Abstract
Evidence from both human studies and animal models indicates that cocaine elicits more behavioral stimulation in females than males. The present study sought to determine whether sex-specific responses to cocaine emerge during adolescence and to determine if gonadal steroid action during puberty affects adult responsiveness to cocaine. We administered cocaine using an escalating dose model in male and female rats at ages postnatal (PN) 28, 42, and 65 days. To assess the effects of pubertal gonadal steroid action, we compared the effects of binge cocaine administration on intact and prepubertally gonadectomized male and female rats in adulthood. Cocaine responses changed in opposite directions in males and females as they progressed through adolescence. At most doses, adolescent males were more responsive than adult males whereas adult females were more responsive than adolescent females. Ambulatory activity was age-dependent in males whereas non-ambulatory activity was age-dependent in females. Prepubertal gonadectomy increased behavioral responsiveness to the highest dose of cocaine in males whereas it decreased behavioral responsiveness to lower doses of cocaine in females. We conclude that sex differences in behavioral responses to cocaine arise during adolescence from a concurrent decrease in male responsiveness and increase in female responsiveness. Our results suggest that gonadal steroids exert lasting and opposing effects on the sensitivity of males and females to psychostimulants during development.
Keywords: Adolescence, Cocaine, Behavior, Sex, Ontogeny, Stimulants
1: Introduction
Females demonstrate unique vulnerabilities to cocaine when compared to males (McCance-Katz et al. 1999; Robbins et al. 1999). Although more men than women are current users (SAMHSA 2005), women prefer more highly addictive routes of administration such as smoking or intravenous injections rather than intranasal use (McCance-Katz et al. 1999). Women report greater substance-dependence associated symptoms (Chen and Kandel 2002), higher anxiety following cocaine use (Kosten et al. 1996) and increased cue-induced craving compared to men (Robbins et al. 1999). Animal models of addiction have provided evidence that these differences reflect in part biological differences. Adult female rats consistently show greater behavioral responses to cocaine or amphetamine than adult males (Becker et al. 1982; Camp et al. 1986; Camp and Robinson 1988; Chin et al. 2002; Glick et al. 1983; Schneider and Norton 1979; Sell at al. 2000; Van Haaren and Meyer 1991; Walker et al. 2001). Female rats may also initiate cocaine self-administration more rapidly and achieve higher break point values than males when provided with equal access to drug (Carroll et al. 2002) although contradictory results also exist (Caine et al. 2004; reviewed in Roth et al. 2004). Sex differences in dopaminergic function have been identified and likely contribute to these effects (Walker et al. 2000; 2006).
Numerous reports suggest that gonadal hormones modulate behavioral responsiveness to cocaine. Cocaine-induced locomotor activity varies across the estrus cycle of female rats: cocaine responses are blunted during diestrus compared to the proestrus and estrus phases (Quinones-Jenab et al. 1999; Sell et al. 2000; Walker et al. 2002). Ovariectomy decreases female responsiveness to cocaine (Chin et al. 2002; Walker et al. 2001). Castration may slightly increase stimulant-induced behavioral responsiveness in males although specific results have been inconsistent and highly mixed (Chin et al. 2002; Dluzen et al. 1986; Hu and Becker 2003; Savageau and Beatty 1981; Van Luijtelaar et al. 1996; Walker et al. 2001).
The studies cited above that were conducted in animals gonadectomized as adults provide valuable information about activational hormonal effects. However, they cannot demonstrate if gonadal hormones influence cocaine responsiveness through organizational effects at specific windows during development. Two studies in females show that either prepubertal ovariectomy or androgen treatment at birth both lower locomotor responses to amphetamine during adulthood (Forgie and Stewart 1993, 1994). However, in females, it is difficult to discriminate between the contributions of activational and organizational effects from these studies, as estradiol has such a marked effect on locomotor responses to amphetamine (Becker 1999, Becker and Beer 1986). Furthermore, similar studies have not been conducted in males. Organizational effects of gonadal steroids throughout adolescence may contribute significantly to the sex differences in stimulant responsiveness observed in adulthood but these have not been compared in males and females.
Organizational effects during puberty may be particularly relevant to addiction as adolescence has been identified as a critical period for drug abuse (Chambers et al. 2003; Laviola et al. 1999; Spear 2000). The onset of drug taking behavior typically occurs during adolescence (Chambers et al. 2003; Spear 2000). Adolescents may also progress from first substance use to dependence faster than adults (Clark et al. 1998). In animal models, subtle differences in dosing, drug administration paradigms and methods of data collection have made it difficult to ascertain how adolescents respond to stimulants in comparison to adults. Several studies suggest that adolescents may respond more (Caster et al. 2005; Catlow and Kirstein 2005) or less (Lanier and Isaacson. 1977; Laviola et al. 1995) than adults to acute stimulant treatment. Additionally, It is unclear whether the sex differences seen in adulthood result from a developmental increase in female responsiveness, a decrease in male responsiveness or a combination of the two.
The present study characterized the emergence of sex differences in cocaine-stimulated behavioral responsiveness across adolescence by treating PN 28, 42, and 65 male and female rats with a progressively increasing dose regimen of cocaine during a single session to simulate a binge pattern of drug taking. We have previously observed robust age-related differences in males using this paradigm (Caster et al 2005). Differences in behavioral response were determined by an analysis of automated open-field horizontal activity and trained observer recorded stereotyped behaviors. To assess the role of gonadal hormones in this process, shamoperated and prepubertally gonadectomized animals were subjected to the same binge cocaine paradigm after reaching adulthood. We hypothesized that the behavioral response to cocaine would decrease across adolescence in males but increase in females and that gonadectomy would exert sex-specific effects by increasing male responsiveness but reducing female responsiveness.
2: Methods
2.1 Subjects
Male and female Sprague-Dawley rats were acquired from Charles River Laboratories (Raleigh, NC) and separated into plastic self-ventilated cages by age and sex. Animals were housed in a vivarium with a 12 hour light:dark cycle and given ad libitum access to food and water. For experiment 1, rats PN 28, 42, and 65 were used to correspond to early adolescence, mid adolescence, and adulthood, respectively (Spear 2000). These animals were shipped and received on PN 21, 35, and 58 and given one week to acclimate following transportation. For experiment 2, PN 25 male and female rats underwent either gonadectomy or sham-gonadectomy. These animals were allowed to recover and were tested 6 weeks after surgery. Females were used without regard to estrous state to avoid potential behavioral disruptions, because we have shown that the estrous cycle testing by vaginal lavage affects cocaine-stimulated motor behavior (Walker et al. 2002). Completeness of castration in males was confirmed by palpating the testes post surgery. For females, trunk blood samples (taken immediately post experimentation) were analyzed for progesterone and estradiol content by radioimmunoassay to verify surgery status. Sham females had average progesterone and estradiol levels of 13.5 +/− 5.3 ng/ml and 39.4 +/− 13.3 pg/ml, respectively. Ovariectomized females had average progesterone and estradiol levels of 1.6 +/− 0.5 ng/ml and 4.1 +/− 1.2 pg/ml, respectively. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
2.2 Drugs
Cocaine HCl (Sigma-Aldrich, St Louis, MO, Lot 074K1738) was diluted in saline (final concentration of 5, 10, or 25 mg/ml) just prior to experimentation. All injections were given intraperitoneally (i.p) to ensure rapid absorption.
2.3 Automated Behavioral Measurements
Behavioral activity was measured using open field boxes (Kinder Scientific, Inc., Poway, CA). The apparatus consisted of a 41 cm × 41 cm open central chamber layered with corncob bedding and walled by Plexiglas. Motion was recorded by the interruption of photobeams spaced 2.5 cm apart along both axes of two surrounding metal frames. The lower frame is 2 5 cm high and the upper frame is 5.5 cm high. Breaking of beams on the lower bar was recorded as horizontal activity. Horizontal activity was resolved into ambulatory and non-ambulatory (fine movements) activity to determine more precisely which behaviors may be age or sex dependent. Ambulatory activity refers to moving from place to place and is recorded as the breaking of a forward beam with concurrent cessation of breaking of an anchor beam. Fine movements are recorded as the breaking of any beams not counted as ambulations. Fine movements include a spectrum of behaviors such as grooming, stretching, and sniffing as well as some stereotypies such as head-scanning or head-bobbing. Assignment of animals to locomotor boxes was counterbalanced for age, sex, and surgery status.
2.4 Observational Behavioral Measurements
The occurrence of specific behaviors was recorded by an observer according to a noncontinuous 6 point behavioral rating scale that has been previously described (Walker et al. 2001). This scale provides a relative measure of behavioral activity with higher numbers denoting more intense behavioral activity than lower numbers. All observers were trained to a 95% inter-rater reliability. Observations of specific behaviors were made starting 5 minutes after each behavioral session that followed drug injection. These observations were made over 3 consecutive 15s intervals once every 5 minutes for each animal and scored post-hoc. Scores from the 15s intervals were then averaged to produce a final score for each 5-minute period. Numerical values were assigned as follows: 1, inactive; 2, grooming, locomotion, sniffing or rearing; 3, continuous sniffing or sniffing in combination with either locomotion or rearing or both; 4, continuous sniffing and continuous locomotion; 5, frequent stereotyped movements with locomotion or clear pattern locomotion; 6, stereotyped movements largely confined to one area of the chamber. In order to determine stereotypy frequency, we divided the total number of 15 sec intervals observed in stereotypy (level 5 or 6) and divided by the total number of intervals (36).
2.5 Experiment 1: Behavioral response to cocaine across adolescence
PN 28, 42, and 65 male and female rats were given cocaine using an escalating-dose “binge” administration paradigm that models one human method of drug administration. This produces age-specific responses in male rats (Caster et al. 2005). All animals were habituated to the open-field boxes for 1 hr, during which time ambulations and fine movements were recorded. Animals were not observed for stereotypy during habituation. Behavior during habituation provided information about sex differences in the non-drug stimulated condition. Following the habituation period, each animal received three i.p. injections of cocaine spaced at one-hour intervals at doses of 5, 10, and 25 mg/kg (respectively). Both automated and experimenter observed data were collected during these sessions.
2.6 Experiment 2: Behavioral response to cocaine after prepubertal gonadectomy
The same cocaine administration paradigm used in the first experiment was repeated with prepubertally gonadactomized or sham-gonadectomized adult (PN 65) rats.
2.7 Data analysis
Ambulations, fine movements, and stereotypy scores were averaged across subjects at 5 minute intervals and graphed as the mean + SEM using Graphpad Prism 5.0 software (GraphPad Software, San Diego California USA, www.graphpad.com) to display a time course of behavior. Statistical analysis by 4 factor repeated measures ANOVAs were performed using NCSS 2000 software (NCSS, Inc, Kaysville, UT) with age and sex as factors with repeated measures on dose and time. For the gonadectomy experiments, 4 factor ANOVAs were performed using sex and surgery as factors with repeated measures on dose and time. Effects were considered to be significant at p<0.05. Significant interactions were examined further by additional ANOVAs filtered by the interacting terms. Group differences were determined post-hoc with Newman-Keuls Multiple Comparison Test.
3: Results
Habituation and Novelty-Induced Behavior
Horizontal activity during habituation to the novel open field test chamber was resolved as ambulatory and non-ambulatory (fine movements) activity. Figure 1 shows the behaviors over time and session totals. Consistent with previous reports, females exhibited more exploratory behavior than males during the one hr test session. ANOVA indicated a main effect of sex for ambulations (F(1,63)=20.0, P<0.001) and fine movements (F(1,63)=11.8, P<0.001) and post-hoc analysis indicated females did more of each than males. ANOVA indicated no main effect of age or age interactions. ANOVA also indicated a main effect of time for both ambulations (F(11,693)=175.6, P<0.001) and fine movements (F(11,693)=116.6, P<0.001) and a sex x time interaction for ambulations (F(11,693)=4.8, P<0.001) and fine movements (F(11,693)=2.4, P<0.01). Male and female animals showed high activity during the first few intervals followed by habituation to the test cage. The magnitude of sex differences was most pronounced during the initial exploratory phase (time <15 min). However, even when the analysis only includes the last half hour as a measure of habituation (to exclude locomotor responses to novelty), ANOVA still indicates a main effect of sex for ambulations and fine movements (P<0.05 for both). These data demonstrate that compared to males, females have greater behavioral responses to novelty (time<10), greater activity during habituation (time>30), and that age does not affect these differences.
Figure 1.
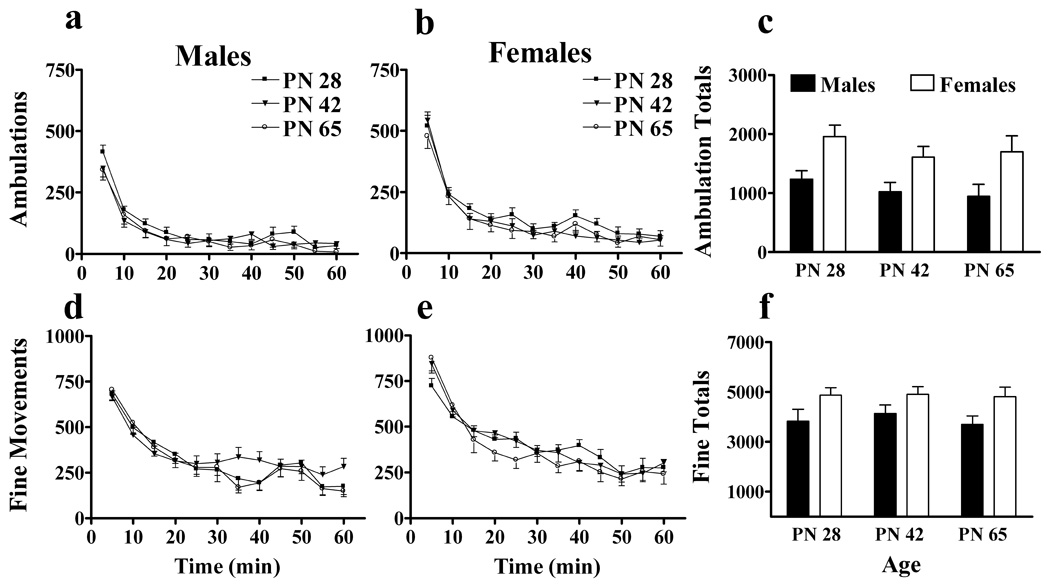
Age and sex differences in horizontal activity during habituation of rats to novel, open-field test chambers. The time courses of horizontal activity of males (left panels) and females (center panels) across adolescence were resolved in terms of ambulations (a–b) and fine movements (d–e). Session totals for ambulations (c) and fine movements (f) are also shown. Titles and labels for the y and x axes apply to all panels in the same rows and columns, respectively. N=12–15 for each age x sex cohort.
Responses to Binge Cocaine
After the habituation session, rats were administered the escalating dose cocaine binge treatment (figure 2). We used the escalating dose binge (3 hourly injections of 5,10, and 25 mg/kg cocaine, respectively) to measure the effects of age and sex on locomotor responses in individual animals across a range of locomotor activating doses. As expected, ANOVA also indicated main effects of dose, time, and a dose x time interaction for all measures as higher dose injections induced more locomotor stimulation for longer periods of time (P<0.001 for all).
Figure 2.
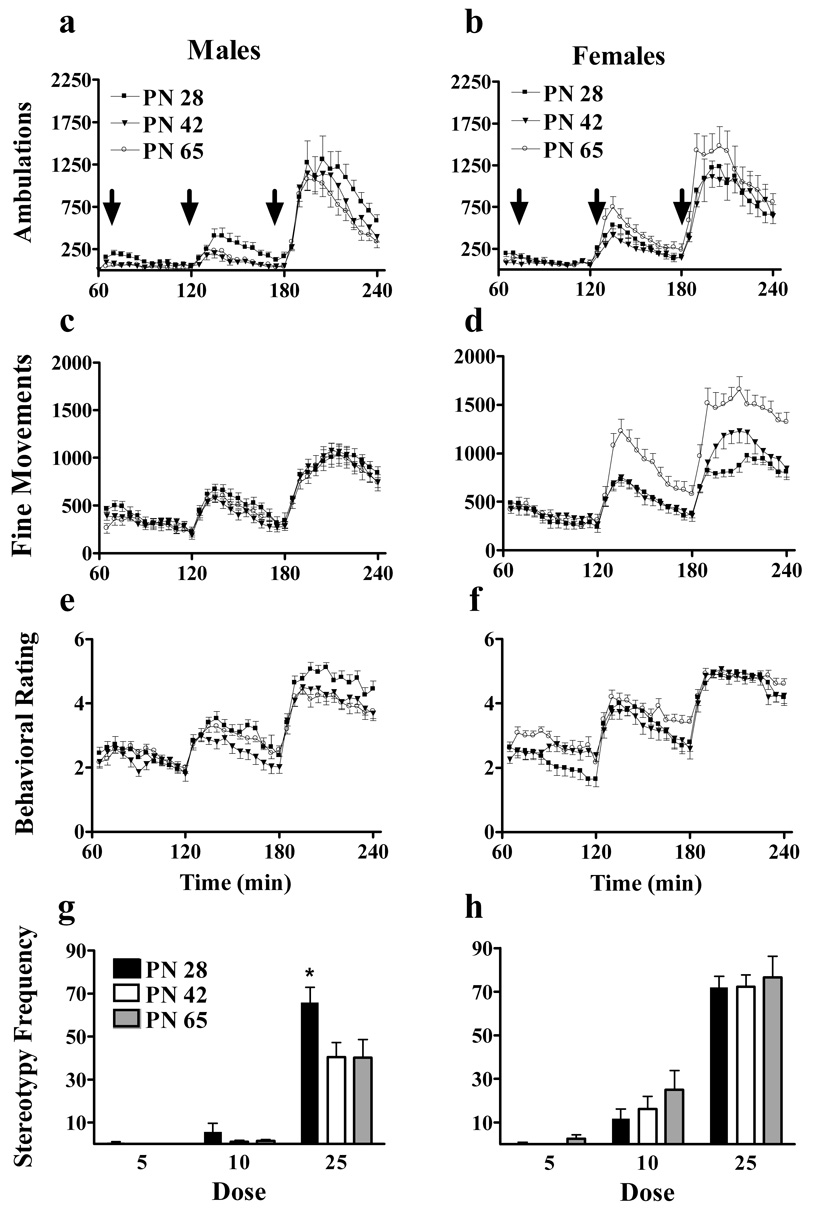
Escalating binge cocaine induces age differences in horizontal activity and experimenter observed behavior in male and female rats. Cocaine (5, 10 and 25 mg/kg) was injected i.p. at hourly intervals. Behavioral observations were made simultaneously with the automated activity measurements. Panels a–f show the temporal patterns of behavior following the cocaine treatments. Ambulations (a–b), fine movements (c–d) and average behavioral rating (e–f) are shown for males (left panels) and females (right panels). Injections were given after times 60, 120, and 180 minutes as denoted by arrows in panels a and b. The frequency of intervals in which animals engaged in stereotypies following each injection are shown in g–h. N=12–15 for each age x sex cohort. * indicates greater response in PN 28 males than older males (P<0.05
Ambulatory responses to binge cocaine for each sex can be seen in figure 2a–b. We analyzed ambulations following the first and second injections (5 and 10 mg/kg) separately from the third injection. Following 25 mg/kg cocaine, many animals begin to transition from forward ambulatory activity to repetitive behaviors that can displace ambulations. This biological transition introduces variability. Further, 25 mg/kg represents a near maximal dose for ambulatory activity. We chose to investigate the effects of age on lower doses near the threshold for ambulatory activity.
Age and sex affected both the temporal pattern and magnitude of ambulatory activity during the escalating dose binge. Following the first two injections of cocaine, ANOVA indicated that both age and sex affect the temporal pattern of activity by interactions of age x time (F(22,781)=3.0, P<0.001) and sex x time (F(11,781)=3.3, P<0.001). Post-hoc analysis demonstrated that cocaine-stimulated activity was more prolonged in females than males. As for the magnitude of ambulatory activity, ANOVA indicated a main effect of age (F(2,71)=4.4, P<0.02), sex (F(1,71)=15.6, P<0.001), and an age x sex interaction (F(2,71)=3.3, P<0.05). Consistent with previous findings, post-hoc analyses indicated that females had greater ambulatory responses than males and that PN 28 animals had greater ambulatory responses than PN 42. We then further evaluated the age x sex interaction by analyzing the sexes separately. Within males, ANOVA indicated a main effect of age (F(2,37)=6.6, P<0.005). Post-hoc analyses indicated that PN 28 males had greater ambulatory responses than older males. In contrast, ANOVA indicated no main effect or interactions with age in females.
Following the third and highest dose of cocaine (time >180 min), ANOVA indicated no main effects or interactions of age or sex with time. Animals of all ages and sexes showed high levels of ambulatory activity to this dose of cocaine. It is likely that this represents a maximal dose for ambulatory stimulation. Again, some animals begin to transition into repetitive stereotypies than can displace ambulatory activity, thereby increasing the variance and decreasing statistical power. The age and sex effects in terms of ambulations are detectable at low but not high doses of cocaine.
Fine movement responses to escalating dose binge cocaine can be seen in figure 2c–d. In contrast to ambulations, fine movements dose-dependently increased with evenly distributed variance at both low and high doses. As with ambulations, age affected both the temporal pattern and magnitude of fine movements. ANOVA indicated an age x time interaction (F(22,792)=1.8, P<0.02) and post-hoc analysis indicated that cocaine stimulated fine movements for longer durations in adults than adolescents. Both age and sex affected the magnitude of cocaine-stimulated fine movements. ANOVA indicated a main effect of age (F(2,72)=6.6, P<0.001), sex (F(1,72)=25.7, P<0.001), dose (F(2,137)=387.3, P<0.001), an age x sex interaction (F(2,72)=12.8, P<0.001), and an age x sex x dose interaction (F(4,137)=5.5, P<0.001). Post-hoc analyses indicated that PN 65 animals had greater fine movement responses than younger animals and that females had greater responses than males. These analyses also demonstrated that PN 65 females had greater fine movement responses than all other age x sex cohorts following the 10 and 25 mg/kg doses. When we analyzed the sexes separately, ANOVA did not indicate any main effect or interactions with age in males. However, ANVOA indicated a main effect of age (F(2,35)=17.7, P<0.001) and an age x dose interaction (F(4,66)=12.8, P<0.001) in females. Post-hoc analyses indicated that PN 65 females had greater fine movement responses than younger females following the second and third doses. The age x sex effects for fine movements are directly opposite those we observed for ambulations: ambulatory responses to cocaine decrease with age in males but fine movements increase with age in females.
We also observed animals for specific behaviors to generate an average behavioral rating (Figure 2e–f). While neither age nor sex affected the temporal pattern of behavioral rating, sex did significantly affect the magnitude of behavioral ratings. ANOVA indicated a main effect of sex (F(1,76)=23.8, P<0.001), and a sex x dose interaction (F(2,151)=6.5, P<0.02). Post-hoc analysis indicated that females had greater average behavioral ratings than males following the second and third injections. In addition to generating an average behavioral rating, we also measured the frequency of intervals that animals were observed in stereotypies (Figure 2g–h). ANOVA indicated a main effect of sex (F(1,68)=30.8, P<0.001), dose (F(2,136)=351.9, P<0.001), a sex x dose interaction (F(2,136)=14.11, P<0.001) and an age x sex interaction (F(2,68)=4.8, P<0.02). Post-hoc analysis indicated that females were more frequently observed in stereotypies than males following the second and third injections. We analyzed the sexes separately to investigate the age x sex interaction. Within males, ANOVA indicated a main effect of age (F(2,36)=4.4, P<0.02) and post-hoc analysis indicated PN 28 males were more frequently observed in stereotypies than older males. ANOVA indicated no main effect or interactions of age within females.
Effects of Prepubertal Gonadectomy on Habituation
To examine the effects of gonadal hormones in mediating changes in behavioral responsiveness to stimulants during adolescence, we tested the effects of binge cocaine administration in sham operated and prepubertally castrated males and females in adulthood. Figure 3 shows the effects of prepubertal gonadectomy on ambulatory (a–b) and fine movement c–d) responses in males (left panels) and females (right panels) during habituation. Prepubertal gonadectomy had no effect on the magnitude or temporal pattern of ambulatory responses during habituation as ANOVA indicated no main effects or interactions of sex or gonadectomy (Figure 3a–b). The lack of a main effect of sex as in the development experiment is likely attributable to the smaller population size. Prepubertal gonadectomy did reduce the number of fine movements made during habituation as ANOVA indicated a main effect of gonadectomy (F(1,28)=12.4, P<0.002) (Figure 3c–d). Post-hoc analysis indicated that gonadectomized animals made fewer fine movements than intact animals.
Figure 3.
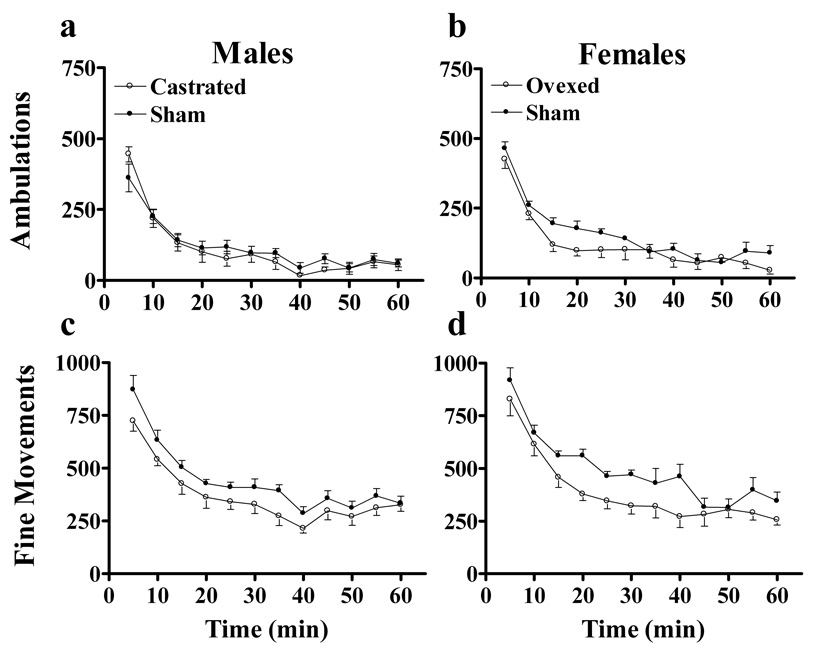
Effects of prepubertal gonadectomy on the horizontal activity of male and female rats during habituation to novel, open-field test chambers. Activities of males are shown in the right panels and the activities of females are shown in the left panels. Behaviors are reported as ambulations (a–b) and fine movements (c–d). N=8 for each age x surgery group.
Effects of Prepubertal Gonadectomy on Cocaine Responsiveness in Adulthood
Figure 4 shows the effects of prepubertal gonadectomy on ambulations (a–b), fine movements (c–d) behavioral ratings (e–f) and stereotypy responses to escalating dose binge cocaine in males (right panels) and females (left panels). Prepubertal gonadectomy disparately affected behavioral responses to escalating dose cocaine in males and females. For ambulations (Figure 4a–b), we again analyzed the third and highest dose of cocaine separately from the first two injections. Following 5 and 10 mg/kg, ANOVA indicated a main effect of sex (F(1,27)=7.6, P<0.02), dose (F(1,27)=50.4, P<0.001), time (F(11,297)=10.9, P<0.001), a sex x surgery interaction (F(1,27)=7.4, P<0.02), a sex x dose (F(1,27)=7.8, P<0.01) and a sex x surgery x dose interaction (F(1,27)=6.1, P<0.02). As expected, post-hoc analysis indicated that females had greater ambulatory responses than males to cocaine. To explore the sex x surgery interaction, we analyzed the effects of surgery within each sex. ANOVA indicated a main effect of gonadectomy in females and post-hoc analysis indicated that sham females had greater ambulatory responses to 5 and 10 mg/kg cocaine than gonadectomized females. ANOVA indicated no effect of gonadectomy or gonadectomy x dose interaction in males. Adult males have relatively minimal ambulatory responses to 5 and 10 mg/kg cocaine regardless of surgical status.
Figure 4.
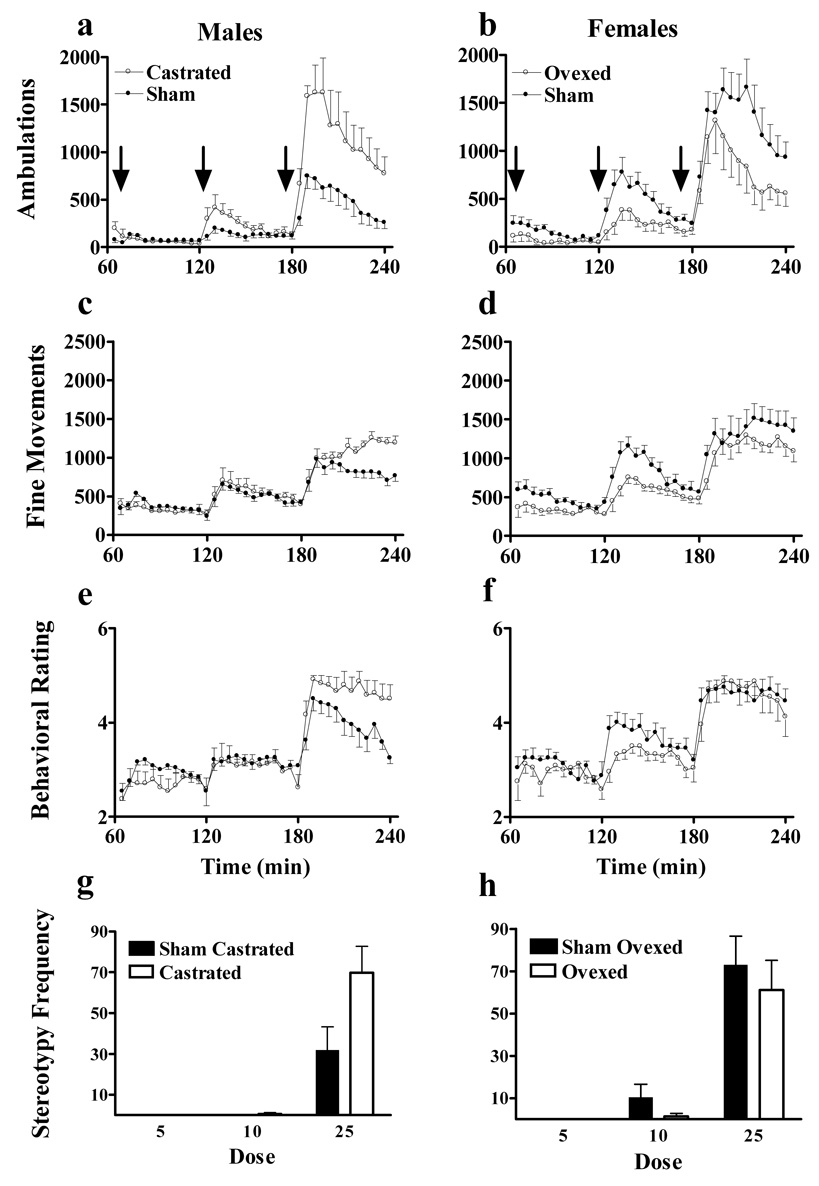
Effects of prepubertal gonadectomy of male and female rats on horizontal activity following binge cocaine administration. Drug administration and data collection were identical to that described in Figure 2. Data from each sex are presented in columns and individual behavioral topographies are shown in rows. N=8 for each age x surgery group.
Following 25 mg/kg cocaine, ANOVA indicated main effects of time (F(11,297)=108.5, P<0.001) and a sex x gonadectomy interaction (F(1,27)=9.8, P<0.01). Post-hoc analysis demonstrated that gonadectomy had opposite effects in males and females: gonadectomized males had greater ambulatory responses compared to cocaine than sham males whereas gonadectomized females had blunted ambulatory responses to cocaine compared to shame females. The disparate effects of gonadectomy were sufficient to ablate the consistently-demonstrated main effect of sex at this dose. Neither sex nor gonadectomy affected the temporal pattern of activity.
We obtained similar results when we analyzed fine movements (Figure 4c–d). ANOVA indicated a main effect of sex (F(1,27)=12.8, P<0.002), dose (F(2,54)=108.6, P<0.001), time (F(11,297)=7.2, P<0.001) and a sex x surgery interaction (F(1,27)=12.3, P<0.002). Post-hoc analysis again demonstrated that females had greater fine movement responses than males. Additionally, we observed a main effect of surgery within each sex (P<0.001 for each). Within males, the gonadectomized animals had greater fine movement responses than sham controls whereas sham control females had greater fine movement responses to escalating dose cocaine than gonadectomized females.
Animals were observed for specific behaviors to generate an average behavioral rating and measure cocaine induced stereotypies. Gonadectomizing the animals had little effect on their average behavioral rating (Figure 4e–f). ANOVA indicated a main effect of dose (F(2,54)=138.3, P<0.001), time (F(11,297)=11.8, P<0.001) and a surgery x dose interaction (F(2,54)=5.5, P<0.01). Post-hoc analysis indicated that there was no effect of gonadectomy following the lowest dose of the escalating dose binge. For stereotypy frequency (Figure 4g–h) ANOVA indicated a sex x surgery interaction (F(1,28)=4.4, P<0.05). Within males, ANOVA indicated a main effect of surgery (F(1,14)=4.9, P<0.05) and post-hoc analysis indicated that castrated males were more frequently observed in stereotypy than sham castrated males. ANOVA indicated no main effect of surgery on stereotypy frequency in females.
4: Discussion
The present study demonstrates that sex differences in cocaine-induced behavioral activation arise during adolescence. Females had greater responses to cocaine in all behavioral measures in adulthood. These differences in cocaine-stimulated behavior in adulthood result from increased activation with age in females and decreased activation with age in males. Additionally, different behavioral profiles changed with age for males and females. While ambulatory activity decreased with age in males, fine movements increased with age in females. Finally, we showed that prepubertal gonadectomy produced opposite effects on behavioral responsiveness to cocaine in males and females. Prepubertal castration increased behavioral responsiveness in males while prepubertal ovariectomy decreased responsiveness in females. These data suggest that gonadal hormones have significant and opposing effects on behavioral responsiveness to cocaine across adolescence. This finding raises the possibility that gonadal steroids modulate the structure/function of developing dopamine systems during adolescence and thus produce many of the lasting sex differences in sensitivity to cocaine observed in adulthood.
The greater behavioral responsiveness of females to cocaine and the developmental fall in males observed in this study are largely supported by previous data. We have previously treated adolescent and adult male rats with the same escalating dose paradigm (Caster et al. 2005). In this and the previous study we found that the youngest adolescents had exaggerated stereotypy responses following the third and highest dose of cocaine (25 mg/kg). Previous studies have consistently demonstrated that adult female rats have greater behavioral responses to psychostimulants than adult males (Becker et al. 1982; Camp et al. 1986; Camp and Robinson 1988; Chin et al. 2002; Glick et al. 1983; Schneider and Norton 1979; Sell at al. 2000; Van Haaren and Meyer 1991; Walker et al. 2001). In the present study, we found that intact adult females have greater cocaine-induced behavioral responses than intact adult males for all behavioral measures. Several previous studies have reported higher basal (habituation) activity in females than in age-matched males (Leret et al. 1994; Tropp and Markus 2001) as was also observed in this study.
The changing pattern (ambulatory vs. non-ambulatory) of cocaine-induced behaviors across adolescence is sex-specific. Earlier studies, including our own, have primarily utilized equipment that separates automated behavioral activity into vertical and horizontal activity. The present study utilized software that further resolves horizontal locomotor activity into ambulations and fine movements. This enabled a finer discrimination of how cocaine-stimulated behavior changed during adolescence in a sex-specific way. We observed that young adolescent males have greater ambulatory responses than older animals, while there is no effect of age at any dose for ambulations in females: adult females retain the high activity of adolescence. In contrast, adult females had greater non-ambulatory (fine movement) responses than younger females, but there was no effect of age for fine movements in males. Fine movements include all non-ambulatory behaviors including (but not limited to) stereotypies. Several studies have demonstrated that lesions to the nucleus accumbens primarily attenuate locomotor responses to cocaine and amphetamine whereas lesions to the caudate (striatum) primarily attenuate stereotyped responses (Kelly et al. 1975; Kelly and Iverson 1976). The sex-specific developmental changes in topography suggest that male and female hormones could differentially affect the activity and/or development of dopamine projections to nucleus accumbens and caudate/putamen.
Gonadal steroid hormones work by a combination of classical genomic effects mediated by hormone/receptor complex-mediated transcription and rapid non-genomic actions (McEwen and Alves 1999). Their effects can require the presence of the hormone (activational) or result from differentiation of brain structures during specific periods of development (organizational). The robust and persistent effects of gonadectomy in males observed in the present study may reflect organizational effects of gonadal steroids during puberty. The significant enhancement of cocaine-stimulated activity observed here after prepubertal gonadectomy contrast with the generally slight effects of castration in adulthood. Our results are the first to demonstrate that prepubertal castration robustly increased the behavioral responsiveness (locomotion, fine movements and stereotypy scores) to cocaine in males. This contrasts with the modest activational effects of testosterone on acute responsiveness observed following adult castration (reviewed in Festa and Quinones-Jenab 2004). Several groups, including our own, have reported that adult castration had a slight effect on stereotyped behavior (Dluzen et al. 1986; Van Luijtelaar et al. 1996; Walker et al. 2001), but most have reported no significant effect of adult castration on acute locomotor responsiveness to stimulants (Chin et al. 2002; Hu and Becker 2003; Savageau and Beatty 1981). Our laboratory found that castration increased cocaine-stimulated horizontal activity, only when statistical power was increased by combining multiple experiments (Walker et al. 2001a). The ability of prepubertal castration to substantially increase cocaine-stimulated behavior in relation to the modest effects of adult castration, suggests that testosterone may induce significant organizational effects during puberty. Beatty et al. (1982) also demonstrated that prepubertal gonadectomy produced greater increases in amphetamine-induced stereotyped behavior than adult castration. Androgen effects on amphetamine metabolism may likely have contributed to that effect. The results of the present and previous studies provide evidence that androgen activity during puberty is likely a critical mediator in the development of sex-specific responses to stimulants.
The decrease in female cocaine responsiveness with prepubertal ovariectomy in this study is similar to previously documented decreases following ovariectomy in adulthood (Walker et al. 2001; reviewed in Festa and Quinones-Jenab 2004). Therefore it is likely that activational effects contribute to this effect. A large literature suggests that both genomic and nongenomic effects of estrogen contribute to this response. Estrogen augments dopaminergic function both presynaptically and postsynaptically (Becker 1999; Becker and Beer 1986; Lynch et al. 2002; Walker et al. 2006). Estrogen enhances stimulant-induced dopamine release and dopamine receptor sensitivity (Becker 1999). Estradiol administration has been shown to increase dopamine receptor density over a period of days (Hruska 1986). Adult ovariectomy has also been shown to reduce the density of the dopamine transporter (DAT) in the nucleus accumbens and in the striatum (Bosse et al. 1997), a region where DAT density is reduced in males compared to females (Rivest et al. 1995). Further, cocaine-induced behavioral activity varies with alterations in estrogen levels across the estrus cycle in adult females: cocaine responses are blunted during diestrus compared to the proestrus and estrus phases (Becker and Cha 1989; Quinones-Jenab 1999; Walker et al. 2002). The enhanced cocaine responsiveness of adult females compared to younger animals could largely be mediated by higher circulating levels of estrogen in adult female rats.
The similarities between our present results and previous studies in females suggest that activational effects of estrogen largely mediate the decreases in stimulant-induced locomotor activity in both pre- and postpubertally ovexed females. However, the potential for organizational effects of estrogen during adolescence can not be excluded based on our results as we did not replace estrogen in any prepubertally ovexed females after adolescence but prior to cocaine testing. In fact, at least one study supports the role of activational effects of estrogens by demonstrating the importance of the timing of gonadectomy with respect to puberty and amphetamine-stimulated behavioral activity in females (Forgie and Stewart 1994). In that study, prepubertally ovexed females had lower amphetamine-stimulated activities scores than those ovexed as adults. Thus, it is likely that changes in cocaine-responsiveness across adolescence in females represent the sum of both activational and organizational effects of estrogens and the exact contribution of each remains presently unclear.
The cocaine-induced behavioral profile observed after prepubertal gonadectomy did not completely match the emerging profile observed across normal development in either sex. During normal development, there is no effect of age for fine movements in males and no effect of age for ambulations in females. However, prepubertal gonadectomy affected locomotor and fine movements in both males and females. Similarly, there are differences in the doses at which age and gonadectomy affect cocaine-stimulated behaviors in each sex. These observations are not surprising as the presence or absence of sex hormones is only one factor that could affect behavioral responsiveness to stimulants across adolescence. Forebrain dopamine systems continue to mature and reorganize across adolescence (Haycock et al. 2003; Montague et al. 1999; Teicher et al. 1995; reviewed in Spear 2000). For example, it has been shown that there is an overproduction and regressive pruning of striatal dopamine receptors during adolescence (Teicher et al. 1995). Such maturational events in dopamine systems could likely affect the behavioral responsiveness to stimulants across adolescence independent of sex hormones. While this study did not specifically examine the relative contribution of sex hormones and other events in modulating behavioral responsiveness to stimulants across adolescence it did clearly demonstrate that the presence of male and female sex hormones during adolescence exerts opposite and lasting effects on cocaine-stimulated behavior in adulthood.
Age and sex differences in stimulant responsiveness are not likely due to differences in drug metabolism. While this study did not directly examine blood and brain cocaine levels in animals following each injection of the escalating dose paradigm, several lines of evidence suggest differences in pharmacokinetics are unlikely to account for these observations. We have previously examined brain cocaine levels in adolescent and adult males during a repeated dose model and found no significant differences across age (Caster et al. 2005). At least within males, age-related differences in acute cocaine metabolism have not been identified. Similarly, blood and brain cocaine levels are comparable in male and female rats and primates (Bowman et al. 1999; Mendelson et al. 1999). While sex differences in the metabolism of amphetamine do exist in rats, sex differences in behavioral stimulation still exist when the doses are adjusted (Becker et al. 1982). Mechanisms other than changes in drug metabolism must underlie the sex and age-specific behavioral responses to stimulants.
The results of this study demonstrate the importance of sex hormone exposure during adolescence on cocaine sensitivity in adulthood. The presence of male and female hormones during adolescence exerts opposite and lasting effects on cocaine responsiveness. The actions of sex hormones during adolescence appear to be critical in mediating the lasting sex differences observed in adulthood, particularly in males. The underlying mechanisms of these observations may account for the sex-specific sensitivity to stimulants in humans.
Acknowledgments
This work was generously supported by NIDA grant #DA09079. These experiments comply with all applicable United States laws and regulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beatty WW, Dodge AM, Traylor KL. Stereotyped behavior elicited by amphetamine in the rat: influences of the testes. Pharmacol Biochem Behav. 1982;16:565–568. doi: 10.1016/0091-3057(82)90416-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha J. Estrous cycle-dependant variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharm Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bosse R, Rivest R, DiPaolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Mol Brain Res. 1997;46:343–346. doi: 10.1016/s0169-328x(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of gender and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Camp DM, Becker JB, Robinson TE. Sex differences in the effect of gonadectomy on amphetamine-induced rotational behavior in rats. Behav Neural Bio. 1986;46:491–495. doi: 10.1016/s0163-1047(86)90527-3. [DOI] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology. 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J Psychopharm. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HBK, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Green MA, Ramirez VD. The effect of hormonal condition on dose-dependent amphetamine-stimulated behaviors. Hormones Behav. 1986;20:1–6. doi: 10.1016/0018-506x(86)90024-3. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Hormones Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Sex differences in amphetamine-induced locomotor activity in adolescent rats: role of testosterone exposure in the neonatal period. Pharmacol Biochem Behav. 1993;46:637–645. doi: 10.1016/0091-3057(93)90555-8. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Stewart J. Effect of prepubertal ovariectomy on amphetamine-induced locomotor activity in adult female rats. Hormones Behav. 1994;28:241–260. doi: 10.1006/hbeh.1994.1021. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA, Shapiro RM. Cocaine-induced rotation: sex-dependent differences between left-and right-sided rats. Science. 1983;221:775–777. doi: 10.1126/science.6879177. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Hruska RE. Elevation of striatal dopamine receptors by estrogen: dose and time studies. J Neurochem. 1986;47:1908–1915. doi: 10.1111/j.1471-4159.1986.tb13106.x. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. Nuerons. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Bio Psych. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animals models. Neurosci and Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn CM, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharm Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Leret ML, Molina-Holgado F, Gonzalez MI. The effect of perinatal exposure to estrogens on the sexually dimorphic response to novelty. Physiol Behav. 1994;55:371–373. doi: 10.1016/0031-9384(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Rivest R, Falardeau P, Di Paolo T. Brain dopamine transporter – gender differences and effect of chronic haloperidol. Brain Res. 1995;692:269–272. doi: 10.1016/0006-8993(95)00611-s. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci and Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Savageau MM, Beatty WW. Gonadectomy and sex differences in responses to amphetamine and apomorphine of rats. Pharmacol Biochem Behav. 1981;14:17–21. doi: 10.1016/0091-3057(81)90097-6. [DOI] [PubMed] [Google Scholar]
- Schneider BF, Norton S. Circadian and sex differences in hyperactivity produced by amphetamine in rats. Physiol Behav. 1979;22:47–51. doi: 10.1016/0031-9384(79)90402-5. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Results from the 2004 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-28, DHHS Publication No. SMA 05-4062) 2005
- Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum nut not nucleus accumbens. Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tropp J, Markus J. Sex differences in the dynamics of cue utilization and exploratory behavior. Behav Brain Res. 2001;119:143–154. doi: 10.1016/s0166-4328(00)00345-4. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar LJM, Dirksen R, Vreet B, Van Haaren F. Effects of acute and chronic cocaine administration on EEG and behaviour in intact and castrated male and intact and ovariectomized female rats. Brain Res Bull. 1996;40:43–50. doi: 10.1016/0361-9230(96)00005-6. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan K, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]


