Abstract
We report here syntenic loci in humans and mice incorporating gene clusters coding for secreted proteins each comprising 10 cysteine residues. These conform to three-fingered protein/Ly-6/urokinase-type plasminogen activator receptor (uPAR) domains that shape three-fingered proteins (TFPs). The founding gene is PATE, expressed primarily in prostate and less in testis. We have identified additional human PATE-like genes (PATE-M, PATE-DJ, and PATE-B) that co-localize with the PATE locus, code for novel secreted PATE-like proteins, and show selective expression in prostate and/or testis. Anti-PATE-B-specific antibodies demonstrated the presence of PATE-B in the region of the sperm acrosome and at high levels on malignant prostatic epithelial cells. The syntenic mouse Pate-like locus encompasses 14 active genes coding for secreted proteins, which are all, except for Pate-P and Pate-Q, expressed primarily in prostate and/or testis. Pate-P and Pate-Q are expressed solely in placental tissue. Castration up-regulates prostate expression of mouse Pate-B and Pate-E, whereas testosterone ablates this induced expression. The sequence similarity between TFP/Ly-6/uPAR proteins that modulate activity of nicotinic acetylcholine receptors and the PATE (Pate)-like proteins stimulated us to see whether these proteins possess analogous activity. Pharmacological studies showed significant modulation of the nicotinic acetylcholines by the PATE-B, Pate-C, and Pate-P proteins. In concert with these findings, certain PATE (Pate)-like genes were extensively expressed in neuron-rich tissues. Taken together, our findings indicate that in addition to participation of the PATE (Pate)-like genes in functions related to fertility and reproduction, some of them likely act as important modulators of neural transmission.
A functional genomic approach has identified a gene, designated PATE, that codes for a secreted protein with preferential prostate and testis expression (1). PATE protein includes 10 cysteine residues, with the C-terminal cysteine residue positioned within a cysteine-asparagine (CN) dipeptide sequence. The distribution of cysteine residues conforms to a consensus cysteine pattern found in a large family of three-fingered proteins (TFPs),3 characterized by a distinct disulfide bonding pattern between 8 and 10 cysteine residues (2, 3). This domain is additionally found in uPAR and murine Ly-6 GPI-anchored proteins, and is also called an Ly-6/uPAR domain (3). Interestingly, the TFP architecture is seen also in the transforming growth factor-β receptor family of proteins, including BMP2 and activin receptors (4).
A large protein family encompassing an extensive group of GPI-anchored, transmembrane, and secreted proteins contains this domain. The prototype-secreted protein members of this family include short chain snake and frog toxins, which in many cases bind with high affinity to neuronal receptors and block their activity (5-7).
Until recently, the only recognized secreted mammalian TFP/Ly-6/uPAR proteins were SLURP-1 (secreted mammalian Ly-6/uPAR-related protein) (8) and SLURP-2 (9), which are located on human chromosome 8q24.3 within a cluster of Ly-6-like human genes that otherwise code for GPI-linked proteins. Consistent with the putative ligand function of some secreted TFP/Ly-6/uPAR proteins, SLURP-1 was recently identified as a neuromodulator of the α7 nicotinic receptor (α7 nAChR), suggesting that it may regulate calcium homeostasis (10). It is clear that members of the secreted TFP/Ly-6/uPAR protein family, to which the PATE protein belongs, interact with partner proteins that, in many cases, are membrane-tethered receptors.
The human PATE gene is telomerically juxtaposed to the gene encoding acrosomal vesicle protein 1 (ACRV1), also known as the SP10 gene (11). Interestingly, the ACRV1 protein also contains 10 cysteine residues that conform to the TFP/Ly-6/uPAR domain, suggesting that the two genes ACRV1 and PATE may be part of a single chromosomal locus comprising TFP/Ly-6/uPAR genes.
In this study, we report the identification and expression patterns of three additional human PATE-like genes (PATE-M, PATE-DJ, and PATE-B) that co-localize with the ACRV1 and PATE genomic locus. These novel PATE-like genes code for secreted proteins containing the typical TFP/Ly-6/uPAR domain. Significantly, all show selective expression in prostate and/or testis. We have identified the orthologous murine Pate, Pate-M, Pate-DJ, and Pate-B genes that localize centromerically to the mouse Acrv1/sp10 gene. Remarkably, the mouse Pate-like genomic locus includes an additional nine transcriptionally active Pate-like genes, which all encode secreted TFP/Ly-6/uPAR-domain-containing proteins, whereas in the human genome these mouse Pate-like genes are either inactive (two genes) or completely absent (the remaining seven genes). Selective expression of the mouse Pate-like genes in prostate, testis, placenta as well as specific effects of castration and subsequent testosterone administration on their expression in prostate all indicate that these genes function in both male- and female-related reproductive activities and are likely hormonally regulated. Furthermore, because of the sequence similarity between TFP/Ly-6/uPAR proteins that modulate activity of nicotinic acetylcholine receptors (nAChRs) and the PATE (Pate)-like proteins, we conducted experiments to see whether these proteins also possess comparable activity. These analyses showed that certain PATE (Pate)-like proteins modulate the activity of nAChRs. Taken together, our findings indicate that in addition to participation of the PATE (Pate)-like genes in functions related to fertility and reproduction, they are likely to function as important modulators of neural activity.
EXPERIMENTAL PROCEDURES
Materials and Antibodies—Unless otherwise specified, chemicals and reagents were obtained from Sigma. The anti-FLAG antibodies were affinity-purified rabbit polyclonal antibodies. Fresh ACh stock solutions were made daily in Ringer's solution and diluted.
RT-PCR Analyses of the Human and Mouse PATE (Pate)-like Genes—Forward and reverse oligonucleotide primers were synthesized using the DNA sequences obtained from the PATE (Pate)-like sequences (see under “Bioinformatic Strategies for Identification of PATE (Pate)-like Genes”). RT-PCR analysis of the human PATE-like genes (and flanking genes) as well as the mouse Pate-like genes was performed with cDNAs obtained from different human or mouse tissues (Clontech) as indicated. Forward and reverse primers were chosen such that they always spanned an intron, and the observed RT-PCR product at all times corresponded to the size expected of a spliced mRNA.
Sequencing of PATE (Pate)-like cDNAs—All human PATE-like cDNAs were either directly sequenced from gel-purified RT-PCR DNAs or alternatively the gel-purified cDNAs were cloned into TOPO4 (Invitrogen) plasmids and then sequenced. For the mouse Pate-like cDNAs, RT-PCR generated DNAs were gel-purified and directly sequenced.
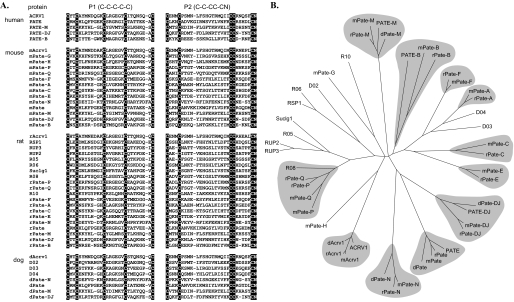
Bioinformatic Strategies for Identification of PATE (Pate)-like Genes—The sequence homology among the TFP family of proteins is generally low except for the common pattern of cysteines in the sequence, and an initial attempt to identify PATE-like genes by using conventional protein homolog search programs such as BLAST or BLAT was not successful. However, a careful inspection of protein sequences and exon structures of initially identified PATE and PATE-like genes enabled us to devise a method to detect PATE-like genes in the genomic sequences. We developed two protein sequence patterns, P1 and P2, to represent C1-C5 and C6-C10N patterns, respectively, found in PATE-like proteins. The two patterns are “CX(2)CX(5,10)CX(3,8)CX(4,9)C” for P1 and “CX(2,4)CX(11,20)CCX(2,7)C” for P2, where X(n,m) denotes a stretch of any amino acids ranging in length from n to m. The patterns were reverse-translated and transformed to Perl regular expressions. We searched the genomic locus bounded by PKNOX2 and CDON genes in the human genome (May 2004 freeze) and orthologous loci in the mouse genome (May 2004 freeze), in the rat genome (June 2003 freeze), and in the dog genome (July 2004 freeze). Two exons bearing P1 and P2, respectively, were separately searched. Then, closely (less than 10 kb) located P1 and P2 pairs were joined to form single genes. In human and mouse, the precise exon boundaries were predicted by manually inspecting all possible intron-exon boundaries for those that will maintain the open reading frame and the experimentally determined protein sequence. The corresponding signal peptide-bearing exon was located in the 5′-flanking region of each gene. The putative gene structure was verified by expressed sequence tag search, by comparing each gene with a respective ortholog in human or mouse, or experimentally by RT-PCR, cloning, and sequencing. Possible orthologous relationships among PATE-like genes from human, mouse, rat, and dog were inferred by a phylogenetic analysis using MEGA3 program (12) based on a multiple sequence alignment of P1 and P2 sequences obtained by using T-Coffee program (13).
Generation of Eucaryotic Expression Constructs and Fusion Proteins—Cloning was conducted with the eucaryotic expression vector pCMV3 (Sigma) via selected restriction sites. This vector codes for the preprotrypsin signal peptide followed by sequences coding for the FLAG epitope. DNA coding for the human Fc fragment (hFc) was inserted 3′ to the FLAG epitope. A cleavage site for the highly specific tomato etch virus (TEV) protease was also introduced between the C terminus of the Pate-like proteins and the hFc segment. cDNA fragments encoding the Pate-like proteins were subcloned in-frame into the pCMV3 (5′FLAG—hFc3′) vector to render pCMV3 as 5′FLAG-Pate-like-TEV-hFc3′.
Generation of HEK293 Transfectants Expressing FLAG-Pate-TEV-hFc Proteins—HEK293 (human kidney) cells were transiently transfected with the eucaryotic pCMV3 expression vectors (6 μg of DNA/25-cm2 flask) coding for the FLAG-(Pate-like)-TEV-hFc fusion proteins. The secreted C-terminally hFc tagged FLAG-Pate-like-TEV-hFc proteins contained in the conditioned media (CM) were collected on protein A-Sepharose 4 Fast Flow resin (Amersham Biosciences), and the N-terminal Pate-like proteins were released by incubating the protein A beads with TEV protease.
Preparation of RNA—Human nAChR clones were obtained from Dr. Jon Lindstrom. After linearization and purification of cloned cDNAs, RNA transcripts were prepared in vitro using the appropriate mMessage mMachine kit from Ambion Inc. (Austin, TX).
Expression in Xenopus Oocytes—Mature (>9 cm) female Xenopus laevis African toads (Nasco, Ft. Atkinson, WI) were used as a source of oocytes. Prior to surgery, frogs were anesthetized by placing the animal in a 1.5 g/liter solution of MS222 (3-aminobenzoic acid ethyl ester; Sigma) for 30 min. Oocytes were removed from an incision made in the abdomen.
To remove the follicular cell layer, harvested oocytes were treated with 1.25 mg/ml collagenase (Worthington) for 2 h at room temperature in calcium-free Barth's solution (88 mm NaCl, 1 mm KCl, 2.38 mm NaHCO3, 0.82 mm MgSO4, 15 mm HEPES (pH 7.6), 0.1 mg/ml gentamicin sulfate). Subsequently, stage 5 oocytes were isolated and injected with 50 nl (5-20 ng) each of the appropriate subunit cRNAs. Recordings were made 3-5 days after injection.
Electrophysiology—Experiments were conducted using OpusXpress 6000A (Axon Instruments, Union City, CA) as reported previously (14). OpusXpress is an integrated system that provides automated impalement and voltage clamp of up to eight oocytes in parallel. Cells were automatically perfused with bath solution, and agonist solutions were delivered from a 96-well plate. Both the voltage and current electrodes were filled with 3 m KCl. The agonist solutions were applied via disposable tips, which eliminated any possibility of cross-contamination. Cells were voltage-clamped at a holding potential of -60 mV. Data were collected at 50 Hz and filtered at 20 Hz. ACh applications were 8 s with 241-s wash periods.
Each oocyte received two initial control applications of ACh, and then the PATE-like peptides were pre-applied for 241 s at the indicated concentrations through an alternative supply of bath solution. Subsequently the PATEs were co-applied with ACh at the control concentration. The control ACh concentrations for a7 and a4b2 receptors were 60 and 30 μm, respectively. Responses to the ACh PATE co-application were calculated relative to the preceding ACh control responses based on net charge (15). Net charge was integrated for the entire response, i.e. until the currents return to base line, such that the total time window for the net charge measurement was 120 s, beginning 2 s prior to the ACh delivery. Responses of at least four oocytes were measured for each experimental concentration. Statistical analyses of PATE effects were based on pairwise t test between the responses of each oocyte to ACh alone or ACh plus PATE peptide, following preincubation with the PATEs. All of the statistics were based on pairwise t tests, where responses of cells recorded under control conditions were compared on a cell by cell basis with those obtained after PATE treatment. The calculations of p values were made with Stateview version 4.01, (ABACUS Concepts, Berkeley, CA).
SDS-PAGE—SDS-polyacrylamide gel for protein separation was performed as described previously (16), and blots were reacted with polyclonal rabbit anti-FLAG primary antibody. For glycosidase treatment, protein A-purified proteins were incubated with 1 unit of PNGase F (New England Biolabs).
RESULTS
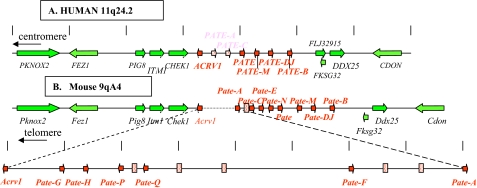
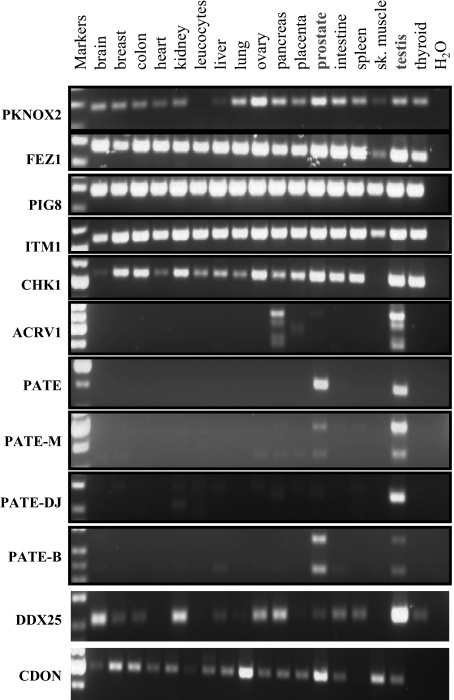
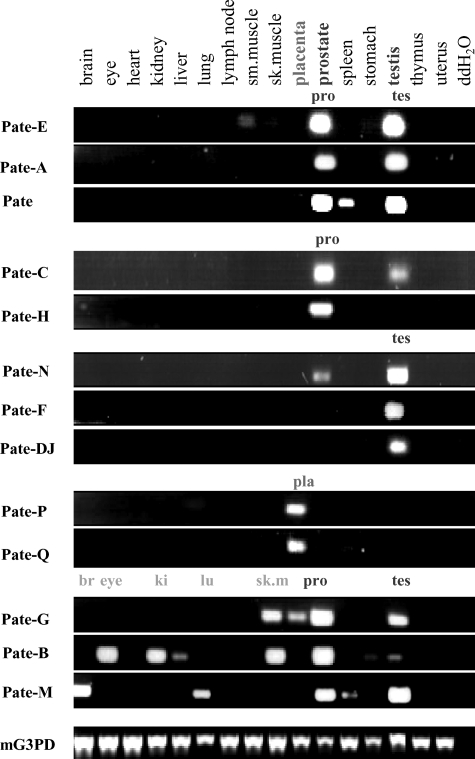
Identification of a Human PATE-like Gene Cluster—The PATE gene codes for a small, cysteine-rich protein selectively expressed in human male reproductive tissues, including prostate, testis, epididymis, and seminal vesicle (1, 17). Patternsearch techniques (see “Experimental Procedures”) revealed three additional PATE-like genes, designated PATE-B, PATE-M, and PATE-DJ, which localized to the same 11q24 genomic locus as the PATE gene (Fig. 1). Expression analyses of these genes by RT-PCR in 17 different human tissues demonstrated selective expression in prostatic and/or testicular tissue with negligible expression in all other tissues (Fig. 2). The PATE-B gene was expressed primarily in prostate with lesser expression in testis, whereas the PATE-M and PATE-DJ genes showed a reverse pattern of expression (Fig. 2). RT-PCR analyses of all known genes within a stretch of 700 kbp comprising the human PATE-like genes demonstrated that the non-PATE-like genes did not show preferential expression in reproductive tissues (Fig. 2). Consistent with previously published data, ACRV1 (acrosomal vesicle protein 1 gene also known as SP10) demonstrated multiple splice isoforms, expressed primarily in testicular tissue (11).
FIGURE 1.
Arrangement of genes within the locus comprising the human PATE-like genes and the corresponding syntenic murine genomic locus. A, known human genes lie within a genomic segment on chromosome 11q24 initiating at the centromeric side with PKNOX2 at nucleotide 124,726,419 (numbering as in the Genome Browser May 2004 release), and terminating at the telomeric side with CDON at nucleotide 125,438,397. Arrows indicate direction of transcription. The genes coding for proteins containing the distinctive 10-cysteine motif are shown in red, and inactive pseudogenes are stippled. Accession numbers are as follows: ACRV1, NM_001612; PATE, NM_138294; PATE-M, NM_212555; PATE-B, AK123042. Accession number for PATE-DJ is EU703625. B, syntenic murine (chromosome 9qA4) genomic locus. Note the 0.8-Mbp insertion in the mouse genome between Acrv1 and Pate-A. Accession numbers are as follows: Acrv1, NM_007391; Pate-H, BY721155; Pate-Q, BQ032923; Pate-F, BY721010; Pate-A, AK020329; Pate-C, NM_026593; Pate-E:, AV379335; Pate, AK033745; Pate-M, BY721028; Pate-B (svs7), NM_020264. Accession numbers for Pate-G, Pate-P, Pate-N, and Pate-DJ are EU703627, EU703629, EU703628, and EU703626, respectively.
FIGURE 2.
RT-PCR expression analysis of human PATE-like genes and flanking genes. RT-PCR analysis of the human PATE-like genes and flanking genes was performed with cDNAs obtained from the indicated human tissues. Forward and reverse primers were chosen such that they always spanned an intron, and the observed RT-PCR product at all times corresponded to the size expected of a spliced mRNA. For the ACRV1, PATE, PATE-M, PATE-DJ, and PATE-B genes, the forward and reverse primers were located in the first and third exons (coding for the signal peptide and cysteines 6-10, respectively). PCR was performed for 35 cycles. Note that PIG8 (p53 induced gene 8) is ubiquitously expressed in all tissues, serving as a convenient internal control for cDNA integrity.
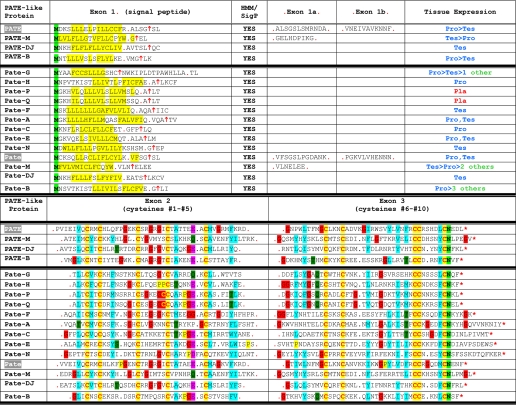
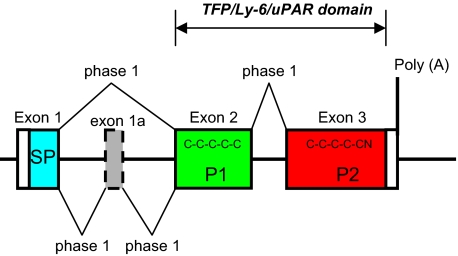
Sequencing full-length cDNAs showed that the human PATE-like genes code for similar proteins that all include a putative N-terminal signal peptide (see below) and 10 conserved cysteine residues (Table 1). Comparison of genomic and cDNA sequences showed that in all PATE-like proteins the N-terminal signal peptide is encoded by the first exon (exon 1), whereas protein domains containing cysteines 1-5 and cysteines 6-10 are encoded by two separate 3′ exons (exons 2 and 3, respectively, shown in Fig. 3). For PATE and PATE-M genes, 1-2 additional exons, designated 1a and 1b, are present between exons 1 and 2 and code for a small number of amino acids. The upstream ACRV1 gene shows a similar exon structure to the PATE-like genes.
TABLE 1.
Sequences of the human PATE-like and mouse Pate-like proteins and signal peptide prediction
The amino acid sequences of the human PATE-like (and mouse Pate-like) proteins are presented starting with the initiating methionine. Upper panel, sequences were subjected to SignalP analysis-signal peptide probabilities, and the predicted signal peptide cleavage sites are presented. The upward facing arrow designates the predicted cleavage site of the signal peptide, and the red dots indicate the exon boundaries. Hydrophobic amino acid residues downstream to the initiating methionine, and likely comprising the signal peptide, are highlighted. Lower panel, exons 2 and 3 include cysteines 1-5 and cysteines 6-10, respectively.
FIGURE 3.
Exon structure of PATE (Pate)-like genes. The canonical exon structure of the PATE (Pate)-like genes is presented. Optional extra exons are represented as dotted boxes. The coding region is in color: blue, signal peptide (SP); green, pattern 1 (P1) containing C1 to C5; red, pattern 2 (P2) containing C6 to C10N.
All splice events in the PATE-like genes use a +1 phase (Fig. 3); thus alternative splicing involving exon skipping will not alter downstream reading frames. Interestingly, PATE-B and PATE-M generate two splice isoforms (Fig. 2). Cloning and sequencing of the smaller transcripts confirmed that they derive from exon 2 skipping. Both isoforms include the putative N-terminal signal peptide, but although the larger transcript codes for proteins comprising all 10 cysteine residues, the smaller transcript codes only for cysteines 6-10.
Prediction of Signal Peptides at the N Terminus of All Human PATE-like Genes—As expected of signal peptide sequences, analysis of all human PATE-like genes reveals clustering of hydrophobic amino acid residues just distal to the initiating methionine (Table 1). Analysis by the SignalP (signal peptide prediction) algorithm predicts with very high probability a signal peptide and accompanying cleavage site for each PATE-like protein (Table 1). That this N-terminal region is in fact a signal peptide directing protein for secretion has been functionally demonstrated for PATE (17) and ACRV1 (SP10) (18), and it is very likely that these regions serve the same function in the PATE-B, PATE-DJ, and PATE-M proteins. By transfecting human HEK293 cells with cDNA coding for the native PATE-B protein and analyzing spent culture medium for the presence of PATE-B protein, we have experimentally confirmed (data not shown) that the native PATE-B protein is indeed secreted from the cell.
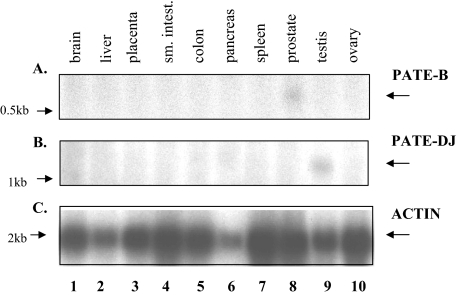
Northern Blot Analysis of PATE-B and PATE-M Expression—To extend and confirm the RT-PCR analyses, Northern blot analyses of PATE-B and PATE-DJ were performed (Fig. 4). This demonstrated exclusive expression, albeit at low levels, of PATE-B and PATE-DJ in prostate and testis, respectively, confirming the RT-PCR analyses; no other tissues expressed these genes.
FIGURE 4.
Northern blot analyses of PATE-B and PATE/DJ expression. PATE-B, PATE/DJ, and actin cDNAs were radioactively (32P) labeled and each used to sequentially probe, under stringent wash conditions, a Northern blot (Clontech) of total RNA derived from the indicated human tissues. The blot was stripped between sequential probing. Expression of PATE-B and PATE/DJ mRNAs is clearly visible in prostatic and testicular tissues (lanes 8 and 9, respectively); no signal was seen in any other tissues examined.
Additional Vestigial Inactive PATE-like Genes in the Human PATE-like Gene Cluster—Searches for additional human PATE-like genes within the human gene cluster revealed two potential PATE-like genes, designated PATE-A and PATE-C (Fig. 1). Extensive RT-PCR analyses using a number of forward and reverse primers based on these genomic sequences failed, in all tissues examined, to reveal expression of these genes.
Identification of 14 Active Mouse Pate-like Genes within the Mouse Genome Syntenic to the Human PATE-like Genomic Locus—The human PATE-like gene locus extends from the ACRV1 gene to the PATE-B gene, encompassing about 180 kbp. The human genes adjacent to the PATE-like gene locus are (centromeric → telomeric, Fig. 1A) as follows: PKNOX2, FEZ1, PIG8, ITM1, CHEK1, ACRV1, (PATE-A), (PATE-C), PATE, PATE-M, PATE-DJ, PATE-B, DDX2,5 and CDON, and this region stretches for about 700 kbp. The syntenic mouse genomic segment was identified and demonstrated the following arrangement (→ centromeric, Fig. 1B): Pknox2, Fez1, Pig8, Itm1, Chk1, Acrv1 (sp10), Svs7 (seminal vesicle secretion protein 7 (19) also called caltrin (20), identified here as the mouse Pate-B ortholog), Ddx25 and Cdon (Fig. 1). Further analyses designed to identify mouse orthologs revealed the mouse Pate-A, Pate-C, Pate, Pate-M, Pate-DJ, and Pate-B (svs7) genes (Fig. 1B). An additional two potential mouse Pate-like genes, designated Pate-E and Pate-N, were identified between Pate-C and Pate. Unexpectedly, inspection of the mouse genomic Pate-like gene locus revealed 0.8 Mbp of genomic DNA situated between the mouse Acrv1 and Pate-A genes. Analysis of this segment revealed several additional Pate-like genes (Fig. 1 and Table 1). The expected human equivalent to the 0.8-Mbp mouse fragment was completely absent.
All mouse Pate-like genes code for putative Pate-like proteins comprising a hydrophobic N-terminal signal peptide (Table 1). RT-PCR analyses (Fig. 5) utilizing oligonucleotide primers located in exons 2 and 3 revealed that the mouse Pate-A and Pate-C mouse genes (corresponding to the inactive human PATE-C and PATE-A genes) were clearly expressed, in addition to expression of the mouse gene orthologs Pate-B, Pate-DJ, Pate-M, and Pate. Even more striking was the discovery of an additional seven transcriptionally active mouse Pate-like genes, designated Pate-E, Pate-H, Pate-N, Pate-F, Pate-P, Pate-Q, and Pate-G (Figs. 1 and 5 and Table 1).
FIGURE 5.
RT-PCR expression analyses of mouse Pate-like genes. RT-PCR analyses of the mouse Pate-like genes were performed with cDNAs (Clontech) obtained from different mouse tissues as indicated. Forward and reverse primers were chosen such that they always spanned an intron, and all RT-PCR products corresponded to the sizes expected of spliced mRNA. Results presented here used forward and reverse primers located in the second and third exons (coding for cysteines 1-5 and cysteines 6-10, respectively); similar results were obtained when the analysis was repeated with forward and reverse primers located in the first and third exons (data not shown). PCR was performed for 35 cycles, and the PCR products were analyzed as described under “Experimental Procedures.” The ubiquitously expressed mouse glyceraldehyde-3-phosphate dehydrogenase (mG3PDH) served as a control for cDNA integrity.
We do not know why the syntenic mouse locus harbors so many more active Pate-like genes as compared with the human genome, but this does suggest a significantly more complex role for the Pate-like proteins in rodents.
Constituent Pate-like genes could be segregated according to their tissue expression profiles (Fig. 5). Genes Pate-E, Pate-A, and Pate were predominantly expressed in both prostate and testis, whereas Pate-C and Pate-H expression was limited to prostatic tissue. Pate-N, Pate-F, and Pate-DJ showed almost exclusive expression in testis. Pate-G, Pate-B, and Pate-M all showed major expression in either prostate or testis but, in addition, showed significant expression levels in skeletal muscle (Pate-G), eye, kidney, skeletal muscle (Pate-B), and brain and lung (Pate-M). Several mouse Pate-like pseudogenes were scattered among the active mouse Pate-like genes (Fig. 1).
Expression in Placental Tissue of Two Mouse Pate-like Genes—Two murine Pate-like genes, Pate-P and Pate-Q, were expressed exclusively in the female-restricted organ, mouse placenta (Fig. 5). Notably, these genes (a) are genomically adjacent to each other (Fig. 1), (b) code for highly similar proteins (Table 1), and (c) both include an 11th cysteine residue in addition to the consensus Pate-like 10 cysteine residues. Supporting placental expression reported here is a trophoblast cDNA library EST (BQ032923) representing a partial Pate-Q sequence.
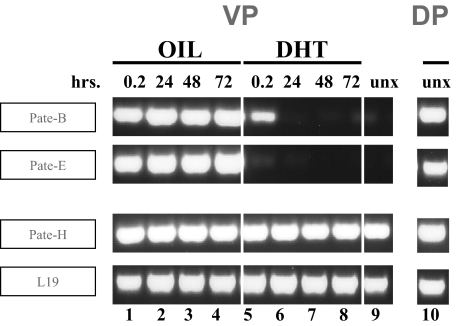
Castration Induces Pate-like Gene Expression in the Ventral Prostate That Is Ablated by Subsequent Dihydroxytestosterone Administration—The selective tissue distribution of Pate-like gene expression in the testis, prostate, and placenta indicated that the Pate-like proteins are likely involved in reproductive-related behavior.
To see whether in vivo hormonal changes may affect Pate- like gene expression, we investigated effects of castration and subsequent dihydroxytestosterone (DHT) administration on prostate expression of the Pate-like genes. As differential gene expression has been documented in the anatomically discrete dorsal and ventral prostate lobes, we investigated Pate-like gene expression in each separate lobe. The dorsal lobe in uncastrated mice clearly expressed the two Pate-like genes, Pate-B and Pate-E (Fig. 6, DP). Dorsal lobe expression remained high, irrespective of castration and subsequent DHT administration (data not shown). In contrast, the Pate-B and Pate-E genes were not expressed in the uncastrated ventral lobe (Fig. 6, lane 9). Following castration, however, both Pate-B and Pate-E were clearly expressed in the ventral lobe (Fig. 6, lanes 1-4). DHT administration subsequent to castration ablated this expression (Fig. 6, lanes 5-8). The DHT-mediated suppression of castration-induced ventral lobe expression occurred swiftly (Fig. 6, lane 5); 0.2 h of DHT administration completely extinguished Pate-E expression and led to the partial suppression of Pate-B expression that was complete following 24 h of DHT treatment. In contrast to the differential dorsal and ventral lobe expression of Pate-B and Pate-E, the Pate-H gene demonstrated high expression in both ventral and dorsal lobes (Fig. 6, lanes 9 and 10, respectively). Neither castration nor subsequent DHT treatment altered this expression (Fig. 6, lanes 1-8). In fact, the universal expression of the Pate-H gene served as a convenient internal control for integrity of prostate cDNAs as did the housekeeping L19 gene (Fig. 6, lanes 1-10).
FIGURE 6.
Effect of castration and subsequent DHT administration on Pate-like gene expression in the ventral and dorsal lobes of the mouse prostate. Mice were castrated and 14 days later injected (subcutaneously) at time 0, 24, and 48 h either with oil or with DHT dissolved in the oil. Mice were sacrificed 12 min and 24, 48, and 72 h, respectively, and the ventral and dorsal prostate lobes were isolated, followed by RNA isolation and cDNA preparation. RT-PCR analyses were performed using forward and reverse primers located in the first and third exons. PCR was performed for 35 cycles, and the PCR products were analyzed as described under “Experimental Procedures.” The ubiquitously expressed mouse L19 gene served as a control for cDNA integrity. unx, uncastrated.
The PATE-B Protein Localizes to the Acrosomal Region of Human Sperm and Is Expressed in Discrete Normal and Malignant Epithelial Prostate Cells—The discovery of clusters of human and mouse PATE (Pate)-like genes expressed in reproductive tissues suggested involvement in functions related to reproductive activities. To further understand their function, we generated antibodies directed against the human PATE-B protein. With these anti-PATE-B reagents we investigated the following: (a) whether the PATE-B protein localizes to sperm cells and (b) whether the PATE-B protein product can be detected in normal and/or malignant prostate tissue.
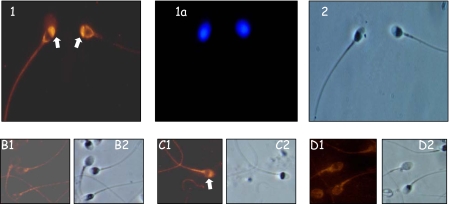
Incubation with anti-PATE-B antibodies demonstrated intense staining of sperm cells primarily in the acrosomal region (Fig. 7, panel 1). Specificity of staining was confirmed by use of preimmune serum (Fig. 7, panel B1, no staining) addition to the anti-PATE-B antibodies of either a nonspecific protein or competing PATE-B protein (staining retained and absence of staining, Fig. 7, panels C and D, respectively).
FIGURE 7.
Localization of the PATE-B protein to the acrosomal region of human sperm. Panels 1, 1a, and 2, human sperm was incubated with anti-PATE-B antisera followed by fluorescently labeled anti-rabbit antibodies. Specific staining was observed in the region of the sperm acrosome (panel 1). Staining with 4′,6-diamidino-2-phenylindole demonstrated the sperm nucleus (panel 1a), and Nomarski optics showed the whole sperm (panel 2). Panels B-D, human sperm similarly investigated with preimmune serum (panel B1) or with immune anti-PATE-B antisera plus a nonrelevant protein (panel C1) or competing soluble PATE-B protein (panel D1) attested to the specificity of staining, indicated by the white arrow. Nomarski optics demonstrated the sperm cells (panels B2, C2, and D2).
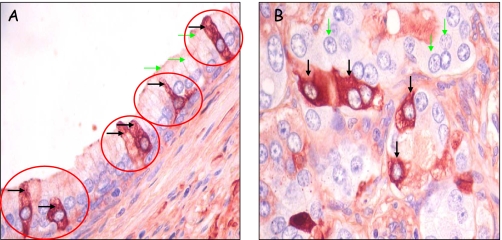
To investigate PATE-B protein expression in the prostate, we performed immunohistochemical analyses of prostatic tissue sections (Fig. 8). Analyzed samples include both normal ducts as well as clusters of malignant epithelial cells that had spread into prostate stroma. Substantial anti-PATE-B immunoreactivity localized in the normal ducts to the cytoplasm of apical epithelial cells (Fig. 8A). Surprisingly, only a discrete subpopulation of apical epithelial cells demonstrated the PATE-B protein (Fig. 8A). Furthermore, PATE-B staining was granular, and the select anti-PATE-B positive cells included distinctive dendritic-like projections.
FIGURE 8.
Human PATE-B protein expression in discrete prostatic apical epithelial cells and in malignant epithelial cells. A, tissue sections of paraffin-embedded prostate tissue containing both normal and malignant prostate cells were reacted with anti-PATE-B rabbit polyclonal antisera, and antibody binding was detected with polymer-horseradish peroxidase-conjugated anti-rabbit antibodies followed by 3-amino-9-ethylcarbazole chromagen. Staining is seen in isolatedcells (blackarrows) suggesting that a specific subpopulation of the ductal epithelial cells express PATE-B; other cells in this duct (green arrows indicating selected cells) are barely stained indicating very low PATE-B expression. B, malignant prostate cells from a different region but contained in the section as in A. Discrete malignant cells are very intensely stained (black arrows) indicating high PATE-B expression, whereas other malignant cells (green arrows indicating selected cells) show very little staining.
Malignant epithelial cells forming cell clusters in the prostatic stroma (Fig. 8B) showed a more intense PATE-B immunoreactivity as compared with that seen in the normal, apical epithelial cells (compare Fig. 8, A and B). As in the case of normal prostatic epithelial cells, only a discrete subpopulation of malignant cells demonstrated PATE-B expression (Fig. 8B). Specificity of staining was confirmed by use of preimmune serum and addition of competing specific PATE-B protein, in both of these controls no staining was observed, as expected (data not shown).
Presence in Rat and Dog Genomes of Pate-like Genes—Investigation of the rat syntenic locus revealed a 2.5-Mbp insertion, corresponding to that of the mouse 0.8-Mbp insert, located between the rat Acrv1 (sp10) and Pate-A genes. This analysis showed that the mouse Pate-like genes Acrv1, Pate-P, Pate-Q, Pate-F, Pate-A, Pate-C, Pate-E, Pate-N, Pate, Pate-M, Pate-DJ, and Pate-B all have rat orthologs (Fig. 9). We could not assign rat orthologs to the mouse Pate-G and Pate-H genes. The rat locus includes several Pate-like genes that code for proteins that appear rat-specific, including RSP1 (rat spleen protein 1), RUP2 (rat urinary protein 2), RUP3 (rat urinary protein 3) (21), Suc1g1, and an additional five rat Pate-like genes that could not be assigned a mouse ortholog. Analysis of the dog Pate-like gene locus revealed dog (d)Acrv1, dPate, dPate-M, and dPate-DJ. Four additional dog Pate-like genes located between dAcrv1 and dPate (Fig. 9) could not be clearly assigned to any human, mouse, or rat orthologs.
FIGURE 9.
Multiple sequence alignment of C10N motif and a phylogenetic tree of PATE (Pate)-like proteins from human, mouse, rat, and dog. A, the amino acid sequences of the human, mouse, rat, and dog PATE (Pate)-like proteins are presented. The first (P1) and second (P2) blocks show sequences extending from C1 to C5 and C6 to C10N, respectively. The C10N motifs are highlighted. Where possible Pate-like nomenclature has been used for the interspecies orthologs. Otherwise arbitrary designations, such as R05, were used for illustration. Expression of all human and mouse PATE (Pate)-like genes presented have been demonstrated experimentally by RT-PCR analyses. B, same multiple sequence alignment was used to build a phylogenetic tree. The tree is a Neighbor-joining consensus tree based on 1000 replicates. Apparent orthologous groups are marked with a gray background.
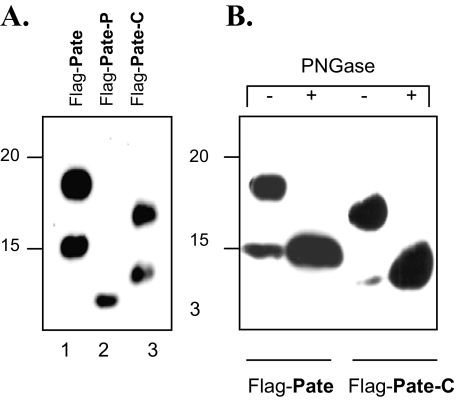
Analysis by SDS-PAGE of Recombinant Pate-like Proteins—To see whether we could synthetically produce recombinant Pate-like proteins in mammalian cells and to investigate whether these proteins undergo post-translational modifications, we expressed mouse Pate, Pate-C, and Pate-P proteins in a mammalian cell expression system. Immunoblot analyses revealed (Fig. 10) that whereas Pate-P protein migrated with its expected mobility (Fig. 10A, lane 2), the Pate and Pate-C proteins each displayed two bands (Fig. 10A, lanes 1 and 3, respectively). Treatment with PNGase F, resulted in increased mobility of the Pate and Pate-C proteins (Fig. 10B, compare 1st and 2nd, and 3rd and 4th lanes, respectively), demonstrating that both these proteins are modified with N-linked sugars. The Pate protein showed a single consensus N-glycosylation site, NCT, whereas Pate-C harbors an NSC sequence, an alternative N-linked glycosylation site (22).
FIGURE 10.
Expression of Pate, Pate-P, and Pate-C proteins, N-glycosylation of Pate and Pate-C in HEK293 (human kidney) cells. A, HEK293 cells were transfected with constructs encoding the proteins that comprised the Pate, Pate-P, or Pate-C sequences, tagged at their N termini with a FLAG epitope, and the secreted Pate-like proteins purified as described under “Experimental Procedures.” The purified proteins were analyzed by 11% SDS-PAGE, Western-blotted, and probed with anti-FLAG antibodies (A and B). B, Western blot analysis of FLAG-tagged Pate-like proteins treated with PNGase F. Proteins were treated as described in A, with the difference that the proteins were treated with PNGase F to remove N-linked sugars (lanes indicated by +).
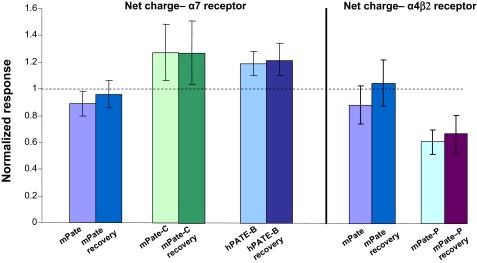
Human PATE (Pate)-like Proteins Modulate Activity of nAChRs—Having established expression of the PATE (Pate)-like genes and their hormonal regulation in reproductive tissues, having identified the homologous clusters of human, mouse, rat, and dog genes, and following the successful synthesis of the PATE (Pate)-like proteins in mammalian cells, we wished to understand what functions they may perform. The striking similarity in molecular makeup between snake toxins that bind to and modulate the activity of nAChRs, the recently discovered mammalian TFP/Ly-6/uPAR-like proteins SLURP1, SLURP2 (23), and Lynx1 all experimentally shown to modulate nAChR activity and the PATE (Pate)-like proteins prompted us to investigate whether these latter proteins possess similar activities. To test for this, we expressed various nAChRs using the Xenopus oocyte cell expression system, and we assessed evoked changes in channel activation that occur in response to application of the recombinant PATE (Pate)-like proteins. In this study we evaluated measurement of net charge accumulation over the entire period of drug administration. This analysis produces results essentially identical to those obtained by more complicated concentration-correction methods. The net charge is a particularly attractive parameter (15) as it represents the time integration of all activated channels responding to drug administration. This analysis (Fig. 11) showed that application of mouse Pate (mPate) to both α4β2 and α7 nAChRs or human PATE-B (hPATE-B) to the α4β2 nAChR did not evoke any changes in net charge, based on pairwise t tests of control ACh-evoked responses recorded prior to PATE application to those responses evoked by the application of ACh in the continued presence of the PATE. In contrast, an increase in the net charge was measured after application of hPATE-B to the α7 nAChR, a change that was statistically significant (p < 0.05, based on pairwise t test analysis of responses obtained from the same cells in the absence and presence of hPATE-B) and persisted even after a 4-min wash (Fig. 11). Additionally, mPate-C increased the net charge when applied to human α7 nAChRs, a statistically significant change (p < 0.05 based on pairwise t tests) that also persisted after a 4-min wash (Fig. 11). Interestingly, the mPate-P protein did not elicit any changes when applied to the human α7 nAChRs but did evoke a statistically significant decrease in net charge when added to the α4β2 nAChR (p < 0.05 based on pairwise t tests), a change also persisting after a 4-min wash (Fig. 11). In summary, of the four recombinant PATE (Pate)-like proteins we synthesized and assayed, three (hPATE-B, mPate-C, and mPate-P) showed modulatory activity of the nAChRs.
FIGURE 11.
Modulation of the activity of nAChRs by PATE (Pate)-like proteins. The indicated nAChRs were expressed in Xenopus oocytes as described under “Experimental Procedures.” After obtaining initial control responses to 8-s applications of ACh, cells were washed for 4 min with either control Ringer or Ringer's plus the PATE-like proteins (60 or 200 nm) and then challenged with ACh or ACh plus the PATE-like proteins, respectively. Responses to ACh plus the PATE-like proteins were normalized to the net charge responses to ACh alone for each oocyte. ACh concentrations were 30 μm for α4β2-expressing cells and 60 μm for α7-expressing cells. To test for recovery, oocytes were then washed for an additional 4 min in the absence of acetylcholine and the PATE-like proteins, and then acetylcholine (30 or 60 μm) was applied for 8 s and the net charge calculated to give the recovery values.
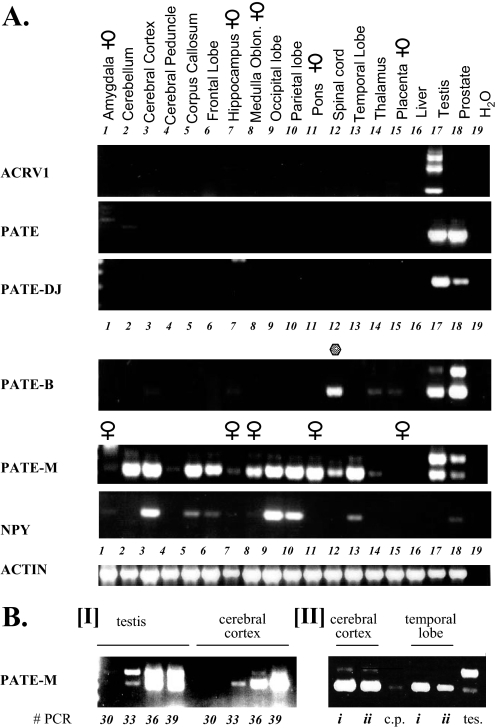
Expression of PATE (Pate)-like Proteins in Neuron-rich Tissues—As shown above certain PATE (Pate)-like proteins clearly modulate the activity of nicotinic AChRs. This matches well with the similarity between the PATE-like protein family and the nAChR neuromodulators, the secreted, mammalian TFP/Ly-6/uPAR SLURP1 and SLURP2 proteins. Functioning as neuromodulators, one would expect that neuron-rich tissues should also express PATE (Pate)-like genes. Consistent with this, analyses of mouse Pate-like gene expression indeed show that mouse Pate-B, in addition to its extensive expression in prostate tissue, is strongly expressed in the eye and skeletal muscle both heavily innervated tissues (Fig. 5). Additionally, besides its expression in prostate and testis, mPate-M is extensively expressed in brain (Fig. 5). Viewed in this context, it was surprising to note the lack of human PATE-like gene expression in neuron-rich tissues, such as brain. This could be due to the following: (a) low expression levels in brain requiring increased sensitivity for detection, and/or (b) expression only in discrete regions of the brain such that specific PATE-like cDNAs are diluted out in total brain cDNAs, proscribing their detection. To test these options and to enhance sensitivity, we repeated the RT-PCR analyses with an increased number of PCR cycles (40 instead of 35). This clearly showed low levels of PATE-M expression in the brain (data not shown). A subsequent analysis of cDNA prepared not from total brain but from different individual regions of brain unambiguously resolved this issue (Fig. 12). Whereas PATE and PATE-DJ were not expressed in any of the tested regions, PATE-B was expressed in spinal cord tissue. Particularly striking was the extensive expression of PATE-M. This was seen in the cerebellum, cerebral cortex, corpus callosum, occipital, parietal and temporal lobes, and pons (Fig. 12, lanes 2, 3, 5, 9, 10, 13, and 11, respectively). Lesser expression levels were observed in the frontal lobe and medulla oblongata with low levels in the spinal cord (Fig. 12, lanes 6, 8, and 12). PATE-M was not expressed in the amygdala, cerebral peduncle, hippocampus, and thalamus (Fig. 12, lanes 1, 4, 7, and 12). Remarkably, PATE-M expression in the various central nervous system locations was exclusively restricted to the exon 2-deleted PATE-M isoform. This stands in contrast to the predominant expression of the exons 1, 2, and 3 isoform in the testis (Fig. 12B, panel I, lower panel, compare lanes labeled testis and cerebral cortex). Brain samples derived from female donors also clearly expressed PATE-M (Fig. 12A, lanes 8 and 11), indicating that its expression in neuronal tissues is not gender-specific.
FIGURE 12.
Expression of human PATE-like genes in different regions of the brain. A, upper panel, cDNAs prepared from different individual regions of the brain as indicated, were subjected to RT-PCR analyses. Expression analysis of the human PATE-like genes was performed with forward and reverse primers that always spanned an intron and the observed RT-PCR product at all times corresponded to the size expected of a spliced mRNA. For the ACRV1, PATE, PATE-M, PATE-DJ, and PATE-B genes, the forward and reverse primers were located in the first and third exons (coding for the signal peptide and cysteines 6-10, respectively). In addition, cDNAs derived from testes and prostate on the one hand and liver and placenta on the other hand were also analyzed for expression of the PATE-like genes and served as positive and negative controls, respectively. PCR was performed for 35 cycles. Control for cDNA integrity was provided by the actin primers, as indicated, and expression analysis of the brain neuropeptide NPY served as a positive control for expression of a brain neuropeptide in specific regions of the brain. B, panel I, to assess the relative PATE-M expression levels in testis and cerebral cortex, a semiquantitative RT-PCR analysis was performed wherein samples were taken following 30, 33, 36, and 39 PCR cycles and subjected to agarose gel analysis. The fact that there are two differently sized PATE-M isoforms, each possessing a distinct duplex melting temperature, precluded real time PCR analysis for this gene. Panel II, to assess the generality of PATE-M gene expression in the brain, cDNA samples deriving from cerebral cortex and temporal lobe isolated from different individuals (i and ii) were subjected to PATE-M RT-PCR analysis. cDNAs prepared from testis (tes) and cerebral peduncle (c.p.) served as positive and negative controls, respectively.
A semi-quantitative analysis comparing testis with cerebral cortex showed comparable levels of PATE-M expression in both tissues (Fig. 12B). This is significant because it means that the level of PATE-M expression in the cerebral cortex is at least equivalent to that seen in testis which, of all non-central nervous system tissues examined (Fig. 2) is by far the highest PATE-M expressing tissue.
DISCUSSION
We report here syntenic human and mouse genomic loci that contain clusters of genes coding for secreted proteins each comprising a typical TFP/Ly-6/uPAR domain. Their unique expression patterns both in humans and mice provide compelling evidence for critical roles of the PATE (Pate)-like proteins in activities related not only to fertility and reproduction but also to neuronal activity.
The mouse and human PATE (Pate)-like genes all code for proteins that harbor N-terminal sequences conforming to signal peptides that direct protein for secretion. This is consistent with the functionally demonstrated secretion of ACRV1 (SP10) (18), PATE (17), and Pate-B (svs7, caltrin (19)). No other hydrophobic regions corresponding either to transmembrane domains or to signals for addition of GPI tails are present in the PATE (Pate)-like proteins.
Similar to the PATE-like gene locus, a cluster of human Ly-6 orthologous genes resides on chromosome 8q24, and each of these genes codes for a protein that includes the distinctive 10-cysteine pattern seen in the PATE (Pate)-like proteins. With the exception of the secreted proteins SLURP-1 (8) and SLURP-2 (9), this 8q24 locus codes only for GPI-linked, membrane-tethered proteins. In contrast, all active genes residing in both the human (11q24) and mouse (9qA4) PATE (Pate)-like loci described here, code exclusively for secreted TFP/Ly-6/uPAR proteins. They are thus similar to the secreted SLURP-1 and SLURP-2 proteins and to the extensive group of secreted snake and frog toxins bearing the TFP/Ly-6/uPAR domain.
As such, the PATE (Pate)-like proteins are likely to be signaling molecules that bind to target cells, thereby modulating their activity. Indeed, SLURP-1 and SLURP-2, both secreted TFP/Ly-6/uPAR-like proteins similar in structure to the PATE-(Pate)-like proteins, bind to and modulate the activity of nicotinic acetylcholine receptors (10, 23) present on the membrane of target cells. Just as these TFP/Ly-6/uPAR-like proteins modulate nAChR activity, we now demonstrate that in fact the PATE (Pate)-like proteins possess similar activities and also modulate the activity of nAChRs.
Our investigations demonstrate that all human PATE-like genes are expressed in male reproductive tissues such as prostate and testis. This does not mean that the PATE (Pate)-like genes function solely in male-restricted reproductive activities. Indeed the mouse Pate-Q and Pate-P genes show very selective expression in placenta, an exclusively female-specific tissue. Furthermore, expression of a discrete group of Pate-like genes in the mammary glands of pregnant and/or lactating mice (data not shown) also suggests that the Pate-like proteins are likely to function in reproductive activities that are not exclusively male-limited.
Hormonal governance of expression is sustained by the unambiguous castration-induced expression in the ventral prostate of Pate-B and Pate-E, both of which undergo swift down-regulation following DHT administration. Additionally, expression of certain Pate-like genes was observed only in mammary glands from pregnant or lactating mice and not from virgin mice (data not shown). Taken as a whole these results clearly implicate hormonal participation in the regulation of Pate-like gene expression.
Immunostaining with antibodies demonstrated human PATE-B protein localizing at the sperm acrosomal region. The secreted TFP/Ly-6/uPAR domain-containing proteins Pate-B, PATE, and ACRV1 (Acrv1), all bind to sites on the sperm cell (17, 19, 24), suggesting specific receptors for these proteins present on the sperm membrane. These results also tie in with what is known about the mouse Pate-B homolog (caltrin or svs7), which has been postulated to modulate calcium permeability in sperm cells (20). It is thus not unreasonable to assume that specific receptors for the PATE-B protein reside on human sperm.
Our analyses also showed that the human PATE-B protein localized to both normal and malignant prostatic epithelial cells. Both their morphology and the fact that only discrete, ductal, apical cells stained positive suggest that these PATE-B-positive cells may in fact represent neuroendocrine cells, a specialized subset of epithelial cells. Prostatic neuroendocrine cells (i) are intraglandular and intraductal hybrid epithelial/neural/endocrine cells, (ii) are generally widely scattered throughout the prostate with only an occasional cell per gland/duct, and (iii) express and secrete serotonin and numerous neuropeptides. Both afferent and efferent nerves innervate prostatic neuroendocrine cells. That the PATE-B positive cells are of the neuroendocrine cell type is especially appealing in light of our findings on the neuromodulatory function of the PATE-like proteins (below).
It was also striking that malignant prostatic epithelial cells showed PATE-B expression. As seen with normal epithelium, only certain cancer cells stained PATE-B-positive. This expression was more intense than that seen in the normal cell counterparts. Further investigations will be required to elucidate whether PATE-B expression in these malignant prostate cells actually contributes to their transformed phenotype. Whatever the case may be, localization of PATE-B protein in both sperm and prostatic cells underscores its likely participation in reproductive functions.
All PATE (Pate)-like proteins probably adopt a “three-fingered” fold similar to that of the snake neuro- and cytotoxins (25, 26). There are extensively documented, highly specific interactions between the TFP/Ly-6/uPAR domain-containing snake toxins and the nAChR (5), a ligand-gated five-subunit ion channel. Interestingly, mammalian Lynx1, a GPI-anchored TFP/Ly-6/uPAR domain-containing protein, modulates cellular calcium permeability by interacting with neuronal nicotinic acetylcholine receptors (27). More pertinent to the secreted PATE (Pate)-like proteins, the secreted SLURP-1 protein potentiates the human α7 nicotinic AChRs (10). Thus there is not only structural likeness between snake neurotoxins and the mammalian TFP/Ly-6/uPAR domain-containing proteins SLURP-1 and Lynx1 but also a functional similarity. Experiments designed to assess whether PATE (Pate)-like proteins possess similar functions, demonstrated that certain PATE (Pate)-like proteins do in fact modulate the activity of nicotinic acetylcholine receptors. Three of the four recombinant PATE (Pate)-like proteins we synthesized and assayed modulated the activity of nAChRs. Two (hPATE-B and mPate-C) up-regulated the homomeric α7 nAChR and one (mPate-P) down-regulated the a4β2 heteromeric nAChR. Because of the multitude of the PATE (Pate)-like proteins, to date only four have been investigated for their neuromodulatory activity. We anticipate that additional PATE (Pate)-like proteins also possess neuro-modulatory activity, and experiments are in progress to check for this.
The diversity of nAChRs engendered by the combinatorial assembly of various nicotinic subunits (α1-α10, β1-β4, δ, γ, and ε) indicates different electrical and binding properties for each combinatorial subtype (28). One would thus naturally expect that modulations of their activity would necessitate a diversity of interacting modulators. For the diversity that we have observed for the PATE (Pate)-like proteins, wherein each particular protein interacts with and modulates in a different manner, specific nAChRs may provide this multiplicity of interacting modulators. Support for such a scenario is provided by our findings with human PATE-B, which on the one hand up-regulates activity of a homomeric α7 nAChR but is without effect on an α4β2 combination. In a similar manner, Pate-P down-regulates the α4β2 configuration but does not affect a homomeric α7 nAChR. Gross nAChR inactivation as mediated by snake toxins could not be tolerated by the organism, and one would thus further predict that naturally occurring endogenous proteins modulating nAChR activity will do so only moderately. That the PATE (Pate)-like proteins mediate modest, yet statistically significant, alterations in nAChR activity fits in well with this postulated fine-tuning.
Taken together our data are consistent with a neuromodulatory function for the PATE (Pate)-like proteins. This firmly places them in the family of the neuromodulatory TFP/Ly-6/uPAR domain-containing proteins SLURP-1 and Lynx1.
How are the neuromodulatory functions of the PATE (Pate)-like proteins to be reconciled with their extensive expression in reproductive tissues? It is well known that neurons and sperm share many common “neuron-specific” receptors. For example, the nAChR subunits α7, α9, α3, α5, and β4 have all been demonstrated to be present in human sperm, and the α7 subunit likely exists as a homomer in the posterior post-acrosomal and neck regions of sperm (29, 30). Furthermore, the human acrosome reaction initiated by acetylcholine or recombinant egg zona pellucida protein ZP3 involves the α7 nAChR (31, 32), and it has been proposed that the sperm nAChRs may be key regulators of signaling pathways important to the acrosome reaction and sperm motility (29, 33). This nAChR commonality in both sperm and neurons has even led to the classification of a sperm cell as “a neuron with a tail” (34). Although provocative, this definition does attest to the unequivocal participation of what was previously thought to be neuron-specific receptors and ligands in the functioning of cells pertaining to the reproductive system. This common functionality of nAChRs in both reproductive and neuronal tissues thus reconciles the neuromodulatory activity of the PATE (Pate)-like proteins and rationalizes their expression in both neuronal and reproductive tissues.
In summary, we have identified a new family of secreted TFP/Ly-6/uPAR domain-containing proteins, designated the PATE (Pate)-like proteins, that likely play important roles in reproductive and neuronal physiology. Further clarification of their normal functions will likely contribute to new therapeutics for pathologies of both the central nervous system and the reproductive system.
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-Bank™/EBI Data Bank with accession number(s) EU703625, EU703626, EU703627, EU703628, and EU703629.
This work was supported, in whole or in part, by a National Institutes of Health grant (Intramural Research Program, NCI, Center for Cancer Research). This work was also supported by a Union Internationale Contre Cancer translational research fellowship (to D. H. W.) and Frances Williams Preston Laboratories of the T. J. Martell Foundation (to R. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TFP, three-fingered protein; uPAR, urokinase-type plasminogen activator receptor; ACh, acetylcholine; nAChR, nicotinic acetylcholine receptor; RT, reverse transcription; GPI, glycosylphosphatidylinositol; h, human; m, mouse; d, dog; TEV, tomato etch virus; PNGase F, peptide:N-glycosidase F; DHT, dihydroxytestosterone.
References
- 1.Bera, T. K., Maitra, R., Iavarone, C., Salvatore, G., Kumar, V., Vincent, J. J., Sathyanarayana, B. K., Duray, P., Lee, B. K., and Pastan, I. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 3058-3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fry, B. G., Wuster, W., Kini, R. M., Brusic, V., Khan, A., Venkataraman, D., and Rooney, A. P. (2003) J. Mol. Evol. 57 110-129 [DOI] [PubMed] [Google Scholar]
- 3.Ploug, M., and Ellis, V. (1994) FEBS Lett. 349 163-168 [DOI] [PubMed] [Google Scholar]
- 4.Ploug, M. (2003) Curr. Pharm. Des. 9 1499-1528 [DOI] [PubMed] [Google Scholar]
- 5.Tsetlin, V. I., and Hucho, F. (2004) FEBS Lett. 557 9-13 [DOI] [PubMed] [Google Scholar]
- 6.Tremeau, O., Lemaire, C., Drevet, P., Pinkasfeld, S., Ducancel, F., Boulain, J. C., and Menez, A. (1995) J. Biol. Chem. 270 9362-9369 [DOI] [PubMed] [Google Scholar]
- 7.Kolbe, H. V., Huber, A., Cordier, P., Rasmussen, U. B., Bouchon, B., Jaquinod, M., Vlasak, R., Delot, E. C., and Kreil, G. (1993) J. Biol. Chem. 268 16458-16464 [PubMed] [Google Scholar]
- 8.Adermann, K., Wattler, F., Wattler, S., Heine, G., Meyer, M., Forssmann, W. G., and Nehls, M. (1999) Protein Sci. 8 810-819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji, H., Okamoto, K., Matsuzaka, Y., Iizuka, H., Tamiya, G., and Inoko, H. (2003) Genomics 81 26-33 [DOI] [PubMed] [Google Scholar]
- 10.Chimienti, F., Hogg, R. C., Plantard, L., Lehmann, C., Brakch, N., Fischer, J., Huber, M., Bertrand, D., and Hohl, D. (2003) Hum. Mol. Genet. 12 3017-3024 [DOI] [PubMed] [Google Scholar]
- 11.Freemerman, A. J., Flickinger, C. J., and Herr, J. C. (1995) Mol. Reprod. Dev. 41 100-108 [DOI] [PubMed] [Google Scholar]
- 12.Kumar, S., Tamura, K., and Nei, M. (2004) Brief. Bioinform. 5 150-163 [DOI] [PubMed] [Google Scholar]
- 13.Notredame, C., Higgins, D. G., and Heringa, J. (2000) J. Mol. Biol. 302 205-217 [DOI] [PubMed] [Google Scholar]
- 14.Stokes, C., Papke, J. K. P., McCormack, T., Kem, W. R., Horenstein, N. A., and Papke, R. L. (2004) Mol. Pharmacol. 66 14-24 [DOI] [PubMed] [Google Scholar]
- 15.Papke, R. L., and Porter Papke, J. K. (2002) Br. J. Pharmacol. 137 49-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitin, F., Stern, O., Weiss, M., Gil-Henn, C., Ziv, R., Prokocimer, Z., Smorodinsky, N. I., Rubinstein, D. B., and Wreschner, D. H. (2005) J. Biol. Chem. 280 33374-33386 [DOI] [PubMed] [Google Scholar]
- 17.Soler-Garcia, A. A., Maitra, R., Kumar, V., Ise, T., Nagata, S., Beers, R., Bera, T. K., and Pastan, I. (2005) Reproduction (Camb.) 129 515-524 [DOI] [PubMed] [Google Scholar]
- 18.Herr, J. C., Wright, R. M., John, E., Foster, J., Kays, T., and Flickinger, C. J. (1990) Biol. Reprod. 42 377-382 [DOI] [PubMed] [Google Scholar]
- 19.Luo, C. W., Lin, H. J., and Chen, Y. H. (2001) J. Biol. Chem. 276 6913-6921 [DOI] [PubMed] [Google Scholar]
- 20.Coronel, C. E., Winnica, D. E., Novella, M. L., and Lardy, H. A. (1992) J. Biol. Chem. 267 20909-20915 [PubMed] [Google Scholar]
- 21.Southan, C., Cutler, P., Birrell, H., Connell, J., Fantom, K. G., Sims, M., Shaikh, N., and Schneider, K. (2002) Proteomics 2 187-196 [DOI] [PubMed] [Google Scholar]
- 22.Satomi, Y., Shimonishi, Y., and Takao, T. (2004) FEBS Lett. 576 51-56 [DOI] [PubMed] [Google Scholar]
- 23.Arredondo, J., Chernyavsky, A. I., Jolkovsky, D. L., Webber, R. J., and Grando, S. A. (2006) J. Cell. Physiol. 208 238-245 [DOI] [PubMed] [Google Scholar]
- 24.Foster, J. A., Klotz, K. L., Flickinger, C. J., Thomas, T. S., Wright, R. M., Castillo, J. R., and Herr, J. C. (1994) Biol. Reprod. 51 1222-1231 [DOI] [PubMed] [Google Scholar]
- 25.Menez, A. (1998) Toxicon 36 1557-1572 [DOI] [PubMed] [Google Scholar]
- 26.Tsetlin, V. (1999) Eur. J. Biochem. 264 281-286 [DOI] [PubMed] [Google Scholar]
- 27.Ibanez-Tallon, I., Miwa, J. M., Wang, H. L., Adams, N. C., Crabtree, G. W., Sine, S. M., and Heintz, N. (2002) Neuron 33 893-903 [DOI] [PubMed] [Google Scholar]
- 28.Nirthanan, S., and Gwee, M. C. (2004) J. Pharmacol. Sci. 94 1-17 [DOI] [PubMed] [Google Scholar]
- 29.Bray, C., Son, J. H., Kumar, P., and Meizel, S. (2005) Biol. Reprod. 73 807-814 [DOI] [PubMed] [Google Scholar]
- 30.Kumar, P., and Meizel, S. (2005) J. Biol. Chem. 280 25928-25935 [DOI] [PubMed] [Google Scholar]
- 31.Bray, C., Son, J. H., and Meizel, S. (2002) Biol. Reprod. 67 782-788 [DOI] [PubMed] [Google Scholar]
- 32.Son, J. H., and Meizel, S. (2003) Biol. Reprod. 68 1348-1353 [DOI] [PubMed] [Google Scholar]
- 33.Bray, C., Son, J. H., and Meizel, S. (2005) Mol. Hum. Reprod. 11 881-889 [DOI] [PubMed] [Google Scholar]
- 34.Meizel, S. (2004) Biol. Rev. Camb. Philos. Soc. 79 713-732 [DOI] [PubMed] [Google Scholar]