Abstract
We report the generation and analysis of mutant mice bearing a targeted disruption of the heparan sulfate (HS)-modifying enzyme GlcNAc N-deacetylase/N-sulfotransferase 3 (NDST3). NDST3-/- mice develop normally, are fertile, and show only subtle hematological and behavioral abnormalities in agreement with only moderate HS undersulfation. Compound mutant mice made deficient in NDST2;NDST3 activities also develop normally, showing that both isoforms are not essential for development. In contrast, NDST1-/-;NDST3-/- compound mutant embryos display developmental defects caused by severe HS undersulfation, demonstrating NDST3 contribution to HS synthesis in the absence of NDST1. Moreover, analysis of HS composition in dissected NDST3 mutant adult brain revealed regional changes in HS sulfation, indicating restricted NDST3 activity on nascent HS in defined wild-type tissues. Taken together, we show that NDST3 function is not essential for development or adult homeostasis despite contributing to HS synthesis in a region-specific manner and that the loss of NDST3 function is compensated for by the other NDST isoforms to a varying degree.
Heparan sulfate (HS)2 is produced by most mammalian cells as part of membrane and extracellular matrix proteoglycans (1). The chain grows by the copolymerization of GlcAβ1,4 and GlcNAcα1,4 residues and undergoes modification by one or more of the four NDST isozymes, which remove acetyl groups from subsets of GlcNAc residues and add sulfate to the free amino groups. In vertebrates, ndst1 and ndst2 mRNA are expressed in all embryonic and adult tissues examined, whereas ndst3 and ndst4 transcripts are predominantly expressed during embryonic development and in the adult brain (2). Most subsequent modifications of the HS chain by O-sulfotransferases and a GlcA C5-epimerase depend on the presence of GlcNS residues, making the NDSTs largely responsible for the generation of sulfated ligand binding sites in HS (3-5). In vitro, NDST3 differs biochemically from the other NDST isoforms by possessing a high deacetylase activity but very low sulfotransferase activity (2).
Many growth factors and morphogens bind to HS. In some cases, HS-proteoglycans are thought to act as co-receptors for these ligands. Studies in Drosophila melanogaster demonstrated that HS is crucial for embryonic development (6) and that the fly NDST ortholog, Sulfateless, affects signaling mediated by Wingless (Wg), Hedgehog (HH), and fibroblast growth factor (FGF) (7-9). The ability of HS to regulate the activity of morphogens and growth factors is currently best understood for the FGFs. HS was found to be a necessary component of FGF-FGF receptor binding and assembly (10), and global changes in HS expression regulate FGF and FGF receptor assembly during mouse development (11). Due to the multiple developmental processes regulated by the 23 FGFs, including those of the lung, limbs, heart, skeleton, and brain (reviewed in Ref. 12), perturbed HS synthesis results in the generation of FGF-related phenotypes (13, 14). The crucial role of HS in morphogen transport and on receiving cells has also been demonstrated for vertebrate HH (15-18) and PDGF function during embryonic vascularization (19).
Mouse mutants made deficient in NDST1 have been characterized, demonstrating a crucial role for this isoform for properly modifying HS during development. In the adult mouse, NDST1 and NDST2 also play important roles in the generation of connective-tissue type mast cells, endothelial cell function, and lipid metabolism (15, 18-26).
In this report, we asked to what extent HS function during development and in the adult vertebrate depends on NDST3 function and to what extent NDST3 contributes to the generation of sulfated HS. Moreover, we wished to examine whether the formation of free amino groups present on heparan sulfate is related to NDST3 activity. We describe that NDST3-deficient mice are born at slightly sub-mendelian ratio, are fertile, and show subtle changes in some hematological parameters and in their behavior. No significant overall changes in HS sulfation could be detected in those mice, but microdissection of the adult brain revealed a region-specific activity of NDST3, leading to changes in HS sulfation in the mutant brain. Mice made deficient in both NDST3 and NDST1 function revealed a role of NDST3 in the proper sulfation of nascent HS, resulting in the complete lack of one disulfated disaccharide product. We thus conclude that, although NDST3 is expressed in various tissues and contributes to HS synthesis, its activity can be substituted by the other NDSTs.
EXPERIMENTAL PROCEDURES
Targeted Recombination of the ndst3 Gene—The thymidine-kinase/neomycin-containing targeting vector was constructed by insertion of loxP sites in intron sequences surrounding exon 2 (the first coding exon) of ndst3, including 327 of 873 amino acids of the open reading frame. The final targeting vector was linearized using SalI before transfection of ES cells. R1 ES cells were grown, transfected, and subjected to neomycin G418 selection. Homologous recombinants were identified by Southern blotting and PCR and transfected with a cre-expressing vector, followed by gancyclovir selection. Four type II recombinants were chosen and injected in C57Bl/6J blastocysts. The mouse line obtained was backcrossed into a C57Bl/6 background for >10 generations. The primers employed for genotyping were: 5′-P1: 5′-ggtacccggggatcaattcg-3′; P2: 5′-ccagaaggctaacactgtaaag-3′; P3: 5′-gaaagtgaagtctctgggcgg-3′; and P4: 5′-gcttggatgatttggtcacact-3′. Assessment of the significance of the deviation from mendelian inheritance was performed using the Chi-square test. Compound mutant mice were derived from matings with NDST1 (18)- and NDST2 (22)-deficient mice.
Reverse Transcription-PCR Analysis of mRNA Expression and Protein Detection—For reverse transcription-PCR analysis in human tissues, normalized cDNA was obtained from Clontech (Human MTC Panels I+II). PCR was performed by running 38 cycles for hndst1-4. For the specific amplification of each hndst, eight specific primers were used as follows: hndst1-F (5′-ctggagccctcggcggatgc-3′) and hndst1-R(5′-ccagggtactcgttgtagaag-3′), hndst2-F (5′-aggaacccttgcccctgccc-3′) and hndst2-R (5′-gattgtgtgagtgaagaggc-3′), hndst3-F (5′-tgtgtttcctgtgagtccagatgtgtg-3′) and hndst3-R (5′-attgtcctcctcacttccatcagcctg-3′), hndst4-F (5′-aacaggaaatgacacttattgaaacc-3′), and hndst4-R (5′-actttggggcctttggtaatatg-3′).
Histology and in Situ Detection of RNA—Embryos were fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin for histological analysis. For in situ hybridization, 700 base probes against the most variable N-terminal region of ndst1 and ndst3 and a 500-base probe against the ndst2 3′-untranslated region were employed (DIG RNA Labeling Kit, Roche Applied Science). Quantitation of apoptosis was performed on paraffin sections of two mutant and two wild-type E12.5 embryos, using the TUNEL Assay Kit (Roche Applied Science). Patched expression was detected using anti-PTC1 antiserum (Acris Antibodies, Hiddenhausen, Germany) and secondary fluorescein isothiocyanate-labeled goat anti-rabbit antibodies (Dianova, Hamburg, Germany) on three mutant and wild-type embryos.
Adult Mouse Brain Immunohistochemistry—Bielschowsky stain, Gallays stain, anti-MAC-3, anti-PCNA, and anti-GFAP were employed to detect possible cellular abnormalities in NDST3 mutant brain. Images were taken on a Zeiss Axiophot microscope employing a 10×/0.3, a 20×/0.5, and a 63×/1.25 Zeiss objective and a Leica DFC280 camera. Leica software was used for image capturing and Photoshop 7 software run on Macintosh computers for the generation of figures. Contrast and brightness were adjusted for whole images during figure assembly.
Preparation of HS—Mutant and wild-type tissues were pooled, homogenized using an Ultra-Turrax homogenizer (IKA, Germany), digested with 2 mg/ml Pronase in 320 mm NaCl, 100 mm sodium acetate (pH 5.5) overnight at 40 °C, diluted 1:3 in water, and applied to a 2.5-ml column of DEAE-Sephacel. After washing the column with 0.3 m NaCl, the glycosaminoglycans were eluted with 1 m NaCl. For disaccharide analysis, the GAG pool was β-eliminated overnight at 4 °C (0.5 m NaOH, 1 m NaBH4), neutralized with acetic acid until the pH was ∼6 and applied to a PD-10 (Sephadex G-25) column (Amersham Biosciences). Glycosaminoglycans eluting in the void volume were lyophilized, purified on DEAE as described above, again applied to a PD-10 column, and lyophilized. 100 mg to 1 g of tissue, depending on the source, typically yielded 40-140 μg of GAGs. 10 μg of GAG samples were digested using heparin lyases I, II, and III (1.5 milliunits of each in 100-μl reactions, IBEX, Montreal, Canada) at 37 °C for 1 h, and the resulting disaccharides were separated from undigested chondroitin sulfate using a 3-kDa spin column (Centricon, Bedford, MA). Compositional disaccharide analysis of wild-type and NDST3 E15.5 embryos was then carried out by high-performance liquid chromatography analysis using Carbopac PA1 columns (Dionex). Compositional disaccharide analysis of compound mutant embryos and defined adult brain areas was carried out by liquid chromatography/mass spectrometry (LC/MS). First, disaccharides were separated on a C18 reverse phase column (0.46 × 25 cm, Vydac) with the ion pairing reagent dibutylamine (Sigma), and eluted species were evaluated using a quantitative mass spectrometric method. Analysis of the disaccharide composition by post-column derivatization with 2-cyanoacetamide (27) or by the LC/MS method gave comparable results.3 A comparison of the two methods using 0.5 μg of commercial porcine heparin showed an error of 2% for abundant disaccharides to 20% for minor species.
Cell Proliferation—Cell proliferation was measured using fibroblasts derived from E14.5 embryos. Cells were labeled using 100 mm bromodeoxyuridine in medium for 5 h, fixed with 4% paraformaldehyde in phosphate-buffered saline, and detected using anti-bromodeoxyuridine antibodies (Zymed Laboratories Inc.). Analysis of FGF2-dependent MAPK pathway activation was performed using anti-ERK1/2 and anti-phospho-ERK1/2 polyclonal antibodies (Promega, Madison, WI). Fibroblasts derived from the heads of E14.5 wild-type and mutant embryos (n = 4) were cultured in DMEM plus 10% FBS, starved for 20 h in DMEM without FBS, incubated in complete medium, DMEM without FBS, or 10 ng/ml FGF2 in DMEM without FBS for 5 min, and lysed. Analyses were done in duplicates.
Behavior—For behavioral tests, 13 male and 13 female NDST3 mutant mice were compared with 12 male and 10 female wt controls. Mice were kept under a 12-h/12-h light dark cycle for 3 weeks before testing began. Food and water was available ad libitum. All procedures and protocols met the guidelines for animal care and experiments in accordance with national and European (86/609/EEC) legislation. General health and neurological status were assessed using a protocol, including tests as described elsewhere (28). Animals were inspected for physical appearance and underwent neurological testing, including acoustic startle, visual placing, grip strength, and reflex functions to ensure that behavioral findings were not the result of deteriorating physical conditions of the animals. The Barrier test was employed to assess spontaneous exploratory behavior, the Open-field test to assess exploration and fear of open spaces, and the Elevated plus-maze, consisting of elevated open stages that mice are reluctant to enter, to assess anxietyrelated behavior. All tests were conducted as blind studies. For a detailed description of behavioral tests see Ref. 29. Data analysis was conducted using the statistical software “R” (The R Project for Statistical Computing, available on the web) using non-parametric statistics. Comparison of two samples was done using the two-sample Wilcoxon test (Mann-Whitney U test).
Hematology—Hematological assays involved the analysis of white blood cell count, numbers of neutrophils, lymphocytes, monocytes, platelets, eosinophils, basophils, and red blood cells and assessment of hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red cell distribution width, mean platelet volume, glucose, protein C, CO2, aspartate transaminase levels, alanine aminotransferase levels, alkaline phosphatase levels, urea levels, and potassium levels. ApiZym assays (BioMerieux) were used to assess the presence of alkaline phosphatase, esterase (C 4), lipase (C 8 and C 14), leucine arylamidase, valine arylamidase, cystine arylamidase, trypsin, α-chymotrypsin, acid phosphatase, α-galactosidase, β-galactosidase, β-glucuronidase α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase.
RESULTS
Targeted Disruption of ndst3—To study the function of the HS biosynthetic GlcNAc N-deacetylase/N-sulfotransferase (NDST) isozymes in mammalian biology, conditional knockout mice for Ndst3 were generated using the cre-loxP system and homologous recombination in embryonic stem cells (Fig. 1). In the targeting vector, CRE-recombination sequences (loxP sites) were positioned in intron sequences surrounding the second exon of ndst3, which included most of the 5′-untranslated region and 327 of 873 amino acids of the open reading frame, containing the signal peptide, cytoplasmic tail, transmembrane region, and part of the catalytic domain (Fig. 1A). Chimeric mice were generated by blastocyst injections of four embryonic stem cell clones. The resulting type II ndst3 mouse line was crossed with ZP3-cre mice, deleting the “floxed” allele in the oocyte and generating mice with a systemic deletion of ndst3 (Type I, Fig. 1B). Type I Ndst3 mice showed only an insignificant deviation from the expected Mendelian distribution (28% NDST3+/+, 50% NDST3+/-, and 22% NDST3-/-, n = 283), were fertile, and appeared normal.
FIGURE 1.
Disruption of the ndst3 gene by targeted recombination and ndst3 expression analysis. A, maps of the wild-type ndst3 locus, the Type II “floxed” allele, and a Type I deletion allele, obtained after breeding of Type II mice with ZP3-CRE mice. Lox sites located in intron sequences are shown as triangles, and arrows denote fragment sizes following HindIII restriction. B, PCR analysis. Employing primers p3 and p4, deletion of exon 2 in NDST3-/- mice yielded a 670-bp product, whereas primers p1 and p2 produced a 500-bp amplification product in wild-type mice. Heterozygous mice yield both amplification products. C, Southern blot analysis of DNA. DNA digestion using restriction endonuclease HindIII yielded a 6.0-kbp (wt) and a 4.2-kbp (NDST3-/-) band. D and E, PCR analysis of the same samples as in C. Employing primers p3 and p4 yielded a 670-bp product from the type I mutant (-/-) allele (D), p1 and p2 yielded a 500-bp product from the wild-type (+/+) allele (E). F, semiquantitative reverse transcription-PCR analysis of ndst1-ndst4 expression using a human cDNA panel (Clontech). ndst1 and ndst2 are abundantly expressed, whereas ndst3 expression is strongest in brain, kidney, liver, pancreas, spleen, testis, and thymus.
Expression of ndst3 in the Mouse and in Human Tissues—To investigate ndst3 expression in adult human tissues, semiquantitative reverse transcription-PCR analysis using cDNA derived from various tissues was conducted (Fig. 1F). Only after 38 cycles of amplification, could ndst3 expression be detected in the brain, kidney, liver, pancreas, spleen, testis, and thymus. This expression pattern was more restricted than that of ndst1 and ndst2. Due to the lack of an isoform-specific anti-NDST3 antibody, ndst3 in situ hybridization was next conducted to detect areas of ndst3 transcription. Strongest ndst3 transcription was detected in cerebellar granule cells, the hippocampus, the brain stem, and the cortex/olfactory bulb (Fig. 2, C and G). ndst1 and ndst2 in situ analysis revealed non-overlapping expression of ndst1 restricted to cerebellar Purkinje cells (Fig. 2A and inset), whereas ndst2 showed overlapping expression in the granule cell layer (Fig. 2B). In the hippocampus, ndst1-3 were all strongly expressed (Fig. 2, E-G). ndst3 expression in the developing embryo was also analyzed at various stages. In the E10.5 embryonic head, ndst3 expression was detected in trigeminal (V) neural crest tissue (supplemental Fig. S1, A-C). In the E12.5 embryonic skull, ndst3 was still expressed in the trigeminal ganglion and additionally in restricted areas of the fourth ventricle, the metencephalic/myelencephalic part of the rhombencephalon, the developing telencephalon, and the spinal cord (supplemental Fig. S1, D-I). ndst3 expression was found to be more widespread in the E15.5 embryo (supplemental Fig. S1, J-L). Strongest expression was found in neural tissue such as the telencephalon (J), the spinal cord (K), as well as in hind brain (L). ndst3 was also strongly expressed in the developing lung and the frontonasal process that forms much of the face (supplemental Fig. S1, J and K).
FIGURE 2.
Detection of ndst expression in the adult mouse brain. A-D, ndst in situ hybridization shows strong ndst1 expression (dark blue stain) in cerebellar Purkinje neurons (A, arrowhead and inset). Ndst2 and ndst3 show overlapping expression in cerebellar granule cells (B and C, arrow) but not in the molecular layer or Purkinje cell layer. D, ndst3 sense control. The arrowhead indicates the granule layer. E-H, ndst1, ndst2, and ndst3 are all expressed in the CA1-3 and dentate gyrus of the hippocampus (E-G, arrows) and the cortex. H, ndst3 sense control. The arrowheads indicate the dentate gyrus and CA1-3. m, molecular layer; w, white matter; g, granule cell layer; dg, dentate gyrus; co, cortex; CA, pyramidal cell layer.
HS Composition in Total Adult Mouse Brain and Embryo—Heparan sulfate can be depolymerized to constituent disaccharides using a combination of three heparin lyases. The individual disaccharides containing one, two, or three sulfate groups can then be separated and quantitated using high-performance liquid chromatography analysis or by mass spectrometry. Disaccharide analysis of HS derived from E15.5 embryos by high-performance liquid chromatography revealed a slight increase in the amount of non-sulfated UA-GlcNAc and monosulfated UA-GlcNAc6S, whereas the amount of UA-GlcNS and UA2S-GlcNS6S was decreased, demonstrating some NDST3 activity in the embryo (Fig. 3). We next analyzed disaccharide composition of purified HS from mutant and wild-type P50 mouse brain by quantitative LC/MS. As shown in Fig. 4A and in Table 1, the amount of sulfation varied in different regions of wild-type mouse brain. Most notably, the cerebellum had reduced levels of all sulfated disaccharides (69 sulfates per 100 disaccharides). The highest overall sulfation was detected in hippocampus, cortex, and brain stem (112, 103, and 96 sulfates per 100 disaccharides, respectively). Generally, the difference in overall sulfation of wild-type tissues was due to parallel differences in N-, 6-O-, and 2-O-sulfation (Table 1).
FIGURE 3.
NDST3-/- mutant heparan sulfate is undersulfated. HS was isolated from E15.5 NDST3-/- embryos and wild-type littermates, and samples were digested with heparin lyases. The resulting disaccharides were analyzed by fast protein liquid chromatography. Values denote the percent of total disaccharide. HS from NDST3-/- embryos showed a slight increase in the amount of non-sulfated UA-GlcNAc and monosulfated UA-GlcNAc6S and reduced amounts of UA-GlcNS and UA2S-GlcNS6S. The relative amounts of UA2S-GlcNH6S, UA-GlcNS6S, UA2S-GlcNS, and UA2S-GlcNAc6S remained unchanged.
FIGURE 4.
Disaccharide analysis of mutant embryo and various regions of the mouse brain. HS was isolated from microdissected NDST3 mutant and wild-type brain or embryo after samples were digested with heparin lyases. The resulting disaccharides were analyzed by quantitative LC/MS. Values denote the mean % of total disaccharide. A, overall sulfation and relative amounts of disaccharides are highly variable among various wild-type brain regions and the wild-type embryo. Wild-type cortex and hippocampus show high levels of sulfation and wild-type cerebellum, and embryo showed lower levels. B-F, compositional disaccharide analysis in brain stem (pons and medulla, B), cerebellum (C), hippocampus (D), and cortex (E) derived from wild-type and NDST3 mutant mice. Relatively unchanged disaccharide composition in the brain stem and hippocampus (B and D) indicate low NDST3 activity in those tissues, or compensatory activity of other NDST isoforms. However, the relative amounts of mono-, di-, and trisulfated disaccharides in the cortex (E) were reduced, and the relative amount of non-sulfated UA-GlcNAc was strongly increased, indicating that NDST3 contributes to HS synthesis in that brain area. In contrast, an increase in the relative amount of trisulfated UA2S-GlcNS6S and decrease in non-sulfated UA-GlcNAc in the cerebellum (C) upon NDST3 deletion indicates overcompensation by another Ndst isoform, possibly by NDST2, which mediates synthesis of highly sulfated heparin in mast cells and is highly expressed in cerebellar granule cells. Results are presented as percent of total disaccharide. F, quantitative LC/MS results of B-E. Disaccharides UA2S-GlcNAc, UA-GlcNH6S, UA-GlcNH, and UA2S-GlcNH were not detected in any tissue investigated.
TABLE 1.
Total amount of sulfates per 100 disaccharides in various brain regions
Disaccharides were analyzed by quantitative LC/MS, and sulfation was calculated from those results. The highest overall sulfation was detected in hippocampus, cortex, and brain stem; lower sulfation levels were detected in the cerebellum. Generally, the difference in overall sulfation was due to parallel differences in N-, 6-O-, and 2-O-sulfation. In the NDST3−/− brain stem, hippocampus, and cortex, the total amount of sulfation decreased or was unaltered. In the cerebellum, a significant increase in N-, 6-O-, and 2-O-sulfation was observed, possibly by compensatory NDST2 activity that results in increased “heparin-like” HS sulfation.
|
Brain stem |
Cerebellum |
Hippocampus |
Cortex |
|||||
|---|---|---|---|---|---|---|---|---|
| KO | WT | KO | WT | KO | WT | KO | WT | |
| Total amount of sulfate per disaccharide | ||||||||
| NS | 40 | 48 | 37 | 33 | 51 | 57 | 35 | 53 |
| 6 S | 25 | 26 | 25 | 19 | 31 | 30 | 19 | 25 |
| 2 S | 17 | 22 | 24 | 17 | 26 | 25 | 13 | 25 |
| Total S | 82 | 96 | 86 | 69 | 108 | 112 | 68 | 103 |
Like in the embryo, NDST3 deletion did not lead to large changes in HS sulfation in various parts of the brain (Fig. 4, B-E), with two notable exceptions. In the cerebellum, the amount of trisulfated disaccharide UA2S-GlcNS6S increased ∼2-fold, whereas the amount of nonsulfated UA-GlcNAc decreased (Fig. 4C). In the cortex, all of the N-sulfated disaccharides decreased with the exception of UA-GlcNS6S, and UA-GlcNAc increased strongly (Fig. 4E). Detection of disaccharides containing free amino groups was also included in the analysis to determine the role of NDST3 in their generation. UA-GlcNH6S and UA2S-GlcNH were not detected in wild-type or mutant samples (data not shown). UA2S-GlcNH6S was detected in wild-type and mutant cerebellum and hippocampus, and no reduction was noted in NDST3-/- animals (Fig. 4F). These results indicate that deletion of NDST3 in the adult brain results in a variable and region-specific change in sulfation patterns.
Histology and Immunohistochemistry of Mutant Tissues—Anti-HS HepSS1 and 10E4 antibody stainings were comparable on adult tissue sections and cultured embryonic fibroblasts. HepSS1 staining was detected in all mutant and wild-type tissues at all stages (supplemental Fig. S2, A and B). Despite high ndst3 expression in the embryo, but consistent with only moderate changes in HS sulfation, no significant reduction in FGF2-dependent MAPK signaling as judged by ERK1/2 phosphorylation was observed in E14.5 cultured mouse embryonic fibroblasts (wt: 100% ± 9% versus NDST3-/-: 90% ± 19%, p ≤ 0.4, n = 4). No reduction in the expression of the hedgehog receptor Patched (PTC) could be observed in the developing mouse head, indicating normal Hedgehog signaling in the mutant (supplemental Fig. S2, A and B).
Based on the high ndst3 expression and the altered HS profile in the adult mouse brain, histological analysis of the brain and immunohistochemical analysis of prominent cell types was conducted. Adult mouse brain analysis using Bielschowsky stain (to stain reticular fibers, neurofibrils, axons, and dendrites), Gallays stain (diffuse and neuritic plaques, amyloid in the central core of neuritic plaques and neurofibrillary tangles), anti-MAC-3 (macrophages), anti-PCNA (cell proliferation) and anti-GFAP (glia) did not detect significant differences between the NDST3 mutant and wild-type brains (data not shown). Neurofilament staining at E12.5 was also conducted to investigate whether the observed ndst3 expression in trigeminal neural crest tissue was indicative of NDST3 function in the development of the peripheral nervous system. Again, no difference in the staining of neurofilament-expressing nerves and in the fasciculation of peripheral nerves could be observed between mutant mice and wild-type littermate controls (supplemental Fig. S2, C and D). We thus conclude that, in the NDST3 mutant embryo and in adult brain, both of which normally express high levels of NDST3, no cellular changes occur in NDST3 mutant mice despite a variable change in overall sulfation and disaccharide composition.
Immunology, Urine Analysis, and Hematology—Because ndst3 expression was found in adult human and mouse kidney, we next assessed kidney function by urine analysis. No significant changes in the levels of glucose, bilirubin, ketones, blood, protein, nitrites, and pH were found in the mutant mouse. Subsequent urine analysis employing an ApiZym assay also revealed no significant changes. Kidney morphology as assessed by histological analysis did not reveal any change in size or any dysmorphology (not shown). ndst3 expression in the thymus and spleen also prompted us to investigate immunological parameters. Again, cellularity counts of the lymph node, bone marrow, and thymus revealed no significant differences (Fig. 5). The bone marrow cellularity count revealed no significant difference between B cells, T cells, myeloid cells (Mac1), and erythroid cells (Ter119) in wild-type and NDST3 mutant mice. In the thymus, the relative number of CD4 single-positive cells, CD8 single-positive cells, CD4/CD8 double-positive cells, and CD4/CD8 double-negative cells was unchanged. The lymph node cellularity count did not reveal differences between ndst3 mutant mice and wild-type littermate controls (CD4, CD8, B220, B220/B7.2, B220/L-Sel, B220/CD44, Gr1, IgM/IgD, IgD/B220, IgM/B220, CD22/CD21, CD22/B220, CD21/B220, CD23/CD40, CD23/B220, CD40/B220, CD79b/B220, and NK1.1 cells were measured).
FIGURE 5.
NDST3 mutants show mostly normal immunological parameters. Analysis of bone marrow (A), thymus (B), and lymph nodes (C) does not indicate significant changes in the relative number of immune cells. In the spleen, the relative number of CD8+ T cells was reduced (D). The relative percentage of wild-type and NDST3-/--derived cells is shown. 25 wild-type and mutant mice were investigated.
Relative amounts of cell subpopulations in the spleen were also similar (T-helper cells (CD4), ProB cells (B220), activated B cells (B220/B7.2), peripheral B cells (B220/L-Sel), B220/CD44, and Gr1 B cells, mature B cells, non-activated B cells, or plasma cells (CD22/CD21, CD22/B220, CD21/B220, CD23/CD40, CD23/B220, CD40B220, and CD79b/B220) as well as NK1.1 cells were investigated; however, the relative number of T-cytotoxic cells (CD8) was strongly reduced (Fig. 5D). This prompted us to investigate the relative amount of circulating lymphocytes. Indeed, hematological analysis showed a 35% reduction in relative lymphocyte numbers in the mutant (64% of wild-type numbers, n = 10 NDST3+/+, 12 NDST3+/-, 8 NDST3-/-, p ≤ 0.01).
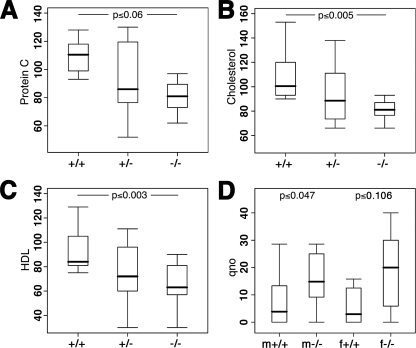
Further hematological assays showed a slight reduction in Protein C levels (20% decrease, p < 0.06, n = 10 wt versus 8 mutant mice) (Fig. 6A). However, no significant change in bleeding time (25 s ± 44 s (wt) versus 31 s ± 17 s (mutant)) could be associated with it. We also found reduced levels of total cholesterol (23% decrease, p ≤ 0.005) and of high density lipoprotein (31% decrease, p ≤ 0.003, n = 10 wt versus 9 mutant mice) (Fig. 6, B and C). Cholesterol reduction was again found in an independently reproduced experiment (20% reduction in females, p ≤ 0.001, n = 8 wt mice versus 4 mutants). Taken together, although subtle but significant changes in the levels of Protein C and cholesterol were detected in NDST3 mutant mice, those mutants show otherwise normal hematological parameters.
FIGURE 6.
NDST3 mutant mice show a moderate hematological phenotype. A, quantification of protein C activity (% inhibition) present in blood samples of NDST3-/- and wild-type mice. The average value of 107% ± 19% inhibition in wild-type mice was reduced to 86% ± 24% inhibition in mutants (n = 17). B, reduced blood cholesterol levels in NDST3-/- mice. The average amount of cholesterol in wild-type mice (109 ± 21 mg/dl) was significantly higher than the amount in NDST3 mutant mice (84 ± 12 mg/dl, n = 19). C, high density lipoprotein (HDL) levels were reduced in NDST3-/- mice. The average amount of high density lipoprotein in wild-type mice (93 ± 18 mg/dl) was significantly higher than the amount in NDST3 mutant mice (64 ± 18.5 mg/dl, n = 19). Triglyceride levels are comparable in both groups. D, reduced anxiety-related behavior in mutants if compared with the wild-type mice. Reduced anxiety-related behavior in mutants was compared with wild-type mice and measured as the quotient of open arm entries (qno) in the Elevated plus-maze. A significant difference is found in male mice (n = 22) but only a trend in females (n = 26).
Behavior—The finding that HS sulfation was altered in the adult brain led us to investigate behavioral changes possibly resulting from subtle defects in brain morphology and function. No differences were observed in locomotor behavior as measured by path length and velocity in the open-field test. Also, no differences in exploration and the ability to climb obstacles could be measured (not shown). However, in the Elevated plus-maze, we detected reduced anxiety-related behavior in mutants if compared with the wild-type mice (p < 0.01, n = 48, not shown). Splitting the data demonstrated a significant difference in male mice (p < 0.05, n = 22) but only a trend in females (Fig. 6D).
NDST2-/-;NDST3-/- and NDST1-/-;NDST3-/- Compound Mutant Mice—NDST2-/-;NDST3-/- and NDST1-/-; NDST3-/- compound mutant mice were produced to examine possible compensation for the loss of NDST3 by other Ndsts. Mice lacking NDST2 have defective connective tissue-type mast cells due to aberrant production of heparin (22, 23), and most NDST1 mutant mice die postnatally (18, 20, 21). NDST1/NDST2 compound null mice die early in development (∼E7) (30) and resemble mutations in EXT1, which is required for HS polymerization, indicating a role of NDST2 for HS synthesis in the absence of NDST1 (31). Strikingly, compound mutant mice for NDST2 and NDST3 appeared normal, showing that expression of NDST1 and NDST4 is fully sufficient for mouse development. Of n = 47 mice derived from breeding of compound heterozygous mice, 6.4% were wild type (expected 6.25%), 8.5% were NDST2-/-;NDST3-/- (6.25%), 13% were NDST2-/-; NDST3+/- (12.5%), 8.5% were NDST2+/-;NDST3-/- (12.5%), 25.5% were NDST2+/-;NDST3+/- (25%), 4.2% were NDST2+/+; NDST3-/- (6.25%), 2% were NDST2-/-;NDST3+/+ (6.25%), 25.5% were NDST2+/+;NDST3+/- (12.5%), and 6.4% were NDST2+/-;NDST3+/+ (12.5%), indicating normal mendelian inheritance. We also analyzed NDST1;NDST3 compound mutant mice to examine possible compensatory activities of NDST1 and NDST3 isoforms during development.
Compound homozygous null animals for NDST1 and NDST3 did not survive birth and had severe brain and frontonasal defects comparable to NDST1 null mice, but often exceeding those in severity and frequency (penetrance) (Fig. 7, A and B). Eyes were mostly absent in the compound knockouts, facial primordia were severely underdeveloped, and the forebrain formed a single, undivided holosphere (Fig. 7C). The maxillary processes and the frontonasal process were also extremely underdeveloped, but the remaining body still showed no obvious dysmorphology. Of n = 91 E13.5-E18.5 embryos derived from matings of NDST3-/-;NDST1+/- mice, n = 18 were NDST1-/-;NDST3-/- (expected: 23), n = 39 were NDST1+/-;NDST3-/- (46 were expected), and n = 34 were NDST1+/+;NDST3-/- (23 were expected), indicating a moderate sub-mendelian ratio and thus implying some degree of early embryonic lethality. Of the 18 compound mutant embryos derived, 7 (39%) displayed severe facial clefting, lack of lower jaw (agnathia), lack of eyes and holoprosencephaly/hypoplastic forebrain, and 11 (61%) showed eye defects as well as hypoplastic frontonasal and maxillary prominences. About 14% of NDST1 mutant embryos showed similar defects, indicating a role of NDST3 in the development of these structures in the absence of NDST1.
FIGURE 7.
NDST1-/-;NDST3-/- mutants show severe developmental defects of the skull and elevated levels of programmed cell death. A, NDST1-/-;NDST3-/- embryos (right) show severe developmental defects of the skull if compared with NDST3-/- littermate controls (left). B, coronal section through the skull of an E12.5 NDST3-/- embryo (left) and an NDST1-/-;NDST3-/- compound mutant (right). Brain and skull development is strongly perturbed. H&E-stained sections. t, telencephalon; mp, maxillary prominences. C, horizontal section through the skull of an E12.5 NDST3-/- mutant embryo (left) and a NDST1-/-;NDST3-/- compound mutant (right). The mutant forebrain forms a single holosphere. 4, fourth ventricle; t, telencephalon. D, TUNEL staining reveals strongly enhanced levels of programmed cell death in maxillary prominences of E12.5 NDST1;NDST3 compound mutant embryos (right) if compared with NDST3 mutant littermate controls. E, quantification of apoptotic cells found in the cortex, hindbrain (hb), thalamus, and maxillary prominences (mp). Significantly elevated levels of apoptosis are found in hindbrain (+1000%, p < 0.01) and maxillary prominences (+380%, p = 0.015). Values are expressed as % relative to wild-type levels.
TUNEL staining of strongly affected NDST1-/-;NDST3-/- compound mutant E13.5 maxillary prominences revealed enhanced cell death (380% of wild-type control levels, n = 3, p < 0.01) (Fig. 7, D and E). Analysis of the forebrain, thalamus, and hindbrain revealed strongly enhanced cell death restricted to the hindbrain (∼1000% of wild-type control, n = 3, p < 0.01, Fig. 7E), whereas apoptosis in the forebrain and thalamus was not significantly affected.
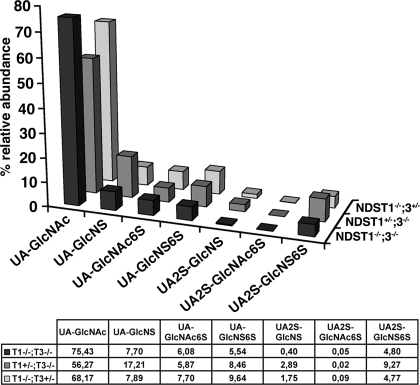
HS Composition in Compound Mutant Embryo—To examine whether NDST1 and NDST3 modify an overlapping set of HS motifs, HS-disaccharide analysis in the compound mutant E16.5 embryos was performed by LC/MS, and the result was compared with each one of the single mutants (Fig. 8). Both NDST1 and NDST3 contribute to UA2S-GlcNS and UA-GlcNS6S production. However, the relative amounts of UA-GlcNS, UA-GlcNAc6S, and UA2S-GlcNS6S were not further reduced in the compound mutant embryo if compared with the NDST1-/-;NDST3+/- embryo. In total, 43.73% sulfated disaccharides were detected in NDST1+/-;NDST3-/-, 31.83% in NDST1-/-;NDST3+/-, and 24.57% in NDST1-/-; NDST3-/- mutants, indicating partial compensatory activities of NDST1 and NDST3. These results show that, in the embryo, NDST3 deficiency impaired downstream sulfation reactions to a small and varying extent. They also demonstrate preferential activity of NDST3 on UA2S-GlcNS and UA-GlcNS6S containing HS motifs and partially overlapping activities of NDST1 and NDST3. The latter finding was confirmed by the severe phenotypes of NDST1-/-;NDST3-/- compound mutant embryos, indicating that NDST3 contributes to the development of the skull, brain, and eyes.
FIGURE 8.
NDST3-/-;NDST1-/- compound mutant heparan sulfate is undersulfated. HS was isolated from NDST3-/-;NDST1-/- embryos and NDST3-/-;NDST1+/- as well as NDST3+/-;NDST1-/- littermates, and samples were digested with heparin lyases. The resulting disaccharides were analyzed by quantitative LC/MS. Values denote the percent total disaccharide. HS from NDST3-/-;NDST1-/- embryos show general undersulfation and a total lack of UA2S-GlcNS.
We also investigated proliferation of isolated E14.5 fibroblasts derived from NDST1;NDST3 compound mutant embryos, NDST3 mutant embryos, and wild-type embryos under normal serum conditions immunohistochemically after bromodeoxyuridine incorporation. Again, no differences could be observed between mutant and wild-type fibroblasts (25% ± 4% proliferating wild-type cells, 27% ± 9% proliferating mutant cells, n = 30, p < 0.25). Likewise, no differences were found between NDST1-/-;NDST3-/- and NDST1+/+;NDST3-/- fibroblasts (NDST1+/+;NDST3-/-: 15% ± 7%, NDST1-/-; NDST3-/-: 15% ± 6%, p = 0.48) indicating that proliferation under normal culture conditions was not reduced in the compound mutant cells. Migration of isolated fibroblasts was also examined but again showed a moderate reduction (NDST1+/+; NDST3+/+ 100% ± 10%, NDST1+/+;NDST3-/- 86% ± 16% of wild-type levels (p < 0.04), NDST1+/-;NDST3-/- 71% ± 13% of wild-type levels (p < 0.02), and NDST1-/-;NDST3-/- 84% ± 29% of wild-type levels (p < 0.1)).
DISCUSSION
In this report, we show that development and physiology of NDST3 mutant mice were not significantly impaired despite strong ndst3 expression in the embryo and in the adult brain, kidney, liver, pancreas, spleen, testis, and thymus. Only moderate phenotypes could be associated with NDST3 deficiency, including small changes in high density lipoprotein and total cholesterol and altered anxiety related behavior. In mouse development, it appears to play no essential role, because all the major organ systems, including the brain, were unaffected morphologically, and no delay in development was obvious. This is in agreement with our finding that NDST3 deficiency did not result in dramatic differences in tissue HS composition. In this regard, NDST3 behaves much like NDST2, which when mutated also does not cause large changes in HS composition (32, 33). The simplest interpretation of these findings is that NDST3, although being expressed, does either not contribute to HS sulfation in non-affected tissues, or that other Ndst isoforms may compensate for NDST3 deficiency in a tissue-dependent context.
This idea is in agreement with the analysis of HS in different parts of the brain. Here, inactivation of NDST3 had highly variable effects, decreasing overall sulfation in the cortex dramatically while having little effect on brain stem or hippocampus. The former finding is reminiscent of HS sulfation in NDST1-deficient mice, which show a general HS undersulfation (18, 32), whereas the latter finding is reminiscent of the behavior of NDST2-deficient mice, which only show alterations in synthesis of highly sulfated heparin in connective tissue mast cells; other tissues were not affected (22, 23, 32). However, HS from the cerebellum showed an increase in N- and O-sulfation, which raises the enigmatic question of how inactivation of a sulfotransferase can cause an increase in overall sulfation.
One model of HS biosynthesis suggests that several of the enzymes are present in a multienzyme complex termed the GAGosome (4). In this model, the GAGosome could vary in composition of specific Ndst isoforms dependent on their levels of expression or of other proteins that act as chaperones or scaffolding proteins in the system. Thus, one can imagine that in the cerebellar granule cells the GAGosome might preferentially contain NDST3, whereas in other parts of the brain other Ndst isozymes predominate. If the capacity to N-deacetylate and N-sulfate N-acetylglucosamine residues varies across the different isozymes, as has been shown (2), then altering the composition of the GAGosome could affect the composition of HS in an unpredictable way. Thus, substitution of NDST3 by other isozymes with greater capacity to sulfate the chain could explain the enhanced sulfation of HS observed in the cerebellum. Indeed, in situ hybridization showed strong overlapping expression of ndst2 in cerebellar granule cells, raising the possibility that NDST2 incorporation in granule cell GAGosomes results in HS oversulfation. Although this model is attractive, it is also possible that changes in expression of NDST3 affect other metabolic pathways, e.g. signaling reactions that then affect metabolism.
Analysis of NDST1-/-;NDST3-/- compound mutant embryos showed a strongly reduced overall sulfation level and more dramatic changes in HS structure than observed in NDST1-/- animals, such as the complete loss of the UA2S-GlcNS disaccharide and strong reduction of the relative amount of the UA-GlcNS6S disaccharide. This indicates a partial ability of NDST1 to compensate for the loss of NDST3 and vice versa, and the potential of both isoforms to generate specific HS modifications. Consistent with this, doubly deficient embryos resembled NDST1 single mutants phenotypically (brain hypoplasia and facial dysmorphia (18, 20)) but showed a higher frequency and severity of deficiencies (39% severe defects in the compound versus 14% in the NDST1 single mutant). Both findings thus suggest that NDST1 and NDST3 participate in the regulation of common pathways required for neural crest and forebrain development, e.g. FGF and HH signaling. The predominant role of NDST1 in mouse development is furthermore supported by the finding that NDST2-/-; NDST3-/- mutant mice develop normally and are viable and fertile. The relative importance of the fourth member of the family, NDST4, awaits characterization of mutant mice lacking this isozyme.
Supplementary Material
Acknowledgments
We thank Dr. Nissi Varki (University of California, San Diego, CA) for histological work and helpful discussions and Dr. L. Kjellén (Uppsala University, Sweden) for NDST2 mutant mice.
This work was supported, in whole or in part, by National Institutes of Health Grants GM33063 and HL57345 (to J. D. E.). This work was also supported by DFG (German Research Council) Grants GR1748 and SFB 492-B15 (to K. G.). The authors state that they have no competing interests. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: HS, heparan sulfate; NDST, GlcNAc N-deacetylase/N-sulfotransferase; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; GAG, glycosaminoglycan; LC/MS, liquid chromatography/mass spectrometry; MAPK, mitogen-activated protein kinase; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; wt, wild type.
R. Lawrence, R. Cummings, and J. E. Esko, submitted for publication.
References
- 1.Esko, J. D., and Lindahl, U. (2001) J. Clin. Invest. 108 169-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aikawa, J., Grobe, K., Tsujimoto, M., and Esko, J. D. (2001) J. Biol. Chem. 276 5876-5882 [DOI] [PubMed] [Google Scholar]
- 3.Lindahl, U., Kusche-Gullberg, M., and Kjellén, L. (1998) J. Biol. Chem. 273 24979-24982 [DOI] [PubMed] [Google Scholar]
- 4.Esko, J. D., and Selleck, S. B. (2002) Annu. Rev. Biochem. 71 435-471 [DOI] [PubMed] [Google Scholar]
- 5.Holmborn, K., Ledin, J., Smeds, E., Eriksson, I., Kusche-Gullberg, M., and Kjellen, L. (2004) J. Biol. Chem. 279 42355-42358 [DOI] [PubMed] [Google Scholar]
- 6.Perrimon, N., and Bernfield, M. (2000) Nature 404 725-728 [DOI] [PubMed] [Google Scholar]
- 7.Lin, X. H., Buff, E. M., Perrimon, N., and Michelson, A. M. (1999) Development 126 3715-3723 [DOI] [PubMed] [Google Scholar]
- 8.The, I., Bellaiche, Y., and Perrimon, N. (1999) Mol. Cell 4 633-639 [DOI] [PubMed] [Google Scholar]
- 9.Lin, X. H., and Perrimon, N. (1999) Nature 400 281-284 [DOI] [PubMed] [Google Scholar]
- 10.Schlessinger, J., Plotnikov, A. N., Ibrahimi, O. A., Eliseenkova, A. V., Yeh, B. K., Yayon, A., Linhardt, R. J., and Mohammadi, M. (2000) Mol. Cell 6 743-750 [DOI] [PubMed] [Google Scholar]
- 11.Allen, B. L., and Rapraeger, A. C. (2003) J. Cell Biol. 163 637-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ornitz, D. M., and Itoh, N. (2001) Genome Biol. 2 3005.1-3005.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inatani, M., Irie, F., Plump, A. S., Tessier-Lavigne, M., and Yamaguchi, Y. (2003) Science 302 1044-1046 [DOI] [PubMed] [Google Scholar]
- 14.Pan, Y., Woodbury, A., Esko, J. D., Grobe, K., and Zhang, X. (2006) Development 133 4933-4944 [DOI] [PubMed] [Google Scholar]
- 15.Pallerla, S. R., Pan, Y., Zhang, X., Esko, J. D., and Grobe, K. (2007) Dev. Dyn. 236 556-563 [DOI] [PubMed] [Google Scholar]
- 16.Carrasco, H., Olivares, G. H., Faunes, F., Oliva, C., and Larrain, J. (2005) J. Cell. Biochem. 96 831-838 [DOI] [PubMed] [Google Scholar]
- 17.McLellan, J. S., Yao, S., Zheng, X., Geisbrecht, B. V., Ghirlando, R., Beachy, P. A., and Leahy, D. J. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 17208-17213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobe, K., Inatani, M., Pallerla, S. R., Castagnola, J., Yamaguchi, Y., and Esko, J. D. (2005) Development 132 3777-3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramsson, A., Kurup, S., Busse, M., Yamada, S., Lindblom, P., Schallmeiner, E., Stenzel, D., Sauvaget, D., Ledin, J., Ringvall, M., Landegren, U., Kjellen, L., Bondjers, G., Li, J. P., Lindahl, U., Spillmann, D., Betsholtz, C., and Gerhardt, H. (2007) Genes Dev. 21 316-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringvall, M., Ledin, J., Holmborn, K., Van Kuppevelt, T., Ellin, F., Eriksson, I., Olofsson, A. M., Kjellén, L., and Forsberg, E. (2000) J. Biol. Chem. 275 25926-25930 [DOI] [PubMed] [Google Scholar]
- 21.Fan, G., Xiao, L., Cheng, L., Wang, X., Sun, B., and Hu, G. (2000) FEBS Lett. 467 7-11 [DOI] [PubMed] [Google Scholar]
- 22.Forsberg, E., Pejler, G., Ringvall, M., Lunderius, C., Tomasini-Johansson, B., Kusche-Gullberg, M., Eriksson, I., Ledin, J., Hellman, L., and Kjellén, L. (1999) Nature 400 773-776 [DOI] [PubMed] [Google Scholar]
- 23.Humphries, D. E., Wong, G. W., Friend, D. S., Gurish, M. F., Qiu, W. T., Huang, C. F., Sharpe, A. H., and Stevens, R. L. (1999) Nature 400 769-772 [DOI] [PubMed] [Google Scholar]
- 24.Wang, L., Fuster, M., Sriramarao, P., and Esko, J. D. (2005) Nat. Immunol. 6 902-910 [DOI] [PubMed] [Google Scholar]
- 25.Fuster, M. M., Wang, L., Castagnola, J., Sikora, L., Reddi, K., Lee, P. H., Radek, K. A., Schuksz, M., Bishop, J. R., Gallo, R. L., Sriramarao, P., and Esko, J. D. (2007) J. Cell Biol. 177 539-549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacArthur, J. M., Bishop, J. R., Stanford, K. I., Wang, L., Bensadoun, A., Witztum, J. L., and Esko, J. D. (2007) J. Clin. Invest. 117 153-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toyoda, H., Nagashima, T., Hirata, R., Toida, T., and Imanari, T. (1997) J. Chromatogr. B Biomed Sci. Appl. 704 19-24 [DOI] [PubMed] [Google Scholar]
- 28.Rogers, D. C., Peters, J., Martin, J. E., Ball, S., Nicholson, S. J., Witherden, A. S., Hafezparast, M., Latcham, J., Robinson, T. L., Quilter, C. A., and Fisher, E. M. (2001) Neurosci. Lett. 306 89-92 [DOI] [PubMed] [Google Scholar]
- 29.Lewejohann, L., Reinhard, C., Schrewe, A., Brandewiede, J., Haemisch, A., Gortz, N., Schachner, M., and Sachser, N. (2006) Genes Brain Behav. 5 64-72 [DOI] [PubMed] [Google Scholar]
- 30.Grobe, K., Ledin, J., Ringvall, M., Holborn, K., Forsberg, E., Esko, J. D., and Kjellen, L. (2002) Biochim. Biophys. Acta 1573 209-215 [DOI] [PubMed] [Google Scholar]
- 31.Lin, X., Wei, G., Shi, Z. Z., Dryer, L., Esko, J. D., Wells, D. E., and Matzuk, M. M. (2000) Dev. Biol. 224 299-311 [DOI] [PubMed] [Google Scholar]
- 32.Ledin, J., Staatz, W., Li, J. P., Gotte, M., Selleck, S., Kjellen, L., and Spillmann, D. (2004) J. Biol. Chem. 279 42732-42741 [DOI] [PubMed] [Google Scholar]
- 33.Ledin, J., Ringvall, M., Thuveson, M., Eriksson, I., Wilen, M., Kusche-Gullberg, M., Forsberg, E., and Kjellen, L. (2006) J. Biol. Chem. 281 35727-35734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.