Abstract
Phenylketonuria (PKU) is an autosomal recessive inborn error of phenylalanine (Phe) metabolism resulting from deficiency of phenylalanine hydroxylase (PAH). Most forms of PKU and hyperphenylalaninaemia (HPA) are caused by mutations in the PAH gene on chromosome 12q23.2. Untreated PKU is associated with an abnormal phenotype which includes growth failure, poor skin pigmentation, microcephaly, seizures, global developmental delay and severe intellectual impairment. However, since the introduction of newborn screening programs and with early dietary intervention, children born with PKU can now expect to lead relatively normal lives. A better understanding of the biochemistry, genetics and molecular basis of PKU, as well as the need for improved treatment options, has led to the development of new therapeutic strategies.
Introduction
PKU (OMIM 261600) and its milder variant HPA are genetic disorders characterised by a deficiency in PAH (EC 1.14.16.1), an enzyme that is required to metabolise l-Phe to l-Tyrosine (l-Tyr). On the basis of blood Phe concentrations, PAH deficiency can be classified into classic PKU (Phe >1200 μmol/L), mild PKU (Phe = 600 1200 μmol/L) and mild HPA, where blood Phe is elevated above upper reference limit, but <600 μmol/L.1 The decreased PAH activity found in most forms of PKU and HPA are caused by mutations in the PAH gene, resulting in a non-functional PAH enzyme.
Untreated PKU is associated with an abnormal phenotype including growth failure, microcephaly, seizures and intellectual impairment caused by the accumulation of toxic by-products of Phe metabolism. The incidence of PKU or HPA is highest amongst Caucasians, occurring in approximately 1 in 10,000 births. PKU can be detected in newborn screening as performed in most Western countries, and early dietary treatment consisting of a low protein diet with Phe restriction can prevent the development of metabolic and pathological sequelae, including intellectual impairment.
History of PKU
PKU was first described by Asbjørn Følling, one of the first Norwegian physicians to apply chemical methods to the study of medicine.2 In 1934, the mother of two intellectually impaired children approached Følling to ascertain whether the strange musty odour of her children’s urine might be related to their intellectual impairment.3 The urine samples were tested for a number of substances including ketones. When ketones are present, urine usually develops a red-brown colour upon the addition of ferric chloride, but in this instance the urine yielded a dark-green colour.3 After confirming that the unusual result was not due to any medications and repeating the test every other day for two months, Følling proceeded with a more detailed chemical analysis involving organic extraction and purification of the responsible compound, and determination of its melting point.3 The basic elements were quantitated by combustion, and an empiric formula of C9H8O3 derived.3 Mild oxidation of the purified substance produced a compound which smelled of benzoic acid, leading Følling to postulate that the compound was phenylpyruvic acid.3 There was no change in the melting point upon mixing of the unknown compound with phenylpyruvic acid thus confirming the mystery compound was indeed phenylpyruvic acid.
Følling subsequently requested urine samples from 430 intellectually impaired patients from a number of local institutions and observed a similar result upon addition of ferric chloride, in a further eight individuals.3 These eight individuals all presented with a mild complexion (often with eczema), stooping figure with broad shoulders, a spastic gait, and severe intellectual impairment.3 Family studies of the affected individuals led to the suggestion of an inherited recessive autosomal trait. Dr Følling published his findings and suggested the name ‘imbecillitas phenylpyruvica’ relating the intellectual impairment to the excreted substance,4 thereafter renamed ‘phenylketonuria’.5
Our understanding has changed dramatically in the 70 years that have elapsed since the discovery of PKU. Jervis established the metabolic block and enzyme deficiency,6,7 and at about the same time, the link between reduced Phe intake and improved prognosis was shown.8 After the birth of his intellectually impaired son and a niece with PKU, the Canadian Paediatrician Robert Guthrie, changed his research interests and developed screening tests for PKU.9,10 In the late 1970s, various groups began investigating the molecular basis of PKU. The most notable recent advance in the study of PKU was the establishment, in 1996, of the PAH Mutation Analysis Consortium Database.11
The discovery of PKU by Dr Asbjørn Følling was an important milestone in medicine. The PKU model was used to illustrate how metabolic abnormalities could have neurological effects and how treatment could dramatically affect the clinical manifestations of the disorder. The development of Guthrie’s screening test, and dietary treatment, led to the prevention of intellectual impairment in affected children throughout the world. Futhermore, the PKU model has since been used as a template to shed light on over 200 other inborn errors of metabolism.12,13
Biochemistry of PKU
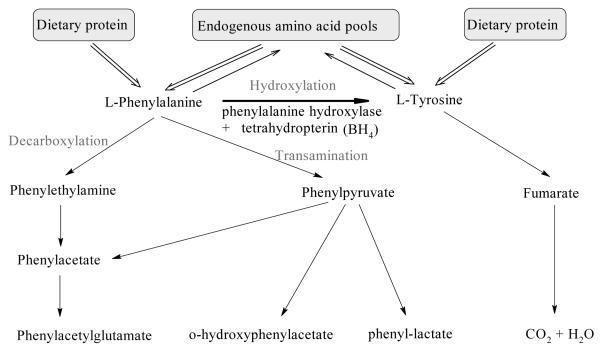
Phe exists as d and l enantiomers, and l-Phe is an essential amino acid required for protein synthesis in humans.14 Figure 1 shows the many processes which contribute to the flux of l-Phe in humans. As with many other metabolites, Phe concentrations are regulated to a steady state level with dynamic input and runout flux. Persistent disturbance to the flux will eventually result in alteration of the steady state concentrations. Dietary intake of Phe along with endogenous recycling of amino acid stores are the major sources of Phe, whereas, utilisation or runout of Phe occurs via integration into proteins, oxidation to Tyr, or conversion to other metabolites.15
Figure 1.
Phe metabolism in humans. Intake of l-Phe is via the diet and it is recycled through amino acid pools. Hydroxylation by PAH with its cofactor BH4, in the presence of molecular O2, produces L-Tyr. Alternative metabolism of L-Phe by decarboxylation or transamination produces various metabolites which are excreted in urine. Based on Scriver CR and Kaufman S (2001).15
Metabolic Pathways
The conversion of Phe to Tyr occurs by a hydroxylating system consisting of:
PAH
the unconjugated pterin cofactor, tetrahydrobiopterin (BH4)
enzymes which serve to regenerate BH4, namely dihydropteridine reductase and 4α-carbinolamine dehydratase
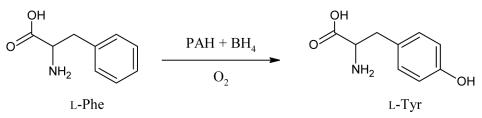
While the para-hydroxylation of Phe is essential for the rupture of the benzene ring, it is not required for further metabolism of the alanine side chain.16 This alternative pathway of transamination and decarboxylation leads to the formation of metabolites such as phenylpyruvate, phenyllactate, and o-hydroxyphenylacetate which are excreted in urine. Conversion of Phe to Tyr (Figure 2) has two outcomes. First, it drives the endogenous production of the non-essential amino acid Tyr.16 Second, the hydroxylation reaction is the rate limiting step for complete oxidation of Phe to CO2 and H2O and contributes to the pool of glucose and 2-carbon metabolites.15,17
Figure 2.
Conversion of Phe to Tyr is via a pathway involving the para-hydroxylation of the benzene by PAH, the cofactor BH4 and molecular oxygen.
A number of rare, related disorders due to defects in the BH4 regeneration system can also affect Phe homeostasis, and catecholamine and serotonin biosynthesis, as this cofactor is common to the Phe, Tyr and tryptophan (Trp) hydroxylating enzymes.18
Phenylalanine Hydroxylase
PAH catalyses the stereospecific hydroxylation of l-Phe, the committed step in the degradation of this amino acid. Phe catabolism and PAH activity is mainly associated with the liver, although minor activity has been demonstrated in rat kidney.19 In humans, the PAH enzyme exists as a mixture of tetramers and dimers; the monomer is about 50 kDa in size and is comprised of 452 amino acids.17 The enzyme PAH requires BH4 as a cofactor, as well as molecular oxygen for its activity.
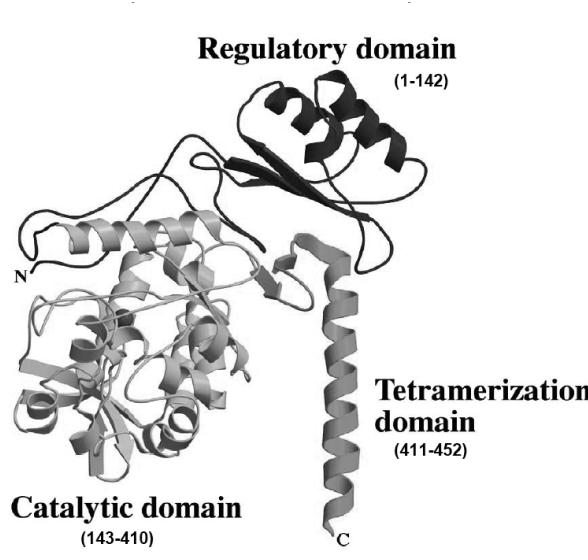
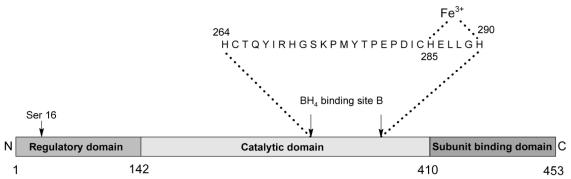
PAH can be divided into a number of functional domains (Figure 3, Figure 4). The regulatory domain contains a serine residue which is thought to be involved in activation by phosphorylation. The catalytic domain contains a motif of 26 or 27 amino acids responsible for cofactor and ferric iron binding. The C-terminal domain is thought to be associated with inter-subunit binding.17
Figure 3.
The domain structure of PAH. Each PAH subunit is classified into three functional domains which are involved with regulation, catalytic activity, and subunit binding. Reprinted from Molecular Genetics and Metabolism, 68, Erlandsen H and Stevens RC, The structural basis of phenylketonuria, 103–25, Copyright (1999), with permission from Elsevier.
Figure 4.
Structural components of PAH. The catalytic domain of PAH contains a motif of 26 or 27 amino acids which are responsible for ferric iron and cofactor (BH4) binding. Adapted from Huften IG et al (1995).17
PAH is regulated by a number of possible mechanisms. After a protein meal, it is postulated that the increased Phe in the amino acid pool causes a release of glucagon from the pancreas.21 Hepatic PAH is subject to control by cAMP-dependent protein kinase and α-andrenergic agent stimulated Ca2+/calmodulin-dependent protein kinase phosphorylation dephosphorylation processes.19 It has been further reported that these control mechanisms influence BH4 co-factor interaction with PAH.19 In addition, there is evidence that Phe may also be able to cause a conformational change in PAH, as well as up-regulate cAMP activity.17 X-ray crystallographic studies are consistent with these mechanisms.22 Taken together, these mechanisms enable fine regulation of Phe concentrations by balancing levels sufficient for maintenance of protein biosynthesis while minimising tissue exposure to high concentrations of Phe.
Genetics of PKU
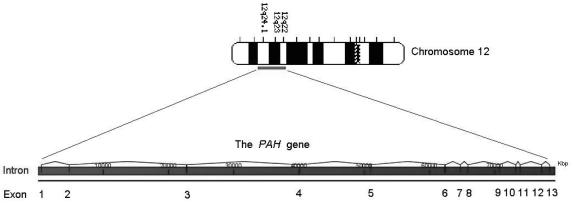
The human PAH gene (Figure 5) is located on chromosome 12q23.2, spans about 171 kb and contains 13 exons.23,24 The total cDNA length is about 2.4 kb; it encodes for a polypeptide of 452 amino acids of near identical sequence to the human PAH protein, indicating little post translational modification.24 HPA can be caused by either mutations at the PAH locus, which results in PKU, or from mutations in a number of loci which effect BH4 synthesis and regeneration resulting in non- PKU HPA.15
Figure 5.
The basic structure of the human PAH gene. Found on the long arm of chromosome 12 (12q23.2), the human PAH gene contains 13 exons which encode a polypeptide of 452 amino acids.
Mutations can either be neutral with respect to phenotype, or pathogenic due to their disruption to enzyme structure and function. More than 500 disease-causing mutations have been identified in patients with PKU or HPA and recorded on the mutation database for PAH.25 The human PAH gene shows great allelic variation and pathogenic mutations have been described in all 13 exons of the PAH gene and its flanking region. The mutations can be of various types:25
missense mutations: 62% of PAH alleles
small or large deletions: 13%
splicing defects: 11%
silent polymorphisms: 6%
nonsense mutations: 5%
insertions: 2%
Since the PAH gene is biallelic, and there are many disease causing mutations, most patients are compound heterozygotes.26 Some PAH mutations are more severe than others depending upon their effect on enzyme structure and function. However, the effect of PAH mutations on the clinical phenotype is variable.27,28 Compliance of dietary treatment is the primary factor which dictates blood Phe concentration and so plays a pivotal role in the severity of the clinical manifestations of PKU in an individual.
The effect of a specific mutation on enzyme function can be assessed through a number of approaches including enzyme assays, in vivo isotopic studies, or by analysis of in vitro gene expression.28 Given that the crystal structures of the catalytic and regulatory domains have now been resolved to 2 Å, a fourth approach by “virtual” molecular modelling techniques (in silico) is also possible.22
Large studies have shown a good correlation between the severity of mutation and hydroxylation rates in most individuals.29 However, exceptions do occur and this is to be expected since hydroxylation by PAH is also dependent on availability of its cofactor BH4. In this regard, some PAH genotypes are known to be more BH4-responsive than others.30 Although intelligence quotient (IQ) score is one of the most complex of human traits, some correlation between the severity of PAH mutations and IQ has been observed.31
Regional Difference in PKU Incidence
The incidence of PKU in Caucasian populations is between 1 in 10,000 and 1 in 15,000 people. Table 1 shows the variability in incidence in various countries and regions. It has been suggested that the high incidence of PKU in Turkey is due to the high prevalence of consanguinity and the low incidence seen in Finland and Japan is due to a pronounced negative founder effect in Finland and genetic drift in the founding of the Japanese island population.32,33
Table 1.
Incidence of PKU by population. Adapted from Scriver and Kaufman (2001).15
| Region / Country | Incidence of PKU | |
|---|---|---|
| Asian Populations | China | 1 : 17,000 |
| Japan | 1 : 125,000 | |
| Turkey | 1 : 2,600 | |
| Yemenite Jews (in Israel) | 1 : 5,300 | |
| Scotland | 1 : 5,300 | |
| Czechoslovakia | 1 : 7,000 | |
| Hungary | 1 : 11,000 | |
| Denmark | 1 : 12,000 | |
| European Populations | France | 1 : 13,500 |
| Norway | 1 : 14,500 | |
| United Kingdom | 1 : 14,300 | |
| Italy | 1 : 17,000 | |
| Canada | 1 : 22,000 | |
| Finland | 1 : 200,000 | |
| Arabic Populations | Up to 1 : 6,000 | |
| Oceania | Australia | 1: 10,000 |
Clinical Manifestations of PKU
Untreated PKU is associated with an abnormal phenotype including growth failure, microcephaly, seizures and intellectual impairment caused by the accumulation of toxic by-products of Phe. Moreover, decreased or absent PAH activity can lead to a deficiency of Tyr and its downstream products, including melanin, l-thyroxine and the cate-cholamine neurotransmitters.15
Pathogenesis of Intellectual Impairment in PKU
The effect of PAH mutations on hepatic enzyme function and the resultant disruption to Phe homeostasis has been well described, but the major clinical effect in PKU relates to brain development and cognitive function.28 The mechanism by which high Phe concentrations result in intellectual impairment is yet to be clarified, but the hallmark of the neuropathology seen in both treated and untreated PKU seems to involve hypomyelination and demyelination.34
A number of factors have been proposed as contributing to the neurotoxicity in PKU, including: Tyr deficiency, the effect of elevated Phe concentrations on transport of other metabolites across the blood brain barrier, and the effects of a potential and relative Tyr deficiency on neurochemistry and metabolism. Tyr is converted to l-DOPA (3,4-dihydroxy-l-Phe), a precursor of dopamine and other catecholamine neurochemicals. It has been suggested that the lack of PAH activity results in relative Tyr deficiency for the foetus, with mothers that are carriers for PKU possibly having limited supply of Tyr.15 However, there are observations which do not support this suggestion: a) restriction of Phe alone should not prevent the development of the phenotype, and b) supplementation of Tyr alone in the post-natal period does not prevent developmental defects nor improve neuropsychological functions.
The l-type amino acid carrier is the sole transporter of large neutral amino acids (LNAA), which includes Phe, across the blood brain barrier. The competition for this carrier when elevated concentrations of Phe exist, may have the effect of blocking transport of the serotonin and catecholamine precursors Tyr and Trp.35 Whilst some have proposed a strong causal link between these disrupted transport mechanisms and PKU, others dispute this claim.35,36
Neurological and Neurophysiological Impact in Adulthood
Magnetic resonance imaging (MRI) has shown white matter lesions in the brain of adult PKU patients, the size and number of which directly relate with blood Phe concentration.37 Such changes have been reversed by lowering blood Phe levels.38,39 This has led to the establishment of Phe treatment targets (birth to 8 years, Phe 100–350 μmol/L; older children and adults, Phe <700 μmol/L).40,41 A recent meta-analysis of the cognitive profile of adolescents and adults with PKU compared with control subjects showed significantly reduced Full Scale IQ, processing speed, motor control and inhibitory abilities, and reduced performance on tests of attention in the PKU groups.42 The link between MRI changes and cognition in PKU remains elusive, although the use of electroencephalogram and event-related potentials has been shown to be a useful tool in identifying the loci of neurophysiological deficiencies during cognitive tasks in PKU.43
Screening and Diagnosis of PKU
Guthrie Card Screening
Since the late 1960s, and following the development of Guthrie’s biochemical assay for diagnosis of PKU and a number of other diseases, Australian health services have been conducting newborn screening programs as part of a worldwide initiative.44 Collection of newborn blood by heel-prick onto filter paper cards has become an accepted facet of newborn care throughout the modern world.45 These blood spots can be analysed by a variety of methods to (in the case of PKU) measure the Phe concentration in the blood.15 More recently, tandem mass spectrometry has been developed as a routine method for newborn screening tests. The cost effectiveness of this new approach has enabled an increase in the number of disorders screened in these programs from four (PKU, congenital hypothyroidism, galactosaemia and cystic fibrosis) to over 20.46 The filter paper spots are stable for many years and the PKU screening tests have been reported to have a low error rate.15
A positive screening result identifies an infant with HPA, but it is possible that the increased Phe concentrations may be transient due to other non-PKU disease (e.g. transient 4α-carbinolamine dehydratase deficiency) or be a result of maternal HPA. Of the children with persistent HPA, over 98% have the condition because of mutations at the PAH locus.15 Diagnosis of HPA is made on the basis of an elevated blood Phe concentration on a repeat blood sample. The upper reference limit for Phe in whole blood or plasma in neonates is <150 μmol/L and slightly lower (<120 μmol/L) in older children.15 The measurement of Phe metabolites in urine is not an accepted PKU screening method as excretion depends upon transaminase activity (which can be low in neonates) and great variation between blood Phe and urine metabolite concentrations have been demonstrated.47
Differential diagnosis of PKU from the disorders of synthesis or recycling of BH4 may involve various testing regimes including BH4 loading tests, measurement of urine and plasma pterin metabolites and neurotransmitter metabolites as well as blood spot dihydropteridine reductase measurement.15 Tests targeting direct enzyme measurement (PAH, 4α-carbinolamine dehydratase) would require tissue biopsy.
Molecular Diagnosis of PKU
As discussed above, conventional PKU diagnosis is based on the aberrant metabolic phenotype, disease causing mutations and associated polymorphic haplotypes can be analysed at the PAH locus. PKU mutation analysis is particularly useful in the detection of carriers, for prenatal diagnosis. A wide variety of molecular genetic techniques have been utilised including Southern blotting, restriction enzyme digestion, detection of mutations by sequencing and multiplex ligation probe amplification.48,49
Treatment of PKU
The foundation of PKU treatment is a low Phe diet which, by reducing or normalising Phe concentrations, prevents the development of the neurological and psychological changes. Since neurological changes have been demonstrated within one month of birth, it is recommended that dietary restriction should be started early and be continued through childhood when neural development is maximal.23 Clinical neurological abnormalities, affected neuropsychological performance and brain imaging in adults with PKU has led to a consensus opinion that the PKU diet should be followed for life.50,51 As discussed later, an even more stringent regime of Phe restriction is required for women with PKU contemplating starting a family, particularly during pregnancy, as elevated blood Phe concentrations are teratogenic towards the developing foetus.
Dietary Restriction
A low Phe diet is used for treatment with, initially, the small amounts of Phe coming from breast milk or commercial infant formula considered sufficient intake in babies. In older children, protein intake is calculated each day, whereby a child is allocated a certain number of grams or units of daily protein, depending upon longitudinal plasma Phe concentrations. Foods such as eggs, milk, cheese, meat, poultry, fish, dried beans and legumes which are high in protein are excluded from the diet.26 However, this regime would not normally provide enough protein for growth requirements and therefore, commercially available supplements of essential amino acids, lacking Phe, need to be taken on a daily basis. Children undergo regular blood Phe testing which, in concert with complete food diaries, are used by dieticians to make adjustments to the recommended diets.26 A special problem persists in the PKU diet with aspartame (l-aspartyl-l-Phe methyl ester) an artificial sweetener which, when metabolised, releases Phe, l-aspartic acid and methanol.
The benefits of the Phe-restricted, low protein diet, are clear and include: avoidance of the biochemical abnormality (increased Phe concentrations), improved neurological and psychological performance, and prevention of neurological damage. However, dietary treatment does not come without challenges such as: compliance with the diet, the requirement of social support, and risk of imbalances in essential dietary nutrients.1
Phe Monitoring in PKU
Blood Phe concentrations are determined by both the severity of the PAH mutation, and by intake of Phe through dietary protein. In milder forms of PKU (non-PKU HPA), where there is residual enzyme activity, blood Phe concentrations may be only mildly elevated even under conditions of poor treatment diet compliance. It is accepted that the best indicator of dietary compliance in classical PKU is regular monitoring of blood Phe concentrations.
Potential Nutritional Deficiencies in PKU
The Phe-restricted diet with semi-synthetic supplementation is not without risk. PKU patients under dietary treatment can have low concentrations of trace elements and cholesterol, and some disturbance to folate metabolism as well as distortion of their fatty acid profile.52–54 However, it remains controversial as to whether all of these dietary imbalances occur as a result of insufficient intake and supplementation, or are due to endogenous disturbances in the biosynthesis of these essential constituents.
The finding of low serum cholesterol concentrations in PKU patients, whilst having been clearly established by various groups (presumably across a wide genotypic spectrum) remains unexplained.54–59 This association could be due to dietary influences, variation in lipoprotein metabolism between individuals due to their lipid controlling mechanisms, or perhaps due to influence of increased concentrations of Phe or its metabolites. While much work remains to be done, the low cholesterol concentrations in PKU may have broader implications to both the pathophysiology of PKU and the development of cardiovascular disease (CVD).
Phe and Cholesterol Biosynthesis
Animal models have been used to demonstrate inhibition of sterol synthesis by various phenolic compounds.60,61 This led to work that investigated inhibition of a number of important enzymes involved in cholesterol biosynthesis; again in animal models under conditions of induced HPA.62 Dietary supplementation of l-Phe has been shown to be associated with inhibition of the rate determining enzyme for cholesterol biosynthesis, 3-hydroxy-3-methylglutaryl coenzyme A reductase (EC 1.1.1.88), in the liver and brain and decreased concentrations of mevalonic acid.
Decreased coenzyme Q10 (ubiquinone-10; CoQ10) concentrations have been found in both plasma and in lymphocytes from patients with PKU.63,64 CoQ10 shares a common biosynthetic pathway (the mevalonate pathway) to cholesterol. Apart from being a cofactor in the mitochondrial electron transport chain, the reduced form of CoQ10 has an important role as an antioxidant in both the mitochondria and lipid membranes as well as in preventing LDL oxidation.63 The effect of decreased CoQ10 concentrations and, presumably increased oxidative stress in PKU would support an inhibitory effect of Phe upon the cholesterol, and CoQ10 pathways.
Cardiovascular Disease in PKU
There is a paucity of studies investigating CVD risk and cardiovascular events in PKU. Vascular risk factors and homocysteine were assessed in children with PKU under dietary treatment and they were found to have low total cholesterol, vitamin B6, B12 and folate concentrations resulting in moderate hyperhomocysteinaemia.65 It is possible, that the hypocholesterolaemia in adult PKU patients may confer a protective effect against CVD. However, an assessment of adult PKU patients using biochemical markers along with non-invasive imaging techniques is needed to test this hypothesis.
Maternal PKU
Highly elevated concentrations of Phe are teratogenic and are a cause of increased risk of miscarriage.66 Specifically, the foetus can be affected by elevated Phe concentrations which lead to intrauterine growth retardation, facial dysmorphism, microcephaly, congenital heart disease and developmental delay and has led to families consisting of multiple children with birth defects and intellectual impairment.67–69 Foetal exposure to HPA in PKU affected pregnancies is exacerbated by the transplacental gradient for Phe; an average foetal/ maternal ratio of 1.5 is suggested although ratios up to 2.9 can be seen in early pregnancy when foetal development is maximal.15 Because of the foetal morbidities associated with HPA, a more stringent control of blood Phe concentrations is required in mothers with PKU. A preconception diet is required with a Phe target interval of between 100 and 360 μmol/L in affected mothers.68 In addition, weekly monitoring of the Phe concentrations is advised to aid in achieving low baseline levels.40
Emerging PKU Therapies
Although dietary restriction of Phe is the cornerstone of treatment for PKU, the practicalities of following the strict diet have led to trials of additional therapies.
BH4 Therapy
Recent clinical trials have shown that a subset of ‘classical’ PKU children respond to BH4 therapy, dependent upon their PAH gene mutation(s).70 Sapropterin dihydrochloride (Kuvan, Biomarin Pharma) is an orally active synthetic form of BH4 that has received Orphan Drug status and Fast Track designation for the treatment of PKU. Phase II and III clinical trials have shown that Kuvan is a safe and effective therapy in selected patients with HPA and mild-to-moderate PKU who responded to a BH4 loading test.71
Enzyme Replacement Therapy
Unfortunately, patients with more severe forms of classic PKU and some non-PKU HPA do not respond to BH4 treatment, presumably because these individuals lack sufficient residual PAH activity for stimulation by BH4. Such nonresponders could benefit most from enzyme replacement therapy. Unlike BH4 treatment, enzyme replacement is not dependent on the PAH genotype. Replacement of the enzyme could be facilitated by partial liver or normal hepatocyte transplantation. Although liver transplantation would correct the metabolic phenotype in PKU, the high risk of major surgery and lifelong immunosuppression precludes its routine use.72
An alternative enzyme therapy for PKU has been trialled which involves the substitution of PAH with Phe ammonia-lyase (PAL, EC 4.3.1.5), a non-cofactor dependent plant protein involved in Phe degradation. This treatment has been shown to be effective in mouse models causing modest but short-lived falls in Phe concentrations.73 Indeed, PhenylaseTM (PAL), Biomarin Pharma is currently under investigation for the potential treatment of patients with PKU who do not respond to BH4.
Large Neutral Amino Acid Therapy
As discussed previously, it is hypothesised that competition for the l-type amino acid carrier by Phe with other LNAAs may occur in PKU. This hypothesis has led to LNAA supplementation trials.35 Increasing the blood concentrations of various LNAAs has led to reduced brain concentrations of Phe.35 Furthermore, the increased Tyr and Trp intake may be of benefit in disorders of BH4 regeneration. A new LNAA formulation (NeoPhe, Solace Nutrition) has been effective in reducing blood Phe concentrations.74
Gene Therapy
Complete and persistent correction of HPA has been reported in the Pahenu2 mouse using somatic gene therapy, site specific genome integration of Pah cDNA and by liver directed recombinant adeno-associated virus vectors.75–77 Early work on gene therapy for children with PKU was considered inappropriate as the therapy involved administration of immunosuppressant agents to block the immune response to the vector so as to prolong the therapeutic effect.76 These trials involved the use of recombinant adenoviral vectors. Other trials involving the use of recombinant retroviral vectors have been abandoned following the observation that these vectors may induce leukaemia-like disorders.76
PAH-deficient (Pahenu2) mouse models have been used to trial gene transfer via a recombinant adeno-associated virus vector encoding the human PAH gene with promising results.78 This vector appears to be a safer mode of transfer as it possesses minimal antigenicity and showed no signs of inducing liver damage.78 However, administration of the vector would ideally be facilitated by a less intrusive means than via the hepatic portal vein, which was used in the aforementioned study.
Conclusion
PKU is a historically significant inborn error of metabolism. Its discovery over 70 years ago and scientific investigation has established the link between metabolic disease and intellectual impairment, led to the development of neonatal screening programs across the globe, and demonstrated how effective treatment can lead to a near normal outcome for affected individuals. However, despite the intensive study, the mechanism by which the aberrant Phe metabolism leads to intellectual impairment is yet to be explained. The successes of the PKU story lie squarely in the hands of the health professionals who diagnose PKU through establishing and managing neonatal screening programs, and the paediatricians, dieticians and carers who then supervise PKU treatment. The study of PKU has revealed genomic components of health and disease.76 A better understanding of the biochemistry, genetics and molecular basis of PKU, as well as the need for improved treatment options, has led to the development of new therapeutic strategies.
Footnotes
Competing Interests: None declared.
References
- 1.Hanley WB. Adult Phenylketonuria. Am J Med. 2004;117:590–5. doi: 10.1016/j.amjmed.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Centerwall SA, Centerwall WR. The discovery of phenylketonuria: the story of a young couple, two retarded children, and a scientist. Pediatrics. 2000;105:89–103. doi: 10.1542/peds.105.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Følling I. The discovery of phenylketonuria. Acta Pediatr Suppl. 1994;407:4–10. doi: 10.1111/j.1651-2227.1994.tb13440.x. [DOI] [PubMed] [Google Scholar]
- 4.Følling A. Über ausscheidung von phenylbrenztraubensäure in den harn als stoffwechselanomalie in verbindung mit imbezillität. Hoppe-Seylers Z Physiol Chem. 1934;227:169–76. [Google Scholar]
- 5.Penrose L, Quastel JH. Metabolic studies in phenylketonuria. Biochem J. 1937;31:266–74. doi: 10.1042/bj0310266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jervis GA. Studies on phenylpyruvic oligophrenia: the position of the metabolic error. J Biol Chem. 1947;169:651–6. [PubMed] [Google Scholar]
- 7.Jervis GA. Phenylpyruvic oligophrenia deficiency of phenylalanine-oxidizing system. Proc Soc Exp Biol Med. 1953;82:514–5. [PubMed] [Google Scholar]
- 8.Bickel H, Gerrard J, Hickmans EM. Influence of phenylalanine intake on phenylketonuria. Lancet. 1953;265:812–3. doi: 10.1016/s0140-6736(53)90473-5. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–43. [PubMed] [Google Scholar]
- 10.Koch JH. Robert Guthrie- the PKU story: A crusade against mental retardation. Pasadena, USA: Hope Publishing House; 1997. [Google Scholar]
- 11.Hoang L, Byck S, Prevost L, Scriver CR. PAH Mutation Analysis Consortium Database: a database for disease-producing and other allelic variation at the human PAH locus. Nucleic Acids Res. 1996;24:127–31. doi: 10.1093/nar/24.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969–1996. Pediatrics. 2000;105:e10. doi: 10.1542/peds.105.1.e10. [DOI] [PubMed] [Google Scholar]
- 13.Raghuveer TS, Garg U, Graf WD. Inborn errors of metabolism in infancy and early childhood: an update. Am Fam Physician. 2006;73:1981–90. [PubMed] [Google Scholar]
- 14.Young VR, Pellett PL. Protein intake and requirements with reference to diet and health. Am J Clin Nutr. 1987;45:1323–43. doi: 10.1093/ajcn/45.5.1323. [DOI] [PubMed] [Google Scholar]
- 15.Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler K, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 1667–724. [Google Scholar]
- 16.Kaufman S. A model of human phenylalanine metabolism in normal subjects and in phenylketonuric patients. Proc Natl Acad Sci USA. 1999;96:3160–4. doi: 10.1073/pnas.96.6.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hufton SE, Jennings IG, Cotton RG. Structure and function of the aromatic amino acid hydroxylases. Biochem J. 1995;311:353–66. doi: 10.1042/bj3110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blau N, Thöny B, Cotton RG, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler K, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 1725–76. [Google Scholar]
- 19.Richardson SC, Aspbury RA, Fisher MJ. The role of reversible phosphorylation in the hormonal control of phenylalanine hydroxylase in isolated rat proximal kidney tubules. Biochem J. 1993;292:419–24. doi: 10.1042/bj2920419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlandsen H, Stevens RC. The structural basis of phenylketonuria. Mol Gen Metab. 1999;68:103–25. doi: 10.1006/mgme.1999.2922. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman S. Regulation of the activity of hepatic phenylalanine hydroxylase. Adv Enzyme Regul. 1986;25:37–64. doi: 10.1016/0065-2571(86)90007-5. [DOI] [PubMed] [Google Scholar]
- 22.Andersen OA, Flatmark T, Hough E. Crystal structure of the ternary complex of the catalytic domain of human phenylalanine hydroxylase with tetrahydrobiopterin and 3-(2-thienyl)-L-alanine, and its implications for the mechanism of catalysis and substrate activation. J Mol Biol. 2002;320:1095–108. doi: 10.1016/s0022-2836(02)00560-0. [DOI] [PubMed] [Google Scholar]
- 23.Konecki DS, Wang Y, Trefz FK, Lichter-Konecki U, Woo SL. Structural characterization of the 5’ regions of the human phenylalanine hydroxylase gene. Biochemistry. 1992;31:8363–8. doi: 10.1021/bi00150a033. [DOI] [PubMed] [Google Scholar]
- 24.Lidsky AS, Law ML, Morse HG, Kao FT, Rabin M, Ruddle FH, et al. Regional mapping of the phenylalanine hydroxylase gene and the phenylketonuria locus in the human genome. Proc Natl Acad Sci USA. 1985;82:6221–5. doi: 10.1073/pnas.82.18.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PAH: Phenylalanine hydroxylase locus knowledgebase. http://www.pahdb.mcgill.ca/ (Accessed 12 April 2007)
- 26.Michals-Matalon K. Developments in phenylketonuria. Topics Clin Nutr. 2001;16:41–50. [Google Scholar]
- 27.Gizewska M, Cabalska B, Cyrytowski L, Nowacki P, Zekanowski C, Walczak M, et al. Different presentations of late-detected phenylketonuria in two brothers with the same R408W/R111X genotype in the PAH gene. J Intellect Disabil Res. 2003;47:146–52. doi: 10.1046/j.1365-2788.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 28.Kayaalp E, Treacy E, Waters PJ, Byck S, Nowacki P, Scriver CR. Human phenylalanine hydroxylase mutations and hyperphenylalaninemia phenotypes: a metanalysis of genotype-phenotype correlations. Am J Hum Genet. 1997;61:1309–17. doi: 10.1086/301638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treacy EP, Delente JJ, Elkas G, Carter K, Lambert M, Waters PJ, et al. Analysis of phenylalanine hydroxylase genotypes and hyperphenylalaninemia phenotypes using L-[l-13C]phenylalanine oxidation rates in vivo: a pilot study. Pediatr Res. 1997;42:430–5. doi: 10.1203/00006450-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 30. [(Accessed 12 April 2007)];BH4 - Tetrahydrobiopterin. http://www.bh4.org.
- 31.Ramus SJ, Forrest SM, Pitt DB, Saleeba JA, Cotton RG. Comparison of genotype and intellectual phenotype in untreated PKU patients. J Med Genet. 1993;30:401–5. doi: 10.1136/jmg.30.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guldberg P, Henriksen KF, Sipila I, Guttler F, de la Chapelle A. Phenylketonuria in a low incidence population: molecular characterisation of mutations in Finland. J Med Genet. 1995;32:976–8. doi: 10.1136/jmg.32.12.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okano Y, Hase Y, Lee DH, Furuyama J, Shintaku H, Oura T, et al. Frequency and distribution of phenylketonuric mutations in Orientals. Hum Mutat. 1992;1:216–20. doi: 10.1002/humu.1380010307. [DOI] [PubMed] [Google Scholar]
- 34.Dyer CA. Pathophysiology of phenylketonuria. Ment Retard Dev Disab Res Rev. 1999;5:104–12. [Google Scholar]
- 35.Pietz J, Kreis R, Rupp A, Mayatepek E, Rating D, Boesch C, et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest. 1999;103:1169–78. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hommes FA. The role of the blood-brain barrier in the aetiology of permanent brain dysfunction in hyperphenylalaninaemia. J Inherit Metab Dis. 1989;12:41–6. doi: 10.1007/BF01805529. [DOI] [PubMed] [Google Scholar]
- 37.Thompson AJ, Tillotson S, Smith I, Kendall B, Moore SG, Brenton DP. Brain MRI changes in phenylketonuria. Association with dietary status. Brain. 1993;116:811–21. doi: 10.1093/brain/116.4.811. [DOI] [PubMed] [Google Scholar]
- 38.Cleary MA, Walter JH, Wraith JE, White F, Tyler K, Jenkins JP. Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J Pediatr. 1995;127:251–5. doi: 10.1016/s0022-3476(95)70303-9. [DOI] [PubMed] [Google Scholar]
- 39.Walter JH, White F, Wraith JE, Jenkins JP, Wilson BP. Complete reversal of moderate/severe brain MRI abnormalities in a patient with classical phenylketonuria. J Inherit Metab Dis. 1997;20:367–9. doi: 10.1023/a:1005330012574. [DOI] [PubMed] [Google Scholar]
- 40.Dennison B, editor. Australian Society for Inborn Errors of Metabolism. PKU handbook. Alexandra, Australia: Human Genetics Society of Australasia; 2005. [Google Scholar]
- 41.Blau N, Burgard P. Disorders of Phenylalanine and Tetrahydrobiopterin Metabolism. In: Blau N, Leonard J, Hoffmann G, Clarke J, editors. Physician’s Guide to the Treatment and Follow-up of Metabolic Diseases. Heidelberg: Springer; 2006. pp. 25–34. [Google Scholar]
- 42.Moyle JJ, Fox AM, Arthur M, Bynevelt M, Burnett JR. Meta-analysis of neuropsychological symptoms of adolescents and adults with PKU. Neuropsychol Rev. 2007;17:91–101. doi: 10.1007/s11065-007-9021-2. [DOI] [PubMed] [Google Scholar]
- 43.Moyle JJ, Fox AM, Bynevelt M, Arthur M, Burnett JR. Event-related potentials elicited during a visual Go-Nogo task in adults with phenylketonuria. Clin Neurophysiol. 2006;117:2154–60. doi: 10.1016/j.clinph.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Muchamore I, Morphett L, Barlow-Stewart K. Exploring existing and deliberated community perspectives of newborn screening: informing the development of state and national policy standards in newborn screening and the use of dried blood spots. Australia and New Zealand Health Policy. 2006;3:14. doi: 10.1186/1743-8462-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AAP Newborn Screening Task Force. Newborn screening: a blueprint for the future. A call for a national agenda on state newborn screening programs. Pediatrics. 2000;106(Suppl):389–422. [PubMed] [Google Scholar]
- 46.WA Newborn Screening Program. Expanded newborn screening: information for health professionals. Perth, Australia: Department of Health, Government of Western Australia; 2004. [Google Scholar]
- 47.Knox WE. PKU newsletter. 1970. Retrospective study of phenylketonuria: relation of phenylpyruvate excretion to plasma phenylalanine; p. 2. [Google Scholar]
- 48.Desviat LR, Perez B, Ugarte M. Identification of exonic deletions in the PAH gene causing phenylketonuria by MLPA analysis. Clin Chim Acta. 2006;373:164–7. doi: 10.1016/j.cca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Kozak L, Hrabincova E, Kintr J, Horky O, Zapletalova P, Blahakova I, et al. Identification and characterization of large deletions in the phenylalanine hydroxylase (PAH) gene by MLPA: evidence for both homologous and non-homologous mechanisms of rearrangement. Mol Genet Metab. 2006;89:300–9. doi: 10.1016/j.ymgme.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Phenylketonuria (PKU): screening and management. NIH Consensus statement. 2000;17:1–33. [PubMed] [Google Scholar]
- 51.Management of PKU: A consensus document for the diagnosis and management of children, adolescents and adults with phenylketonuria, The National Society for Phenylketonuria (United Kingdom) Ltd; 2004.
- 52.Lucock M, Yates Z, Hall K, Leeming R, Rylance G, MacDonald A, et al. The impact of phenylketonuria on folate metabolism. Mol Genet Metab. 2002;76:305–12. doi: 10.1016/s1096-7192(02)00113-0. [DOI] [PubMed] [Google Scholar]
- 53.Moseley K, Koch R, Moser AB. Lipid status and long-chain polyunsaturated fatty acid concentrations in adults and adolescents with phenylketonuria on phenylalanine-restricted diet. J Inherit Metab Dis. 2002;25:56–64. doi: 10.1023/a:1015142001578. [DOI] [PubMed] [Google Scholar]
- 54.Schulpis KH, Karakonstantakis T, Bartzeliotou A, Karikas GA, Papassotiriou I. The association of serum lipids, lipoproteins and apolipoproteins with selected trace elements and minerals in phenylketonuric patients on diet. Clin Nutr. 2004;23:401–7. doi: 10.1016/j.clnu.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Acosta PB, Alfin-Slater RB, Koch R. Serum lipids in children with phenylketonuria (PKU) J Am Diet Assoc. 1973;63:631–5. [PubMed] [Google Scholar]
- 56.Colome C, Artuch R, Lambruschini N, Cambra FJ, Campistol J, Vilaseca M. Is there a relationship between plasma phenylalanine and cholesterol in phenylketonuric patients under dietary treatment? Clin Biochem. 2001;34:373–6. doi: 10.1016/s0009-9120(01)00249-1. [DOI] [PubMed] [Google Scholar]
- 57.DeClue TJ, Davis J, Schocken DM, Kangas R, Benford SA. Serum lipid concentrations in subjects with phenylketonuria and their families. Am J Dis Child. 1991;145:1266–8. doi: 10.1001/archpedi.1991.02160110058020. [DOI] [PubMed] [Google Scholar]
- 58.Galluzzo CR, Ortisi MT, Castelli L, Agostoni C, Longhi R. Plasma lipid concentrations in 42 treated phenylketonuric children. J Inherit Metab Dis. 1985;8(Suppl):129. doi: 10.1007/BF01811492. [DOI] [PubMed] [Google Scholar]
- 59.Verduci E, Agostoni C, Biondi ML, Radaelli G, Giovannini M, Riva E. Apolipoprotein B gene polymorphism and plasma lipid levels in phenylketonuric children. Prostaglandins Leukot Essent Fatty Acids. 2004;71:117–20. doi: 10.1016/j.plefa.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 60.Ranganathan S, Ramasarma T. Inhibition of the biosynthesis of sterols by phenyl and phenolic compounds in rat liver. Biochem J. 1973;134:737–43. doi: 10.1042/bj1340737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shama Bhat C, Ramasarma T. Inhibition of rat liver mevalonate pyrophosphate decarboxylase and mevalonate phosphate kinase by phenyl and phenolic compounds. Biochem J. 1979;181:143–51. doi: 10.1042/bj1810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castillo M, Zafra MF, Garcia-Peregrin E. Inhibition of brain and liver 3-hydroxy-3-methylglutaryl-CoA reductase and mevalonate-5-pyrophosphate decarboxylase in experimental hyperphenylalaninemia. Neurochem Res. 1988;13:551–5. doi: 10.1007/BF00973296. [DOI] [PubMed] [Google Scholar]
- 63.Colome C, Artuch R, Vilaseca MA, Sierra C, Brandi N, Cambra FJ, et al. Ubiquinone-10 content in lymphocytes of phenylketonuric patients. Clin Biochem. 2002;35:81–4. doi: 10.1016/s0009-9120(02)00278-3. [DOI] [PubMed] [Google Scholar]
- 64.Artuch R, Vilaseca MA, Moreno J, Lambruschini N, Cambra FJ, Campistol J. Decreased serum ubiquinone-10 concentrations in phenylketonuria. Am J Clin Nutr. 1999;70:892–5. doi: 10.1093/ajcn/70.5.892. [DOI] [PubMed] [Google Scholar]
- 65.Schulpis KH, Karikas GA, Papakonstantinou E. Homocysteine and other vascular risk factors in patients with phenylketonuria on a diet. Acta Pediatr. 2002;91:905–9. doi: 10.1080/080352502760148612. [DOI] [PubMed] [Google Scholar]
- 66.American Academy of Pediatrics: Committee on Genetics. Maternal phenylketonuria. Pediatrics. 2001;107:427–8. [PubMed] [Google Scholar]
- 67.Knerr I, Zschocke J, Schellmoser S, Topf HG, Weigel C, Dötsch J, et al. An exceptional Albanian family with seven children presenting with dysmorphic features and mental retardation: maternal phenylketonuria. BMC Pediatr. 2005;5:5. doi: 10.1186/1471-2431-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee PJ, Ridout D, Walter JH, Cockburn F. Maternal phenylketonuria: report from the United Kingdom Registry 1978–97. Arch Dis Child. 2005;90:143–6. doi: 10.1136/adc.2003.037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaw-Smith C, Hogg SL, Reading R, Calvin J, Trump D. Learning and behavioural difficulties but not microcephaly in three brothers resulting from undiagnosed maternal phenylketonuria. Child Care Health Dev. 2004;30:551–5. doi: 10.1111/j.1365-2214.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 70.Hennermann JB, Bührer C, Blau N, Vetter B, Mönch E. Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in children with severe phenotype of phenylketonuria. Mol Genet Metab. 2005;86(Suppl):S86–90. doi: 10.1016/j.ymgme.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Burnett JR. Sapropterin dihydrochloride (Kuvan/ Phenoptin), an orally active synthetic form of BH4 for the treatment of phenylketonuria. IDrugs. 2007;10:805–13. [PubMed] [Google Scholar]
- 72.Vajro P, Strisciuglio P, Houssin D, Huault G, Laurent J, Alvarez F, et al. Correction of phenylketonuria after liver transplantation in a child with cirrhosis. N Engl J Med. 1993;329:363. doi: 10.1056/NEJM199307293290517. [DOI] [PubMed] [Google Scholar]
- 73.Sarkissian CN, Shao Z, Blain F, Peevers R, Su H, Heft R, et al. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc Natl Acad Sci USA. 1999;96:2339–44. doi: 10.1073/pnas.96.5.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matalon R, Michals-Matalon K, Bhatia G, Grechanina E, Novikov P, McDonald JD, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU) J Inherit Metab Dis. 2006;29:732–8. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Woo SL. Complete and persistent phenotypic correction of phenylketonuria in mice by site-specific genome integration of murine phenylalanine hydroxylase cDNA. Proc Natl Acad Sci USA. 2005;102:15581–6. doi: 10.1073/pnas.0503877102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Ding Z, Harding CO, Thöny B. State-of-the-art 2003 on PKU gene therapy. Mol Genet Metab. 2004;81:3–8. doi: 10.1016/j.ymgme.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harding C, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–62. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–84. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]