMed] [Google Scholar]
- 69.Yang, J.-Y., Della-Fera, M. A., Nelson-Dooley, C., and Baile, C. A. (2006) Obesity 14 388-397 [DOI] [PubMed] [Google Scholar]
- 70.Daval, M., Foufelle, F., and Ferre, P. (2006) J. Physiol. (Lond.) 574 55-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan, J. E., Brocklehurst, K. J., Marley, A. E., Carey, F., Carling, D., and Beri, R. K. (1994) FEBS Lett. 353 33-36 [DOI] [PubMed] [Google Scholar]
- 72.Gaidhu, M. P., Fediuc, S., and Ceddia, R. B. (2006) J. Biol. Chem. 281 25956-25964 [DOI] [PubMed] [Google Scholar]
- 73.An, Z., Wang, H., Song, P., Zhang, M., Gong, X., and Zou, M.-H. (2007) J. Biol. Chem. 282 26793-26801 [DOI] [PubMed] [Google Scholar]
- 74.Dagon, Y., Avraham, Y., and Berry, E. M. (2006) Biochem. Biophys. Res. Commun. 340 43-47 [DOI] [PubMed] [Google Scholar]
- 75.Luo, B., Parker, G. J., Cooksey, R. C., Soesanto, Y., Evans, M., Jones, D., and McClain, D. A. (2007) J. Biol. Chem. 282 7172-7180 [DOI] [PubMed] [Google Scholar]
- 76.Yamaguchi, S., Katahira, H., Ozawa, S., Nakamichi, Y., Tanaka, T., Shimoyama, T., Takahashi, K., Yoshimoto, K., Imaizumi, M. O., Nagamatsu, S., and Ishida, H. (2005) Am. J. Physiol. 289 E643-E649 [DOI] [PubMed] [Google Scholar]
- 77.Salt, I., Connell, J., and Gould, G. (2000) Diabetes 49 1649-1656 [DOI] [PubMed] [Google Scholar]
- 78.Garton, A. J., Campbell, D. G., Carling, D., Hardie, D. G., Colbran, R. J., and Yeaman, S. J. (1989) Eur. J. Biochem. 179 249-254 [DOI] [PubMed] [Google Scholar]
- 79.Watt, M. J., Holmes, A. G., Pinnamaneni, S. K., Garnham, A. P., Steinberg, G. R., Kemp, B. E., and Febbraio, M. A. (2006) Am. J. Physiol. 290 E500-E508 [DOI] [PubMed] [Google Scholar]
- 80.Viollet, B., Foretz, M., Guigas, B., Horman, S., Dentin, R., Bertrand, L., Hue, L., and Andreelli, F. (2006) J. Physiol. (Lond.) 574 41-53 [DOI] [PMC free article] [PubMed] [Google Scholar]