Abstract
A common biological pathway may contribute to the comorbidity of atherosclerosis and depression. Increased activity of the enzymatic 5-lipoxygenase (5-LOX; 5LO) pathway is a contributing factor in atherosclerosis and a 5-LOX inhibitor, MK-886, is beneficial in animal models of atherosclerosis. In the brain, MK-886 increases phosphorylation of the glutamate receptor subunit GluR1, and the increased phosphorylation of this receptor has been associated with antidepressant treatment. In this work, we evaluated the behavioral effects of MK-886 in an automated assay of mouse forced swimming, which identifies antidepressant activity as increased climbing behavior and/or decreased rest time. Whereas a single injection of MK-886 (3 and 10 mg/kg) did not affect forced swimming behaviors assayed 30 min later, 6 daily injections of 3 mg/kg MK-886 slightly increased climbing and significantly reduced rest time in wild-type mice but not in 5-LOX-deficient mice. A diet delivery of MK-886, 4 μg per 100 mg body-weight per day, required three weeks to affect forced swimming; it increased climbing behavior. Climbing behavior was also increased in naive 5-LOX-deficient mice compared to naive wild-type controls. These results suggest that 5-LOX inhibition and deficiency may be associated with antidepressant activity. Increased climbing in a forced swimming assay is a typical outcome of antidepressants that increase noradrenergic and dopaminergic activity. Interestingly, 5-LOX deficiency and MK-886 treatment have been shown to be capable of increasing the behavioral effects of a noradrenaline/dopamine-potentiating drug, cocaine. Future research is needed to evaluate the clinical relevance of our findings.
Keywords: 5-lipoxygenase (5-LOX, 5LO); antidepressant; depression; atherosclerosis; GluR1 cardiovascular
Recent studies pointed out an association between depressive symptoms and the progression of atherosclerosis [7, 26]. It was proposed that a common biological mechanism contributes to triggering and/or maintenance of these two pathological conditions [19].
A prominent role in the pathobiology of atherosclerosis is played by 5-lipoxygenase (5-LOX; 5LO), an enzyme involved in the metabolism of arachidonic acid in 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and leukotrienes [16, 25]. Whereas an upregulated 5-LOX pathway promotes atherosclerosis, prolonged treatment with the 5-LOX inhibitor MK-886 decreased atherosclerosis in an in-vivo mouse model [13]. The 5-LOX pathway is also active in the mammalian brain [2, 8]. 5-LOX inhibitors, including MK-886, stimulate phosphorylation and membrane insertion of the GluR1 subtype of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) glutamate receptors [12, 23]. Drug-induced increases of GluR1 phosphorylation have been linked to antidepressant activity [5, 18, 21].
Thus, it was hypothesized that by interfering with GluR1 phosphorylation, 5-LOX might be involved in depression and also in antidepressant therapy [20]. In this study, we used a mouse model of forced swimming to evaluate the putative antidepressant action of MK-886. Since this drug may affect systems other than the 5-LOX pathway [22], we performed experiments in wild-type and 5-LOX-deficient (knockout) mice.
Male 5-LOX deficient mice and their wild type controls were purchased from Jackson Laboratories (B6.129S2-Alox5tm1Fun/J; Stock #004155; Bar Harbor, ME). Male mice were selected to reduce the confounding variables associated with the estrous cycle of females, including possible hormonal changes associated with chronic administration of 5-LOX inhibitors. Animals (2-3 months old) were housed in groups of three and had free access to laboratory chow and water except during the experiments. They were kept in a temperature-controlled room on a 14 h light/10 h dark cycle (lights off at 7 PM). All behavioral experiments were performed between 10 AM and 3 PM. The experimental protocol was approved by the Institutional Animal Care and Ethics Committee. MK-886 (Sigma Chemical St. Louis, MO, USA) was dissolved in sterile 5% dimethylsulfoxide (DMSO; Sigma) saline and 3 mg/kg (except in one experiment 10 mg/kg) of this drug was administered intraperitoneally (i.p.). This dose was selected based on prior results; administered repeatedly it increases GluR1 phosphorylation in the mouse brain [12]. Controls were treated with the vehicle. The food delivery of MK-886 was performed as reported previously [13]. Briefly, mice received a diet (TD.06348, Harlan-Teklad, Madison, WI, USA) mixed with MK-886 (Sigma) at a dosage of 4 μg per 100 mg body-weight per day for three weeks. The drug was mixed with the diet without heating by the manufacturer. Controls received the same diet without MK-886. Mice treated with MK-886 had a similar food intake and body-weight after the experiment as the control mice.
Automated forced swimming experiments were conducted using the computer controlled Hamilton-Kinder forced swim test device (Hamilton-Kinder; Poway, CA, USA), which consists of two clear water-filled tanks, each equipped with two photobeam arrays that allow monitoring of swimming and climbing behaviors [14]. Two mice at a time were individually dropped from approximately 10 cm above the water surface into tanks filled with 1l of water (24°C) and left there for six minutes. The water was replaced between each test. The device was operated with the manufacturer's software (MotorMonitor version 4.11). The collected data included: a) basic movements, which corrrespond to any kind of horizontal beam block, b) climbing, which corresponds to movements vertical to the water surface, and c) rest time, which is the time the animal remains inactive (i.e., did not change any beam status). The values for each of the above-noted parameter are automatically calculated and expressed as units/session (a and b) and sec/6 min (c). In this assay, antidepressant drugs increase climbing and/or shorten rest time. Data (mean ± S.E.M.) obtained from automated swim tests were evaluated by an independent-samples t-test. Significance was accepted at p<0.05.
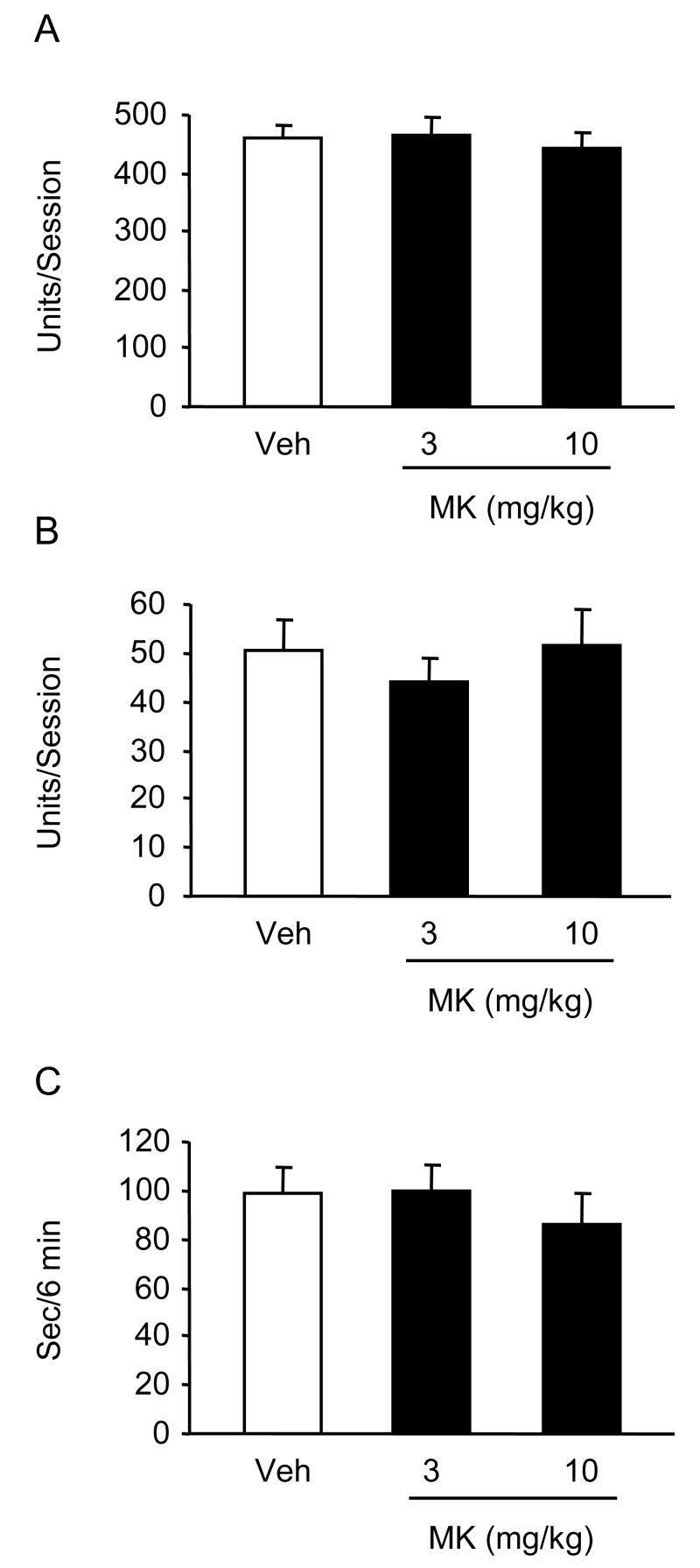
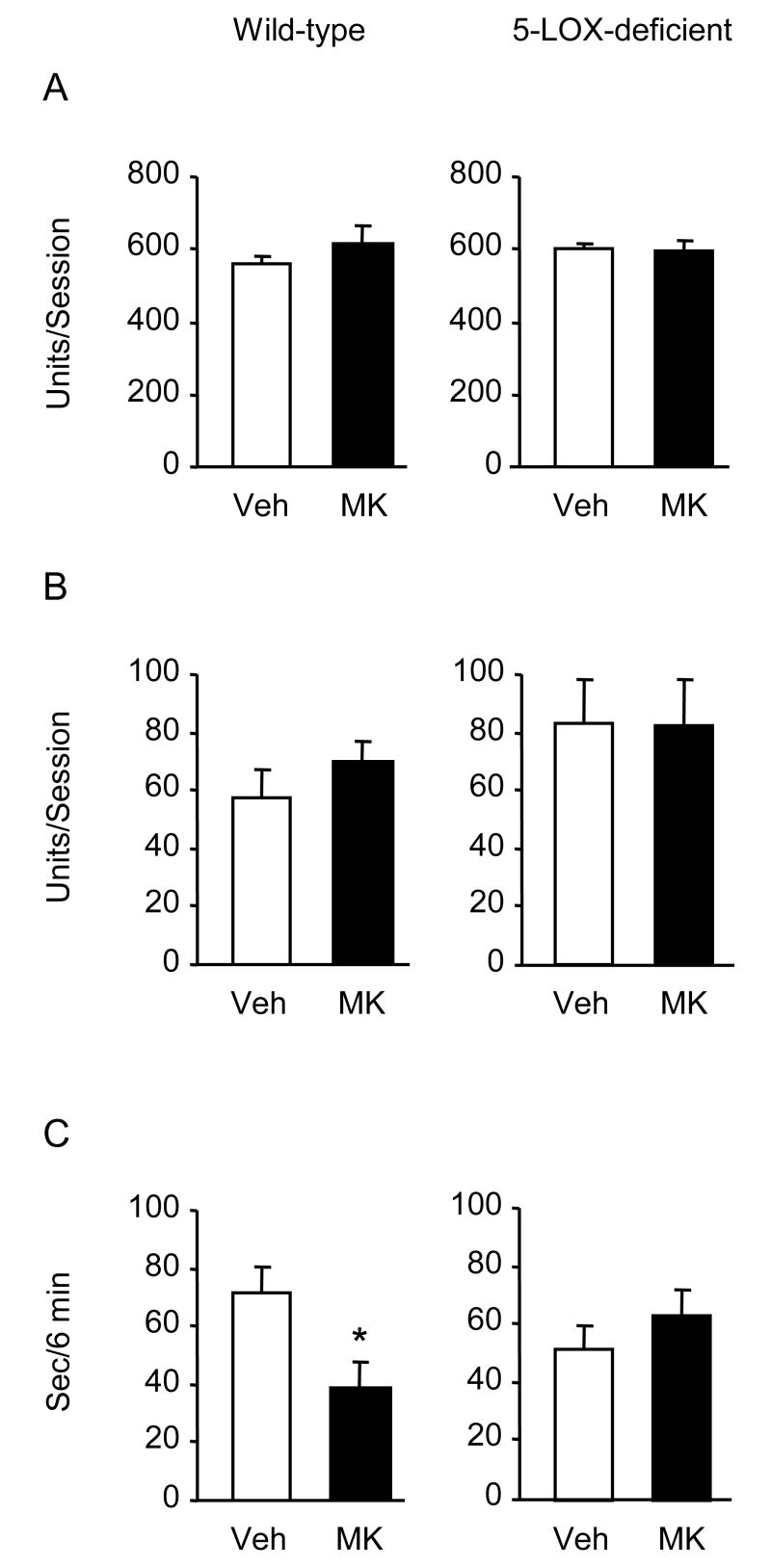
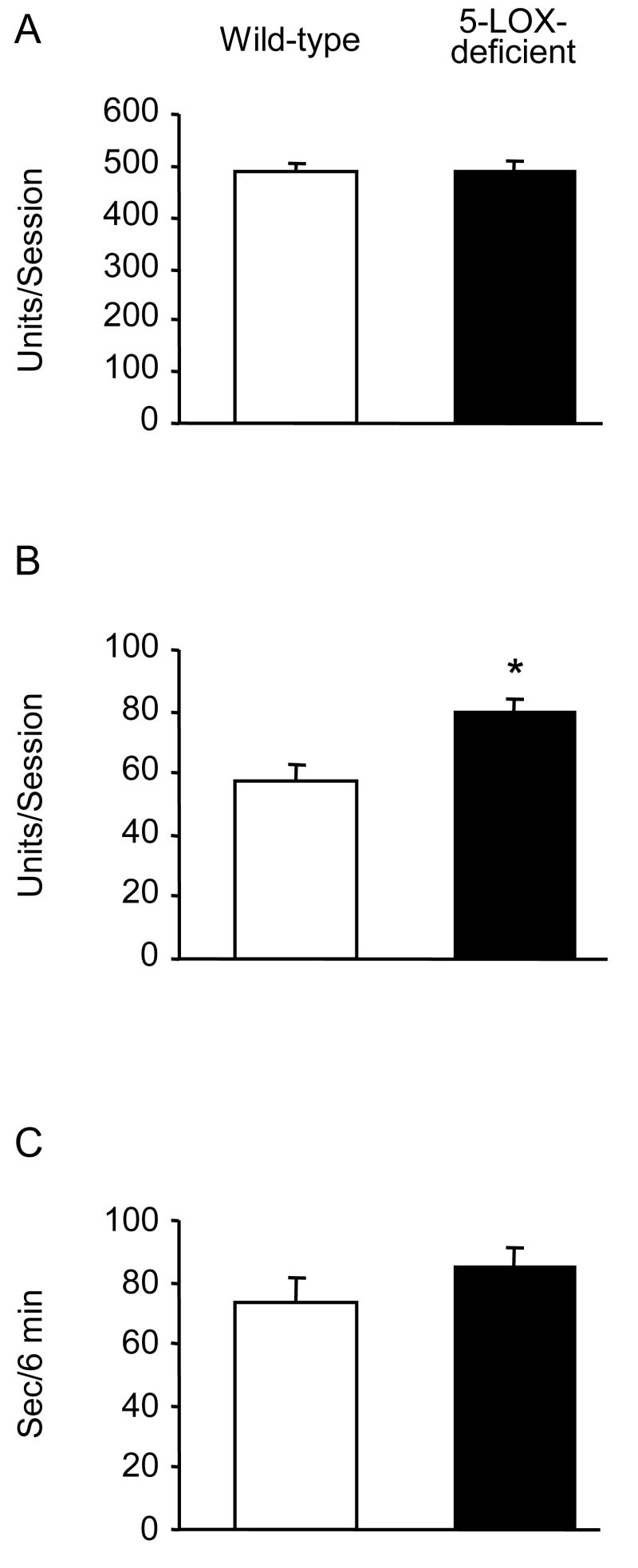
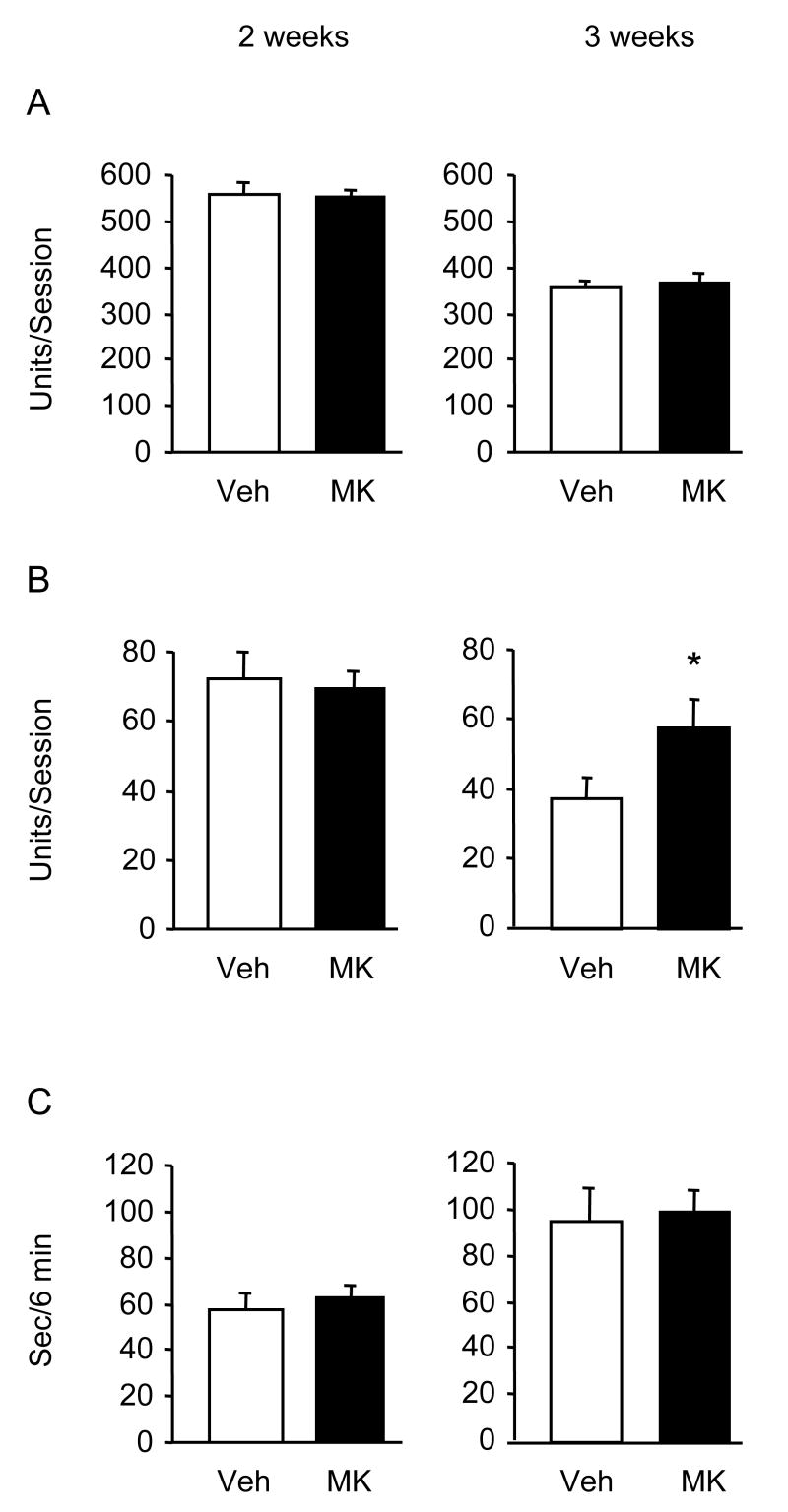
A single injection of MK-886 (3 and 10 mg/kg) did not alter the behavior of mice in the swim test (Fig. 1). Repeated injections of 3 mg/kg produced a slight (21 %) increase of climbing and a significant (45 %) reduction of rest time in wild type mice but not in 5-LOX-deficient mice (Fig. 2). In these experiments, vehicle-injected 5-LOX-deficient mice showed a trend to increased climbing compared to vehicle-treated wild-type controls. To investigate the contribution of a 5-LOX deficiency to the behavior of mice in the swim test, we used naive 5-LOX-deficient and wild type mice and found significantly elevated climbing in the 5-LOX deficient group (Fig. 3). Previous research in atherosclerosis has established a diet MK-886 delivery schedule and dose for prolonged treatment [13]. We used this treatment schedule for 3 weeks. Mice were tested at the end of weeks 2 and 3. After 3 weeks on the diet containing MK-886, climbing behavior was significantly increased compared to mice on the control diet (Fig. 4).
Fig. 1.
Effects of a single MK-886 injection on mouse behavior in the forced swimming test. Mice (n = 6) received i.p. injections of 3 and 10 mg/kg MK-886 or vehicle 30 min prior to testing. Results are shown as mean ± S.E.M.; open bars = vehicle (Veh), closed bars = MK-886 (MK). (A) basic movements, (B) climbing, (C) rest time. There were no significant differences between drug treated groups and vehicle treated controls.
Fig. 2.
Effects of repeated MK-886 injections on mouse behavior in the forced swimming test. Mice (wild-type and 5-LOX-deficient; n = 8) received either 3 mg/kg MK-886 or vehicle (saline with 5% DMSO) i.p. once a day for six days. Thirty min after the last injection, mice were assayed in an automated swim test apparatus. Results are shown as mean ± S.E.M.; open bars = vehicle (Veh), closed bars = MK-886 (MK). (A) basic movements, (B) climbing, (C) rest time. Rest time was significantly reduced after drug treatment only in wild-type mice (p<0.01).
Fig. 3.
Effects of 5-LOX deficiency on mouse behavior in the forced swimming test. Naive wild-type and 5-LOX-deficient mice (n = 26) were assayed in an automated swim test apparatus. Data are shown as mean ± S.E.M.; open bars = wild-type, closed bars = 5-LOX-deficient. (A) basic movements, (B) climbing, (C) rest time. Climbing behavior was significantly greater in 5-LOX-deficient mice (p<0.01).
Fig. 4.
Effects of prolonged diet delivery of MK-886 on mouse behavior in the forced swimming test. Mice (n = 12) on TD.06348 Harlan-Teklad diet with and without MK-886 were assayed in an automated swim test apparatus after two and three weeks of exposure to this diet. Results are shown as mean ± S.E.M.; open bars = vehicle (Veh), closed bars = MK-886 (MK). (A) basic movements, (B) climbing, (C) rest time. Climbing behavior was significantly increased in mice on a diet supplemented with MK-886 for three weeks compared to mice on a drug-free diet (p<0.05).
The main finding of this study is that prolonged MK-886 treatment alters the behavior of mice in the forced swimming assay in a manner indicative of antidepressant activity. Antidepressant drugs that primarily increase noradrenergic and dopaminergic activity increase climbing whereas drugs acting primarily on serotonergic transmission affect swimming [4]. Both long-term diet delivery of MK-886 and 5-LOX gene disruption significantly increased the climbing behavior, suggesting that a long-lasting 5-LOX deficiency may promote noradrenergic and dopaminergic activity. Interestingly, it has been noted that 5-LOX deficiency potentiates the behavioral effects of cocaine, a drug that primarily stimulates the noradrenergic/dopaminergic neurotransmitter systems [15].
Activation of dopamine D1 receptors contributes to mouse antidepressant-like behaviors induced by drugs such as imipramine [11] and also increases the phosphorylation of GluR1 receptors [27]. Recent studies suggest that a drug-induced increase of GluR1 phosphorylation may contribute to antidepressant activity [5, 18, 21]. A single MK-886 injection, which does not affect brain GluR1 phosphorylation [12], did not produce antidepressant-like behavior, whereas this behavior was induced by repeated injections of MK-886, which also increase GluR1 phosphorylation [12]. These findings, along with our observation that several weeks of diet-delivery of MK-886 are needed to alter behavior, suggest that protracted 5-LOX inhibition or certain brain levels of MK-886 are a prerequisite for increased GluR1 phosphorylation and antidepressant-like effects. Although it is has been reported earlier that a systemic administration of MK-886 is capable of delivering this drug into the brain [3], further studies are needed to evaluate the pharmacokinetics and bioavailability of MK-886.
The lack of antidepressant-like action of MK-886 in 5-LOX-deficient mice indicates that this behavior may involve MK-886-mediated inhibition of 5-LOX activity. Interestingly, in contrast to the stimulation of GluR1 phosphorylation in wild-type mice [12], repeated injections of MK-886 did not increase brain GluR1 phosphorylation in 5-LOX-deficient mice (unpublished observation). Hence, it is possible that 5-LOX inhibition produces behavioral effects by increasing GluR1 phosphorylation via the dopamineric system and/or through dopamine-independent [12, 23] mechanisms.
Our previous research found that glucocorticoids stimulate 5-LOX expression and activation in the brain [28]. Hence, it is possible that stress-induced glucocorticoid surge, i.e., due to forced swimming, could stimulate the 5-LOX system. 5-LOX activity leads to conversion of arachidonic acid into 5-HPETE and leukotrienes, and MK-886 disrupts this process by inhibiting the 5-lipoxygenase-activating protein (FLAP) [9]. 5-HPETE is a potent inhibitor of neuronal Na+, K+-ATPase activity [10] and it remains to be clarified whether MK-886 treatment and/or 5-LOX gene disruption alter Na+, K+-ATPase activity in brain regions involved in antidepressant effects. On the other hand, leukotrienes act through specific leukotriene receptors, some of which are expressed in the brain [6, 29]. The exact function of brain leukotriene receptors is poorly understood and their putative role in the antidepressant action of 5-LOX inhibition is currently unclear. For example, in endothelial cells, activation of cysteinyl leukotriene receptor 2 by 5-LOX products leads to significant upregulation of cyclooxygenase-2 (COX-2) [17]. If similar action occurs in the brain, 5-LOX inhibition could lead to antidepressant effects by preventing COX-2 upregulation. Namely, hippocampal COX-2 is upregulated in a rat model of depression [1] and a COX-2 inhibitor, celecoxib, demonstrated therapeutic effects in major depression [24].
In conclusion, our results suggest that the pharmacological inhibition of 5-LOX and 5-LOX deficiency created by 5-LOX gene disruption trigger antidepressant-like behaviors in mice. It remains to be investigated whether our findings have clinical value. Interestingly, a dietary administration of MK-886 to mice, which previously was shown capable of decreasing atherosclerosis [13], also produced antidepressant-like behavior. Considering the known comorbidity of atherosclerosis and depression [7], we propose that focusing on mechanisms common to both disorders, e.g., 5-LOX, could lead to discovery of novel and better therapeutic options.
Acknowledgments
This work was supported by NIH grant R21 MH074139 (R.M.) and by the UIC Psychiatric Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cassano P, Hidalgo A, Burgos V, Adris S, Argibay P. Hippocampal upregulation of the cyclooxygenase-2 gene following neonatal clomipramine treatment (a model of depression) Pharmacogenomics J. 2006;6:381–387. doi: 10.1038/sj.tpj.6500385. [DOI] [PubMed] [Google Scholar]
- 2.Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol. 2001;133:1323–1329. doi: 10.1038/sj.bjp.0704189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA, Jr, Manji HK. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- 6.Fang SH, Zhou Y, Chu LS, Zhang WP, Wang ML, Yu GL, Peng F, Wei EQ. Spatio-temporal expression of cysteinyl leukotriene receptor-2 mRNA in rat brain after focal cerebral ischemia. Neurosci Lett. 2007;412:78–83. doi: 10.1016/j.neulet.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 7.Faramawi MF, Gustat J, Wildman RP, Rice J, Johnson E, Sherwin R. Relation between depressive symptoms and common carotid artery atherosclerosis in American persons ≤65 years of age. Am J Cardiol. 2007;99:1610–1613. doi: 10.1016/j.amjcard.2006.12.090. [DOI] [PubMed] [Google Scholar]
- 8.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-β pathology in a mouse model of Alzheimer's disease. FASEB J. 2007 doi: 10.1096/fj.07-9131.com. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer L, Hornig M, Pergola C, Meindl N, Franke L, Tanrikulu Y, Dodt G, Schneider G, Steinhilber D, Werz O. The molecular mechanism of the inhibition by licofelone of the biosynthesis of 5-lipoxygenase products. Br J Pharmacol. 2007;152:471–480. doi: 10.1038/sj.bjp.0707416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley TD. 5-HPETE is a potent inhibitor of neuronal Na+, K+-ATPase activity. Biochem Biophys Res Commun. 1997;235:374–376. doi: 10.1006/bbrc.1997.6790. [DOI] [PubMed] [Google Scholar]
- 11.Hirano S, Miyata S, Onodera K, Kamei J. Involvement of dopamine D1 receptors and alpha1-adrenoceptors in the antidepressant-like effect of chlorpheniramine in the mouse tail suspension test. Eur J Pharmacol. 2007;562:72–76. doi: 10.1016/j.ejphar.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 12.Imbesi M, Zavoreo I, Uz T, Sharma RP, Dimitrijevic N, Manev H, Manev R. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Res. 2007;1147:148–153. doi: 10.1016/j.brainres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Jawien J, Gajda M, Rudling M, Mateuszuk L, Olszanecki R, Guzik TJ, Cichocki T, Chlopicki S, Korbut R. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Invest. 2006;36:141–146. doi: 10.1111/j.1365-2362.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 14.Kurtuncu M, Luka LJ, Dimitrijevic N, Uz T, Manev H. Reliability assessment of an automated forced swim test device using two mouse strains. J Neurosci Methods. 2005;149:26–30. doi: 10.1016/j.jneumeth.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kurtuncu M, Battista N, Uz T, D'Agostino A, Dimitrijevic N, Pasquariello N, Manev R, Maccarrone M, Manev H. Effects of cocaine in 5-lipoxygenase-deficient mice. J Neural Transm. doi: 10.1007/s00702-007-0848-8. in press. [DOI] [PubMed] [Google Scholar]
- 16.Lötzer K, Funk CD, Habenicht AJ. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim Biophys Acta. 2005;1736:30–37. doi: 10.1016/j.bbalip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Lötzer K, Jahn S, Kramer C, Hildner M, Nüsing R, Funk CD, Habenicht AJ. 5-Lipoxygenase/cyclooxygenase-2 cross-talk through cysteinyl leukotriene receptor 2 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;84:108–115. doi: 10.1016/j.prostaglandins.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.05.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Manev H, Manev R. 5-lipoxygenase as a possible biological link between depressive symptoms and atherosclerosis. Arch Gen Psychiatry. 2007;64:1333. doi: 10.1001/archpsyc.64.11.1333. [DOI] [PubMed] [Google Scholar]
- 20.Manev R, Mrazovac D, Manev H. Possible role for interactions between 5-lipoxygenase (5-LOX) and AMPA GluR1 receptors in depression and in antidepressant therapy. Med Hypotheses. 2007;69:1076–1079. doi: 10.1016/j.mehy.2007.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Turrillas R, Del Río J, Frechilla D. Neuronal proteins involved in synaptic targeting of AMPA receptors in rat hippocampus by antidepressant drugs. Biochem Biophys Res Commun. 2007;353:750–755. doi: 10.1016/j.bbrc.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 22.Mayburd AL, Martlínez A, Sackett D, Liu H, Shih J, Tauler J, Avis I, Mulshine JL. Ingenuity network-assisted transcription profiling: Identification of a new pharmacologic mechanism for MK886. Clin Cancer Res. 2006;12:1820–1827. doi: 10.1158/1078-0432.CCR-05-2149. [DOI] [PubMed] [Google Scholar]
- 23.Ménard C, Valastro B, Martel MA, Chartier E, Marineau A, Baudry M, Massicotte G. AMPA receptor phosphorylation is selectively regulated by constitutive phospholipase A(2) and 5-lipoxygenase activities. Hippocampus. 2005;15:370–380. doi: 10.1002/hipo.20061. [DOI] [PubMed] [Google Scholar]
- 24.Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Möller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, addon pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 25.Rådmark O, Samuelsson B. 5-lipoxygenase: regulation and possible involvement in atherosclerosis. Prostaglandins Other Lipid Mediat. 2007;83:162–174. doi: 10.1016/j.prostaglandins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell MF, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 27.Swayze RD, Lisé MF, Levinson JN, Phillips A, El-Husseini A. Modulation of dopamine mediated phosphorylation of AMPA receptors by PSD-95 and AKAP79/150. Neuropharmacology. 2004;47:764–778. doi: 10.1016/j.neuropharm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Uz T, Dwivedi Y, Savani PD, Impagnatiello F, Pandey G, Manev H. Glucocorticoids stimulate inflammatory 5-lipoxygenase gene expression and protein translocation in the brain. J Neurochem. 1999;73:693–699. doi: 10.1046/j.1471-4159.1999.0730693.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang WP, Hu H, Zhang L, Ding W, Yao HT, Chen KD, Sheng WW, Chen Z, Wei EQ. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci Lett. 2004;363:247–251. doi: 10.1016/j.neulet.2004.03.088. [DOI] [PubMed] [Google Scholar]