Abstract
In this study, we demonstrate that treatment of human lung adenocarcinoma H460 cells with farnesol induces the expression of a number of immune response and inflammatory genes, including IL-6, CXCL3, IL-1α, and COX-2. This response was dependent on the activation of the NF-κB signaling pathway. Farnesol treatment reduces the level of IκBα and induces translocation of p65/RelA to the nucleus, its phosphorylation at Ser276, and transactivation of NF-κB-dependent transcription. Moreover, overexpression of IκBα or treatment with the NF-κB inhibitor caffeic acid phenethyl ester greatly diminishes the induction of inflammatory gene expression by farnesol. We provide evidence indicating that the farnesol-induced phosphorylation of p65/RelA at Ser276 is important for optimal transcriptional activity of NF-κB. The MEK1/2 inhibitor U0126 and knockdown of MEK1/2 expression with small interfering RNAs effectively blocked the phosphorylation of p65/RelA(Ser276) but not that of Ser536, suggesting that this phosphorylation is dependent on the activation of the MEK1/2-ERK1/2 pathway. We further show that inhibition of MSK1, a kinase acting downstream of MEK1/2-ERK1/2, by H89 or knockdown of MSK1 expression also inhibited phosphorylation of p65/RelA(Ser276), suggesting that this phosphorylation is dependent on MSK1. Knockdown of MEK1/2 or MSK1 expression inhibits farnesol-induced expression of CXCL3, IL-1α, and COX-2 mRNA. Our results indicate that the induction of inflammatory genes by farnesol is mediated by the activation of the NF-κB pathway and involves MEK1/2-ERK1/2-MSK1-dependent phosphorylation of p65/RelA(Ser276). The activation of the NF-κB pathway by farnesol might be part of a prosurvival response during farnesol-induced ER stress.
Isoprenoids are intermediates of the cholesterol/sterol biosynthetic pathway and are formed from mevalonate, which is synthesized from acetyl-CoA by the rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase, a major target for statins in the treatment of cardiovascular disease (1–3). Isoprenoids are important in the regulation of cell proliferation, apoptosis, and differentiation (4–10).
Farnesol and the related isoprenoids perillyl alcohol, geranylgeraniol, and geraniol have been reported to be effective in chemopreventative and -therapeutic strategies in several in vivo cancer models, including melanoma, colon, and pancreatic cancer (11–18). In addition, these isoprenoids inhibit proliferation and induce cell death in a variety of neoplastic cell lines (5, 7, 10, 13, 15, 19–21). The mechanisms by which these agents mediate their actions are not yet fully understood. In human pancreatic carcinoma cells, the antiproliferative response by these isoprenoids involves a p21- and p27-dependent mechanism (21). Farnesol has been reported to be able to weakly activate the farnesoid X-activated receptor (22) and inhibit phospholipase D (23), 3-hydroxy-3-methylglutaryl-CoA reductase activity (10), and the CDP-choline pathway (24). Study of farnesol-induced toxicity in yeast has indicated an important role for mitochondria and the PKC signaling pathway in the generation of reactive oxygen species (25).
Recently, we reported that a large number of genes associated with the endoplasmic reticulum (ER)2 stress response are rapidly induced by farnesol treatment, suggesting that farnesol-induced apoptosis is coupled to ER stress (26). Disturbance of ER homeostasis results in the activation of the unfolded protein response (27–30). During this response, several prosurvival and proapoptotic signals are activated, and, depending on the extent of the ER stress, cells survive or undergo apoptosis. We demonstrated that farnesol induces activation of several MAPK pathways, including p38, MEK1/2-ERK1/2, and JNK1/2 (26) and provided evidence indicating that activation of MEK/ERK is an early and upstream event in farnesol-induced ER stress signaling cascade.
In this study, we demonstrate that treatment of human lung adenocarcinoma H460 cells with farnesol induces the expression of a number of immune response and inflammatory-related genes, including COX-2 and several chemo/cytokines, and examine the signaling pathway involved in the induction of several of these genes. We show that this induction involves activation of the NF-κB signaling pathway by farnesol and that this activation, as well as the induction of the expression of immune and inflammatory genes, is dependent on the activation of p65/RelA by the MEK1/2-ERK1/2-MSK1 (mitogen- and stress-activated kinase-1) signaling pathway. The activation of the NF-κB might be part of a prosurvival pathway in the farnesol-induced ER stress response.
EXPERIMENTAL PROCEDURES
Materials—Trans,trans-farnesol (farnesol) was purchased from Sigma. Caffeic acid phenethyl ester (CAPE), SB203580, and SP600125 were obtained from Calbiochem, and U0126 was from Promega (Madison, WI).
Cell Line and Culture—Human lung adenocarcinoma H460 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and 100 units/ml each of penicillin and streptomycin. Cells were treated with 250 μm farnesol, a dose that was previously shown to be optimal (26).
Microarray Analysis—Microarray analysis with RNA from vehicle- and farnesol-treated cells was performed in duplicate by the NIEHS Microarray Group on Agilent whole human genome microarrays (Agilent Technologies, Palo Alto, CA), and data were analyzed as described previously (26).
Western Blot Analysis—Cells were harvested and lysed in lysis buffer containing 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, and 0.1% SDS, supplemented with protease and phosphatase inhibitor mixtures I and II (Sigma). After centrifugation, proteins were examined by Western blot analysis with the following antibodies: anti-phospho-ERK1/2 (catalog number 9101), anti-ERK1/2 (catalog number 9102), anti-phospho-MEK1/2 (catalog number 9121), anti-MEK1/2 (catalog number 9122), anti-IκBα (catalog number 9242), anti-phospho-NF-κB p65 Ser536 (catalog number 3031), anti-phospho-NF-κB p65 Ser276 (catalog number 3037), anti-phospho-MSK1 (catalog number 9595), and anti-NF-κB p65 (catalog number 3034) from Cell Signaling Technology (Beverly, MA) and anti-MSK1 (catalog number sc-9392) and anti-actin (catalog number sc-1615) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The blots were developed using a peroxidase-conjugated secondary antibody and ECL Detection Reagent (GE Healthcare Life Sciences).
Small Interfering RNA (siRNA) Knockdown—Knockdown of MEK1/2 and MSK1 expression in H460 cells was achieved by transfection of siRNA. The siRNAs of human MEK-1 (catalog number sc-29396) and MEK-2 (catalog number sc-35905) were purchased from Santa Cruz Biotechnology. The siMSK1 (catalog number N2006S) and silencer-negative control siRNA (catalog number 4611) were purchased from New England BioLabs (Ipswich, MA) and Ambion (Austin, TX), respectively. Transfection of siRNA was performed using DharmaFECT 4 transfection reagent (Dharmacon, Chicago, IL). H460 cells were plated in 6-well dishes at a density 3.3 × 105 cells/well. The next day, cells were treated with the siRNA transfection mixtures following the DharmaFECT General Transfection Protocol. After 48 h of incubation, cells were treated with or without farnesol as indicated and harvested for Western and Northern blot analysis.
Electrophoretic Mobility Shift Assay (EMSA)—Preparation of nuclear extracts and EMSA were performed as described previously (31). Briefly, 2 × 106 H460 cells were harvested, washed two times in ice-cold PBS, and then resuspended in 400 μlof cold cell lysis buffer (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 1 mm dithiothreitol, and 1% (v/v) protease inhibitor mixture). After a 15-min incubation on ice, 12.5 μl of 10% Nonidet P-40 was added, and the mixture was centrifuged at 10,000 × g for 30 s at 4 °C. The nuclear pellet was resuspended in 25 μl of ice-cold nuclear extraction buffer (20 mm HEPES, pH 7.9, 0.4 m NaCl, 1 mm EDTA, 1 mm dithiothreitol, 1% (v/v) protease inhibitor mixture), incubated on ice for 30 min, and then centrifuged for 5 min at 4 °C. The nuclear extracts were stored at –80 °C. For EMSA, double-stranded NF-κB consensus and mutant oligonucleotide (catalog numbers sc-2505 and sc-2511; Santa Cruz Biotechnology) were labeled with [γ-32]ATP using T4 polynucleotide kinase (Roche Applied Science). The DNA-protein binding reactions were performed in 10 μl of binding buffer (10 mm Tris-HCl, pH 7.5, 100 mm KCl, 1 mm dithiothreitol, 1 mm EDTA, 12.5% glycerol, 0.1% Triton X-100, and 0.5 μg/ml bovine serum albumin) with 5 μgof nuclear extract, 105 cpm of the radiolabeled oligonucleotide, and 1 μg of poly(dI-dC) for 30 min at room temperature. The samples were electrophoresed through 6% polyacrylamide gels in Tris (89 mm)-boric acid (89 mm)-EDTA (2 mm) buffer. For supershift assays, nuclear proteins were incubated with anti-p65 antibody (catalog number sc-109; Santa Cruz Biotechnology) for 20 min at room temperature prior to the addition of labeled oligonucleotide.
IκBα-overexpressing Stable Cell Line—To generate clonal cell lines stably overexpressing wild-type IκBα, H460 cells were transfected with 10 μg of pCMW-IκBα carrying the wild-type IκBα gene (Clontech) or empty plasmid vector using Fugene 6 transfection reagent (Roche Applied Science). Single cell colonies were isolated after selection with G418 (800 μg/ml; Invitrogen) and then tested for overexpression of IκBα by Western blot analysis. H460 cells overexpressing IκBα or containing the empty vector are referred to as H460(IκBα) and H460(Empty), respectively.
Quantitative Real Time PCR (QRT-PCR)—RNA was isolated using TriReagent (Sigma) following the manufacturer's protocol. Synthesis of cDNAs was performed using oligo(dT) primer and murine leukemia virus reverse transcriptase according to the manufacturer's instructions (Invitrogen). Each cDNA sample was analyzed for the expression of IL-6, IL-1β, CXCL3, and COX-2 using the POWER SYBER® Green PCR master mix (Applied Biosystems, Foster City, CA). The forward and reverse oligonucleotide primers for IL-6 (5′-CCTGAGAAAGGAGACATGTAACAAGA, 5′-GGCAAGTCTCCTCATTGAATCC) (32), CXCL3 (5′-GTTTGACTATTTCTTACGAGGGTTC, 5′-ACCCATCATATTCCAATTAAATAATCAGG) (33), IL-1α (5′-TCAAGGAGAGCATGGTGGTAGTAG, 5′-TGGCTTAAACTCAACCGTCTCTT), and COX-2 (5′-CATTCTTTGCCCAGCACTTCAC, 5′-GACCAGGCACCAGACCAAAGAC) (34) were purchased from Sigma. PCR assays were performed using the 7300 Real Time PCR System (Applied Biosystems). Gene expression level was normalized to 18 S RNA, and relative mRNA expression was presented relative to the control. Data were analyzed using Sequence Detection software (version 1.2.2; Applied Biosystems) and are presented as mean ± S.D. of three independent experiments.
NF-κB Reporter Assay—The NF-κB reporter vector, pNF-κB-Luc (Stratagene, La Jolla, CA) containing five copies of consensus NF-κB binding sequence linked to the minimal E1B promoter-luciferase gene, was used to monitor NF-κB-dependent transcriptional activity. A constitutive active SV40 promoter-Renilla luciferase construct, pRL-SV40 (Promega, Madison, WI), was used to normalize transfection efficiency. Plasmids expressing FLAG-p65 (wild type), FLAG-p65/S276A, FLAG-p65/S529A, FLAG-p65/S536A, and FLAG-p65/S276/529/536A (triple mutant) were generously provided by Dr. James M. Samet (University of North Carolina, Chapel Hill, NC) (35). H460 cells (1 × 105 cells/well) were seeded in 12-well tissue culture dishes and 12 h later co-transfected with pNF-κB-Luc (0.4 μg), pRL-SV40 (50 ng), and one of the FLAG-p65 expression vectors (0.4 μg) using Fugene 6. Forty-eight h later, cultures were treated with 250 μm farnesol for an additional 24 h before luciferase activities were determined.
Statistics—Values are presented as means ± S.D. Statistical analysis was performed with Student's t test.
RESULTS
Farnesol Induces Immune Response and Inflammatory-related Genes—Comparison of the gene expression profiles of untreated and farnesol-treated (250 μm for 4 h) H460 cells showed that expression of many immune response and inflammatory genes are greatly enhanced in farnesol-treated cells (Table 1). IL-8 (interleukin-8) expression was the most induced, about 275-fold, whereas IL-1α, IL-6, IL-1β, and IL-32 were up-regulated 58-, 24-, 4-, and 2-fold, respectively. In addition, the expression of several chemokines, including CXCL3, CXCL2, CCL20, and CXCL1, were increased 155-, 83-, 42-, and 20-fold, respectively. The expression of the cyclooxygenase COX-2, which is involved in various inflammatory processes, is elevated 79-fold in farnesol-treated H460 cells. Expression of the NF-κB inhibitor IκBα (NFKBIA) is up-regulated 23-fold. Several transcription factors with established roles in inflammation were found to be highly up-regulated, including FOS (144-fold) and JUN (108-fold), the basic leucine zipper protein NFIL3 (14-fold), and the CCAAT/enhancer-binding protein CEBPβ (3-fold). Other genes with established roles in inflammation that are up-regulated include PTX3, GDF15, SOCS3, and IRF7. The expression of a few inflammatory genes, including IL-13RA and IRAK1BP1, was slightly down-regulated.
TABLE 1.
Changes in the expression of several immune response and inflammatory genes by farnesol
| Gene name | Description | GenBank™ | Change |
|---|---|---|---|
| -fold | |||
| IL-8 | Interleukin-8 | NM_000584 | 275.04 |
| CXCL3 | Chemokine (CXC motif) ligand 3 (MIP2B) | NM_002090 | 155.15 |
| FOS | c-fos protooncogene | NM_005252 | 144.50 |
| JUN | c-jun protooncogene | NM_002228 | 108.20 |
| CXCL2 | Chemokine (CXC motif) ligand 2 (MIP2A) | NM_002089 | 83.82 |
| PTGS2 | Protaglandin-endoperoxide synthase 2 (COX-2) | NM_000963 | 79.10 |
| IL-1α | Interleukin 1, α | NM_000575 | 58.62 |
| CCL20 | Chemokine (CC motif) ligand 20 (MIP-3A) | NM_004591 | 42.67 |
| PTX3 | Pentaxin-related gene, rapidly induced by IL-1 β | NM_002852 | 42.36 |
| IL-6 | Interleukin-6 (interferon, β2) | NM_000600 | 24.54 |
| NFKBIA | Nuclear factor of κ light polypeptide gene in B-cells inhibitor, α | NM_020529 | 23.88 |
| CXCL1 | Chemokine (CXC motif) ligand 1 | NM_001511 | 20.11 |
| GDF15 | Growth differentiation factor 15 (MIC-1) | NM_004864 | 14.65 |
| NFIL3 | Nuclear factor interleukin-3-regulated | NM_005384 | 14.63 |
| SOCS3 | Suppressor of cytokine signaling 3 | NM_003955 | 12.54 |
| HRH1 | Histamine receptor H1 | NM_000861 | 7.25 |
| IL-1β | Interleukin 1, β | NM_000576 | 4.92 |
| CEBPβ | CCAAT/enhancer binding protein (C/EBP), beta | NM_005194 | 3.08 |
| IL-32 | Interleukin-32 | NM_004221 | 2.57 |
| IRF7 | Interferon-regulatory factor 7 | NM_004031 | 2.14 |
| CCL5 | Chemokine (CC motif) ligand 5 (RANTES) | NM_002985 | 1.79 |
| IL-13RA1 | Interleukin-13 receptor α 1 | NM_001560 | -1.34 |
| IRAK1BP1 | Interleukin-1 receptor-associated kinase 1-binding protein 1 | AL049321 | -1.98 |
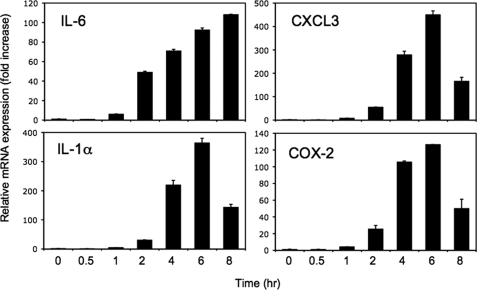
The induction of several genes was confirmed by QRT-PCR analysis. As shown in Fig. 1, farnesol induced IL-6, CXCL3, IL-1α, and COX-2 mRNA expression in a time-dependent manner. Expression levels reached a maximum 6 h after the addition of farnesol and then decreased.
FIGURE 1.
Farnesol induces IL-6, CXCL3, IL-1α, and COX-2 mRNA expression. H460 cells were treated with 250 μm farnesol, and at the times indicated, cells were collected and total RNA was isolated. Levels of IL-6, CXCL3, IL-1α, and COX-2 mRNA expression were evaluated by QRT-PCR as described under “Experimental Procedures.” Each value is the mean ± S.D. of three separate experiments.
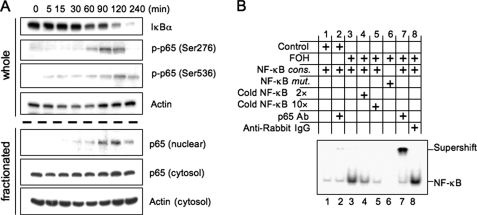
Farnesol Activates NF-κB Transcription Factor—Activation of the NF-κB signaling pathway is frequently involved in the regulation of many immune response and inflammatory genes (36). To determine whether farnesol induces activation of the NF-κB signaling pathway, we examined the effect of farnesol treatment on several steps that are part of this pathway, including reduction in IκBα protein, translocation of p65 to the nucleus, and the phosphorylation of p65. We first determined the effect of farnesol on the level of IκBα protein. H460 cells were treated with 250 μm farnesol, and at different time intervals the level of total cellular IκBα protein was examined by Western blot analysis. As shown in Fig. 2A, the level of IκBα was significantly decreased 60 min after the addition of farnesol, whereas after 4 h of treatment IκBα was barely detectable. We further demonstrated that this decrease was accompanied by increased nuclear localization of p65 protein. These results suggest that farnesol induces degradation of IκBα and subsequently translocation of NF-κB to the nucleus. Examination of the phosphorylation status of p65 at Ser276 and Ser536, sites that play a critical role in regulating the transcriptional activity of NF-κB (35, 37, 38), showed that farnesol treatment induced phosphorylation of p65 at both Ser276 and Ser536 (Fig. 2A) in a time course very similar to that of its nuclear localization. The accumulation of p65 in the nucleus is transient and may at least in part be responsible for the transient induction of gene expression observed in Fig. 1.
FIGURE 2.
Farnesol treatment induces phosphorylation and nuclear localization of p65. A, H460 cells were treated with 250 μm farnesol at the times indicated. Levels of IκBα, phospho-p65(Ser276), and phospho-p65(Ser536) in whole cell lysates were examined by Western blot analysis with the respective antibodies. Levels of p65 were also determined in nuclear and cytosolic fractions. Actin is shown as a control for equal loading. B, EMSA. Nuclear extracts isolated from cells treated for 2 h with vehicle (control) or farnesol (FOH; 250 μm) were incubated with radiolabeled NF-κB consensus oligonucleotide (NF-κB cons.) or NF-κB mutant oligonucleotide (NF-κB mut.) and subjected to EMSA. Antibodies against p65 and anti-rabbit-IgG were used for supershift assay.
Next, we examined the binding of NF-κB complexes, isolated from vehicle or 250 μm farnesol-treated H460 cells, to the consensus NF-κB DNA binding site (Fig. 2B). EMSA analysis showed increased binding of protein complexes to the 32P-labeled NF-κB response element with extracts from farnesol-treated cells (lane 3). Increasing amounts of unlabeled oligonucleotides competed for the binding (lanes 4 and 5). Nuclear proteins from farnesol-treated cells did not bind to a mutant NF-κB response element (lane 6). The addition of an anti-p65 antibody caused a supershift of the p65-DNA complex (lane 7), whereas no shift was observed with anti-rabbit IgG (lane 8). These observations are in agreement with the conclusion that farnesol treatment causes an increase in the level of nuclear p65/RelA protein complexes that are able to bind NF-κB binding sites.
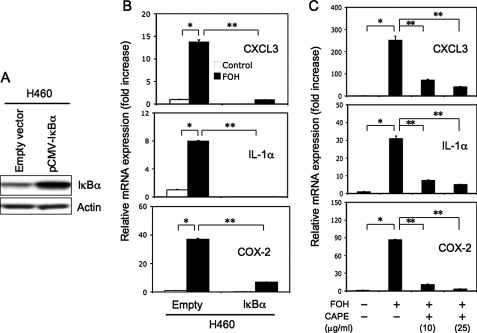
To determine whether the induction of inflammatory genes by farnesol was related to an activation of the NF-κB signaling pathway, we examined the effect of increased expression of IκBα on the expression of these genes. For this purpose, we established a cell line H460(IκBα) that stably overexpressed IκBα protein. Fig. 3A shows that the level of IκBα was elevated in H460(IκBα) cells compared with H460(Empty) cells containing the empty vector. Comparison of the induction of CXCL3, IL-1α, and COX-2 in H460(IκBα) cells indicated that the induction of these genes by farnesol was significantly less than in control H460(empty) cells (Fig. 3B). These observations suggest that activation of the NF-κB signaling pathway is involved in the induction of CXCL3, IL-1α, and COX-2 by farnesol. This was supported by findings examining the effect of CAPE, a potent inhibitor of NF-κB activation (39, 40), on the induction of these inflammatory genes. As shown in Fig. 3C, the expression of CXCL3, IL-1α, and COX-2 mRNA was significantly inhibited by CAPE, in agreement with the conclusion that their induction by farnesol requires activation of the NF-κB pathway.
FIGURE 3.
Inhibition of the NF-κB signaling pathway reduces the induction of CXCL3, IL-1α, and COX-2 mRNA expression by farnesol. A, analysis of IκBα in H460(IκBα) cells overexpressing IκBα. IκBα levels were examined in H460(Empty) and H460(IκBα) cells by Western blot analysis using an anti-IκBα antibody. Actin is shown as a control for equal loading. B, H460(IκBα) and H460(Empty) cells were treated with 250 μm farnesol (FOH) for 6 h. RNA was then isolated, and the expression of CXCL3, IL-1α, and COX-2 was examined by QRT-PCR. C, H460 cells were pretreated with the NF-κB inhibitor, CAPE, at 10 or 25 μg/ml 2 h before 250 μm farnesol was added. After an additional 6 h of incubation, RNA was harvested and analyzed by QRT-PCR for the expression of CXCL3, IL-1α, and COX-2 mRNA. Each value is the mean ± S.D. of three separate PCRs. *, p < 0.0001; **, p < 0.001.
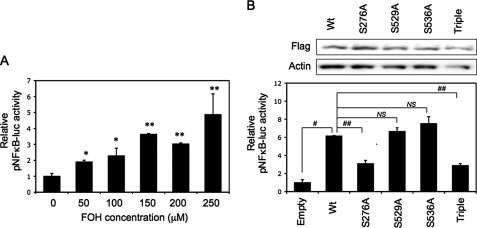
Farnesol-triggered Transcriptional Activation of NF-κB Requires p65(Ser276) Phosphorylation—Above, we showed that farnesol induces translocation of p65 to the nucleus and binding of p65 protein complexes to the NF-κB binding site; we therefore determined whether farnesol enhances NF-κB-dependent transactivation. For this purpose, H460 cells were transfected with a NF-κB-Luc reporter plasmid, and the effect of farnesol on reporter activity was determined. As shown in Fig. 4A, farnesol increased NF-κB-dependent reporter activity about 5-fold in a dose-dependent manner. NF-κB-dependent transcriptional activity has been reported to depend on the phosphorylation status of p65, which can be phosphorylated at multiple sites, including Ser276, Ser529, and Ser536 (36, 37, 41). To determine which phosphorylation site of p65/RelA is important in the induction of NF-κB-dependent transcriptional activation by farnesol, we transfected H460 cells with expression vectors containing wild type p65 or several p65 mutants and compared their transcriptional activity in farnesol-treated H460 cells. Western blot analysis showed that p65 and its mutants were equally expressed (Fig. 4B, top). As shown in Fig. 4B (bottom), transfection of wild type p65 increased reporter activity about 6-fold in farnesol-treated cells compared with cells transfected with empty vector. The mutations (S529A and S536A) had little effect on the transcriptional activity of p65 in farnesol-treated cells; however, activation of the reporter activity was significantly diminished with p65(S276A) and the triple mutant. These results suggest that phosphorylation of p65 at Ser276 is important in the induction of NF-κB transactivation by farnesol.
FIGURE 4.
The Ser276 phosphorylation site in p65-RelA is important in NF-κB-mediated transcriptional activation by farnesol. A, activation of NF-κB-dependent transcriptional activation by farnesol (FOH). H460 cells were transfected with pNF-κB-Luc and subsequently treated with farnesol for 24 h at the concentrations indicated before cells were collected and assayed for luciferase reporter activities. *, p < 0.1; **, p < 0.01 compared with vehicle-treated control (0 μm). B, H460 cells were co-transfected with pNFκB-Luc and expression vectors containing wild type or various point mutants of FLAG-p65-RelA (Empty, empty vector; Wt, FLAG-p65; S276A, FLAG-p65/S276A mutant; S529A, FLAG-p65/S529A; S536A, FLAG-p65/S536A; Triple, FLAG-p65/S276/529/536A triple mutant). After 48 h, cells were treated with 250 μm farnesol, and 24 h later, they were harvested. Cells were assayed for luciferase reporter activities (bottom) or Western blot analysis with anti-FLAG M2 to determine the expression levels of FLAG-p65 proteins (top). Each value is the mean ± S.D. of three separate experiments. #, p < 0.0001; ##, p < 0.001; NS, no significant change.
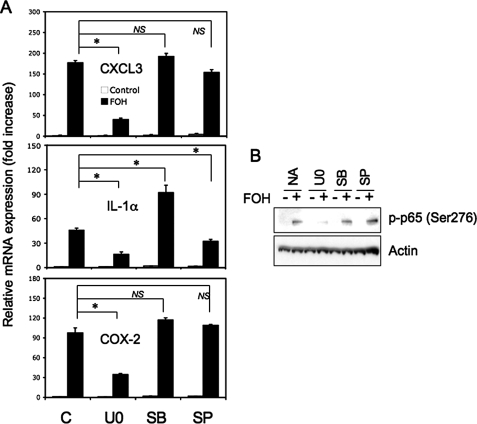
MEK and MSK1-mediated Phosphorylation of p65(Ser276) Plays an Important Role in Farnesol-induced Immune Response and Inflammatory Gene Expression—Our previous studies showed that treatment of H460 cells with farnesol results in the activation of several MAPKs, including MEK1/2, ERK1/2, p38, and JNK (26). Other studies have reported that phosphorylation of p65(Ser276) can be mediated by MEK1/2-ERK1/2 and p38 through activation of MSK1 (37, 42). We therefore examined whether the activation of NF-κB transcriptional activity and the induction of immune response and inflammatory genes by farnesol was dependent on its activation of MAPKs. To obtain insight into the role of MAPKs in the induction of these responses by farnesol, we first examined the effect of several MAPK inhibitors. Fig. 5A shows that the MEK1/2 inhibitor U0126 significantly reduced the induction of CXCL3, IL-1α, and COX-2 mRNA expression by farnesol, whereas the p38 inhibitor SB203580 had little effect on this induction. The JNK inhibitor SP600125 reduced the expression of IL-1α. Next, we examined the effect of these inhibitors on the phosphorylation of p65(Ser276). U0126 inhibited the phosphorylation of p65 at Ser276, whereas the p38 and JNK inhibitors had little effect (Fig. 5B). These data suggest that activation of the MEK1/2-ERK1/2 pathway is involved in the induction of these immune response and inflammatory genes and p65(Ser276) phosphorylation by farnesol.
FIGURE 5.
Optimal induction of CXCL3, IL-1α, and COX-2 mRNA expression by farnesol in H460 cells involves activation of MEK1/2. A, H460 cells were treated for 30 min with vehicle (C), MEK inhibitor U0126 (U0; 5 μm), p38 inhibitor SB203580 (SB; 10 μm), or JNK inhibitor SP600125 (SP; 10 μm) before farnesol (FOH; 250 μm) was added. Cells were collected 6 h later, and the levels of CXCL3, IL-1α, and COX-2 mRNA were analyzed by QRT-PCR. Each value is the mean ± S.D. of three separate PCRs. B, effect of MAPK inhibitors on p65(Ser276) phosphorylation. H460 cells were treated as described for A, except that they were treated for 2 h with farnesol. Protein lysates were then examined by Western blot analysis with an anti-phospho-p65(Ser276) antibody. Actin is shown as a control for equal loading. *, p < 0.01; NS, no significant change.
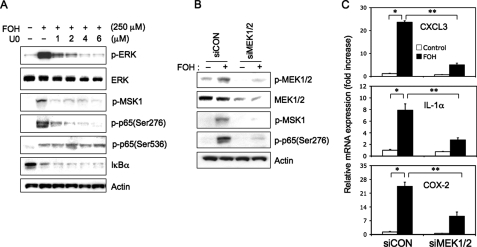
To further investigate the possible link between the activation of MAPKs, MSK-1, and p65, we examined the effect of farnesol on MSK1 phosphorylation and the effect of U0126 on the phosphorylation of MSK1 and p65(Ser276). Fig. 6A shows that farnesol treatment induced phosphorylation of MSK1 and that U0126 inhibited the farnesol-induced phosphorylation of ERK1/2, MSK1, and p65(Ser276) in a dose-dependent manner. However, U0126 did not inhibit the farnesol-induced phosphorylation of p65(Ser536) and the degradation of IκBα. These observations suggest that the phosphorylation of p65(Ser276) by farnesol involves activation of the MEK1/2-ERK1/2-MSK1 cascade. To further support this hypothesis, we examined the effect of MEK1/2 knockdown by siRNAs on the phosphorylation of MSK1 and p65(Ser276). As shown in Fig. 6B, MEK1/2 siRNA knockdown significantly inhibited the phosphorylation of MSK1 and p65(Ser276) by farnesol (Fig. 6B). In addition, MEK1/2 siRNA knockdown greatly reduced the induction of CXCL3, IL-1α, and COX-2 expression by farnesol, in agreement with the role of MEK1/2-ERK1/2 in the regulation of these genes by farnesol (Fig. 6C).
FIGURE 6.
Farnesol-induced phosphorylation of p65(Ser276) is mediated by the MEK1/2 pathway. A, H460 cells were treated for 30 min with the MEK1/2 inhibitor U0126 (U0) at the concentration indicated before farnesol (FOH; 250 μm) was added. Two h later, total protein lysates were isolated and examined by Western blot analysis using antibodies against phospho-p65(Ser276), phospho-p65(Ser536), phospho-MSK1, IκBα, phospho-ERK, and total ERK (ERK). Actin is shown as a control for equal loading. B, H460 cells were transfected with MEK1/2 (siMEK1/2) or scrambled siRNAs (siCON) and 48 h later treated for 2 h with 250 μm farnesol. Total protein lysates were then prepared and examined by Western blot analysis using antibodies against total MEK1/2 (MEK), phospho-MEK1/2, phospho-MSK1, and phospho-p65(Ser276). Actin is shown as a control for equal loading. C, MEK1/2 or control siRNA-transfected cells were treated with 250 μm farnesol for 6 h before RNA was isolated. Expression of CXCL3, IL-1 α, andCOX-2 mRNA were examined by QRT-PCR. Each value is the mean ± S.D. of three separate PCRs. *, p < 0.0001; **, p < 0.001.
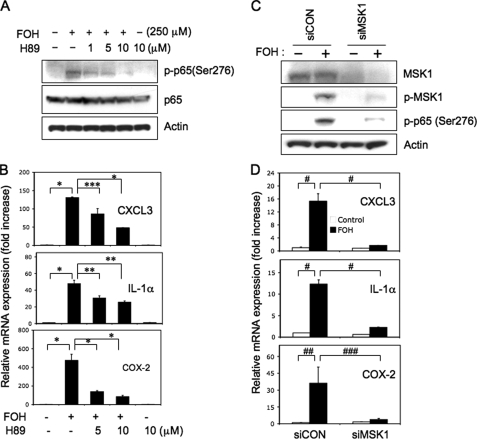
To further strengthen the role of MSK1 in the phosphorylation of p65(Ser276) and induction of immune response and inflammatory genes by farnesol, we examined the effect of the MSK1 inhibitor H89. Fig. 7A shows that H89 inhibits the farnesol-induced phosphorylation of p65(Ser276) and inhibits the induction of CXCL3, IL-1α, and COX-2 mRNA expression by farnesol (Fig. 7B). To confirm that activation of MSK1 is involved in the phosphorylation of p65(Ser276) and the up-regulation of these genes by farnesol, we examined the effect of MSK1 depletion by siRNA. As shown in Fig. 7C, knockdown of MSK1 expression reduced the level of phospho-MSK1 as well as the level of phosphorylated p65(Ser276) in farnesol-treated H460 cells. Moreover, MSK1 siRNA greatly reduced the induction of CXCL3, IL-1α, and COX-2 mRNA expression by farnesol (Fig. 7D). These data further support the conclusion that the phosphorylation of p65(Ser276) by farnesol is mediated by activation of the MEK1/2-ERK1/2-MSK1 cascade and that this cascade is an important part of the mechanism by which farnesol induces the expression of a number of immune response and inflammatory-related genes.
FIGURE 7.
The induction of phosphorylation of p65(Ser276), immune response, and inflammatory genes by farnesol involves activation of MSK1. A, H89 inhibits farnesol-induced phosphorylation of p65(Ser276). H460 cells were treated for 2 h with MSK1 inhibitor H89 before farnesol (FOH) was added. Two h later, protein lysates were examined by Western blot analysis with p-p65(Ser276) and total p65 antibodies. Actin is shown as a control for equal loading. B, H89 inhibits the induction of immune response and inflammatory genes by farnesol. H460 cells were treated H89 as described for A, except that they were treated for 6 h with farnesol before total RNA was isolated. mRNA expression of CXCL3, IL-1α, and COX-2 was then analyzed by QRT-PCR. Each value is the mean ± S.D. of three separate PCRs. *, p < 0.0001; **, p < 0.001; ***, p < 0.01. C, knockdown of MSK1 inhibits farnesol-induced phosphorylation of p65(Ser276). H460 cells were transfected with MSK1 (siMSK1) or scrambled siRNAs (siCON) and 48 h later treated for 2 h with 250 μm farnesol. Total cell lysates were examined by Western blot analysis using antibodies against MSK1, phospho-MSK1, and phospho-p65(Ser276). Actin is shown as a control for equal loading. D, knockdown of MSK1 inhibits the induction of immune response and inflammatory genes by farnesol. H460 cells were transfected with siMSK1 or siCON as described and treated for 6 h with farnesol before total RNA was isolated. CXCL3, IL-1α, and COX-2 mRNA expression was then analyzed by QRT-PCR. Each value is the mean ± S.D. of three separate PCRs. #, p < 0.0001; ##, p < 0.001; ###, p < 0.01.
DISCUSSION
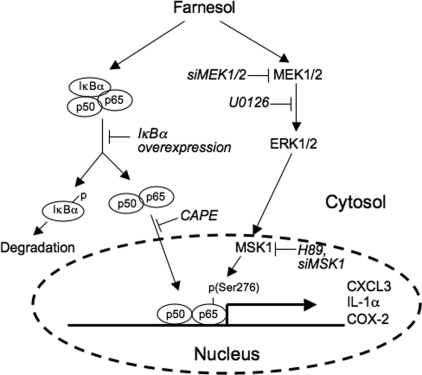
Previously, we reported that farnesol treatment induces an ER stress response in a number of human lung carcinoma cells and that this induction was greatly dependent on the activation of the MEK1/2-ERK1/2 pathway (26). Although activation of the MEK-ERK signaling pathway is often considered as a prosurvival signal, our observations are in agreement with several other reports demonstrating a significant role for activation of MEK1/2-ERK1/2 pathway in the induction of apoptosis (43–45). In this study, we demonstrate that farnesol treatment induces the expression of a number of inflammatory and immune response genes, including IL-1α, IL-6, IL-8, CXCL3, and COX-2, many of which have been reported to be targets of the transcription factor NF-κB (46–48). NF-κB consists of homo- or heterodimeric subunits of the Rel family, including p65 (or RelA), p50, p52, c-Rel, and Rel-B (36). In unstimulated cells, most of the NF-κB is inactive and retained in the cytoplasm in complex with IκB inhibitory proteins. NF-κB activation involves alleviation of the inhibition by IκB, which allows NF-κB to translocate into the nucleus, where it binds NF-κB binding sites in the regulatory region of specific target genes, resulting in their transcriptional activation. In this study, we demonstrated that farnesol induces activation of the NF-κB pathway, as indicated by its translocation to the nucleus. The latter was supported by EMSA that showed a significant increase in the binding of p65 protein complexes to NF-κB binding sites by nuclear extracts from farnesol-treated cells. Activation of the NF-κB pathway was further demonstrated by the induction of NF-κB binding site-dependent transcriptional activation in farnesol-treated cells. These observations suggested that the induction of these immune and inflammatory response genes by farnesol was at least in part related to activation of the NF-κB pathway (Fig. 8). This was supported by observations showing that the NF-κB inhibitor CAPE greatly reduced the induction of the expression of several immune and inflammatory genes by farnesol.
FIGURE 8.
A schematic model of the role of the NF-κB and MEK1/2 pathways in the induction of inflammatory genes by farnesol. Farnesol treatment reduces the level of IκBα and induces translocation of NF-κB transcription factors to the nucleus. Activation of the MEK1/2-ERK1/2 pathway by farnesol results in the activation of MSK1 and subsequently the phosphorylation of p65 at Ser276 and increased p65 activity. Together this leads to increased CXCL3, IL-1α, and COX-2 expression by NF-κB. Inhibition of MEK1/2 by U0126 or by siRNAs results in reduced MSK1 and p65 phosphorylation, whereas inhibition of MSK1 by H89 or siRNAs represses the phosphorylation of p65 and the induction of these inflammatory genes. (→, induction; ⊢, inhibition).
The canonical pathway of NF-κB activation, through the activation of various receptors, including those for TNFα or IL-1β, involves phosphorylation and ubiquitination of IκBα and its subsequent degradation by the proteosome machinery (36). This alleviation of the inhibition of NF-κB activation by IκBα allows translocation of NF-κB into the nucleus and transcriptional activation of target genes. The activation of NF-κB by several stress-inducing agents has been reported to involve a reduction in the level of IκB protein; however, it does not involve phosphorylation and degradation of IκBα but appears to be related to a reduction in the translation of IκB mRNA (49–51). This reduced translation was shown to depend on the phosphorylation of the translation initiation factor 2α (eIF2α) subunit. Our data demonstrate that farnesol treatment also reduces the level of IκBα protein and that overexpression of IκBα inhibits the induction of the expression of several immune response and inflammatory genes by farnesol. Previously, we reported that farnesol also induces eIF2α phosphorylation (26). However, eIF2α phosphorylation appears not to be essential for the reduction in IκBα levels by farnesol, since the MEK1/2 inhibitor U0126 inhibited the phosphorylation of eIF2α but had no effect on the observed decrease in IκBα protein.
Not only degradation of IκB and nuclear translocation of NF-κB but also post-translational modifications of NF-κB, including site-specific phosphorylation, are important for optimal transactivation activity of NF-κB. We demonstrate that farnesol treatment induced phosphorylation of p65/RelA at Ser276 and Ser536 (Fig. 2A) and that the S276A mutation but not the S536A mutation greatly diminished the induction of NF-κB transactivation by farnesol. We show that inhibition of MEK1/2 by U0126 or knockdown of MEK1/2 expression significantly reduced the phosphorylation of p65 at Ser276 but not that of Ser536, whereas the p38 inhibitor SB203580 and the JNK1/2 inhibitor SP600125 did not block farnesol-induced phosphorylation of p65(Ser276) or the induction of immune response and inflammatory genes. These observations suggest that p65(Ser276) phosphorylation is dependent on the activation of the MEK1/2-ERK1/2 pathway. We further show that inhibition of MSK1, a kinase acting downstream of MEK1/2-ERK1/2, by H89 or knockdown of MSK1 expression also inhibited phosphorylation of p65/RelA(Ser276), suggesting that this phosphorylation is dependent on MSK1. The latter is in agreement with previous reports showing MSK1-mediated phosphorylation of p65(Ser276) in TNFα-stimulated mouse fibroblast L929sA and in C2 murine myoblast by oxidative stress (36, 37, 42).
The physiological significance of the activation of the NF-κB pathway may relate to its established role in the regulation of immune and inflammatory responses and the promotion of cell survival (30, 36, 48, 51). Although activation of NF-κB can promote cell death, during development and in response to many signals, including ER stress, it appears to be part of a prosurvival response. Thus, the activation of the NF-κB pathway during farnesol-induced ER stress may be part of a prosurvival response. The reduced (24% lower) cell viability in IκBα-overexpressing H460 cells treated with 200 μm farnesol compared with that of farnesol-treated control cells (data not shown) is in agreement with this hypothesis. This is further supported by the observed increase in expression of several cytokines, including CXCL1 and IL-8, as well as of COX-2, which have been reported to be associated with growth-stimulatory activities. These observations are in agreement with the conclusion that activation of the NF-κB pathway by farnesol is part of a pro-cell survival response. In cells treated with pharmacological doses of farnesol, prosurvival and proapoptosis signals compete among each other (19, 26). Therefore, differences in the susceptibility of cells to the growth-inhibitory and apoptosis-inducing effects of farnesol might be due to differences in the balance between pro-cell survival and proapoptosis responses.
Acknowledgments
We thank Drs. Dori Germolec and Radiah Corn Minor for comments on the manuscript.
This work was supported, in whole or in part, by the National Institutes of Health, NIEHS, Intramural Research Program. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The data discussed in this publication have been deposited in theNCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series Accession number GSE7215.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; NF-κB, nuclear factor-κB; IκB, inhibitory κB; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; CAPE, caffeic acid phenethyl ester; siRNA, small interfering RNA; EMSA, electrophoretic mobility shift assay; QRT, quantitative reverse transcription; eIF2α, eukaryotic initiation factor 2α.
References
- 1.Goldstein, J. L., and Brown, M. S. (1990) Nature 343 425–430 [DOI] [PubMed] [Google Scholar]
- 2.Edwards, P. A., and Ericsson, J. (1999) Annu. Rev. Biochem. 68 157–185 [DOI] [PubMed] [Google Scholar]
- 3.Endres, M., and Laufs, U. (2004) Stroke 35 2708–2711 [DOI] [PubMed] [Google Scholar]
- 4.Hanley, K., Komuves, L. G., Ng, D. C., Schoonjans, K., He, S. S., Lau, P., Bikle, D. D., Williams, M. L., Elias, P. M., Auwerx, J., and Feingold, K. R. (2000) J. Biol. Chem. 275 11484–11491 [DOI] [PubMed] [Google Scholar]
- 5.Rioja, A., Pizzey, A. R., Marson, C. M., and Thomas, N. S. (2000) FEBS Lett. 467 291–295 [DOI] [PubMed] [Google Scholar]
- 6.Bifulco, M. (2005) Life Sci. 77 1740–1749 [DOI] [PubMed] [Google Scholar]
- 7.Miquel, K., Pradines, A., and Favre, G. (1996) Biochem. Biophys. Res. Commun. 225 869–876 [DOI] [PubMed] [Google Scholar]
- 8.Wright, M. M., Henneberry, A. L., Lagace, T. A., Ridgway, N. D., and McMaster, C. R. (2001) J. Biol. Chem. 276 25254–25261 [DOI] [PubMed] [Google Scholar]
- 9.Mo, H., and Elson, C. E. (2004) Exp. Biol. Med. (Maywood) 229 567–585 [DOI] [PubMed] [Google Scholar]
- 10.Ong, T. P., Heidor, R., de Conti, A., Dagli, M. L., and Moreno, F. S. (2006) Carcinogenesis 27 1194–1203 [DOI] [PubMed] [Google Scholar]
- 11.Bailey, H. H., Attia, S., Love, R. R., Fass, T., Chappell, R., Tutsch, K., Harris, L., Jumonville, A., Hansen, R., Shapiro, G. R., and Stewart, J. A. (2008) Cancer Chemother. Pharmacol. 62 149–157 [DOI] [PubMed] [Google Scholar]
- 12.Burke, Y. D., Stark, M. J., Roach, S. L., Sen, S. E., and Crowell, P. L. (1997) Lipids 32 151–156 [DOI] [PubMed] [Google Scholar]
- 13.Burke, Y. D., Ayoubi, A. S., Werner, S. R., McFarland, B. C., Heilman, D. K., Ruggeri, B. A., and Crowell, P. L. (2002) Anticancer Res. 22 3127–3134 [PubMed] [Google Scholar]
- 14.Rao, C. V., Newmark, H. L., and Reddy, B. S. (2002) Cancer Detect. Prev. 26 419–425 [DOI] [PubMed] [Google Scholar]
- 15.He, L., Mo, H., Hadisusilo, S., Qureshi, A. A., and Elson, C. E. (1997) J. Nutr. 127 668–674 [DOI] [PubMed] [Google Scholar]
- 16.Crowell, P. L. (1999) J. Nutr. 129 775S–778S [DOI] [PubMed] [Google Scholar]
- 17.Azzoli, C. G., Miller, V. A., Ng, K. K., Krug, L. M., Spriggs, D. R., Tong, W. P., Riedel, E. R., and Kris, M. G. (2003) Cancer Chemother. Pharmacol. 51 493–498 [DOI] [PubMed] [Google Scholar]
- 18.Hudes, G. R., Szarka, C. E., Adams, A., Ranganathan, S., McCauley, R. A., Weiner, L. M., Langer, C. J., Litwin, S., Yeslow, G., Halberr, T., Qian, M., and Gallo, J. M. (2000) Clin. Cancer Res. 6 3071–3080 [PubMed] [Google Scholar]
- 19.Adany, I., Yazlovitskaya, E. M., Haug, J. S., Voziyan, P. A., and Melnykovych, G. (1994) Cancer Lett. 79 175–179 [DOI] [PubMed] [Google Scholar]
- 20.Gibbs, B. S., Zahn, T. J., Mu, Y., Sebolt-Leopold, J. S., and Gibbs, R. A. (1999) J. Med. Chem. 42 3800–3808 [DOI] [PubMed] [Google Scholar]
- 21.Wiseman, D. A., Werner, S. R., and Crowell, P. L. (2007) J. Pharmacol. Exp. Ther. 320 1163–1170 [DOI] [PubMed] [Google Scholar]
- 22.Forman, B. M., Goode, E., Chen, J., Oro, A. E., Bradley, D. J., Perlmann, T., Noonan, D. J., Burka, L. T., McMorris, T., Lamph, W. W., Evans, R. M., and Weinberger, C. (1995) Cell 81 687–693 [DOI] [PubMed] [Google Scholar]
- 23.Taylor, M. M., Macdonald, K., Morris, A. J., and McMaster, C. R. (2005) FEBS J. 272 5056–5063 [DOI] [PubMed] [Google Scholar]
- 24.Lagace, T. A., Miller, J. R., and Ridgway, N. D. (2002) Mol. Cell. Biol. 22 4851–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairn, G. D., Macdonald, K., and McMaster, C. R. (2007) J. Biol. Chem. 282 4868–4874 [DOI] [PubMed] [Google Scholar]
- 26.Joo, J. H., Liao, G., Collins, J. B., Grissom, S. F., and Jetten, A. M. (2007) Cancer Res. 67 7929–7936 [DOI] [PubMed] [Google Scholar]
- 27.Wu, J., and Kaufman, R. J. (2006) Cell Death Differ. 13 374–384 [DOI] [PubMed] [Google Scholar]
- 28.Szegezdi, E., Logue, S. E., Gorman, A. M., and Samali, A. (2006) EMBO Rep. 7 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder, M., and Kaufman, R. J. (2005) Mutat. Res. 569 29–63 [DOI] [PubMed] [Google Scholar]
- 30.Kim, R., Emi, M., Tanabe, K., and Murakami, S. (2006) Apoptosis 11 5–13 [DOI] [PubMed] [Google Scholar]
- 31.Schreiber, E., Matthias, P., Muller, M. M., and Schaffner, W. (1989) Nucleic Acids Res. 17 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gambichler, T., Skrygan, M., Hyun, J., Bechara, F., Tomi, N. S., Altmeyer, P., and Kreuter, A. (2006) Arch. Dermatol. Res. 298 139–141 [DOI] [PubMed] [Google Scholar]
- 33.Huang, S. J., Schatz, F., Masch, R., Rahman, M., Buchwalder, L., Niven-Fairchild, T., Tang, C., Abrahams, V. M., Krikun, G., and Lockwood, C. J. (2006) J. Reprod. Immunol. 72 60–73 [DOI] [PubMed] [Google Scholar]
- 34.Alique, M., Moreno, V., Kitamura, M., Xu, Q., and Lucio-Cazana, F. J. (2006) Br. J. Pharmacol. 149 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim, Y. M., Cao, D., Reed, W., Wu, W., Jaspers, I., Tal, T., Bromberg, P. A., and Samet, J. M. (2007) Cell. Signal. 19 538–546 [DOI] [PubMed] [Google Scholar]
- 36.Chen, L. F., and Greene, W. C. (2004) Nat. Rev. Mol. Cell. Biol. 5 392–401 [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen, L., De Wilde, G., Van Damme, P., Vanden Berghe, W., and Haegeman, G. (2003) EMBO J. 22 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai, H., Chiba, H., Miyoshi, H., Sugita, T., and Toriumi, W. (1999) J. Biol. Chem. 274 30353–30356 [DOI] [PubMed] [Google Scholar]
- 39.Nicklaus, M. C., Neamati, N., Hong, H., Mazumder, A., Sunder, S., Chen, J., Milne, G. W., and Pommier, Y. (1997) J. Med. Chem. 40 920–929 [DOI] [PubMed] [Google Scholar]
- 40.Natarajan, K., Singh, S., Burke, T. R., Jr., Grunberger, D., and Aggarwal, B. B. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 9090–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen, Y., Feldman, D. E., Deng, C., Brown, J. A., De Giacomo, A. F., Gaw, A. F., Shi, G., Le, Q. T., Brown, J. M., and Koong, A. C. (2005) Mol. Cancer Res. 3 669–677 [DOI] [PubMed] [Google Scholar]
- 42.Kefaloyianni, E., Gaitanaki, C., and Beis, I. (2006) Cell. Signal. 18 2238–2251 [DOI] [PubMed] [Google Scholar]
- 43.Arai, K., Lee, S. R., van Leyen, K., Kurose, H., and Lo, E. H. (2004) J. Neurochem. 89 232–239 [DOI] [PubMed] [Google Scholar]
- 44.Zhuang, S., Yan, Y., Daubert, R. A., Han, J., and Schnellmann, R. G. (2007) Am. J. Physiol. 292 F440–F447 [DOI] [PubMed] [Google Scholar]
- 45.Zhuang, S., and Schnellmann, R. G. (2006) J. Pharmacol. Exp. Ther. 319 991–997 [DOI] [PubMed] [Google Scholar]
- 46.Kucharczak, J., Simmons, M. J., Fan, Y., and Gelinas, C. (2003) Oncogene 22 8961–8982 [DOI] [PubMed] [Google Scholar]
- 47.Liou, H. C., and Hsia, C. Y. (2003) BioEssays 25 767–780 [DOI] [PubMed] [Google Scholar]
- 48.Karin, M., and Greten, F. R. (2005) Nat. Rev. Immunol. 5 749–759 [DOI] [PubMed] [Google Scholar]
- 49.Deng, J., Lu, P. D., Zhang, Y., Scheuner, D., Kaufman, R. J., Sonenberg, N., Harding, H. P., and Ron, D. (2004) Mol. Cell. Biol. 24 10161–10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wek, R. C., Jiang, H. Y., and Anthony, T. G. (2006) Biochem. Soc. Trans. 34 7–11 [DOI] [PubMed] [Google Scholar]
- 51.Marciniak, S. J., and Ron, D. (2006) Physiol. Rev. 86 1133–1149 [DOI] [PubMed] [Google Scholar]