Abstract
Previous studies have shown that substitutions in the Tfg1 or Tfg2 subunits of Saccharomyces cerevisiae transcription factor IIF (TFIIF) can cause upstream shifts in start site utilization, resulting in initiation patterns that more closely resemble those of higher eukaryotes. In this study, we report the results from multiple biochemical assays analyzing the activities of wild-type yeast TFIIF and the TFIIF Tfg1 mutant containing the E346A substitution (Tfg1-E346A). We demonstrate that TFIIF stimulates formation of the first two phosphodiester bonds and dramatically stabilizes a short RNA-DNA hybrid in the RNA polymerase II (RNAPII) active center and, importantly, that the Tfg1-E346A substitution coordinately enhances early bond formation and the processivity of early elongation in vitro. These results are discussed within a proposed model for the role of yeast TFIIF in modulating conformational changes in the RNAPII active center during initiation and early elongation.
Transcription of eukaryotic protein-encoding genes is a multistep process involving the concerted actions of RNA polymerase II (RNAPII) and a host of auxiliary factors that include the general transcription factors (TF)IIB, TFIID, TFIIE, TFIIF, and TFIIH (17, 18, 32). In higher eukaryotes, the architecture of the preinitiation complex (PIC) is believed to be the main determinant for the site of transcription initiation. For promoters that contain a TATA element, transcription typically initiates at a single site within the region that initially undergoes ATP-dependent DNA strand separation approximately 30 base pairs downstream of the TATA element. In striking contrast, mRNA 5′ ends in the yeast Saccharomyces cerevisiae frequently map to multiple sites within an extended window ranging from 45 to 200 base pairs downstream of the TATA element (9, 42). Although the mechanistic basis for this difference remains unknown, results from both in vivo and in vitro studies suggest that both the overall architectures and the relative locations of promoter melting for human and S. cerevisiae PICs are similar (15, 31).
Previous studies in our laboratory and by others have established that RNAPII and the general factors TFIIB and TFIIF play critical roles in determining the positions of the mRNA 5′ ends in S. cerevisiae. Mutations in the highly conserved B finger domain of TFIIB, or in the switch 2 region of the RNAPII Rpb1 subunit, can cause downstream shifts in the positions of mRNA 5′ ends (1, 10, 33, 35, 36, 50). Conversely, mutations in the Rpb2 or the Rpb9 polymerase subunit (5, 21, 43, 52) or in the TFIIF Tfg1 or Tfg2 subunit (14) can confer upstream shifts in start site utilization, resulting in initiation patterns that more closely resemble those of higher eukaryotes.
To date, relatively few studies have been conducted to analyze the functions of S. cerevisiae TFIIF, in contrast to the numerous reports that have demonstrated activities for mammalian TFIIF during multiple steps of the transcription cycle. Mammalian TFIIF has been shown to assist in the recruitment of RNAPII, TFIIE, and TFIIH into the PIC (12, 30), to induce the wrapping of promoter DNA around RNAPII in the PIC (39), to facilitate the efficient escape of RNAPII from the promoter (48), to increase the rate of transcript elongation (22, 44), and in conjunction with the elongation factor TFIIS, to stimulate the rescue of paused elongation complexes (49). Last, the recycling of RNAPII back into the initiation-competent hypophosphorylated form is mediated by TFIIF through its stimulation of the RNAPII C-terminal domain phosphatase Fcp1 (4).
In S. cerevisiae, TFIIF is composed of three protein subunits designated Tfg1, Tfg2, and Tfg3. The largest subunit, Tfg1 (calculated molecular mass, ∼82 kDa), and the second largest subunit, Tfg2 (calculated molecular mass, ∼47 kDa), are homologous to the RAP74 and RAP30 subunits of mammalian TFIIF, respectively, and are essential for cell viability (20). The Tfg3 subunit, also known as Taf14, is associated with at least six yeast nuclear complexes, has no known homologues in higher eukaryotes, and is dispensable for viability (3, 8, 20, 23, 24, 40, 41, 45). As noted above, our previous work identified substitutions in both the Tfg1 and the Tfg2 subunits that cause upstream shifts in start site utilization (14). More recently, we have shown that these TFIIF mutations display genetic interactions with a deletion of the gene encoding the elongation factor TFIIS (PPR2) (11). Although TFIIS has recently been reported to also play a role during initiation (26, 38), these Tfg1 and Tfg2 mutations might coordinately alter TFIIF function during both start site utilization and postinitiation stages of the transcription cycle.
In the work reported here, a series of in vivo and in vitro assays were employed to probe and compare the activities of wild-type and Tfg1-E346A mutant yeast TFIIF during initiation/start site utilization, promoter escape, and early transcript elongation. The results demonstrate two previously unrecognized activities of TFIIF, namely, the stimulation of early phosphodiester bond formation and the stabilization of a short RNA-DNA hybrid in the RNAPII active center. Surprisingly, the Tfg1-E346A substitution was found to coordinately enhance early phosphodiester bond formation and the processivity of early elongation in vitro. We discuss these results within a proposed model for the mechanism of yeast transcription initiation in which TFIIF interaction with RNAPII modulates both early phosphodiester bond formation during start site utilization and the conversion of RNAPII to a more processive elongating form.
MATERIALS AND METHODS
Yeast strains and plasmids.
S. cerevisiae strain FP310 (MATα ura3-52 trp1Δ63 his3Δ200 tfg1-E346A) and its parental strain, FY105 (MATα ura3-52 trp1Δ63 his3Δ200), are derivatives of S288C and were described previously (14). Strain WY9 (MATα ura3-52 his3Δ200 leu2-112 lys2Δ20 ade2 rpb9Δ1::HIS3) (21) was kindly provided by Nancy Woychik.
Plasmid p316/A, containing the yeast ADH1 promoter, has been described previously (10). Plasmid p316/A(−48/43 A→T), containing adenosine-to-thymidine substitutions at positions −48, −46, −45, and −43 (relative to the initiating AUG codon), was generated from the p316/A plasmid using QuikChange mutagenesis (Stratagene). Mutations were confirmed by DNA sequencing (Health Research, Inc., Roswell Park Cancer Institute). The plasmid pADH1/G− was constructed using the megaprimer method of PCR site-directed mutagenesis and contains the first 140 base pairs from the G-less cassette of pAdML-D2 (34) inserted between position −36 of the ADH1 promoter and the downstream EcoRI site in p316/A. A TATA-lacking variant of pADH1/G− was constructed similarly using the p316/D1 plasmid (10). Plasmid pET15b/IIS 1-309 was provided by Caroline Kane.
Purification of yeast transcription factors.
Yeast TBP, TFIIB, TFIIE, and TFIIH and both wild-type and Rpb1-R344A RNAPII were purified as described previously (52). Recombinant hexahistidine-tagged yeast TFIIS was expressed from plasmid pET15b/IIS 1-309 and purified using the same protocol as that for TFIIB. Recombinant yeast wild-type and Tfg1-E346A TFIIFs were expressed in Escherichia coli, and lysates were prepared as described previously (52). The hexahistidine-tagged Tfg1-Tfg2 complexes were then affinity purified from the lysates, using Co2+-activated HiTrap fast-performance liquid chromatography columns (GE Healthcare). A linear imidazole gradient elution from 20 to 250 mM over 10 column volumes was used at a flow rate of 0.5 ml/min, and fractions containing TFIIF were pooled and dialyzed into TFIIF storage buffer F250 (20 mM HEPES-KOH [pH 7.6], 20% glycerol, 250 mM potassium acetate, 1 mM EDTA, 1 mM dithiothreitol [DTT], 500 μg/μl bovine serum albumin, 1 mM phenylmethylsulfonyl fluoride).
Primer extension analysis of in vivo RNA.
For analysis of chromosomal promoters, cultures (50 ml) of strains FY105, FP310, and WY9 were grown at 30°C in YPD medium (1% yeast extract, 2% Bacto peptone, 2% dextrose) or in YPG (1% yeast extract, 2% Bacto peptone, 2% galactose) for GAL1 and GAL10. For analyses of ADH1 promoter mutations, yeast strains FY105 and FP310 were transformed with plasmid p316/A or p316/A(−48/43 A→T), and the transformants were grown at 30°C in CAA-Ura medium (0.6% Casamino acids, 0.68% yeast nitrogen base without amino acids, 2% dextrose, 25 μg/ml of adenine, and 80 μg/ml of tryptophan). Cells were harvested at an optical density at 600 nm of 2.0, and total RNA was isolated as described previously (14). RNA 5′ ends were mapped by primer extension as described previously (37) using AMV reverse transcriptase (Promega) and 32P-labeled primers, as follows: SNR14-PE (5′-ACCAGCAAAAACACAATC-3′), SNR19-PE (5′-TACTATTGGAAGCGCATG-3′), SNR20-PE (5′-AGGTCATTTCAGTTGTTACAC-3′), HTB1-PE (5′-TCAGCTGGGGCTTTGG-3′), GAL1-PE (5′-TCTTCTGAATGAGATTTAGTC-3′), and SPT15-PE (5′-AGCAGTCTAACTAGTAGTCG-3′).
Reconstituted transcription assays.
Transcription reaction mixtures (30 μl) contained 50 mM HEPES-KOH (pH 7.6), 8% glycerol, 5 mM EGTA, 2.5 mM DTT, 80 mM potassium acetate, 10 mM magnesium acetate, 30 mM creatine phosphate, 1.5 units/ml creatine phosphokinase, 0.4% polyvinyl alcohol, 250 ng of plasmid template pADH1/G−, 3.7 pmol TATA-binding protein (TBP), 4 pmol TFIIB, 1 pmol RNAPII, 0.28 pmol TFIIE, 0.12 pmol TFIIH, and 0.87 pmol of either wild-type or Tfg1-E346A TFIIF. Reaction components were incubated at ambient temperature for 15 min before nucleoside triphosphates (NTPs) were added. For direct incorporation reaction mixtures, the NTPs included 50 μM CTP, 10 μCi [α-32P]CTP, 500 μM ATP, UTP, and either 500 μM GTP or 667 μM 3′-O-methyl GTP. Reaction mixtures were incubated for 20 min at ambient temperature, and then reactions were terminated, followed by treatment with RNase T1 and analysis by denaturing electrophoresis as described previously (52). For reactions analyzed by primer extension, labeled CTP was omitted, and the reaction products were analyzed as described previously (52), using a 32P-labeled G-less cassette primer (5′-GTGAGAGTGAATGATGATAGATTTGGGAAA-3′).
Bubble template initiation and runoff assays.
Synthetic bubble80 DNA template was prepared by annealing 1.75 nmol of template strand oligonucleotide with a 10% molar excess of nontemplate strand oligonucleotide (see Fig. 4A) in 20 μl of 25 mM Tris (pH 7.9), 8 mM MgCl2, and 50 mM KCl. Annealing and purification were performed as described previously (25) but with the omission of the Sephadex gel filtration step.
FIG. 4.
Yeast TFIIF stimulates early phosphodiester bond formation, and this activity is increased by the Tfg1-E346A substitution. (A) Structure of the bubble80 template. Highlighted within the bubble are the locations of the template bases that give rise to a 5′-GGA-3′ trinucleotide product in the presence of GTP and radiolabeled ATP. (B) TFIIF stimulation of early phosphodiester bond formation. Reaction mixtures contained the bubble80 template, purified RNAPII, [α-32P]ATP, unlabeled GTP, and purified wild-type or Tfg1-E346A mutant TFIIF as indicated. The 5′-GGA-3′ trinucleotide product is indicated by the arrow. (C) Effect of TFIIF on formation of the third phosphodiester bond. Reactions were performed as described in panel B except that GTP was replaced with the indicated amount of the unlabeled RNA3 oligonucleotide 5′-AGG-3′. The 5′-AGGA-3′ tetranucleotide product is indicated by the arrow. (D) Quantitation of TFIIF effects on early phosphodiester bond formation. Relative band intensities for the 5′-AGG-3′ and 5′-AGGA-3′ products for the gels shown in panels B and C were quantified using Bio-Rad QuantityOne software (n = 3).
Early phosphodiester bond formation reaction mixtures (20 μl) contained 20 mM HEPES-KOH (pH 7.6), 5% glycerol, 50 mM potassium acetate, 7.5 mM magnesium acetate, 2.5 mM DTT, 250 μg/ml bovine serum albumin, 2 units of RiboLock RNase inhibitor (Fermentas), 0.5 pmol bubble80 template, 0.2 pmol RNAPII, and 0.6 pmol of either wild-type or Tfg1-E346A mutant TFIIF. Reactions were initiated by the addition of the indicated concentrations of GTP or RNA3 oligonucleotide (5′-AGG-3′; Dharmacon) and 166 nM [α-32P]ATP, reaction mixtures were incubated for 4 min at ambient temperature, and reactions were terminated with an equal volume of formamide loading buffer. A portion of the reaction products was then immediately loaded onto a denaturing polyacrylamide gel (24% polyacrylamide; 12:1, 7 M urea, 0.6× Tris-borate-EDTA [TBE]), and the gels were run at 45 W constant power for 2.5 h with 0.6× TBE in the upper reservoir and 1.2× TBE in the bottom reservoir.
Bubble template runoff reactions were assembled with the same procedure used for the early phosphodiester bond formation reactions, except they were primed with 0.5 pmol of 5′ end-labeled [32P]RNA5 (5′-AGAGG-3′; ∼1,000,000 dpm/pmol; Dharmacon,). After reaction mixtures were incubated at ambient temperature for 5 min, transcription was initiated by the addition of 50 μM of each NTP and, where indicated, 3 pmol of TFIIS. Transcription was terminated after 20 min, and reaction products were analyzed by denaturing gel electrophoresis, as described above.
Electrophoretic mobility shift assays.
Mobility shift assays were performed as previously described (29), with modifications. Reaction mixtures (20 μl) contained 0.2 pmol RNAPII (wild type or Rpb1-R344A), 0.4 pmol TFIIF (wild type or Tfg1-E346A), and either 0.3 pmol 5′ end-labeled [32P] bubble80 template (∼1,000,000 dpm/pmol) or 0.3 pmol unlabeled template and 0.5 pmol of 5′ end-labeled [32P]RNA5. After a 20-min incubation at ambient temperature, half of each reaction mixture was resolved on a native 5% polyacrylamide gel (30:1), with the gel run at 4°C in 25 mM Tris and 190 mM glycine (pH 8.3) at 200 V for 3 h.
RESULTS
The TFIIF Tfg1-E346A substitution causes upstream shifts in start site utilization on a variety of promoters in vivo.
Our previous characterization of the effects of Tfg1 and Tfg2 mutations on start site utilization patterns involved analyzing the ADH1 and CYC1 promoters (14). Since the effects of TFIIB mutations on start site utilization have been shown to be promoter specific (2, 10), we sought to determine whether upstream shifts conferred by TFIIF mutations, or by deletion of the Rpb9 polymerase subunit, are promoter specific. Using primer extension analyses to map in vivo mRNA 5′ ends for a larger set of genes, the results demonstrated that, in addition to ADH1 and CYC1, both the Tfg1-E346A substitution and the Rpb9 deletion caused upstream shifts on all 10 additional promoters examined, suggesting that the effects of these mutations are not promoter specific (Fig. 1). The promoters analyzed included those for the protein-encoding genes HTB1, GAL1, and SPT15 and, interestingly, for the RNAPII-dependent promoters directing the synthesis of spliceosomal snRNAs (SNR14, SNR19, and SNR20/LSR1). Upstream shifts in start site utilization were also observed with the GAL10, HIS3, HIS4, and SNR7 promoters (data not shown). The degree of the upstream shifts varied with the individual promoters, with the Tfg1-E346A substitution always conferring more pronounced shifts than the Rpb9 deletion. Notably, however, the positions of the upstream start sites (purines) were the same in both the tfg1-E346A and the rpb9Δ strains, suggesting that the underlying mechanism affecting start site utilization in these mutants is related.
FIG. 1.
The TFIIF Tfg1-E346A substitution causes upstream shifts in start site utilization on a variety of promoters in vivo. (A) Primer extension analysis of in vivo RNA. Total RNA isolated from yeast strains containing the indicated TFG1 and RPB9 alleles was analyzed by primer extension utilizing oligonucleotide primers specific for the indicated RNA transcripts. The positions of the major wild-type (WT) transcription start sites are indicated by the filled circles, while the open circles indicate the positions of upstream start sites exhibiting enhanced utilization in the mutant strains. (B) Shown are the promoter sequences for the above-described genes with the TATA elements underlined and the positions of the normal and upstream start sites indicated as in panel A.
Yeast PICs can encounter and utilize downstream start sites by a mechanism that does not require contiguous intervening RNA synthesis and that is not compromised by the Tfg1-E346A substitution.
The mechanism underlying the ability of the S. cerevisiae RNAPII machinery to generate mRNA 5′ ends up to 200 base pairs downstream from the site of the PIC assembly remains unclear. In vivo studies from a number of laboratories support the view that the RNAPII machinery undergoes a unidirectional “scanning” or translocation process that emanates from the site of yeast PIC assembly toward the downstream region (15, 27, 33, 36). However, these in vivo analyses have been unable to distinguish between two potential mechanisms by which the RNAPII machinery comes into contact with the downstream start sites, namely, (i) an energy-dependent but transcription-independent translocation and/or (ii) a transcription-dependent translocation coupled to abortive initiation and reinitiation at the downstream site. To determine whether the sequences upstream of the normal start sites in yeast have to be transcribed in order for RNAPII to arrive at downstream start sites, we analyzed transcription initiation in vitro by using reconstituted yeast PICs and a modified ADH1 template containing a 140-base-pair G-less cassette fused immediately downstream of the normal ADH1 −38 in vivo start site (Fig. 2A). For these assays, two complementary approaches for RNA analysis were used. In the first approach, [α-32P]CTP was used to directly label the nascent transcripts, and the reaction products were treated with RNase T1 so that only the G-less transcripts would be visualized after electrophoresis (Fig. 2C). In the second approach, nonlabeled transcripts were analyzed by primer extension utilizing a radiolabeled primer specific to the G-less cassette (Fig. 2D). Since there are five guanines residing within the region upstream of the −38 start site (Fig. 2A), we reasoned that if yeast PICs are able to translocate promoter DNA in the absence of RNA synthesis in order to arrive at the downstream initiation sites, then the omission of GTP from the NTP mixture should allow for efficient initiation within the G-less cassette. Alternatively, if contiguous transcription of the upstream promoter sequence is absolutely required, then initiation within the G-less cassette should not occur in the absence of GTP. In reconstituted reactions using wild-type TFIIF, efficient initiation at the −38 start site and within the G-less cassette was observed in the absence of GTP and presence of 3′-O-methyl GTP (Fig. 2C and D, lanes 1 and 2). Utilization of these sites was absolutely dependent on the presence of the functional TATA element and TBP to nucleate PIC assembly upstream of the G-less cassette (Fig. 2B). Similar assays utilizing ADH1 promoter U-less cassette fusions also generated downstream U-less cassette transcripts in the absence of UTP and presence of 3′-O-methyl-UTP (C. Yang and A. Ponticelli, unpublished data). Together, these results support the hypothesis that the yeast RNAPII machinery has the ability to utilize a transcription-independent translocation mechanism for initiation at sites far downstream of a TATA element.
FIG. 2.
Yeast PICs can encounter and utilize downstream start sites by a mechanism that does not require contiguous intervening RNA synthesis and that is not compromised by the Tfg1-E346A substitution. (A) Diagram of the ADH1 promoter/G-less cassette fusion template. Shown is the relevant portion of the plasmid template pADH1/G− that contains the ADH1 promoter fused to a 140-base-pair fragment of the G-less cassette (highlighted in black). The positions of the major ADH1 start site (designated −38 where +1 is defined as the A in the translation-initiating ATG codon) and an internal G-less cassette start site (designated with an asterisk) are indicated by the arrows. Highlighted in gray is a naturally occurring 14-base-pair G-less region in the vicinity of the major ADH1 start site, and the positions of upstream guanines are underlined. (B, C, and D) In vitro transcription reactions reconstituted with purified transcription factors. (B) Yeast PICs containing wild-type TFIIF were assembled on template pADH1/G− (lanes 2 to 4) or a TATA-lacking variant (lane 1), as described in Materials and Methods. Reactions were initiated by the addition of GTP-lacking (G−) NTPs in the presence of [α-32P]CTP, and the products were either treated (lanes 1 to 3) or not (lane 4) with RNase T1 prior to electrophoresis. (C and D) Yeast PICs containing either wild-type (WT) or Tfg1-E346A mutant TFIIF were assembled on the pADH1/G− template, and reactions were initiated by the addition of GTP-containing (G+) or lacking (G−) NTPs in the presence of [α-32P]CTP (C) or unlabeled CTP (D). Reaction products from the radiolabeled reactions were treated with RNase T1 prior to electrophoresis; reaction products from the unlabeled reactions were analyzed by primer extension. The numbers to the left of each panel correspond to the positions of the 5′ ends of the indicated transcripts.
Having obtained evidence supporting a transcription-independent translocation mechanism, we next sought to determine whether the upstream shifts conferred by Tfg1-E346A TFIIF might be due to this TFIIF mutant impairing the ability of the PICs to efficiently translocate to the downstream start sites. As anticipated, reconstituted reactions using the Tfg1-E346A TFIIF resulted in increased initiation upstream of the G-less cassette when GTP was present in the reaction (Fig. 2D, lane 2 versus 4). Importantly, however, the Tfg1-E346A mutant TFIIF did not impair the ability of the PICs to arrive and utilize the downstream initiation sites in reaction mixtures lacking GTP and containing 3′-O-methyl GTP (Fig. 2C and D, lane 1 versus 3). Taken together, these results support a mechanism by which upstream shifts in start site utilization caused by mutations in TFIIF are the result of an enhanced ability to utilize potential upstream start site sequences.
The Tfg1-E346A mutant exhibits an enhanced ability to utilize a suboptimal start site in vivo.
The increase in upstream initiation conferred by the Tfg1-E346A substitution could be explained by the mutant TFIIF protein conferring upon the RNAPII machinery an enhanced ability to productively utilize a suboptimal start site encountered early in the translocation process. To directly test this hypothesis, we compared the utilization of a mutated ADH1 −38 start site in a wild-type versus a tfg1-E346A mutant strain. The variant start site (designated −48/43 A→T) contained four adenosine-to-thymidine substitutions between positions −48 and −43 (Fig. 3B), corresponding to the upstream A-rich region of the reported consensus yeast initiator sequence A(Arich)5NYA(A/T)NN(Arich)6 (51). In the wild-type TFIIF strain, utilization of the −48/43 A→T start site was almost completely abolished, consistent with this region's importance as part of the consensus yeast initiator (Fig. 3A, lane 3). Importantly, however, the −48/43 A→T start site was efficiently utilized in the tfg1-E346A mutant strain (Fig. 3A, lane 4). Thus, these results support the view that the Tfg1-E346A mutant TFIIF facilitates more efficient utilization of a suboptimal start site.
FIG. 3.
The Tfg1-E346A mutant exhibits enhanced ability to utilize a suboptimal start site in vivo. (A) Primer extension analysis of in vivo transcripts produced from wild-type and variant ADH1 promoter constructs. Total RNA isolated from the wild-type (WT) or tfg1-E346A mutant strain harboring the indicated ADH1 promoter-HIS3 fusion construct was analyzed by primer extension utilizing a HIS3-specific oligonucleotide primer. The numbers to the left of the panel indicate the position of the transcription start sites, where +1 is defined as the A in the translation-initiating ATG codon. Arrow, position of the ADH1 −38 major transcript. (B) Sequences of the wild-type and variant ADH1 promoter constructs. Shown are the relevant sequences in the vicinity of the major ADH1 start sites for the wild-type promoter construct (plasmid p316/AH) and for the variant construct (plasmid p316/AH −48/43 A→T) that contains the highlighted (black boxes) nucleotide substitutions. The positions of the major start sites in the wild-type TFIIF strain are indicated by the filled circles; the open circles indicate the positions of the start sites in the tfg1-E346A mutant strain.
Yeast TFIIF stimulates early phosphodiester bond formation, and this activity is increased by the Tfg1-E346A substitution.
The precise mechanism by which a consensus yeast initiation sequence is recognized and utilized by the yeast RNAPII machinery remains unknown. One explanation for the ability of the Tfg1-E346A mutant TFIIF to facilitate more efficient usage of a nonconsensus start site is that the TFIIF mutant confers altered sequence recognition properties upon the polymerase. Alternatively, we hypothesized that TFIIF might have a general stimulatory effect on early phosphodiester bond formation and that the Tfg1-E346A substitution might further enhance this activity. Although such an activity for TFIIF has not been reported, we tested this possibility directly by using a simplified in vitro system containing RNAPII and TFIIF and a premelted bubble template (bubble80; Fig. 4A). By utilizing a bubble template to provide an RNAPII entry site, this experimental approach was conceptually similar to the well-established use of dC-tailed templates to test the direct effects of factors such as TFIIS on the activity of purified polymerase. To monitor the efficiency of early phosphodiester bond formation, we first examined the formation of the first two phosphodiester bonds by determining the amount of 5′-GGA-3′ trinucleotide that was generated in reactions using [α-32P]ATP and various concentrations of unlabeled GTP. The results demonstrated that compared to reactions using only RNAPII, the addition of wild-type TFIIF stimulated the production of the GGA trinucleotide by approximately twofold at all GTP concentrations tested (Fig. 4B and D, left panel). Significantly, reactions using the Tfg1-E346A mutant TFIIF showed an even greater stimulation of trinucleotide production, which was most pronounced in the reactions with lower GTP concentrations (Fig. 4B and D, left panel). To test the effects of TFIIF on the formation of the third phosphodiester bond, similar assays were performed, in which the unlabeled GTP was replaced with unlabeled RNA3 5′-AGG-3′, and production of the radiolabeled tetranucleotide 5′-AGGA-3′ was quantified. Using 1 μM RNA3, a modest stimulatory effect was observed in reactions with the wild-type TFIIF that was slightly greater in the reactions using the Tfg1-E346A mutant (Fig. 4C, lanes 1 to 3, and D, right panel). No stimulation of tetranucleotide production was observed for reactions using either wild-type or mutant TFIIF when 10 μM RNA3 was utilized (Fig. 4C, lanes 4 to 6, and D, right panel). Thus, these results demonstrate that TFIIF can interact directly with RNAPII to stimulate early phosphodiester bond formation and that this activity is enhanced by the Tfg1-E346A substitution.
Both wild-type and Tfg1-E346A mutant TFIIFs dramatically stabilize a 5-nucleotide RNA in the RNAPII active center.
The observation that both wild-type and Tfg1-E346A mutant TFIIF can directly stimulate early bond formation in vitro suggests that the interaction of TFIIF with RNAPII may induce structural changes within the RNAPII active center. To test this possibility, we utilized a mobility shift assay similar to one that we employed previously to monitor short RNA-DNA hybrid stability in the active centers of both wild-type and Rpb1 switch 2 mutant polymerases (29). Initially, we determined the relative abilities of wild-type and Tfg1-E346A TFIIF to form stable DNA-RNAPII-TFIIF complexes using a 32P-labeled bubble80 template. As seen previously, the Tfg1-E346A mutant was slightly impaired for stable complex formation relative to that of wild-type TFIIF (Fig. 5B, lanes 2 to 4). We then examined the effects of TFIIF on the retention of a short RNA in the RNAPII active center in reactions using unlabeled bubble80 template and a 32P-labeled RNA5 oligonucleotide complementary to the template strand within the mispaired region (5′-AGAGG-3′). Strikingly, the addition of either wild-type or Tfg1-E346A TFIIF to the reaction mixtures resulted in a dramatic increase in the retention of RNA5 by RNAPII (Fig. 5C, lanes 2 to 4), in which the amounts of DNA-RNAPII-TFIIF-RNA complexes appeared to be comparable for the reactions using wild-type versus mutant TFIIF. Importantly, however, this striking retention of the RNA5 was not observed for either wild-type or Tfg1-E346A TFIIF in reactions using the Rpb1-R344A mutant polymerase (Fig. 5B and C, lanes 5 to 7). Previously, we demonstrated that the R344A substitution, which resides within the switch 2 region of the polymerase active center, confers downstream shifts in start site utilization, diminished elongation processivity, and increased abortive initiation (29). This result suggests that the retention is not a result of TFIIF interacting directly with the RNA to stabilize it in the complex but rather that such retention requires a functional switch 2 in the active center. Thus, these combined results demonstrate another previously unreported activity of TFIIF and support the view that the interaction of TFIIF with RNAPII induces structural changes near the RNAPII active center.
FIG. 5.
Both wild-type and Tfg1-E346A mutant TFIIF dramatically stabilize a 5-nucleotide RNA in the RNAPII active center. (A) Coomassie blue-stained 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel of the recombinant TFIIF preparations used in this study. Tfg1ΔN corresponds to a degradation product of Tfg1 lacking the N-terminal 80 to 90 amino acids. In vivo analyses have shown that the N-terminal 100 amino acids of Tfg1 are dispensable for cell viability and normal start site selection (unpublished data). Two additional minor degradation products of Tfg1 are highlighted with asterisks. (B) Mobility shift assays for DNA-RNAPII-TFIIF complexes. Reaction mixtures contained 32P-labeled bubble80 template, purified wild-type or Rpb1-R344A RNAPII, and purified wild-type or Tfg1-E346A mutant TFIIF, as indicated. The positions of the DNA-RNAPII and the DNA-RNAPII-TFIIF complexes are shown at the left of the panel. (C) Mobility shift assays for DNA-RNAPII-TFIIF-RNA complexes. Reaction mixtures contained unlabeled bubble80 template, 32P-labeled RNA5 oligonucleotide (5′-AGAGG-3′), purified wild-type or Rpb1-R344A RNAPII, and purified wild-type or Tfg1-E346A mutant TFIIF, as indicated. The positions of the DNA-RNAPII-RNA and DNA-RNAPII-TFIIF-RNA complexes are shown at the left of the panel.
The Tfg1-E346A mutant TFIIF enhances processivity to a greater extent than wild-type TFIIF.
Having examined the activities of wild-type and Tfg1-E346A TFIIF during early steps in transcription initiation, we next analyzed their relative activities for elongation efficiency. Reactions using unlabeled bubble80 template and radiolabeled RNA5 were performed similarly to the mobility shift assays to allow for complex formation, and runoff transcription was then initiated by the addition of unlabeled NTPs. In the reaction using only wild-type RNAPII, most of the extended transcripts were paused after reaching approximately 15 nucleotides in length, and almost no full-length products were detected (Fig. 6, lane 1). The addition of wild-type TFIIF stimulated elongation, as shown by an increase in the amount of both the longer and the full-length transcripts extending to the end of the template (Fig. 6, lane 2). The addition of Tfg1-E346A TFIIF resulted in a similar stimulation of elongation, perhaps slightly more so than wild-type TFIIF (Fig. 6, lane 2 versus 3). Importantly, however, Tfg1-E346A TFIIF stimulated elongation to a much greater extent than wild-type TFIIF in reactions that also used the elongation factor TFIIS (Fig. 6, lane 9 versus 10). Additional experiments demonstrated that the efficiency of TFIIS-induced backtracking and RNA cleavage in reactions using wild-type TFIIF was comparable to that in reactions using the mutant TFIIF (data not shown).
FIG. 6.
The Tfg1-E346A mutant enhances processivity to a greater extent than wild-type (WT) TFIIF. Runoff transcription assays were performed as described in Materials and Methods with the bubble80 template; 32P-labeled RNA5 (5′-AGAGG-3′); and purified wild-type or Rpb1-R344A RNAPII, TFIIS, and wild-type or Tfg1-E346A mutant TFIIF as indicated. Transcript lengths are shown at the left of the panel.
In addition to testing the effects of wild-type and Tfg1-E346A on elongation by wild-type RNAPII, we also examined their effects on elongation by the Rpb1-R344A mutant polymerase. In the reaction using only Rpb1-R344A RNAPII, elongation was less efficient than that observed with wild-type RNAPII alone, as expected (Fig. 6, lane 4 versus 1). Addition of wild-type or Tfg1-E346A TFIIF resulted in a very modest stimulation of elongation (Fig. 6, lanes 5 and 6) that was enhanced somewhat in the reactions that also used TFIIS (Fig. 6, lanes 11 and 12). These results demonstrate that the Tfg1-E346A mutant TFIIF stimulates elongation processivity to a greater extent than wild-type TFIIF, and they strongly suggest that this mechanism involves the RNAPII switch 2 region.
DISCUSSION
In this study, in vivo and in vitro analyses were employed to investigate the functions of S. cerevisiae TFIIF during transcription start site utilization. Our previous work identified TFIIF mutations, including the Tfg1-E346A substitution, that conferred upstream shifts in start site utilization on the ADH1 and CYC1 promoters (14). Here, we show that upstream shifts occurred on all 10 additional RNAPII-dependent promoters analyzed, including 6 that direct mRNA synthesis and 4 that direct synthesis of spliceosomal snRNAs (Fig. 1). In all cases, upstream start sites mapped to purines within the region between the TATA elements and the major transcription start sites, with the upstream sites never closer than approximately 50 base pairs downstream of the beginning of the TATA sequence (Fig. 1B). Previous analyses of yeast promoter sequences (28, 47) and our own analysis of 158 S. cerevisiae genes demonstrate that the intervening sequences between the TATA elements and the normal downstream start sites are rich in pyrimidines, especially thymidines (data not shown). Since purines are considered to be preferred initiation sites in both prokaryotes and eukaryotes (16, 19), those that do reside in these upstream regions are presumably not utilized in a wild-type strain because they are not contained within the reported consensus yeast initiator sequence (51). Although the mechanism by which this sequence is recognized and/or utilized remains to be elucidated, our studies show that the Tfg1-E346A substitution facilitates the utilization of suboptimal start sites. This is evident from the start site utilization patterns for the above-described 12 promoters combined with the results demonstrating that the Tfg1-E346A mutant efficiently utilizes a mutated ADH1 start site (Fig. 3) and that the Tfg1 E346A substitution does not impair the ability of the yeast RNAPII machinery to utilize a transcription-independent translocation mechanism for initiation at sites far downstream of a TATA element (Fig. 2).
If alterations in TFIIF can facilitate the utilization of suboptimal start sites, by what mechanism might this occur? As noted above, the mechanism by which a consensus yeast initiator sequence functions remains unclear. It has yet to be determined whether this sequence is actually recognized by the RNAPII machinery to initiate RNA synthesis or whether initiation is attempted but aborted at most purines, and the initiator represents an optimal sequence for efficient early phosphodiester bond formation and short nascent RNA stability. Although direct recognition may occur and be influenced by TFIIF, we tested the hypothesis that the Tfg1-E346A TFIIF might facilitate usage of suboptimal sites through a general stimulation of early bond formation. Results from in vitro assays using the bubble80 template and RNAPII in the absence or presence of TFIIF revealed that the formation of the first two phosphodiester bonds, and to a lesser extent the third bond, was stimulated by the presence of wild-type TFIIF (Fig. 4). To our knowledge, this stimulatory activity of TFIIF on the formation of the first two phosphodiester bonds has not been reported previously. Importantly, this stimulatory activity was further enhanced by the Tfg1-E346A substitution (Fig. 4). The enhanced activity of the mutant TFIIF was not due to a greater fraction of active molecules in the preparation, as evidenced by the results from the mobility shift assays using labeled DNA template to monitor the formation of DNA-RNAPII-TFIIF complexes (Fig. 5B). In addition, the results from the mobility shift assays using labeled RNA5 to monitor the formation of DNA-RNAPII-TFIIF-RNA complexes demonstrated that the presence of either wild-type or mutant TFIIF dramatically increased the stability of RNA5 in the wild-type, but not the switch 2 mutant, RNAPII active center (Fig. 5B, C), supporting the view that interaction between TFIIF and RNAPII results in structural alterations within and/or in the immediate vicinity of the RNAPII active center.
Since TFIIF is known to function during both initiation and elongation, runoff transcription assays were subsequently carried out in order to determine whether the stimulation of early bond formation conferred by the Tfg1-E346A mutant was accompanied by any changes in the processivity of early elongation from the bubble80 template. The results demonstrated that elongation by wild-type RNAPII was stimulated to a greater extent by Tfg1-E346A TFIIF than by wild-type TFIIF and that overall elongation efficiency was significantly impaired in reactions using the Rpb1-R344A switch 2 mutant polymerase (Fig. 6). The dramatically reduced effects of wild-type or Tfg1-E346A TFIIF on the activity of the switch 2 mutant are consistent with our previous genetic results demonstrating that the downstream shifts and the conditional phenotype conferred by the rpb1-R344A allele are dominant to the phenotypes conferred by tfg1-E346A (29).
A model for the role of yeast TFIIF in modulating conformational changes in the RNAPII active center during initiation and early elongation.
Based upon the findings reported here and those previously, we propose a model for the role of S. cerevisiae TFIIF during start site utilization. In building this model, we sought to incorporate and provide plausible explanations for the following observations. (i) The Tfg1-E346A mutant exhibits enhanced ability to utilize a suboptimal start site; (ii) the Tfg1-E346A substitution coordinately stimulates early phosphodiester bond formation and elongation to a greater extent than wild-type TFIIF; (iii) Rpb1 (switch 2) mutations in the polymerase active center are dominant to tfg1-E346A (29); (iv) both wild-type and Tfg1-E346A mutant TFIIFs dramatically stabilize a 5-nucleotide RNA in the RNAPII active center; (v) the region of Tfg1 containing Glu346 has been proposed to reside near the RNAPII active center (13), and recent studies demonstrated that this region interacts with the Rpb2 lobe and that mutations in the Rpb2 lobe can confer upstream shifts in start site utilization (5); (vi) deletion of Rpb9 confers upstream shifts that are similar to yet weaker than those conferred by tfg1-E346; (vii) RNAPII lacking Rpb9 exhibits impaired interaction with TFIIF (52); (viii) Rpb9 is immediately adjacent to the Rpb2 lobe in the reported crystal structure (7); and (ix) TFIIF binding induces significant rearrangements of RNAPII domains surrounding the major cleft, as evidenced by cryo-electron microscopy analysis (6).
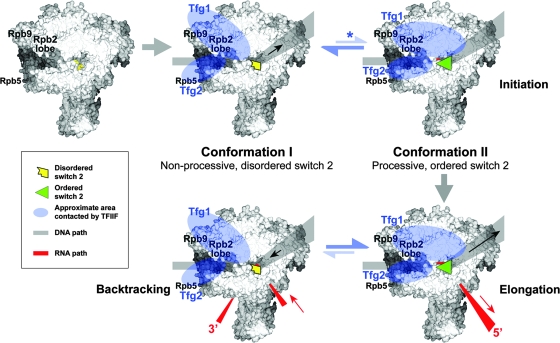
In our model, we propose the existence of two TFIIF-RNAPII conformations that are in dynamic equilibrium and that dictate the structure and activity of the polymerase active center (Fig. 7). In conformation I, the Tfg1 subunit interacts primarily with the Rpb2 lobe, and this interaction is stabilized by the presence of the adjacent Rpb9 subunit. In conformation I, the polymerase clamp is open, and the switch 2 domain is disordered, resulting in inefficient early bond formation and nonprocessive elongation due to poor stabilization of the 3′ end of the RNA-DNA hybrid. In conformation II, the Tfg1 subunit has an altered interaction with the Rpb2 lobe that is accompanied by repositioning of Tfg2 in the central cleft, closure of the clamp, and importantly, positioning of an ordered switch 2 for interaction with the DNA template strand at the site of incoming nucleotide addition (29, 46). Accordingly, conformation II is associated with more efficient early bond formation and processive elongation due to enhanced stabilization of the 3′ end of the RNA-DNA hybrid. Mutations in TFIIF, the Rpb2 lobe, or deletion of Rpb9 that causes upstream shifts is proposed to compromise the stability of conformation I and favor conformation II, resulting in better utilization of suboptimal start sites closer to the TATA element. Continued biochemical and structural analyses are needed to further test this model and to provide additional insights into the functions of S. cerevisiae TFIIF.
FIG. 7.
Model for the role of yeast TFIIF in modulating conformational changes in the RNAPII active center during initiation and early elongation. Two TFIIF-RNAPII conformations are proposed. In conformation I, the binding of TFIIF results in a narrowing of the central cleft and involves key contacts between the Tfg1 subunit and the Rpb2 lobe that are stabilized by the adjacent Rpb9 subunit. Conformation I is characterized by a disordered switch 2, inefficient early bond formation, and nonprocessive elongation due to poor stabilization of the 3′ end of the RNA-DNA hybrid. In conformation II, the Tfg1 subunit has altered interaction with the Rpb2 lobe, and the Tfg2 subunit is repositioned in the central cleft. Importantly, conformation II is characterized by an ordered switch 2 positioned in the active center due to clamp closure, resulting in efficient early bond formation and processive elongation due to enhanced stabilization of the 3′ end of the RNA-DNA hybrid. Mutations in TFIIF and Rpb2 or deletion of Rpb9 that cause upstream shifts are proposed to destabilize conformation I and favor conformation II (highlighted with an asterisk), resulting in better utilization of suboptimal start sites closer to the TATA element. Approximate positions of the TFIIF domains that interact around the major cleft are highlighted in blue; additional potential interactions of TFIIF with other regions of RNAPII are not indicated.
Acknowledgments
We thank Nancy Woychik for yeast strain WY9, Caroline Kane for plasmid pET15b/IIS 1-309, and Steve Hahn for communicating results prior to publication. We also thank Jim Hernandez for technical advice about the analysis of short RNAs and members of the Ponticelli laboratory for helpful discussions and comments on the manuscript.
This research was supported by National Institutes of Health Public Health service grant GM51124 (to A.S.P.).
Footnotes
Published ahead of print on 24 March 2008.
REFERENCES
- 1.Bangur, C. S., T. S. Pardee, and A. S. Ponticelli. 1997. Mutational analysis of the D1/E1 core helices and the conserved N-terminal region of yeast transcription factor IIB (TFIIB): identification of an N-terminal mutant that stabilizes TATA-binding protein-TFIIB-DNA complexes. Mol. Cell. Biol. 176784-6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berroteran, R. W., D. E. Ware, and M. Hampsey. 1994. The sua8 suppressors of Saccharomyces cerevisiae encode replacements of conserved residues within the largest subunit of RNA polymerase II and affect transcription start site selection similarly to sua7 (TFIIB) mutations. Mol. Cell. Biol. 14226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns, B. R., N. L. Henry, and R. D. Kornberg. 1996. TFG/TAF30/ANC1, a component of the yeast SWI/SNF complex that is similar to the leukemogenic proteins ENL and AF-9. Mol. Cell. Biol. 163308-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers, R. S., B. Q. Wang, Z. F. Burton, and M. E. Dahmus. 1995. The activity of COOH-terminal domain phosphatase is regulated by a docking site on RNA polymerase II and by the general transcription factors IIF and IIB. J. Biol. Chem. 27014962-14969. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. T., L. Warfield, and S. Hahn. 2007. The positions of TFIIF and TFIIE in the RNA polymerase II transcription preinitiation complex. Nat. Struct. Mol. Biol. 14696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung, W. H., J. L. Craighead, W. H. Chang, C. Ezeokonkwo, A. Bareket-Samish, R. D. Kornberg, and F. J. Asturias. 2003. RNA polymerase II/TFIIF structure and conserved organization of the initiation complex. Mol. Cell 121003-1013. [DOI] [PubMed] [Google Scholar]
- 7.Cramer, P., D. A. Bushnell, and R. D. Kornberg. 2001. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 2921863-1876. [DOI] [PubMed] [Google Scholar]
- 8.Eberharter, A., S. John, P. A. Grant, R. T. Utley, and J. L. Workman. 1998. Identification and analysis of yeast nucleosomal histone acetyltransferase complexes. Methods 15315-321. [DOI] [PubMed] [Google Scholar]
- 9.Escobar-Henriques, M., B. Daignan-Fornier, and M. A. Collart. 2003. The critical cis-acting element required for IMD2 feedback regulation by GDP is a TATA box located 202 nucleotides upstream of the transcription start site. Mol. Cell. Biol. 236267-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faitar, S. L., S. A. Brodie, and A. S. Ponticelli. 2001. Promoter-specific shifts in transcription initiation conferred by yeast TFIIB mutations are determined by the sequence in the immediate vicinity of the start sites. Mol. Cell. Biol. 214427-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish, R. N., M. L. Ammerman, J. K. Davie, B. F. Lu, C. Pham, L. Howe, A. S. Ponticelli, and C. M. Kane. 2006. Genetic interactions between TFIIF and TFIIS. Genetics 1731871-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores, O., H. Lu, M. Killeen, J. Greenblatt, Z. F. Burton, and D. Reinberg. 1991. The small subunit of transcription factor IIF recruits RNA polymerase II into the preinitiation complex. Proc. Natl. Acad. Sci. USA 889999-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freire-Picos, M. A., S. Krishnamurthy, Z. W. Sun, and M. Hampsey. 2005. Evidence that the Tfg1/Tfg2 dimer interface of TFIIF lies near the active center of the RNA polymerase II initiation complex. Nucleic Acids Res. 335045-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazy, M. A., S. A. Brodie, M. L. Ammerman, L. M. Ziegler, and A. S. Ponticelli. 2004. Amino acid substitutions in yeast TFIIF confer upstream shifts in transcription initiation and altered interaction with RNA polymerase II. Mol. Cell. Biol. 2410975-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardina, C., and J. T. Lis. 1993. DNA melting on yeast RNA polymerase II promoters. Science 261759-762. [DOI] [PubMed] [Google Scholar]
- 16.Grosveld, G. C., C. K. Shewmaker, P. Jat, and R. A. Flavell. 1981. Localization of DNA sequences necessary for transcription of the rabbit beta-globin gene in vitro. Cell 25215-226. [DOI] [PubMed] [Google Scholar]
- 17.Hahn, S. 2004. Structure and mechanism of the RNA polymerase II transcription machinery. Nat. Struct. Mol. Biol. 11394-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampsey, M. 1998. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol. Mol. Biol. Rev. 62465-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 112237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry, N. L., A. M. Campbell, W. J. Feaver, D. Poon, P. A. Weil, and R. D. Kornberg. 1994. TFIIF-TAF-RNA polymerase II connection. Genes Dev. 82868-2878. [DOI] [PubMed] [Google Scholar]
- 21.Hull, M. W., K. McKune, and N. A. Woychik. 1995. RNA polymerase II subunit RPB9 is required for accurate start site selection. Genes Dev. 9481-490. [DOI] [PubMed] [Google Scholar]
- 22.Izban, M. G., and D. S. Luse. 1992. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J. Biol. Chem. 26713647-13655. [PubMed] [Google Scholar]
- 23.John, S., L. Howe, S. T. Tafrov, P. A. Grant, R. Sternglanz, and J. L. Workman. 2000. The something about silencing protein, Sas3, is the catalytic subunit of NuA3, a yTAF(II)30-containing HAT complex that interacts with the Spt16 subunit of the yeast CP (Cdc68/Pob3)-FACT complex. Genes Dev. 141196-1208. [PMC free article] [PubMed] [Google Scholar]
- 24.Kabani, M., K. Michot, C. Boschiero, and M. Werner. 2005. Anc1 interacts with the catalytic subunits of the general transcription factors TFIID and TFIIF, the chromatin remodeling complexes RSC and INO80, and the histone acetyltransferase complex NuA3. Biochem. Biophys. Res. Commun. 332398-403. [DOI] [PubMed] [Google Scholar]
- 25.Keene, R. G., and D. S. Luse. 1999. Initially transcribed sequences strongly affect the extent of abortive initiation by RNA polymerase II. J. Biol. Chem. 27411526-11534. [DOI] [PubMed] [Google Scholar]
- 26.Kim, B., A. I. Nesvizhskii, P. G. Rani, S. Hahn, R. Aebersold, and J. A. Ranish. 2007. The transcription elongation factor TFIIS is a component of RNA polymerase II preinitiation complexes. Proc. Natl. Acad. Sci. USA 10416068-16073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuehner, J. N., and D. A. Brow. 2006. Quantitative analysis of in vivo initiator selection by yeast RNA polymerase II supports a scanning model. J. Biol. Chem. 28114119-14128. [DOI] [PubMed] [Google Scholar]
- 28.Maicas, E., and J. D. Friesen. 1990. A sequence pattern that occurs at the transcription initiation region of yeast RNA polymerase II promoters. Nucleic Acids Res. 183387-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majovski, R. C., D. A. Khaperskyy, M. A. Ghazy, and A. S. Ponticelli. 2005. A functional role for the switch 2 region of yeast RNA polymerase II in transcription start site utilization and abortive initiation. J. Biol. Chem. 28034917-34923. [DOI] [PubMed] [Google Scholar]
- 30.Maxon, M. E., J. A. Goodrich, and R. Tjian. 1994. Transcription factor IIE binds preferentially to RNA polymerase IIa and recruits TFIIH: a model for promoter clearance. Genes Dev. 8515-524. [DOI] [PubMed] [Google Scholar]
- 31.Miller, G., and S. Hahn. 2006. A DNA-tethered cleavage probe reveals the path for promoter DNA in the yeast preinitiation complex. Nat. Struct. Mol. Biol. 13603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orphanides, G., T. Lagrange, and D. Reinberg. 1996. The general transcription factors of RNA polymerase II. Genes Dev. 102657-2683. [DOI] [PubMed] [Google Scholar]
- 33.Pardee, T. S., C. S. Bangur, and A. S. Ponticelli. 1998. The N-terminal region of yeast TFIIB contains two adjacent functional domains involved in stable RNA polymerase II binding and transcription start site selection. J. Biol. Chem. 27317859-17864. [DOI] [PubMed] [Google Scholar]
- 34.Pardee, T. S., M. A. Ghazy, and A. S. Ponticelli. 2003. Yeast and human RNA polymerase II elongation complexes: evidence for functional differences and postinitiation recruitment of factors. Eukaryot. Cell 2318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto, I., D. E. Ware, and M. Hampsey. 1992. The yeast SUA7 gene encodes a homolog of human transcription factor TFIIB and is required for normal start site selection in vivo. Cell 68977-988. [DOI] [PubMed] [Google Scholar]
- 36.Pinto, I., W. H. Wu, J. G. Na, and M. Hampsey. 1994. Characterization of sua7 mutations defines a domain of TFIIB involved in transcription start site selection in yeast. J. Biol. Chem. 26930569-30573. [PubMed] [Google Scholar]
- 37.Ponticelli, A. S., and K. Struhl. 1990. Analysis of Saccharomyces cerevisiae his3 transcription in vitro: biochemical support for multiple mechanisms of transcription. Mol. Cell. Biol. 102832-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prather, D. M., E. Larschan, and F. Winston. 2005. Evidence that the elongation factor TFIIS plays a role in transcription initiation at GAL1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 252650-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert, F., M. Douziech, D. Forget, J. M. Egly, J. Greenblatt, Z. F. Burton, and B. Coulombe. 1998. Wrapping of promoter DNA around the RNA polymerase II initiation complex induced by TFIIF. Mol. Cell 2341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen, X., G. Mizuguchi, A. Hamiche, and C. Wu. 2000. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406541-544. [DOI] [PubMed] [Google Scholar]
- 41.Steger, D. J., A. Eberharter, S. John, P. A. Grant, and J. L. Workman. 1998. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc. Natl. Acad. Sci. USA 9512924-12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Struhl, K. 1995. Yeast transcriptional regulatory mechanisms. Annu. Rev. Genet. 29651-674. [DOI] [PubMed] [Google Scholar]
- 43.Sun, Z. W., A. Tessmer, and M. Hampsey. 1996. Functional interaction between TFIIB and the Rpb9 (Ssu73) subunit of RNA polymerase II in Saccharomyces cerevisiae. Nucleic Acids Res. 242560-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan, S., T. Aso, R. C. Conaway, and J. W. Conaway. 1994. Roles for both the RAP30 and RAP74 subunits of transcription factor IIF in transcription initiation and elongation by RNA polymerase II. J. Biol. Chem. 26925684-25691. [PubMed] [Google Scholar]
- 45.Treich, I., and M. Carlson. 1997. Interaction of a Swi3 homolog with Sth1 provides evidence for a Swi/Snf-related complex with an essential function in Saccharomyces cerevisiae. Mol. Cell. Biol. 171768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westover, K. D., D. A. Bushnell, and R. D. Kornberg. 2004. Structural basis of transcription: separation of RNA from DNA by RNA polymerase II. Science 3031014-1016. [DOI] [PubMed] [Google Scholar]
- 47.Yagil, G. 2004. The over-representation of binary DNA tracts in seven sequenced chromosomes. BMC Genomics 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan, Q., R. J. Moreland, J. W. Conaway, and R. C. Conaway. 1999. Dual roles for transcription factor IIF in promoter escape by RNA polymerase II. J. Biol. Chem. 27435668-35675. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, C., and Z. F. Burton. 2004. Transcription factors IIF and IIS and nucleoside triphosphate substrates as dynamic probes of the human RNA polymerase II mechanism. J. Mol. Biol. 3421085-1099. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, D. Y., D. J. Carson, and J. Ma. 2002. The role of TFIIB-RNA polymerase II interaction in start site selection in yeast cells. Nucleic Acids Res. 303078-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Z., and F. S. Dietrich. 2005. Mapping of transcription start sites in Saccharomyces cerevisiae using 5′ SAGE. Nucleic Acids Res. 332838-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler, L. M., D. A. Khaperskyy, M. L. Ammerman, and A. S. Ponticelli. 2003. Yeast RNA polymerase II lacking the Rpb9 subunit is impaired for interaction with transcription factor IIF. J. Biol. Chem. 27848950-48956. [DOI] [PubMed] [Google Scholar]