Abstract
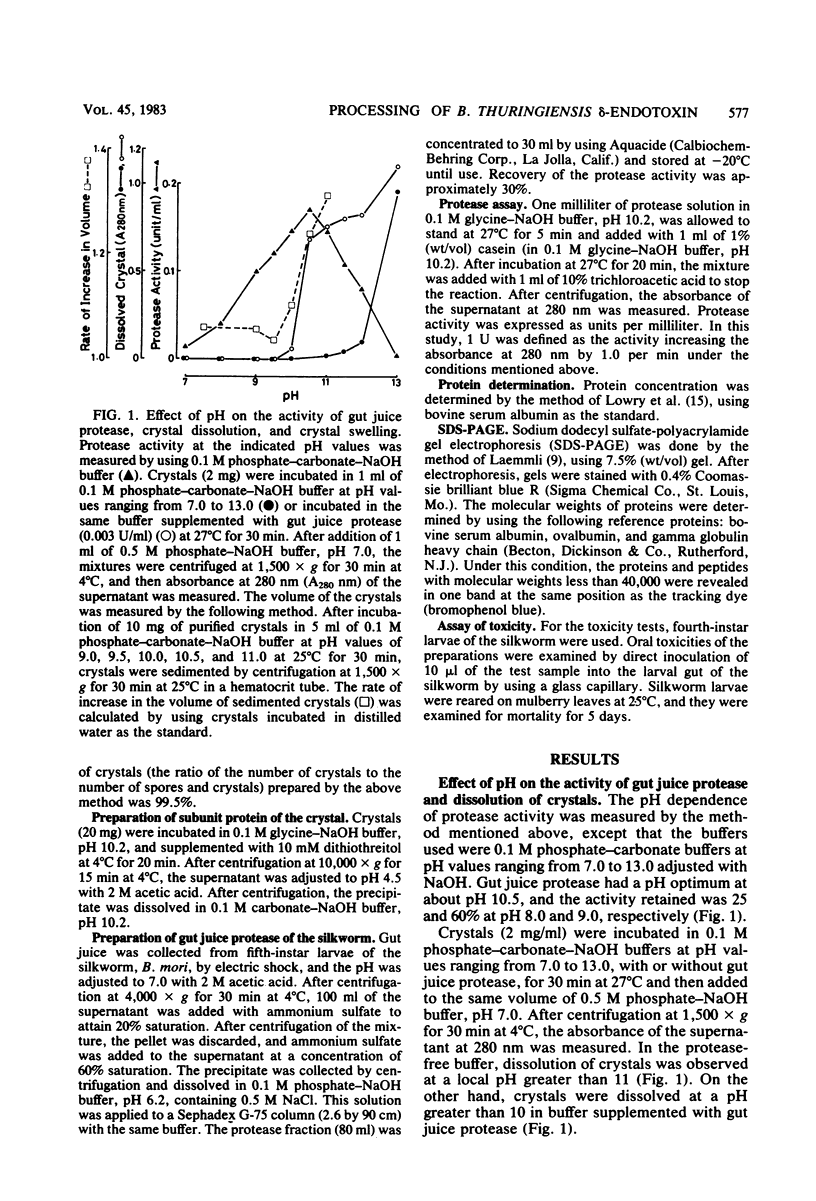
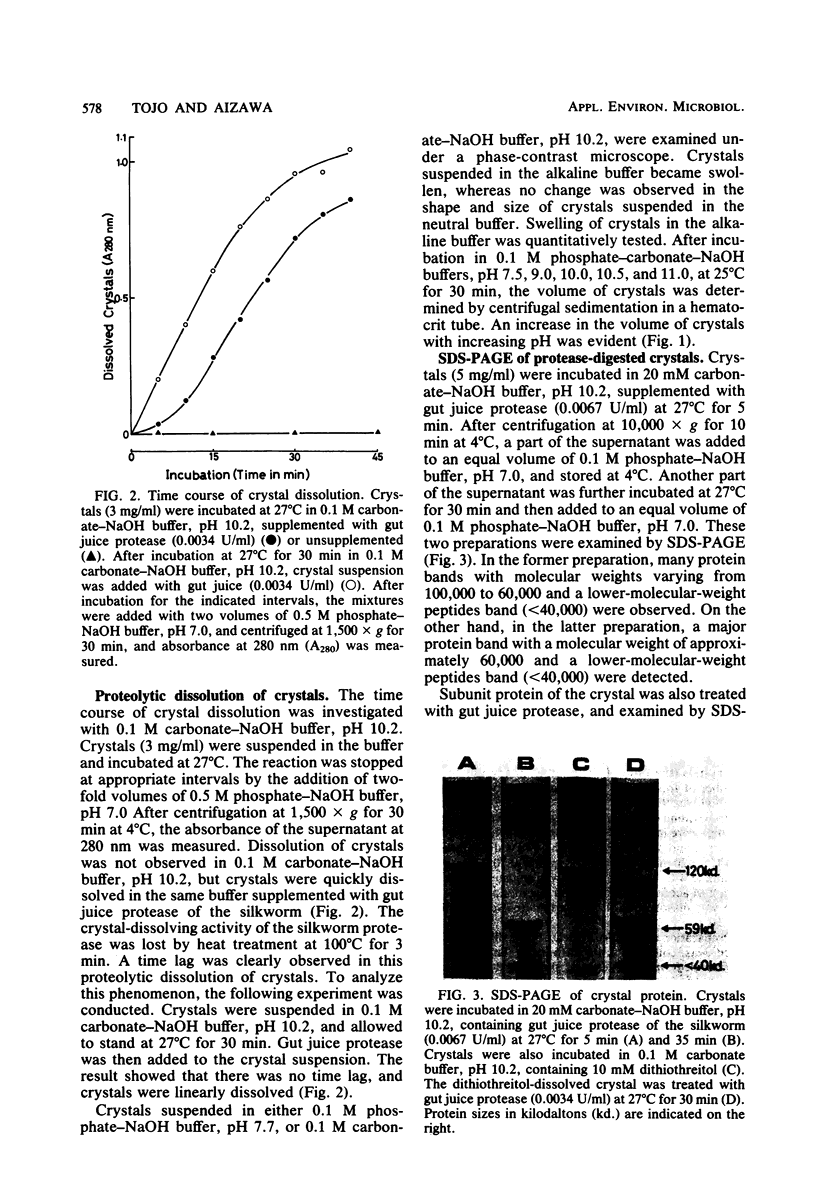
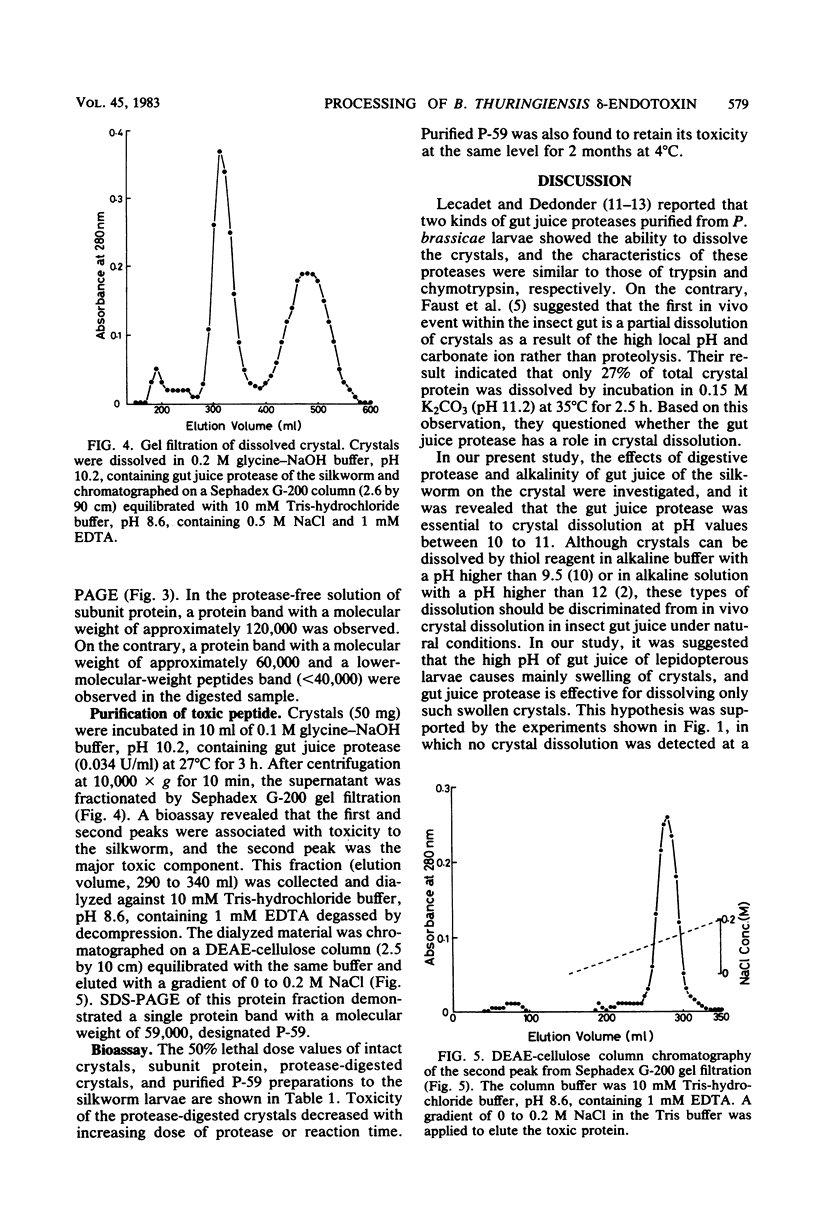
The dissolution and degradation of †-endotoxin (crystal) of Bacillus thuringiensis subsp. kurstaki strain HD-1 were investigated. Crystals were dissolved in 0.1 M phosphate-carbonate-NaOH buffer at pH > 12. Swelling of crystals occurred in the buffer between pH 10 and 11, and crystals dissolved in the same buffer supplemented with gut juice protease of the silkworm Bombyx mori. The proteolytic dissolution of crystals occurred after a time lag of several minutes in 0.1 M carbonate-NaOH buffer, pH 10.2. The time lag was not observed when crystals were suspended in the buffer for 30 min before the addition of protease. After the dissolution of the crystals and further degradation of the solubilized protein, the appearance of a toxic protein with a molecular weight of 59,000, designated P-59, was observed. Lower-molecular-weight peptides (less than 40,000) showed no toxicity to the silkworm larvae on feeding. Digestion of the 120,000-dalton subunit of the crystal by gut juice protease also produced P-59. These observations suggest the occurrence of a similar process in vivo, i.e., the swelling of crystals due to the alkalinity of gut juice and the production of P-59, dependent on the hydrolysis of swollen crystals by gut juice protease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ang B. J., Nickerson K. W. Purification of the protein crystal from Bacillus thuringiensis by zonal gradient centrifugation. Appl Environ Microbiol. 1978 Oct;36(4):625–626. doi: 10.1128/aem.36.4.625-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Jr, Kramer K. J., Davidson L. I. Characterization of the entomocidal parasporal crystal of Bacillus thuringiensis. J Bacteriol. 1977 Apr;130(1):375–383. doi: 10.1128/jb.130.1.375-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey K. E. Purification of a protein from Bacillus thuringiensis toxic to larvae of lepidoptera. Biochem J. 1968 Jan;106(2):445–454. doi: 10.1042/bj1060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust R. M., Hallam G. M., Travers R. S. Degradation of the parasporal crystal produced by Bacillus thuringiensis var. kurstaki. J Invertebr Pathol. 1974 Nov;24(3):365–373. doi: 10.1016/0022-2011(74)90145-1. [DOI] [PubMed] [Google Scholar]
- Goodman N. S., Gottfried R. J., Rogoff M. H. Biphasic system for separation of spores and crystals of Bacillus thuringiensis. J Bacteriol. 1967 Aug;94(2):485–485. doi: 10.1128/jb.94.2.485-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNAY C. L. Crystalline inclusions in aerobic spore-forming bacteria. Nature. 1953 Nov 28;172(4387):1004–1004. doi: 10.1038/1721004a0. [DOI] [PubMed] [Google Scholar]
- LECADET M., DEDONDER R. LES PROT'EASES DU CHYLE DE PIERIS BRASSICAE. PURIFICATION. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3117–3120. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lecadet M. M., Dedonder R. Les protéases de Pieris brassicae. I. Purification et propriétés. Bull Soc Chim Biol (Paris) 1966;48(5):631–660. [PubMed] [Google Scholar]
- Lecadet M. M., Dedonder R. Les protéases de Pieris brassicae. II. Spécificité. Bull Soc Chim Biol (Paris) 1966;48(5):661–691. [PubMed] [Google Scholar]
- Lecadet M. M. La toxine figurée de bacillus thuringiensis. Dissolution par action du thioglycolate ou de la cystéine. C R Acad Sci Hebd Seances Acad Sci D. 1966 Jan 3;262(1):195–198. [PubMed] [Google Scholar]