Abstract
Carbohydrate chip technology has a great potential for the high-throughput evaluation of carbohydrate-protein interactions. Herein, we report syntheses of novel sulfated oligosaccharides possessing heparin and heparan sulfate partial disaccharide structures, their immobilization on gold-coated chips to prepare array-type Sugar Chips, and evaluation of binding potencies of proteins by surface plasmon resonance (SPR) imaging technology. Sulfated oligosaccharides were efficiently synthesized from glucosamine and uronic acid moieties. Synthesized sulfated oligosaccharides were then easily immobilized on gold-coated chips using previously reported methods. The effectiveness of this analytical method was confirmed in binding experiments between the chips and heparin binding proteins, fibronectin and recombinant human von Willebrand factor A1 domain (rh-vWf-A1), where specific partial structures of heparin or heparan sulfate responsible for binding were identified.
Keywords: Sugar, Carbohydrate, chip, heparin, heparan sulfate, carbohydrate-protein interaction, Surface Plasmon Resonance, SPR, SPR-imaging
Carbohydrate chips and related array technologies1–3 have attracted a great deal of attention as a powerful tool for glycomics. Like DNA4 and protein chips5, they can rapidly and simply evaluate carbohydrate–protein interactions in parallel, with a minimum amount of sample. Our ongoing research involves this functional analysis of sulfated polysaccharides such as heparin (HP) and heparan sulfate (HS).3a HP and HS are highly sulfated polysaccharides and belong to the glycosaminoglycan (GAG) superfamily. They are among the most complex of carbohydrates, and play a significant role in biological processes through their binding interactions with numerous proteins,6 such as growth factors, cytokines, viral proteins, and coagulation factors, among others. HP/HS have a basic structure composed of a repeating α or β(1,4)-linked disaccharide moiety which is derived from uronic acid (either glucuronic acid or iduronic acid) and N-acetyl-glucosamine residues. In general, HP/HS chains are very heterogeneous and contain innumerable substitution patterns due in part to some randomness in the multiple enzymatic modifications in their biosynthesis. This heterogeneity makes it difficult to elucidate the structure-function relationships of HP/HS at the molecular level. Therefore, structurally defined HP/HS sequences are necessary for the precise elucidation of the mode of HP/HS actions on their target molecules. So far, many synthetic efforts have been dedicated to the synthesis of HP/HS fragments.3b–d,7,8
Previously, we have reported that a specific disaccharide unit in HP, O-(2-deoxy-2-sulfamido-6-O -sulfo-α-D-glucopyranosyl)-(1–4)-2-O-sulfo-α-L-idopyran osyluronic acid (abbreviated as GlcNS6S-IdoA2S), is a key unit for binding to human platelets9 and von Willebrand factor (vWf),10 and that the clustering of these disaccharides significantly enhanced the interaction.11,12 To systematically investigate heparin’s binding properties, we have developed a method3a for the immobilization the sulfated oligosaccharide onto a gold-coated chip, and have devised an analytical system using surface plasmon resonance (SPR) technology, which permits their real-time study without further labeling. These systems can be applied to the investigation of the binding interactions of a variety of structurally defined oligosaccharides.
To better understand the HP/HS disaccharide structures involved in specific protein interactions, we designed three kinds of sulfated trisaccharide ligand conjugates 2–4 containing the disaccharide units as shown in Figure 1; GlcNS-IdoA2S (2): O-(2-deoxy-2-sulfamido-α-D-glucopyranosyl)-(1–4)-2-O-sulfo-α-L-idopyranosyluronic acid, GlcNS6S-GlcA (3): O-(2-deoxy-2-sulfamido-6-O-sulfo-α-D-glucopyranosyl)-(1–4)-α-D-glucopyranosyluron ic acid, GlcNS-GlcA (4): O-(2-deoxy-2-sulfamido-α-D-glucopyranosyl)-(1–4)-α-D-glucopranosyluronic acid. The disaccharide units contained in ligand conjugates 1–4 of Figure 1 are frequently found in HP/HS disaccharide unit.
Fig. 1.
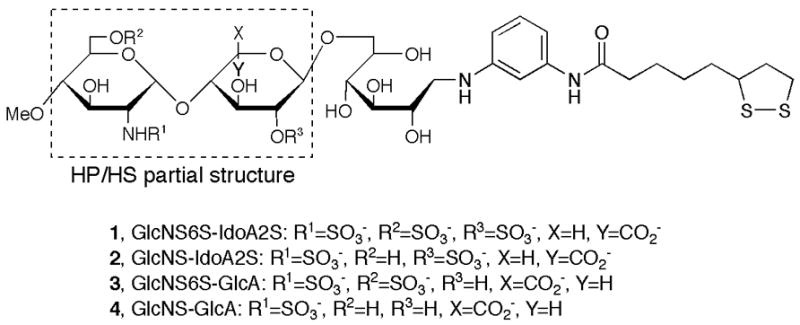
Sulfated disaccharide partial structures of heparin/heparan sulfate.
For efficient synthesis, four monomeric building blocks were prepared. 2-Azido glucose derivative 5, idose derivative 6, and 4,6-benzylidene glucose derivative 17 were used for glucosamine, iduronic acid, and glucuronic acid moieties, respectively. Selective sulfation onto glucosamine and iduronic acid or glucuronic acid moieties can be carried out by an appropriate functionalization. The 6-OH glucose derivative 7 was used as the reducing end for the conjugation to linker molecule 16 after deprotection on the glucose, which works not only as a reducing end donor for reductive amination but also as the hydrophilic moiety in the molecule to minimize any non-specific hydrophobic interactions between the linker and target proteins or cells.
The synthesis of ligand conjugate 2 containing GlcNS-IdoA2S unit was carried out as shown in Scheme 1. Trisaccharide 8, which was prepared according to the method reported previously,12 was selectively protected by t-butyldimethylsilyl (TBDMS) group. The methyl ester of trisaccharide 9 was hydrolyzed and the remaining 2′-hydroxy group was sulfated using sulfur trioxide-pyridine complex at room temperature. After removing the TBDMS group with HF•pyridine complex, the azido group was reduced using a catalytic amount of Pd-C under hydrogen atmosphere and the resulting amino group was N-sulfated. All benzyl protecting groups were removed by hydrogenolysis using catalytic Pd-C to give the desired trisaccharide 15. Finally, the reductive amination of trisaccharide 15 with linker compound 16 was performed using NaBH3CN to afford the desired ligand conjugate 2 in good yield. Compound 2 was purified by gel-filtration chromatography with Sephadex G-25 fine and confirmed by 1H NMR and ESI-TOF/MS analyses.13
Scheme 1.
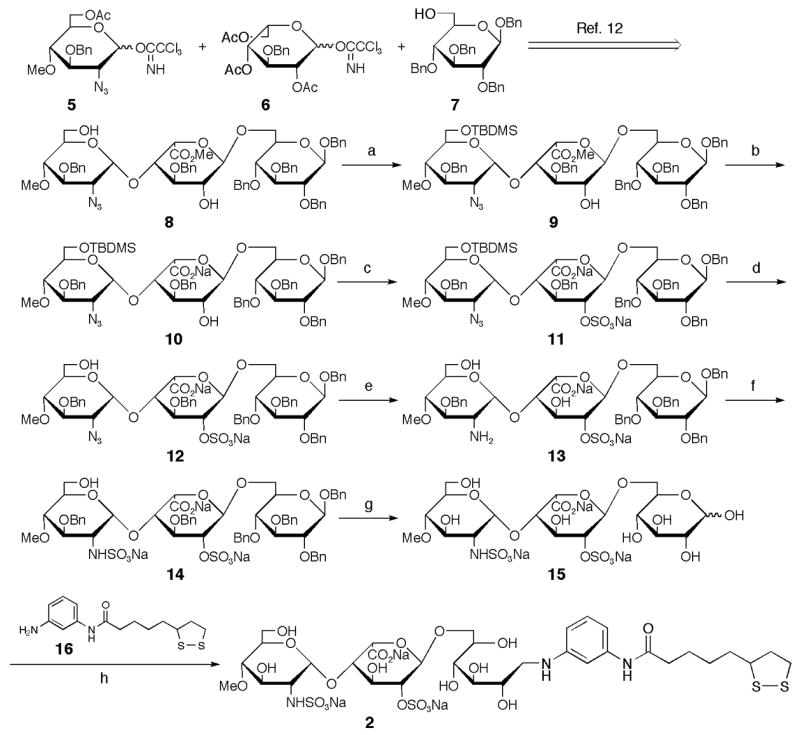
Synthesis of ligand conjugate 2 containing GlcNS-IdoA2S. (a) TBDMSCl, imidazole, MS4AP in CH2Cl2, 45%; (b) 1 M NaOH, MeOH/THF (1:1), 70%; (c) SO3•Pyr in Pyr; (d) HF•Pyr in Pyr; (e) 10% Pd-C, H2 (1 kg/cm2) in THF/MeOH (2:1); (f) SO3•Pyr in H2O; (g) 10% Pd-C, H2 (7 kg/cm2) in H2O/AcOH (5:1), 29% (5 steps); (h) NaBH3CN in DMAc/H2O/AcOH (1:1:0.1), 82%.
The syntheses of ligand conjugates 3 and 4 were carried out in the same fashion as the syntheses of 1 and 2 (Scheme 2). Glycosylation of 6-OH glucose 7 and imidate 17 with trimethylsilyl trifluoromethanesulfonate (TMSOTf) as a promoter and treatment of the resultant with NaOMe gave disaccharide 18 in a good yield. The resulting hydroxy groups of 18 were then protected with a benzyl group. After removal of the benzylidene group, the primary 6′-OH group was selectively oxidized to carboxylic acid using 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO).14 The resulting carboxyl group was esterified with (trimethylsilyl)diazomethane to afford the disaccharide 20. The 2-azido imidate 5 was condensed with disaccharide 20 using TBDMSOTf at −20 °C to give selectively an α-linked trisaccharide 21.11,15 Hydrolysis of the acetyl group and methyl ester was then carried out using aqueous NaOH to give the common intermediate 22 for trisaccharides 23 and 24. The sulfated trisaccharide 23 was obtained by O-sulfation of the 6″-hydoxyl group and reduction and N-sulfation of 2′-azido group was followed by hydrogenolysis. Conversely, the sulfated trisaccharide 24 was prepared by the same method as the synthesis of trisaccharide 23, omitting the O-sulfation. The ligand-conjugates 316 and 417 were synthesized in satisfactory yields as similar to the described procedure for compound 2.
Scheme 2.
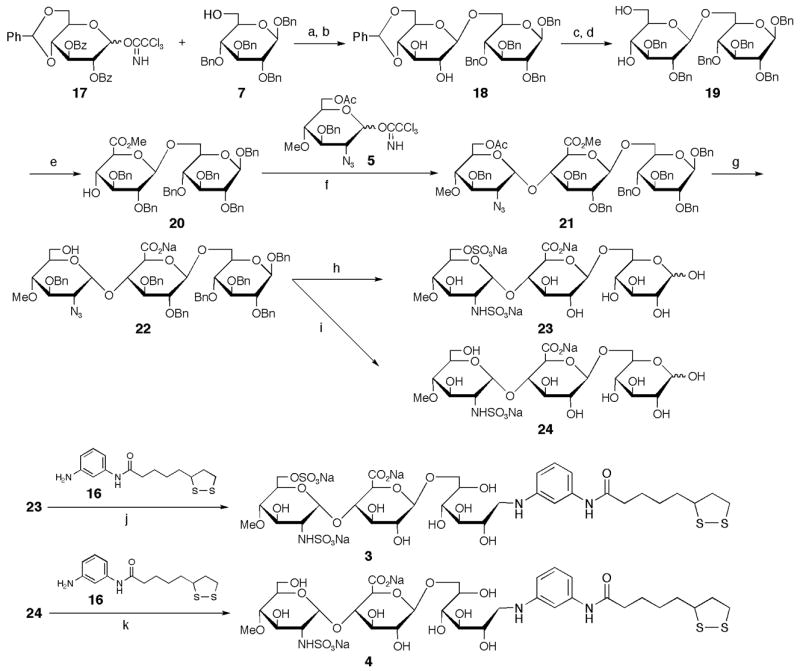
Synthesis of ligand conjugates 3 and 4 containing GlcNS6S-GlcA and GlcNS-GlcA, respectively. (a) BF3•OEt2, MS4AP in CH2Cl2, −20°C; (b) 0.1 M NaOMe, 90% (2 steps); (c) NaH, BnBr in DMF, 0°C→rt, 88%; (d)16% TFA, 8% MeOH in CH2Cl2, 0°Crt, 93%; (e) TEMPO, KBr, NaClO in CH2Cl2; TMSCHN2, 83% (2 steps); (f) TBDMSOTf, MS4AP in toluene, −20°C, 84%; (g) 5 M NaOH in MeOH/THF (1:1), 89%; (h) SO3•Pyr in Pyr; 10% Pd-C, H2 (1 kg/cm2) in THF/H2O (2:1); SO3•Pyr in H2O (pH 9.5); 10% Pd-C, H2 (7 kg/cm2) in H2O/AcOH (5:1), 28% (4 steps); (i) 10% Pd-C, H2 (1 kg/cm2) in THF/H2O (2:1); SO3•Pyr in MeOH/H2O (3:2); 10% Pd-C, H2 (7 kg/cm2) in H2O/MeOH/AcOH (5:5:2), 39% (3 steps); (j) NaBH3CN in DMAc/H2O/AcOH (1:1:0.1), 62%; (k) NaBH3CN in DMAc/H2O/AcOH (1:1:0.1), 50%.
Binding interactions were investigated by use of the SPR imaging sensor.18 When fibronectin was tested (Figure 2), specific binding interactions were clearly observed with compounds 1 (GlcNS6S-IdoA2S, KD = 5.5 nM) and 3 (GlcNS6S-GlcA, KD = 6.5 nM), but not with compounds 2 (GlcNS-IdoA2S, KD = 30 nM) and 4 (GlcNS-GlcA, KD = 33 nM). These results indicate that the N-sulfation and 6-O-sulfation of glucosamine in HP/HS are important for fibronectin binding, while 2-O-sulfation of iduronic acid is less important. Recently, Couchman and coworkers showed that N-sulfation of glucosamine was essential for fibronectin binding and 2-O-sulfation of iduonic acid or 6-O-sulfation of glucosamine has marginal effects.19 Additionally, N-sulfation and 6-O-sulfation of glucosamine were important for focal adhesion formation through syndecan-4, heparan sulfate proteoglycan. Our results are in agreement with those data.
Fig. 2.
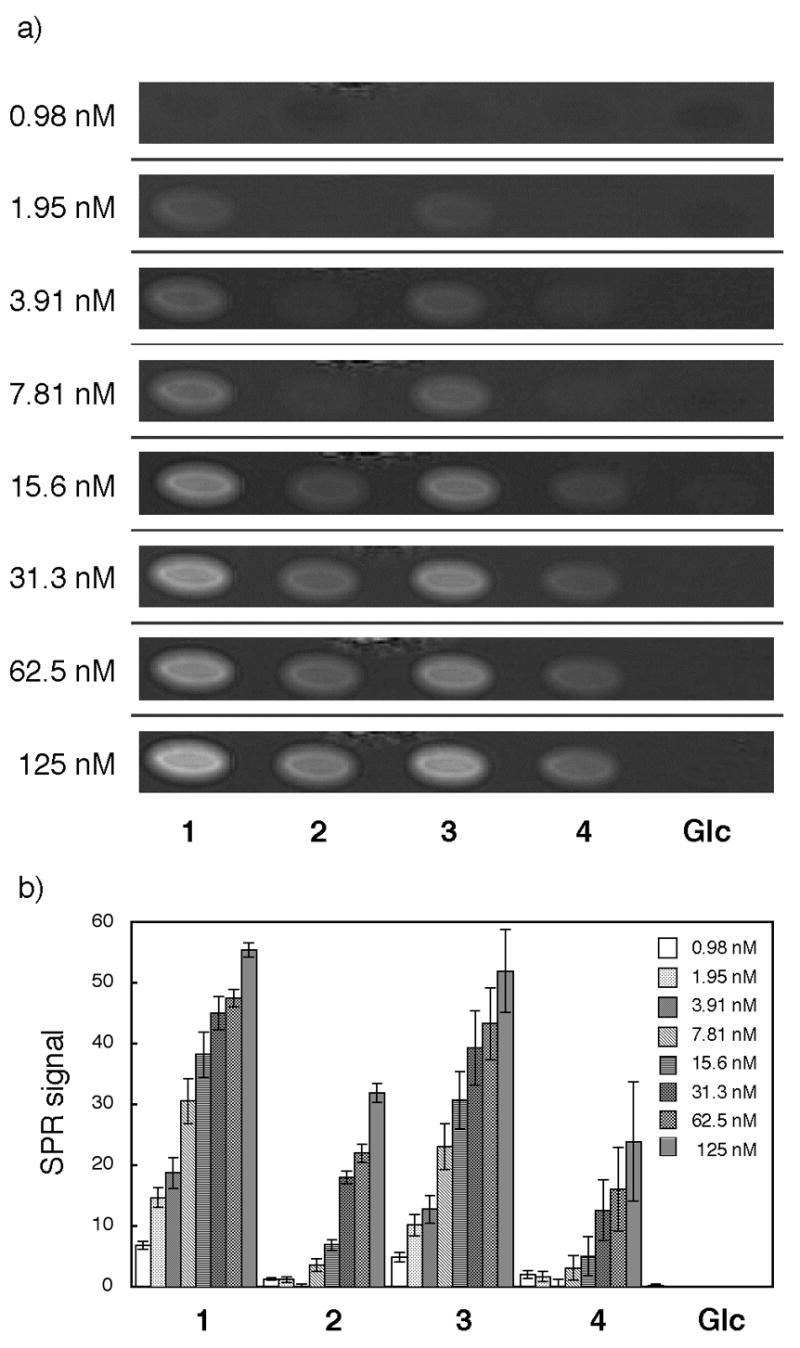
Binding study with fibronectin. a) SPR difference imaging on the chip immobilized with compounds 1, 2, 3, 4, and Glc α(1–6)Glc-mono (Glc). Measurements were carried out with analyte in the range between 0.98 nM and 125 nM. b) Bar graph profiles of different concentrations of protein. The error bars represent +/− SEM.
In contrast, when recombinant human vWf A1 domain (rh-vWf-A1)20 was injected over the chips, a different pattern of oligosaccharide binding preference was noted (Figure 3). A strong interaction was observed with compounds 1 (GlcNS6S-IdoA2S, KD = 1.0 μM) and 2 (GlcNS-IdoA2S, KD = 0.9 μM). Weaker interaction was seen with compound 3 (GlcNS6S-GlcA, KD = 1.4 μM), while distinctly low binding was observed with compound 4 (GlcNS-GlcA, KD = 4.3 μM). Although the GlcNS6S-IdoA2S (1) disaccharide structure was considered a key binding domain of vWf10, the exact disaccharide structure responsible for vWf binding is still unclear. We found previously that clustered compounds containing three units of GlcNS6S-IdoA2S12 possessed higher competitive binding activity compared to compounds containing less than two units of GlcNS6S-IdoA2S (unpublished data). Together with those data, the current results indicate that the tri-sulfated disaccharide binds vWf best, that loss of either the 6-sulfate of GlcN or the 2-sulfate of Ido reduces vWf binding significantly, and that the N-sulfate of GlcN alone is not sufficient for binding vWf.
Fig. 3.
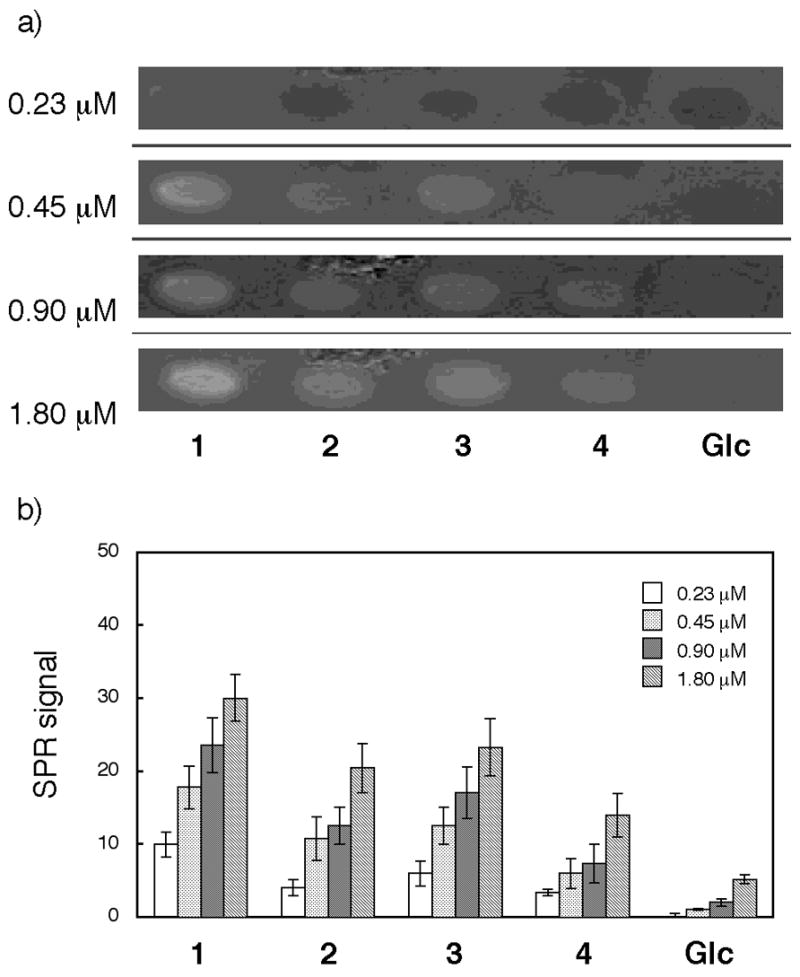
Binding study with rh-vWf-A1. a) SPR difference imaging on the chip immobilized with compounds 1, 2, 3, 4, and Glc α(1–6)Glc-mono (Glc). Measurements were carried out with analyte in the range between 0.23 μM and 1.80 μM. b) Bar graph profiles of different concentrations. The error bars represent +/− SEM.
In conclusion, we have designed new, precisely sulfated oligosaccharides of HP/HS partial structures. These oligosaccharides were efficiently synthesized using appropriate monosaccharide intermediates. Their application in an array type Sugar Chip, using SPR imaging analysis has been shown to be an efficient and specific technology to elucidate the interactions between a protein and multiple sulfated disaccharides, on a real time scale. These techniques can be used for high-throughput screening of protein samples, as well as for solving the structure-function relations of an individual protein-glycosaminoglycan interaction at the molecular and nano-scale.
Supplementary Material
Supplementary data
Supplementary data associated with this article can be found, in the online version, at ????????
Acknowledgments
The present work was financially supported in parts by grants from Japan Science and Technology Agency (Pre-venture program to Y.S., CREST to Y.S.), the National Institutes of Health (Grant HL079182 to M.S. and the Department of Veterans Affairs Research Service (to M.S.).
Footnotes
BRIEFS: Syntheses of sulfated oligosaccharide of heparin and heparan sulfate partial structures and their application to Sugar Chips are described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.For reviews, see: Love KR, Seeberger PH. Angew Chem Int Ed. 2002;41:3583. doi: 10.1002/1521-3773(20021004)41:19<3583::AID-ANIE3583>3.0.CO;2-P.Feizi T, Fazio F, Chai W, Wong CH. Curr Opin Struct Biol. 2003;13:637. doi: 10.1016/j.sbi.2003.09.002.Wang D. Proteomics. 2003;3:2167. doi: 10.1002/pmic.200300601.Shin I, Park S, Lee M. Chem Eur J. 2005;11:2894. doi: 10.1002/chem.200401030.Ortiz Mellet C, Garcia Fernandez JM. ChemBioChem. 2002;3:819. doi: 10.1002/1439-7633(20020902)3:9<819::AID-CBIC819>3.0.CO;2-Z.
- 2.For articles, see: Blixt O, Head S, Mandala T, Scanlan C, Hufflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH. Proc Natl Acad Sci USA. 2004;101:17033. doi: 10.1073/pnas.0407902101.Chevolet Y, Martins J, Milosevic N, Léonard D, Zeng S, Malissard M, Berger EG, Maier P, Mathieu HJ, Crout DHG, Sigrist H. Bioorg Med Chem. 2001;9:2943. doi: 10.1016/s0968-0896(01)00172-9.Park S, Lee MR, Pyo SJ, Shin I. J Am Chem Soc. 2004;126:4812. doi: 10.1021/ja0391661.Burn MA, Disney MD, Seeberger HP. ChemBioChem. 2006;7:421. doi: 10.1002/cbic.200500361.Houseman BT, Mrksich M. Chem Biol. 2002;9:443. doi: 10.1016/s1074-5521(02)00124-2.Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. J Am Chem Soc. 2002;124:14397. doi: 10.1021/ja020887u.Lee M, Shin I. Angew Chem Int Ed. 2005;44:2881. doi: 10.1002/anie.200462720.Schwarz M, Spector L, Gargir A, Shtevi A, Gortler M, Altstock RT, Dukler AA, Dotan N. Glycobiology. 2003;13:749. doi: 10.1093/glycob/cwg091.Zhou XC, Zhou JH. Biosens Bioelectron. 2006;21:1451. doi: 10.1016/j.bios.2005.06.008.Manimala JC, Roach TA, Li ZT, Gildersleeve JC. Angew Chem Int Ed. 2006;45:3607. doi: 10.1002/anie.200600591.Wang D, Liu S, Trummer BJ, Deng C. Nat Biotechnol. 2002;20:275. doi: 10.1038/nbt0302-275.Chevolet Y, Bouillon C, Vidal S, Morvan F, Meyer A, Cloarec JJ, Jochum A, Parly JP, Vasseur JJ, Souteyrand E. Angew Chem Int Ed. 2007;46:2398. doi: 10.1002/anie.200604955.
- 3.Recent carbohydrate chips immobilized sulfated oligosaccharide, see: Suda Y, Arano A, Fukui Y, Koshida S, Wakao M, Nishimura T, Kusumoto S, Sobel M. Bioconjugate Chem. 2006;17:1125. doi: 10.1021/bc0600620.de Paz JL, Noti C, Seeberger PH. J Am Chem Soc. 2006;128:2766. doi: 10.1021/ja057584v.de Paz JL, Spillmann D, Seeberger PH. Chem Commun. 2006:3116. doi: 10.1039/b605318a.Noti C, de Paz JL, Polito L, Seeberger PH. Chem Eur J. 2006;12:8664. doi: 10.1002/chem.200601103.Tully SE, Rawat M, Hsieh-Wilson LC. J Am Chem Soc. 2006;128:7740. doi: 10.1021/ja061906t.
- 4.a) Perou CM. Nature. 2000;406:747. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]; b) Ramsey G. Nat Biotechnol. 1998;16:40. doi: 10.1038/nbt0198-40. [DOI] [PubMed] [Google Scholar]; c) Marshall A, Hodgson J. Nat Biotechnol. 1998;16:27. doi: 10.1038/nbt0198-27. [DOI] [PubMed] [Google Scholar]; d) DeRisi JL, Lyer VR, Brown PO. Science. 1997;278:680. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 5.a) Templin MF, Stoll D, Schrenk M, Traub PC, Vchringer CF, Joos TO. Trends Biotechnol. 2002;20:160. doi: 10.1016/s0167-7799(01)01910-2. [DOI] [PubMed] [Google Scholar]; b) Weinberger SR, Dalmasso EA, Fung ET. Curr Opin Chem Biol. 2002;6:86. doi: 10.1016/s1367-5931(01)00282-4. [DOI] [PubMed] [Google Scholar]; c) Fung ET, Thulasiraman V, Weinberger SR, Dalmasso EA. Curr Opin Biotechnol. 2001;12:65. doi: 10.1016/s0958-1669(00)00167-1. [DOI] [PubMed] [Google Scholar]; d) Zhu H. Science. 2001;293:2101. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]; e) MacBeath G, Schreiber SL. Science. 2000;289:1760. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 6.a) Conrad HE. Heparin-Binding Proteins. Academic Press; San Diego: 1998. [Google Scholar]; b) Capila I, Lindhardt RJ. Angew Chem Int Ed. 2002;41:390. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; c) Turnbull J, Powell A, Guimond S. Trends Cell Biol. 2001;11:75. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]; d) Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Annu Rev Biochem. 1999;68:729. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]; d) Rabenstein DA. Nat Prod Rep. 2002;19:312. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]; e) Casu B, Lindahl U. Adv Carbohydr Chem Biochem. 2001;57:159. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]
- 7.For comprehensive review on the synthesis on GAGs, see: Yeung BKS, Chong PYC, Petillo PA. J Carbohydr Chem. 2002;21:799.
- 8.Recent articles, see: Lu LD, Shie CR, Kulkarni SS, Pan GR, Lu XA, Hung SC. Org Lett. 2006;8:5995. doi: 10.1021/ol062464t.Zhou Y, Lin F, Chen J, Yu B. Carbohydr Res. 2006;341:1619. doi: 10.1016/j.carres.2006.02.020.Codée JDC, Stubba B, Schiattarella M, Overkleeft HS, van Boeckel CAA, van Boom JH, van der Marel GA. J Am, Chem Soc. 2005;127:3767. doi: 10.1021/ja045613g. And references are therein.
- 9.a) Suda Y, Marques D, Kermode JC, Kusumoto S, Sobel M. Thromb Res. 1993;69:501–508. doi: 10.1016/0049-3848(93)90054-r. [DOI] [PubMed] [Google Scholar]; b) Suda Y, Bird K, Shiyama T, Koshida S, Marques D, Fukase K, Sobel M, Kusumoto S. Tetrahedron Lett. 1996;37:1053. [Google Scholar]
- 10.Poletti LF, Bird KE, Marques D, Harris RB, Suda Y, Sobel M. Arterioscler Thromb Vasc Biol. 1997;17:925. doi: 10.1161/01.atv.17.5.925. [DOI] [PubMed] [Google Scholar]
- 11.Koshida S, Suda Y, Fukui Y, Ormsby J, Sobel M, Kusumoto S. Tetrahedron Lett. 1999;40:5725. [Google Scholar]
- 12.Koshida S, Suda Y, Sobel M, Kusumoto S. Tetrahedron Lett. 2001;42:1289. [Google Scholar]
- 13.Spectral data for compound 2: 1H-NMR (600 MHz, D2O), δ 7.11 (1H, t, J = 7.9 Hz), 6.77–6.75 (2H, m), 6.58 (1H, d, J = 7.9 Hz), 5.22 (1H, d, J = 3.4 Hz), 4.97 (1H, brs), 4.14 (1H, brs), 4.05 (1H, brs), 3.92 (1H, brs), 3.82–3.71 (2H, m), 3.70–3.61 (5H, m), 3.59–3.55 (5H, m), 3.38 (3H, s), 3.28 (1H, dd, J = 9.6 and 3.4 Hz), 3.16 (1H, dd, J = 9.6 and 10.3 Hz), 3.09–3.00 (4H, m), 2.34–2.31 (1H, m), 2.27 (2H, t, J = 6.9), 1.87–1.83 (1H, m), 1.63–1.50 (4H, m), 1.37–1.33 (2H, m), ESI-MS (negative mode); Found: m/z 484.62 [(M-3Na+H)2−], Calcd. for C33H50N3O22S4Na3: 1037.15.
- 14.a) Davis NJ, Flitsch SL. Tetrahedron Lett. 1993;34:1181. [Google Scholar]; b) Anelli PL, Biffi C, Montanari F, Quici S. J Org Chem. 1987;52:2559. [Google Scholar]
- 15.Kovensky J, Duchaussoy P, Petitou M, Sinaÿ P. Tetrahedron: Asymmetry. 1996;7:3119. [Google Scholar]
- 16.Spectral data for compound 3: 1H-NMR (600 MHz, D2O), δ 7.13 (1H, t, J = 8.2 Hz), 7.11 (1H, s), 6.77 (1H, d, J = 8.2 Hz), 6.60 (1H, d, J = 8.2 Hz), 5.44 (1H, d, J= 3.4 Hz), 4.30 (1H, d, J = 7.6 Hz), 4.15 (1H, d, J = 10.3 Hz), 4.01 (1H, d, J = 10.3 Hz), 3.90 (1H, d, J = 11.0 Hz), 3.83–3.82 (1H, m), 3.76–3.71 (3H, m), 3.66–3.60 (6H, m), 3.52 (1H, dd, J = 10.3 Hz, J = 9.6 Hz), 3.42 (3H, s), 3.27–3.18 (3H, m), 3.14 (1H, dd, J = 3.4 Hz, J = 10.3 Hz), 3.07–3.02 (3H, m), 2.34–2.30 (1H, m), 2.30–2.27 (2H, m), 1.88–1.80 (1H, m), 1.65–1.46 (4H, m), 1.35–1.33 (2H, m), ESI-MS (negative mode); Found: m/z 484.65 [(M-3Na+H)2−], Calcd. for C33H50N3O22S4Na3: 1037.15.
- 17.Spectral data for compound 4: 1H-NMR (600 MHz, D2O), δ 7.09 (1H, t, J = 7.9 Hz), 7.04 (1H, s), 6.67 (1H, d, J = 7.9 Hz), 6.52 (1H, d, J = 7.9 Hz), 5.43 (1H, d, J= 4.1 Hz), 4.30 (1H, d, J = 8.2 Hz), 3.91 (1H, d, J = 8.9 Hz), 3.83–3.79 (1H, m), 3.79–3.74 (1H, m), 3.70–3.50 (11H, m), 3.38 (3H, s), 3.25–3.18 (2H, m), 3.14 (1H, dd, J = 9.6 and 9.6Hz), 3.11–2.96 (4H, m), 2.35–2.28 (1H, m), 2.26 (2H, t, J = 6.9 Hz), 1.86–1.80 (1H, m), 1.65–1.45 (4H, m), 1.45–1.27 (2H, m), ESI-MS (negative mode); Found: m/z 444.71 [(M-2Na)2−], Calcd. for C33H51N3O19S2Na2: 935.21.
- 18.Binding interactions were measured by use of the SPR imaging sensor, Multi SPRinter (TOYOBO Co. Ltd., Osaka, Japan), under the recommended manufacture’s guidelines with slight modification. Array-type Sugar Chips were prepared with the purified ligand-conjugates 1, 2, 3, and 4. An αGlc-containing ligand-conjugate (Glcα4Glc-mono) was also included in the chips as a non-sulfated control. Typical procedures were as follows. After cleaning the chip surface by UV/O3 treatment, 1 μl of each sample solution (0.5 mM) in H2O containing 10% glycerol was spotted on the chip by a spotter (TOYOBO), and left to stand overnight at room temperature. The resulting chip was then washed with water, treated with TEG conjugate21 to mask the unmodified Au surface, and washed with 0.05% Tween 20 aqueous solution and water in an ultrasonic cleaner. A protein solution in PBS containing 0.05% Tween 20 was injected over the surface at a flow rate of 150 μl/min at various concentrations. The binding interaction was monitored at 25°C as the change in luminance intensity.
- 19.Mahalingam Y, Gallagher JT, Couchman JR. J Biol Chem. 2007;282:3221. doi: 10.1074/jbc.M604938200. [DOI] [PubMed] [Google Scholar]
- 20.Cruz MA, Handin HI, Wise RJ. J Biol Chem. 1993;268:21238. [PubMed] [Google Scholar]
- 21.TEG conjugate is easily prepared by coupling of thioctic acid and 2-{2-[2-(2-hydroxy-ethoxy)-ethoxy]-ethoxy}-ethylamine.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data associated with this article can be found, in the online version, at ????????


