Abstract
Microinjection of neurotensin (NT) into the rostral ventromedial medulla (RVM) produces dose-dependent antinociception. Here we show that antinociception produced by intra RVM microinjection of neurotensin (NT) or the selective NT receptor subtype 1 (NTR1) agonist PD149163 can be partially blocked by intrathecal (i.t.) yohimbine, an α2-adrenoceptor antagonist and by methysergide, a serotonin receptor antagonist. Antinociception produced by the NTR2 agonist beta-lactotensin (β-LT) is blocked by intrathecal (i.t.) yohimbine, but not by methysergide i.t.. It is not known which noradrenergic cell group is involved in this newly identified noradrenergic component of NTR-mediated antinociception.
These experiments provide the first evidence that selective activation of NTR2 in the RVM produces antinociception. These results also provide evidence that activation of NTR1 in the RVM produces antinociception through spinal release of norepinephrine (NE) and serotonin, and that activation of NTR2 in the RVM produces antinociception mediated by spinal release of NE.
INTRODUCTION
Microinjection of neurotensin (NT) into the rostral ventromedial medulla (RVM) has biphasic effects on nociception; facilitation is produced by low doses (0.03 pmol – 0.03 nmol) and inhibition by greater doses (1.3 – 30 nmol) (Behbehani and Pert 1984;Fang et al. 1987;Urban and Smith 1993;Urban et al. 1996;Sarhan et al. 1997;Urban et al. 1999;Holmes et al. 1999), but see (Neubert et al. 2004). It has been shown that the facilitatory effect of intra-RVM NT is mediated through release of cholecystokinin in the spinal dorsal horn (Urban et al. 1996), while at least part of the antinociceptive effect is mediated through activation of NTR1 and release of serotonin in the spinal dorsal horn (Buhler et al. 2005). It has been suggested that an imbalance of facilitation and inhibition may play a role in some chronic pain states (Urban and Gebhart 1999;Porreca et al. 2002).
Of the three cloned NT binding proteins, NTR1 and NTR2 are G-protein coupled receptors (Tanaka et al. 1990;Chalon et al. 1996), while the NTR3 (gp95/sortilin) induces phosphorylation of Erk1/2 and Akt kinases and induces the expression of several chemokines/cytokines, including TNFα and IL-1b (Trudeau 2000). Both NTR1 and NTR2 are expressed in the RVM (Sarret et al. 2003;Buhler et al. 2005) and several studies have shown that NTR1 is involved in antinociception (Wustrow et al. 1995;Urban and Gebhart 1997;Smith et al. 1997;Buhler et al. 2005). There is, however, substantial evidence that non-NTR1 receptors also play a role in RVM NT-mediated antinociception. Thus, block of NT-induced antinociception by the selective NTR1 antagonist SR48692 is incomplete (Dubuc et al. 1994;Smith et al 1997;Buhler et al. 2005), and receptor knock-down strategies utilizing antisense oligonucleotide treatment suggest a role for other NT receptors (Dubuc et al. 1999b;Remaury et al. 2002;Yamauchi et al. 2003a).
Although it is possible that NTR2 may mediate part of the antinociception produced by intra-RVM NT, an NTR2-mediated component has not been directly demonstrated. Previous attempts to identify NTR2-mediated effects in the RVM (Smith et al. 1997) have utilized levocabastine (> 1,000x selectivity NTR2 vs. NTR1; Kitabgi et al. 1987 which has been characterized as a functional agonist at the rat NTR2 (Yamada et al. 1998;Sarret et al. 2002;Gendron et al. 2004). However, levocabastine has several disadvantages including inverse agonist/antagonist activity at the human and mouse NTR2 (Dubuc et al. 1999a;Yamauchi et al. 2003b) and substantial antagonist activity at the histamine H1 receptor (Schotte et al 1986), which is both highly expressed in the rat RVM (Palacios et al. 1981) and modulates nociception (Mobarakeh et al. 2002), but see (Tyler et al. 1998). Further, levocabastine is a functional antagonist of NT effects in rat (Tyler et al. 1998). Because the use of levocabastine to determine NTR2-mediated effects on nociception is likely to produce equivocal results, we used the NTR2-selective agonist β-lactotensin (β-LT) to provide more definitive evidence for the role of NTR2 in RVM-elicited antinociception.
This report describes a series of experiments that examine the neurotransmitters which mediate NTR2- and NTR1-induced antinociception in vivo.
MATERIALS AND METHODS
Nociceptive Testing
Adult male Sprague-Dawley rats (Charles River Sasco, Portage, MI; 250–350 g) were anesthetized with pentobarbital (40 mg/kg; i.p.) for preparation and testing. Animals were maintained at a constant body temperature of 35.5°C using an electronically controlled heater and a thermistor probe inserted 2 cm into the rectum. While deeply anesthetized, a 26 gauge stainless steel guide cannula was guided stereotaxically to a site above the midline RVM (AP – 2.7 mm; ML 0.0 mm; DV −0.7 mm). When anesthesia had lightened, baseline nociceptive tail and paw withdrawal latencies to a noxious thermal stimulus were determined at three time-points before intra-RVM drug administration. Rats were used only if the baseline tail flick latency was between 1.4 and 4.0 sec; animals had an average baseline tail flick latency of 2.9 ± 0.1 sec (n = 178) and there was no significant difference in baseline latencies between groups. Drugs were made up in preservative-free sterile saline, pH 7.4, and delivered in a volume of 0.5 µl via a 33 gauge stainless steel injector inserted into the guide cannula. Movement of a small air bubble in the PE 10 tubing (Intramedic, Becton Dickinson, Sparks, MD) connecting a Hamilton microsyringe to the injector was monitored to ensure accurate delivery of drug. Drug solutions were injected using an electronic syringe pump to drive the microsyringe. Rats were used only once and received only a single dose of drug except when noted. Tail and paw withdrawal response latencies were determined 5, 10, 20, 30, 40, and 50 min after intra-RVM injection of the drug to be studied. Radiant thermal stimulation from a 250 W projection bulb was applied to one of three points on the dorsal surface of the tail and to the plantar surface of each hindpaw, and was terminated at 10 sec to minimize tissue damage. At each time point the three tail response latencies were averaged, as were the two hindpaw latencies. Tail and paw withdrawal response latencies in vehicle-treated rats were stable during the 50 min course of testing, indicating that the plane of anesthesia did not change significantly.
In some experiments, intrathecal catheters were placed in deeply anesthetized rats (pentobarbital, 50 mg/kg, i.p.). Catheters made from PE10 tubing were inserted through an incision in the cisterna magna and threaded 7.5 cm caudally in the intrathecal space. A 10 µl volume of drug was injected through the catheter over 30 sec, and washed through with 10 µl of sterile saline.
After testing, animals were decapitated and the brains rapidly removed and immersed in 10% formalin for 24 hours. The microinjection site was determined using microscopic examination of tissue sections and plotted on drawings of the brainstem section. Previous studies have shown that intracerebral microinjections of 0.5 µl diffuse approximately 0.5 mm from the site of injection (Myers 1966).
Testing of motor performance
Adult male Sprague-Dawley rats (as described above) were anesthetized with pentobarbital (40 mg/kg; i.p.) for surgery. A 26 gauge stainless steel guide cannula (Plastics One, Roanoke, VA) was implanted stereotaxically (AP −2.5 mm; ML 0.0 mm; DV −0.7 mm), secured to the skull by acrylic dental cement and stainless steel screws, and fitted with a solid 25-gauge stainless steel obturator (Plastics One). Animals were allowed to recover for 4–5 days and then tested on the inclined plane 10 min before, and 10 and 20 min after intra-RVM drug administration. Microinjections were performed on loosely restrained animals by lowering a 33-gauge infusion needle (Plastics One) through the guide cannula and delivering 0.5 µl of drug over a 30 sec time period. As above, movement of a small air bubble in the injection tubing was monitored to accurately deliver drug. Rats were placed on a rubber-coated plane and the angle of inclination was increased gradually until the rat could no longer maintain its position on the plane (Rivlin and Tator 1977). Each animal was given three trials on the plane at each of the three testing intervals. After testing, animals were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and the brains were removed and immersed in 10% formalin for 24 hours. The microinjection site was determined using microscopic examination of tissue sections and plotted on drawings of the appropriate brainstem section.
β-LT Binding Assay
The selectivity of β-LT binding to NTR2 was determined using radioligand-binding assays at cloned or endogenously expressed receptors, performed as previously detailed (Shapiro et al. 2003) through the NIMH-PDSP. Detailed protocols are available for all assays at the NIMH-PDSP web site http://kidb.cwru.edu/nimh/binding.php. For non-human receptors, binding assays were performed with cDNA expressed in CHO cells (rat 5-HT1A), NIH-3T3 cells (rat 5-HT2A, rat 5-HT2C), HEK-293 cells (rat D3, rat KOR receptors, mouse α2A) or assayed from guinea pig brain membrane (guinea pig H3) or rat brain membrane (rat BZD). Binding assays on cloned human receptors were performed in CHO cells (α1A, α1B, α2B, α2C, m1, m2) or HEK-293 cells (all others). All assays were performed in quadruplicate with 10 µM β-LT (Chemical Synthesis Branch of NIMH, NIH). Ki was calculated using GraphPad Prizm (GraphPad Software, San Diego, CA) for each ligand that had an affinity of ≤ 10,000 nM.
Statistical analysis
Data are plotted as the mean ± SEM of maximum percent effect (MPE) values at each time point (MPE = [(test latency - baseline latency)/(10 sec - baseline latency)]×100). MPE values greater than 20% at 10 min were considered to indicate antinociception. The area under the curve (AUC) was determined by the trapezoidal method (Sigma Plot 9.0; SPSS Science, Chicago, Ill.). Statistical analyses of differences between groups were determined with a two-way analysis of variance (ANOVA) for repeated measures (treatment and time) and were performed on MPE values as baseline latencies did not differ significantly between groups. When ANOVA results were statistically significant, post hoc analysis (Student-Newman-Keuls test) was performed, and F values with P ≤ 0.05 were considered to be statistically significant. Percent reduction of antinociception was determined from the area under the curve between the 20 and 50 minute time points (post-i.t. injection) by the following formula [1 – (AUC after i.t. drug/AUC after i.t. saline) ] * 100; no reduction in antinociception would be considered 0% block, while an AUC of zero would be considered 100% block
Animal care and use
The experimental protocols were approved by The University of Iowa Animal Care and Use Committee and were in accordance with the guidelines of the International Association for the Study of Pain Committee for Research and Ethical Issues (Zimmermann 1983).
RESULTS
β-LT is a selective agonist for NTR2 in the RVM
Previous studies have characterized β-LT as selective for NTR2 over NTR1 (Yamauchi et al. 2003a;Yamauchi et al. 2003b) and having marginal inhibitory activity at the angiotensin-I converting enzyme (IC50= 1153.2 µM) (Mullally et al. 1996). A more thorough investigation of its binding properties indicates that, with the exception of the α2B adrenergic receptor (Ki = 401.5 nM), which is not expressed in the RVM (see discussion), β-LT displayed negligible binding to all receptors tested (Ki >10,000 nM; Table 1), including NTR1. Because of the binding to the α2B adrenergic receptor, this compound cannot generally be considered a completely selective NTR2 agonist, but is functionally selective in the RVM.
Table 1.
Affinities of β-LT and Reference Compounds at Various Receptors,
| Receptor | 3H-ligand | Ki β-LT (nM) |
|---|---|---|
| NTR1 | NT | >10,000 |
| r5-HT1A | 635 8-OH-DPAT | >10,000 |
| 5-HT1B | GR125743 | >10,000 |
| 5-HT1E | 5HT | >10,000 |
| 5-HT2A | Ketanserin | >10,000 |
| 5-HT2B | LSD | >10,000 |
| r5-HT2C | Mesulergine | >10,000 |
| 5-HT5A | LSD | >10,000 |
| 5-HT6 | LSD | >10,000 |
| 5-HT7 | LSD | >10,000 |
| D1 | SCH23390 | >10,000 |
| D2L | N-methylspiperone | >10,000 |
| rD3 | N-methylspiperone | >10,000 |
| α1A | Prazosin | >10,000 |
| α1B | Prazosin | >10,000 |
| mα2A | Clonidine | >10,000 |
| α2B | Clonidine | 401.5* |
| α2C | Clonidine | >10,000 |
| m1 | QNB | >10,000 |
| m2 | QNB | >10,000 |
| rBZP (A1) | RO 15-1788 | >10,000 |
| MOR | Diprenorphine | >10,000 |
| DOR | DADLE | >10,000 |
| rKOR | U69593 | >10,000 |
| H1 | Pyrilamine | >10,000 |
| H2 | Tiotidine | >10,000 |
| gH3 | α-methyl-histamine | >10,000 |
| H4 | Histamine | >10,000 |
Experiments were performed as described in Materials and Methods, using the radioligands listed above. Data represent the mean of at least four separate experiments. All studies were performed with human cloned cDNAs except where specified; r=rat cloned cDNA, m=mouse cloned cDNA, g=guinea pig cloned cDNA.
β-LT in the RVM produces thermal antinociception that is blocked by a mixed NTR1/2 antagonist, but not by a selective NTR1 antagonist
Microinjection of β-LT in a range of doses (2 pmol - 1 nmol; n = 4–16) into sites in the RVM (similar to those illustrated in Fig. 1C) produced dose-dependent increases in tail-withdrawal responses to a noxious thermal stimulus (Fig. 2A & B). In the tail-withdrawal test the antinociceptive effects of intra-RVM microinjection of β-LT did not vary depending on the site of injection (within the range of −11.5 mm to −10.0 mm caudal to bregma). The duration of the antinociception produced by β-LT was typically shorter (recovery to near baseline values by 50 min) than that produced by NT (minimal recovery by 70 min; Buhler et al. 2005).
Figure 1.
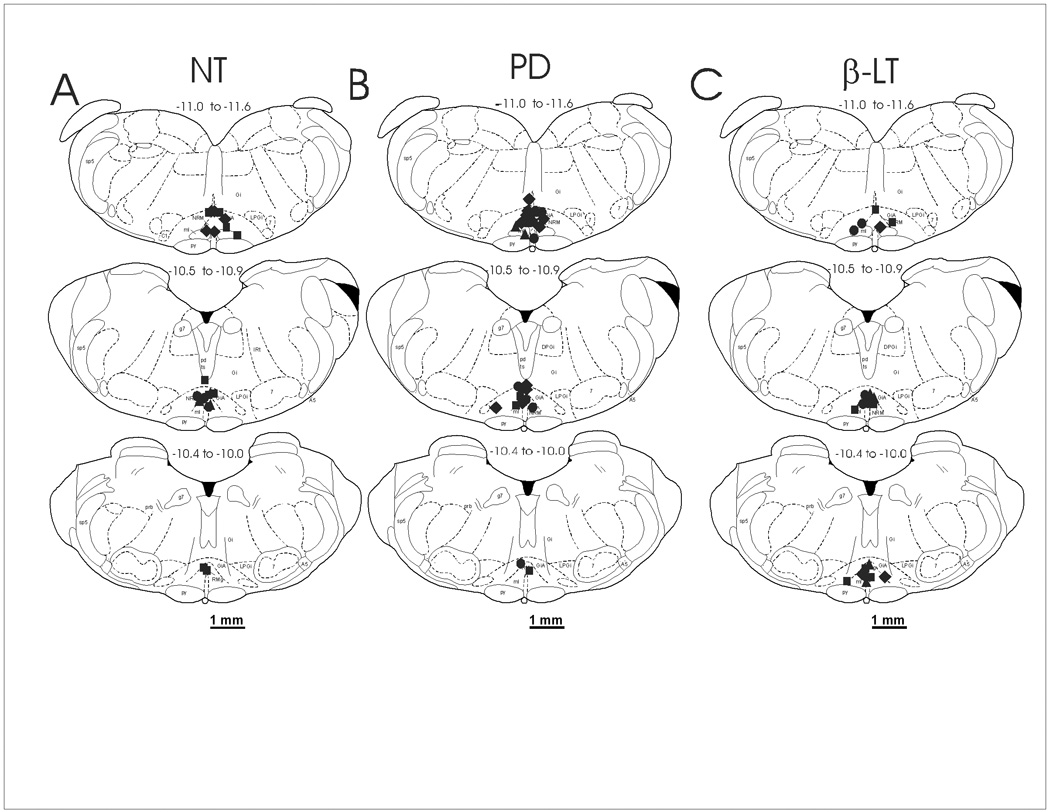
The distribution of microinjection sites in the RVM for A) NT (3 nmol, n = 32), B) PD149163 (0.5 pmol, n = 30), and C) β-LT (1 nmol, n = 22). Sites are plotted between −11.6 mm and −10.0 mm caudal to bregma and the numbers above each section represent the distance caudal to bregma in mm. The intrathecal drug administered after each agonist is indicated by different symbols: circle = saline; diamond = yohimbine; square = methysergide; triangle = yohimbine & methysergide. Diagrams of representative sections were taken from the atlas of Paxinos and Watson, 1997. Abbreviations: 7, facial nerve nucleus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular nucleus, alpha part; LPGi, lateral paragigantocellular nucleus; NRM, nucleus raphe magnus; py, pyramidal tracts. The scale bar = 1 mm.
Figure 2.
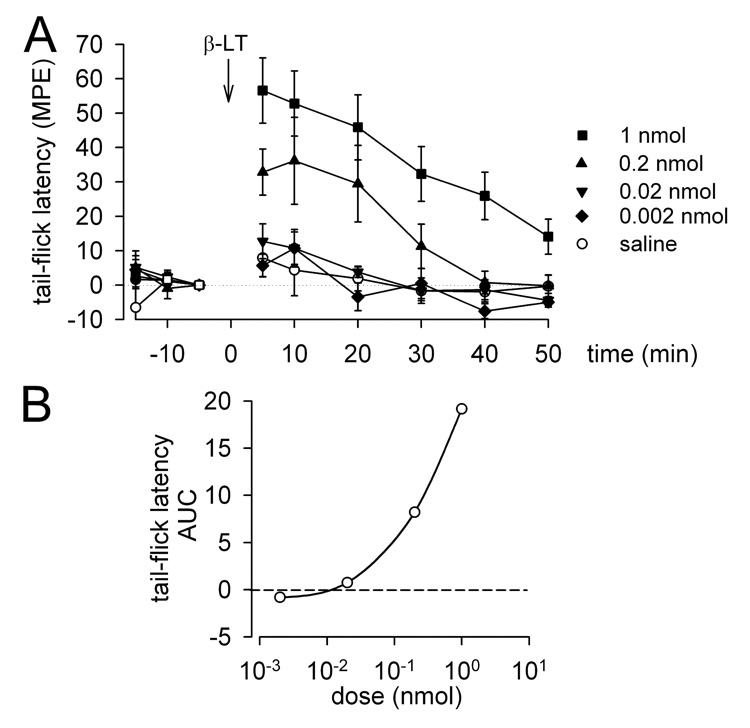
Tail-flick latencies (mean MPE ± SEM) were determined before (−15, −10 and −5 min) and after (5, 10, 20, 30, 40 and 50 min) RVM microinjections. A) Time course of effects produced by saline or β-LT (2 pmol, 20 pmol, 0.2 nmol, or 1 nmol; n = 4–16). B) Dose-response relationship for the effects of β-LT in the tail-flick test represented by mean area under the curve (AUC) between 5 and 50 min.
Microinjection of the NTR1 antagonist SR48692 (3 fmol) into the RVM 5 min before microinjection of β-LT (1 nmol) at the same site did not reduce the antinociceptive effects of β- LT (n = 4) compared to animals in which saline vehicle was microinjected in the RVM 5 min before β-LT (n = 9; P = 0.616; Fig 3A).
Figure 3.
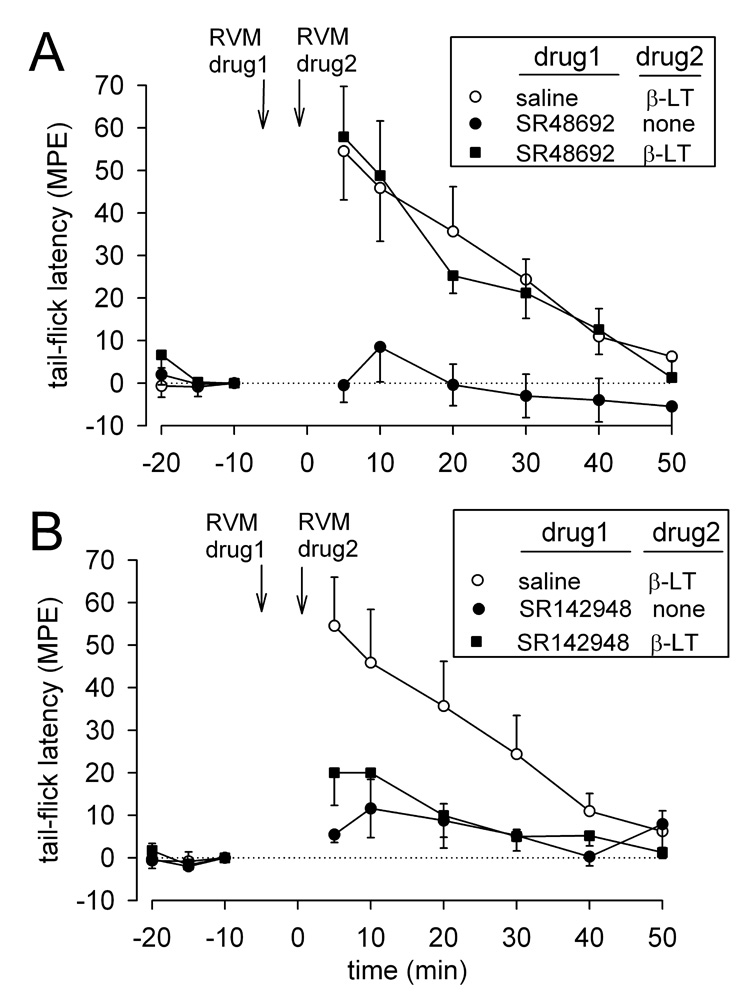
Tail-flick latencies (mean MPE ± SEM ) before (−20, −15 and −10 min) and after (5, 10, 20, 30, 40 and 50 min) pretreatment with A) the NTR1 antagonist SR48692, 3 fmol (n = 4), or B) the mixed NTR 1/2 antagonist SR142948, 0.3 fmol (n = 9), microinjected in the RVM followed by microinjection of β-LT (1 nmol). Pretreatment with saline (n = 9), SR48692 alone (n = 8) and SR142948 alone (n = 4) are also shown.
We also tested the effects of pre-treatment with the NTR1/2 mixed antagonist SR142948 (0.3 fmol) on β-LT-produced antinociception. The 0.3 fmol dose of SR142948 microinjected into the RVM 5 min before microinjection of β-LT (1 nmol) at the same site significantly reduced the antinociceptive effects of β-LT (n = 9) compared to animals in which saline vehicle was microinjected in the RVM 5 min before β-LT (n = 9; P = 0.045; Fig.3B). The 3 fmol dose of SR142948 was not tested against β-LT because the antagonist given alone, at this dose, produced significant increases in response latencies (32.54 % ± 0.09% MPE at 10 min, n = 5).
Because the antinociceptive effects of β-LT could be confounded by motor effects, we examined the effects of β-LT on motor performance. There was no statistically significant difference (Student’s t-test, P = 0.36) in motor performance at 10 and 20 min (the time of peak antinociceptive effect) after microinjection of 1 nmol β-LT (n = 3; −1.67 ± 2.03 degree change in maximum angle of inclination before and after drug injection) compared with saline (n = 5; −0.60 ± 1.33 degree change). However, at greater doses (2 nmol), all animals exhibited statistically significant (Student’s t-test, P = 0.01) motor deficits (n = 3; −12.5 ± 4.5 degree change). Accordingly, the antinociceptive effect of β-LT doses ≤1 nmol are not confounded by potential effects on motor performance.
Microinjection of β-LT in the RVM produces injection-site specific thermal antinociception in the paw
The antinociceptive effect of β-LT was also determined using paw-withdrawal responses to a noxious thermal stimulus. As opposed to the effects seen in the tail flick assay, only minor effects were observed at doses that did not produce motor effects: the mean MPE ± SEM at 10 minutes was 14.5 ±4.3% for 0.2 pmol, 11.9 ± 9.8 % for 2 pmol, 1.9 ± 10.8% for 20 pmol, 20.1 +/− 14.7% for 200 pmol, and 25.3 ± 11.0 for 1 nmol (n = 3 – 15). These smaller effects were due primarily to the fact that while microinjection of β-LT in all regions of the RVM (bregma levels −11.5 to −10.0) consistently produced strong antinociception in the tail flick test, strong antinociception in the paw withdrawal test was not seen at all levels within the RVM. Only 10% (2/20) of β-LT microinjections (0.02-1 nmol) between −10.8 and –10.0 caudal to bregma resulted in antinociception of >40% MPE in the paw withdrawal test, compared to 46% (13/28) in the tail flick test. However, microinjections between −11.5 and −10.8 caudal to bregma produced similar analgesia in both tests, with 43% (10/23) of microinjections producing strong antinociception for paw withdrawal and 57% (16/28) for tail flick. Because of the smaller number of animals responding with antinociception in the paw withdrawal test, intrathecal antagonist experiments were performed using the tail withdrawal test.
β-LT-produced antinociception is blocked by yohimbine, but not by methysergide
I.t. injection of yohimbine (30 µg/10 µl = 76.7 nmol) significantly reduced the increase in tail withdrawal responses by 53% (n = 4; P = 0.05; Fig. 4B) at 15 min after microinjection of β-LT (1 nmol) in the RVM. In contrast, i.t. injection of saline 15 min after microinjection of β- LT (1 nmol, n = 7) in the RVM did not significantly reduce the antinociceptive effect of β-LT (Fig. 4A–C). Significant reversal of β-LT-produced antinociception was also achieved with i.t injection of a combination of methysergide and yohimbine (64% reduction; 30 µg/10 µl each; n = 4; P = 0.022; Fig. 4A), although i.t. injection of methysergide alone (30 µg/10 µl = 63.9 nmol) did not reduce the antinociception produced by β-LT (n = 7; P = 0.653; Fig. 4C). Similarly, i.t. injection of methysergide or the α2-adrenoceptor antagonist yohimbine alone or in combination did not affect tail withdrawal latencies (n = 4–6; P = 0.191; Fig 4–6). Intrathecal antagonist doses were chosen as described in Fang and Proudfit, 1996. Sites in the medulla at which 1 nmol β-LT (Fig 1C) was injected are indicated on illustrations of transverse-sections through the brainstem between −11.5 and −10.0 mm caudal to bregma.
Figure 4.
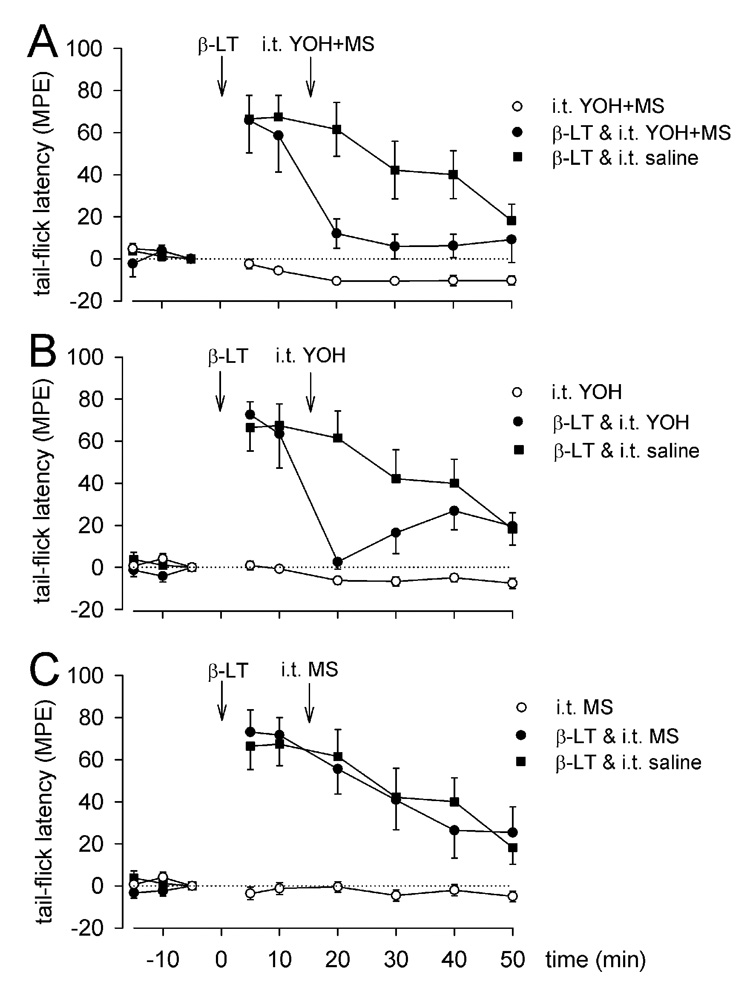
Effect of i.t. injection of selective monoamine antagonists on β-LT-induced antinociception. Mean MPE ± SEM tail-flick latencies before (−15, −10, −5 min) and after (5 – 50 min) microinjection of saline or β-LT (1 nmol) in the RVM and intrathecal administration of saline (n = 7), A) methysergide and yohimbine (n= 4), B) yohimbine (n = 4), or C) methysergide (n = 7). Intrathecal agents were given in a dose of 30µg/10µl each and as intrathecal controls (n = 4–6).
Figure 6.
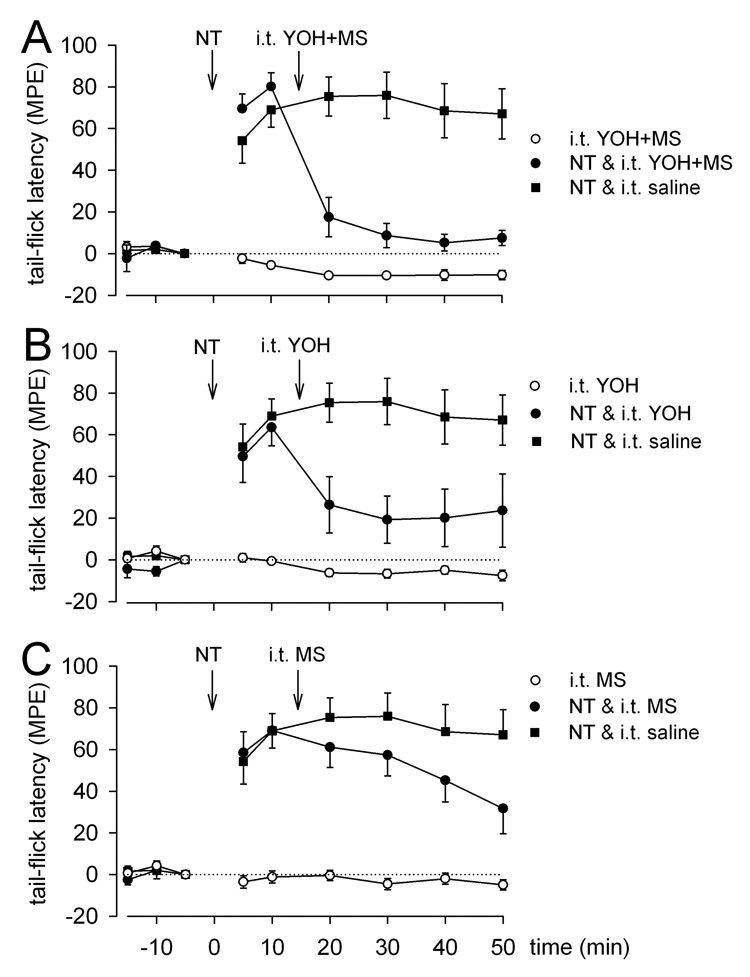
Effect of i.t. injection of selective monoamine antagonists on NT-induced antinociception. Mean MPE ± SEM tail-flick latencies before (−15, −10, −5 min) and after (5 – 50 min) microinjection of saline or NT (3 nmol) in the RVM and intrathecal administration of saline (n = 10) A) methysergide and yohimbine (n = 6), B) yohimbine (n = 7), or C) methysergide (n = 9). Intrathecal agents were given in a dose of 30µg/10µl each and as intrathecal controls (n = 4–6).
A selective NTR1 agonist produces antinociception that is blocked by methysergide and yohimbine
We previously reported that i.t. injection of the serotonin antagonist methysergide partially antagonizes the antinociceptive effect produced by RVM microinjection of NT and the selective NTR1 agonist PD149163 (Buhler et al. 2005). Because this antagonism is not complete, we investigated whether spinal release of norepinephrine contributes to the antinociceptive effect of NTR1 agonists. Sites in the medulla at which 0.5 pmol PD149163 (Fig 1B) was injected are indicated on illustrations of transverse-sections through the brainstem between −11.5 and −10.0 mm caudal to bregma.
Statistically significant reversal of PD149163-produced antinociception was achieved with i.t. injection of methysergide (30 µg/10 µl; n = 7; P = 0.038; Fig. 5C), yohimbine (30 µg/10 µl; n = 9; P = 0.015; Fig. 5B), or a combination of methysergide and yohimbine (30 µg/10 µl each; n = 5; P = 0.001; Fig. 5A), given15 min after microinjection of PD149163 (0.5 pmol) in the RVM. By contrast, i.t. injection of saline 15 min after microinjection of PD149163 (0.5 pmol; n = 9) in the RVM did not reduce the antinociceptive effect of PD149163 (Fig. 5A–C).
Figure 5.
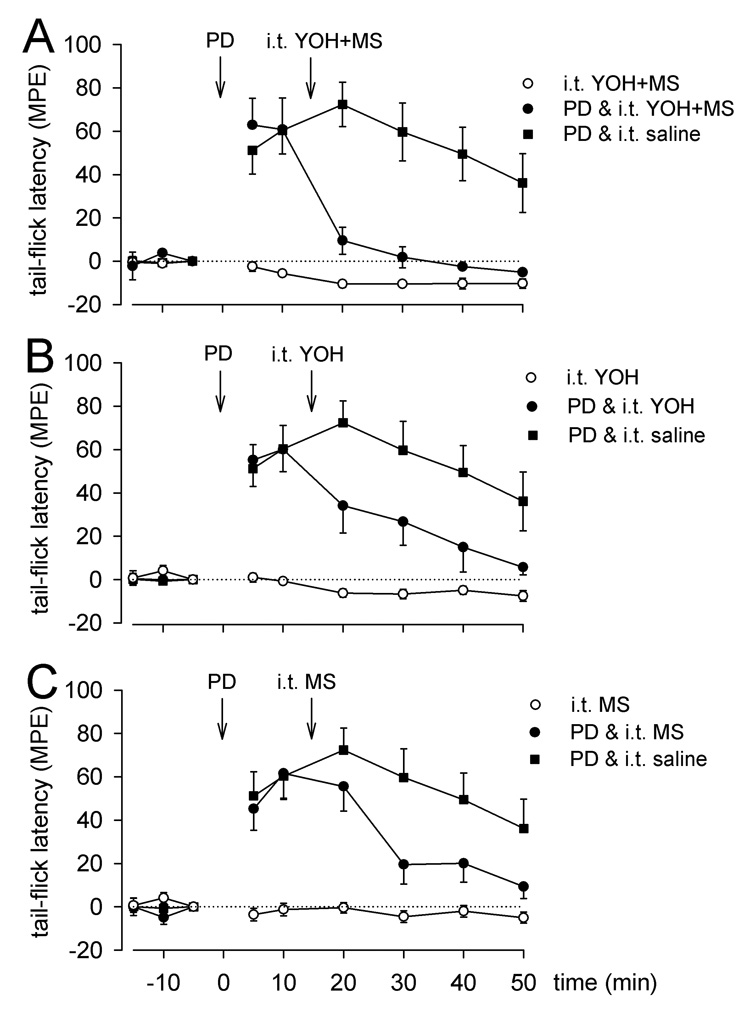
Effect of i.t. injection of selective monoamine antagonists on PD149163-induced antinociception. Mean MPE ± SEM tail-flick latencies before (−15, −10, −5 min) and after (5 – 50 min) microinjection of saline or PD149163 (PD, 0.5 pmol) in the RVM and intrathecal administration of saline (n = 9), A) methysergide and yohimbine (n = 5), B) yohimbine (n = 9), or C) methysergide (n = 7). Intrathecal agents were given in a dose of 30µg/10µl each and as intrathecal controls (n = 4–6).
NT produces antinociception that is blocked by i.t. methysergide and yohimbine
The possibility that spinal cord release of serotonin and/or norepinephrine mediate the antinociception produced by microinjection of NT in the RVM was examined using i.t. injection of selective antagonists of serotonin and/or α2-adrenoceptors. Sites in the medulla at which 3 nmol NT (Fig 1A) was injected are indicated on illustrations of transverse sections through the brainstem between −11.5 and −10.0 mm caudal to bregma. “Statistically significant reversal of NT-produced antinociception was achieved with i.t. injection of yohimbine (30 µg/10 µl; n = 7; P = 0.008; Fig. 6B). In these experiments i.t. injection of methysergide (30 µg/10 µl), 15 min after microinjection of NT (3 nmol) did not produce a significant reversal when measured up to 50 minutes (n = 9; P = 0.125; Fig. 6C). Previous studies have shown, however, that when followed up to 70 minutes, this reversal does reach significance (Buhler et al. 2005). A combination of both methysergide and yohimbine produced a slightly greater reversal of NT-induced antinociception (30 µg/10 µl each; n=6; P = 0.010; Fig. 6A) than yohimbine alone. In contrast, i.t. injection of saline 15 min after microinjection of NT (3 nmol, n = 10) in the RVM did not reverse the antinociceptive effect of NT (Fig. 6A–C).
Methysergide block of the antinociception induced by intra-RVM NTR agonists is not site-dependent
I.t. injection of the serotonin antagonist methysergide reduced the antinociception produced by microinjection of the selective NTR1 agonist PD149163 in the RVM (Fig. 5C), but had no significant effect on the antinociception produced by similar injections of NT (Fig. 6C) or the selective NTR2 agonist β-LT (Fig 4C). It is possible that the differences in methysergide-sensitivity seen for the three NTR agonists tested in the tail-withdrawal test were a result of small differences in the sites in the RVM at which these agonists were microinjected. This possibility was examined by determining the percent inhibition by methysergide of the antinociception produced by microinjection of NTR agonists in different regions of the RVM. The percent inhibition of NTR agonist-induced antinociception was plotted against the level of each injection site (within the limits of bregma −11.5 and −10.0). There was no correlation between site of NTR agonist injection within the RVM and the percent reduction in the agonist-induced responses (R2 = 0.014), which suggests that the location of RVM microinjection sites is not a factor in determining the effectiveness of i.t. methysergide.
DISCUSSION
This study provides evidence that selective agonists of either NTR1 or NTR2 in the RVM both produce antinociception in the thermal tail-withdrawal test, and that while selective activation of NTR1 produces antinociception through pathways that release both serotonin and NE in the spinal cord, selective activation of NTR2 produces antinociception that is predominately mediated by a pathway(s) that releases NE in the spinal cord. These conclusions are supported by anatomical evidence that NTR1, but not NTR2, is expressed on spinally-projecting serotonergic neurons in the RVM (Buhler et al. 2005). These results also suggest that the NE release produced by NTR2 agonists strongly modulates cutaneous nociception in the tail, but only weakly modulates that in the paw, which suggests that nociception in some cutaneous receptive fields can be modulated independently by descending pathways from the brainstem.
β-LT is a selective NTR2 agonist in the RVM
While previous studies have characterized β-LT as selective for NTR2 relative to NTR1 (Yamauchi et al. 2003a;Yamauchi et al. 2003b) and a weak inhibitor of angiotensin-I converting enzyme (IC50= 1153.2 µM) (Mullally et al. 1996), a more thorough investigation of its binding properties has not been undertaken. This study reports that β-LT has no significant binding affinity (Ki <10,000 nM) at any receptor tested other than the α2B adrenoceptor. However, it is unlikely that the antinociceptive actions of β-LT reported here are mediated by α2B adrenoceptors because: 1) multiple mRNA studies have reported that α2B adrenoceptors are not expressed in the rat RVM [see (Winzer-Serhan and Leslie 1997)] and 2) the antinociceptive effect is blocked by an NTR2 antagonist (Zeng and Lynch 1991;Nicholas et al. 1993;Scheinin et al. 1994). However, in the absence of a selective α2B adrenoceptor antagonist, it is not currently feasible to directly disprove the involvement of α2B adrenoceptors in these experiments. Clearly, one must be cautious when attributing NTR2 selective effects to this agent when using systemic administration. For example, it is possible that at least part of the hypertensive effect of intravenous βL (Yamauchi et al. 2003b) is mediated through the α2B adrenoceptor, which mediates the postjunctional pressor response in rodents (Guimaraes and Moura 2001). Although binding studies indicate that β-LT at the concentration used in these studies (1 nmol), has some affinity for the hNTR1, there are several lines of evidence that argue against a role for the adult rNTR1 in the antinociceptive effect of β-LT reported here. First, pretreatment with the selective NTR1 antagonist SR48692 (3 fmol) did not reduce the effect of β-LT, while pretreatment with the NTR1/2 mixed antagonist SR142948A (0.3 fmol) did, suggesting that β-LT-induced antinociception is mediated by NTR2, not NTR1. Second, the antinociceptive effect of β-LT is not altered in NTR1 antisense knock-down mice, but is completely lacking in NTR2 antisense knock-down mice (Yamauchi 1273). Third, if the antinociceptive effect of β-LT is mediated in part by NTR1, such effect should have been reduced by i.t. injection of the serotonin antagonist methysergide, since the antinociception produced by the selective NTR1 agonist PD149163 was reduced by methysergide.
Activation of NTR2 in the RVM produces thermal antinociception in the tail withdrawal test
Microinjection of β-LT into all levels of the RVM produced dose-dependent antinociception in the thermal tail-withdrawal test. The conclusion that NTR2 agonists can produce antinociception is consistent with several previous studies that demonstrated NTinduced antinociception is mediated in part by NTR2: 1) The antinociceptive effect of intracerebroventricular (ICV) injection of β-LT is lost in NTR2 antisense-treated mice with reduced expression of NTR2 (Yamauchi et al. 2003a); 2) Mice deficient in NTR2 expression exhibit reduced NT-mediated antinociception using a noxious chemical stimulus writhing test (Dubuc et al. 1999b;Remaury et al. 2002); and 3) intra-RVM microinjection of the non-selective NTR2 partial agonist levocabastine produces antinociception in the tail flick test ( Smith et al. 1997).
β-LT produces thermal antinociception in the hindpaw only when microinjected in the caudal RVM
The antinociceptive effect of β-LT in the thermal paw-withdrawal test was significantly less than that in the tail withdrawal test and significant antinociception in the hindpaw was evident only when β-LT was microinjected into the caudal RVM. This result is consistent with the report that ICV injection of the non-selective NTR2 partial agonist levocabastine does not produce significant antinociception in the hot plate test (Dubuc et al. 1999a). Similar differences in the modulation of nociceptive responses in the tail and hindpaws have been previously documented. For example, morphine microinjected in the PAG produces substantial antinociception measured by the tail withdrawal test, but only weak antinociception measured by the foot withdrawal test (Fang and Proudfit 1998). These observations were attributed to differences in the modulatory effects of spinally-released norepinephrine on nociceptive responses of the tail and the hindpaws. More specifically, spinal α2–adrenoceptors mediate the antinociceptive effect of morphine injected into the PAG in the tail-withdrawal, but not in the paw-withdrawal test (Brodie and Proudfit 1986;Fang and Proudfit 1996). Similarly, the antinociceptive effect of microinjecting the cholinergic agonist carbachol (Brodie and Proudfit 1986) or morphine (Jensen and Yaksh 1986) into the RVM is mediated by spinal α2– adrenoceptors when the tail withdrawal test is used, but not the hot plate test. Finally, intrathecally injected α2–adrenoceptor agonists are much more efficacious in reducing nociceptive responses from the tail than those from the hindpaws (Graham et al. 1997). These observations indicate that nociceptive responses from cutaneous tissue of the hindpaws are not as effectively modulated by the release of norepinephrine in the spinal cord dorsal horn as those responses from tail skin (Graham et al. 1997). That NE-mediated antinociceptive circuits have minimal effect on paw withdrawal latencies may explain why the (mainly yohimbine sensitive) NTR2 tail antinociception reported here does not generally coincide with paw antinociception.
The antinociception produced by activation of both NTR1 and NTR2 in the RVM is mediated by the spinal cord release of norepinephrine
We previously reported that the antinociception produced by NTR1 activation in the RVM is mediated at least in part by release of serotonin in the spinal cord, but the incomplete block by the serotonin antagonist methysergide suggested that additional spinal transmitters may be involved. While the neurotransmitter identity of NTR2-expressing neurons is still unclear, the results reported here suggest that activation of both NTR1 and NTR2 results in activation of descending noradrenergic neurons and spinal release of NE, as previously suggested by Naranjo (Naranjo et al. 1989).
The source of the NE released by NTR1 and NTR2 agonists is unknown, but since the RVM does not contain noradrenergic neurons, microinjection of NTR agonists in the RVM must activate neurons that project either directly or indirectly to brainstem noradrenergic nuclei such as the A7, A5, or locus coeruleus (Proudfit 2002). Several lines of evidence indicate that spinally-projecting NE neurons in the A7 catecholamine cell group most likely mediate part of the antinociception produced by microinjection of NTR agonists in the RVM. The antinociception produced by stimulation of neurons in the RVM has been proposed to be mediated in part by neurons that project to, and activate, spinally-projecting noradrenergic neurons in the A7 catecholamine cell group (Proudfit HK 1992). More recent anatomical and pharmacological evidence provides additional support for this conclusion. First, anterograde tracer studies combined with immunocytochemical identification of noradrenergic A7 neurons demonstrate that a large number of neurons in the RVM, including the nucleus raphe magnus and the nucleus reticularis gigantocellularis pars α project to the A7 cell group and the surrounding area of the dorsolateral pontine tegmentum (Holden and Proudfit 1998; Clark and Proudfit 1991). Second, electrical (Barbaro et al. 1985;Aimone and Gebhart 1987) or chemical (Jensen and Yaksh 1984;Jensen and Yaksh 1986;Brodie and Proudfit 1986;Iwamoto and Marion 1993) stimulation of neurons in the RVM produces antinociception that can be blocked by intrathecal injection of α2-adrenoceptor antagonists. Finally, the antinociception produced by stimulating sites in the RVM can be reduced by microinjecting tetracaine, a local anesthetic, or cobalt chloride, a divalent cation that blocks synaptic transmission, into the A7 cell group (Nuseir et al. 1999).
The finding that NTR1, but not NTR2-mediated antinociception is in part mediated through serotonin release in the spinal cord is consistent with our previous report that NTR1 is expressed almost exclusively on serotonergic neurons in RVM, about 50% of which project directly to the dorsal horn of the spinal cord, and NTR2 is expressed on non-serotonergic RVM neurons (Buhler et al. 2005). Furthermore, NT excites serotonergic NRM neurons in culture (Li et al. 2001) and NTR agonists would be expected to activate spinally-projecting serotonergic neurons in vivo.
Conclusions
These experiments characterized the antinociceptive activity of the NTR2 agonist β-LT in the RVM and investigated the spinal transmitters involved in NTR1 and NTR2-mediated antinociception. We report that NTR1-mediated antinociception is mediated by spinal release of serotonin and NE, while NTR2-mediated antinociception involves spinal release of NE only. This suggests that while both NTR1 and NTR2 in the RVM can produce thermal antinociception in the tail withdrawal assay, activation of both NTR receptors by non-selective agonists such as NT produces antinociception mediated by at least two distinct descending inhibitory pathways.
ACKNOWLEDGEMENTS
This work was supported by NIH grants DA 02879-27 (GFG), DA 03980-22 (HKP), an ASPET-Merck Fellowship in Integrative Pharmacology (AVB), and an Ortho-McNeil Janssen Scientific Affairs — AFPE — AACP New Investigator Grant for Pharmacy Faculty (AVB). We thank Shirley Knapp, Mohamed Karim and Lynn Burns for their assistance with animal surgeries and immunohistochemistry, and Jamie Driscoll and Bryan Roth at the NIMH Psychoactive Drug Screening Program for their expert work on the binding assays. In addition, we thank Mike Burcham for assistance with graphics and Barbara Olsen for proofreading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Aimone LD, Gebhart GF. Spinal monoamine mediation of stimulation-produced antinociception from the lateral hypothalamus. Brain Research. 1987;403:290–300. doi: 10.1016/0006-8993(87)90066-7. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Hammond DL, Fields HL. Effects of intrathecally administered methysergide and yohimbine on microstimulation-produced antinociception in the rat. Brain Research. 1985;343:223–229. doi: 10.1016/0006-8993(85)90738-3. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Pert A. A mechanism for the analgesic effect of neurotensin as revealed by behavioral and electrophysiological techniques. Brain Research. 1984;324:35–47. doi: 10.1016/0006-8993(84)90619-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Proudfit HK. Antinociception induced by local injections of carbachol into the nucleus raphe magnus in rats: alteration by intrathecal injection of monoaminergic antagonists. Brain Research. 1986;371:70–79. doi: 10.1016/0006-8993(86)90811-5. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Choi J, Proudfit HK, Gebhart GF. Neurotensin activation of the NTR1 on spinally-projecting serotonergic neurons in the rostral ventromedial medulla is antinociceptive. Pain. 2005;114:285–294. doi: 10.1016/j.pain.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Letters. 1996;386:91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- Clark FM, Proudfit HK. Projections of neurons in the ventromedial medulla to pontine catecholamine cell groups involved in the modulation of nociception. Brain Research. 1991;540:105–115. doi: 10.1016/0006-8993(91)90496-i. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Costentin J, Terranova JP, Barnouin MC, Soubrie P, Le Fur G, Rostene W, Kitabgi P. The nonpeptide neurotensin antagonist, SR 48692, used as a tool to reveal putative neurotensin receptor subtypes. British Journal of Pharmacology. 1994;112:352–354. doi: 10.1111/j.1476-5381.1994.tb13077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc I, Remande S, Costentin J. The partial agonist properties of levocabastine in neurotensin-induced analgesia. European Journal of Pharmacology. 1999a;381:9–12. doi: 10.1016/s0014-2999(99)00554-3. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Sarret P, Labbe-Jullie C, Botto JM, Honore E, Bourdel E, Martinez J, Costentin J, Vincent JP, Kitabgi P, Mazella J. Identification of the receptor subtype involved in the analgesic effect of neurotensin. Journal of Neuroscience. 1999b;19:503–510. doi: 10.1523/JNEUROSCI.19-01-00503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Proudfit HK. Spinal cholinergic and monoamine receptors mediate the antinociceptive effect of morphine microinjected in the periaqueductal gray on the rat tail, but not the feet. Brain Research. 1996;722:95–108. doi: 10.1016/0006-8993(96)00198-9. [DOI] [PubMed] [Google Scholar]
- Fang F, Proudfit HK. Antinociception produced by microinjection of morphine in the rat periaqueductal gray is enhanced in the foot, but not the tail, by intrathecal injection of alpha1-adrenoceptor antagonists. Brain Research. 1998;790:14–24. doi: 10.1016/s0006-8993(97)01441-8. [DOI] [PubMed] [Google Scholar]
- Fang FG, Moreau JL, Fields HL. Dose-dependent antinociceptive action of neurotensin microinjected into the rostroventromedial medulla of the rat. Brain Research. 1987;420:171–174. doi: 10.1016/0006-8993(87)90255-1. [DOI] [PubMed] [Google Scholar]
- Gendron L, Perron A, Payet MD, Gallo-Payet N, Sarret P, Beaudet A. Low-affinity neurotensin receptor (NTS2) signaling: internalization-dependent activation of extracellular signal-regulated kinases 1/2. Molecular Pharmacology. 2004;66:1421–1430. doi: 10.1124/mol.104.002303. [DOI] [PubMed] [Google Scholar]
- Graham BA, Hammond DL, Proudfit HK. Differences in the antinociceptive effects of alpha-2 adrenoceptor agonists in two substrains of Sprague-Dawley rats. Journal of pharmacology & Experimental Therapeutics. 1997;283:511–519. [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacological Reviews. 2001;53:319–356. [PubMed] [Google Scholar]
- Holden JE, Proudfit HK. Enkephalin neurons that project to the A7 catecholamine cell group are located in nuclei that modulate nociception: ventromedial medulla. Neuroscience. 1998;83:929–947. doi: 10.1016/s0306-4522(97)00437-5. [DOI] [PubMed] [Google Scholar]
- Holmes BB, Rady JJ, Smith DJ, Fujimoto JM. Supraspinal neurotensin-induced antianalgesia in mice is mediated by spinal cholecystokinin. Japanese Journal of Pharmacology. 1999;79:141–149. doi: 10.1254/jjp.79.141. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Marion L. Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. Journal of Pharmacology & Experimental Therapeutics. 1993;265:777–789. [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Research. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Research. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Kitabgi P, Rostene W, Dussaillant M, Schotte A, Laduron PM, Vincent JP. Two populations of neurotensin binding sites in murine brain: discrimination by the antihistamine levocabastine reveals markedly different radioautographic distribution. European Journal of Pharmacology. 1987;140:285–293. doi: 10.1016/0014-2999(87)90285-8. [DOI] [PubMed] [Google Scholar]
- Li AH, Yeh TH, Tan PP, Hwang HM, Wang HL. Neurotensin excitation of serotonergic neurons in the rat nucleus raphe magnus: ionic and molecular mechanisms. Neuropharmacology. 2001;40:1073–1083. doi: 10.1016/s0028-3908(01)00030-2. [DOI] [PubMed] [Google Scholar]
- Mobarakeh JI, Sakurada S, Hayashi T, Orito T, Okuyama K, Sakurada T, Kuramasu A, Watanabe T, Watanabe T, Yanai K. Enhanced antinociception by intrathecally-administered morphine in histamine H1 receptor gene knockout mice. Neuropharmacology. 2002;42:1079–1088. doi: 10.1016/s0028-3908(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Mullally MM, Meisel H, FitzGerald RJ. Synthetic peptides corresponding to alpha-lactalbumin and beta-lactoglobulin sequences with angiotensin-I-converting enzyme inhibitory activity. Biological Chemistry Hoppe-Seyler. 1996;377:259–260. doi: 10.1515/bchm3.1996.377.4.259. [DOI] [PubMed] [Google Scholar]
- Myers RD. Injection of solutions into cerebral tissue: relation between volume and diffusion. Physiology & Behavior. 1966;1:171–174. [Google Scholar]
- Naranjo JR, Arnedo A, Molinero MT, Del Rio J. Involvement of spinal monoaminergic pathways in antinociception produced by substance P and neurotensin in rodents. Neuropharmacology. 1989;28:291–298. doi: 10.1016/0028-3908(89)90106-8. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Heidenreich BA, Proudfit HK. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic neurons in the A7 catecholamine cell group. Brain Research. 1999;822:1–7. doi: 10.1016/s0006-8993(98)01195-0. [DOI] [PubMed] [Google Scholar]
- Palacios JM, Wamsley JK, Kuhar MJ. The distribution of histamine H1-receptors in the rat brain: an autoradiographic study. Neuroscience. 1981;6:15–37. doi: 10.1016/0306-4522(81)90240-2. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Mallorga PJ, Pascarella DM, Snyder MA, Williams JB, Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. Journal of Pharmacology & Experimental Therapeutics. 2002;300:305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in Neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Proudfit HK. The behavioural pharmacology of the noradrenergic descending system. In: Besson J-M, editor. Toward the Use of Noradrenergic Agonists for the Treatment of Pain. Amsterdam: Elsevier; 1992. pp. 119–136. [Google Scholar]
- Proudfit HK. The Challenge of Defining Brainstem Pain Modulation Circuits. Journal of Pain. 2002;3:350–354. doi: 10.1054/jpai.2002.127777. [DOI] [PubMed] [Google Scholar]
- Remaury A, Vita N, Gendreau S, Jung M, Arnone M, Poncelet M, Culouscou JM, Le Fur G, Soubrie P, Caput D, Shire D, Kopf M, Ferrara P. Targeted inactivation of the neurotensin type 1 receptor reveals its role in body temperature control and feeding behavior but not in analgesia. Brain Research. 2002;953:63–72. doi: 10.1016/s0006-8993(02)03271-7. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. Journal of Neurosurgery. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- Sarhan S, Hitchcock JM, Grauffel CA, Wettstein JG. Comparative antipsychotic profiles of neurotensin and a related systemically active peptide agonist. Peptides. 1997;18:1223–1227. doi: 10.1016/s0196-9781(97)00145-9. [DOI] [PubMed] [Google Scholar]
- Sarret P, Gendron L, Kilian P, Nguyen HM, Gallo-Payet N, Payet MD, Beaudet A. Pharmacology and functional properties of NTS2 neurotensin receptors in cerebellar granule cells. Journal of Biological Chemistry. 2002;277:36233–36243. doi: 10.1074/jbc.M202586200. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical Distribution of NTS2 Neurotensin Receptors in the Rat Central Nervous System. Journal of Comparative Neurology. 2003;461:520–538. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Research. Molecular Brain Research. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schotte A, Leysen JE, Laduron PM. Evidence for a displaceable non-specific [3H]neurotensin binding site in rat brain. Naunyn-Schmiedebergs Archives of Pharmacology. 1986;333:400–405. doi: 10.1007/BF00500016. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Hawranko AA, Monroe PJ, Gully D, Urban MO, Craig CR, Smith JP, Smith DL. Dose-dependent pain-facilitatory and -inhibitory actions of neurotensin are revealed by SR 48692, a nonpeptide neurotensin antagonist: influence on the antinociceptive effect of morphine. Journal of Pharmacology & Experimental Therapeutics. 1997;282:899–908. [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Trudeau LE. Neurotensin regulates intracellular calcium in ventral tegmental area astrocytes: evidence for the involvement of multiple receptors. Neuroscience. 2000;97:293–302. doi: 10.1016/s0306-4522(99)00597-7. [DOI] [PubMed] [Google Scholar]
- Tyler BM, Groshan K, Cusack B, Richelson E. In vivo studies with low doses of levocabastine and diphenhydramine, but not pyrilamine, antagonize neurotensin-mediated antinociception. Brain Research. 1998;787:78–84. doi: 10.1016/s0006-8993(97)01479-0. [DOI] [PubMed] [Google Scholar]
- Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. Journal of Pharmacology & Experimental Therapeutics. 1999;290:207–213. [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Characterization of biphasic modulation of spinal nociceptive transmission by neurotensin in the rat rostral ventromedial medulla. Journal of Neurophysiology. 1997;78:1550–1562. doi: 10.1152/jn.1997.78.3.1550. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Role of neurotensin in the nucleus raphe magnus in opioid-induced antinociception from the periaqueductal gray. Journal of Pharmacology & Experimental Therapeutics. 1993;265:580–586. [PubMed] [Google Scholar]
- Urban MO, Smith DJ, Gebhart GF. Involvement of spinal cholecystokininB receptors in mediating neurotensin hyperalgesia from the medullary nucleus raphe magnus in the rat. Journal of Pharmacology & Experimental Therapeutics. 1996;278:90–96. [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Alpha2B adrenoceptor mRNA expression during rat brain development. Brain Research. Developmental Brain Research. 1997;100:90–100. doi: 10.1016/s0165-3806(97)00035-7. [DOI] [PubMed] [Google Scholar]
- Wustrow DJ, Davis MD, Akunne HC, Corbin AE, Wiley JN, Wise LD, Heffner TG. Reduced amide bond neurotensin 8–13 mimetics with potent in vivo activity. Bioorganic & Medicinal Chemistry Letters. 1995;5:997–1002. [Google Scholar]
- Yamada M, Yamada M, Lombet A, Forgez P, Rostene W. Distinct functional characteristics of levocabastine sensitive rat neurotensin NT2 receptor expressed in chinese hamster ovary cells. Life Sciences. 1998;62:L375–L380. doi: 10.1016/s0024-3205(98)00192-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Sonoda S, Jinsmaa Y, Yoshikawa M. Antinociception induced by beta-lactotensin, a neurotensin agonist peptide derived from beta-lactoglobulin, is mediated by NT2 and D1 receptors. Life Sciences. 2003a;73:1917–1923. doi: 10.1016/s0024-3205(03)00546-0. [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Usui H, Yunden J, Takenaka Y, Tani F, Yoshikawa M. Characterization of beta-lactotensin, a bioactive peptide derived from bovine beta-lactoglobulin, as a neurotensin agonist. Bioscience, Biotechnology & Biochemistry. 2003b;67:940–943. doi: 10.1271/bbb.67.940. [DOI] [PubMed] [Google Scholar]
- Zeng DW, Lynch KR. Distribution of alpha 2-adrenergic receptor mRNAs in the rat CNS. Brain Research. Molecular Brain Research. 1991;10:219–225. doi: 10.1016/0169-328x(91)90064-5. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


