Abstract
Fluorous proline derivatives generated from one-pot, three-component [3+2] cycloaddition of azomethine ylides are employed for different post-condensation reactions to form hydantoin-, piperazinedione-, and benzodiazepinedione-fused tricyclic and tetracyclic ring systems. The high synthetic efficiency is achieved by conducting fast microwave reactions and easy fluorous-solid phase extractions for reaction mixture purifications. Methods developed for these novel drug-like heterocyclic compounds can be applied to diversity-oriented library synthesis.
Keywords: Fluorous synthesis, Microwave reaction, Solid-phase extraction, [3+2] Cycloaddition, Diversity-oriented synthesis
Introduction
Fluorous synthesis employs perfluoroalkyl (Rf) chains as “phase tags”1 to improve the efficiency of reaction mixture purifications.2 This technology shares the characteristics of solution-phase synthesis, which has homogenous reaction enviroment,3 easy intermediate analysis,4 and good compatibility to other synthetic techniques such as microwave5 and multicomponent reactions.6 Compared to its counterpart solid-phase synthesis, fluorous synthesis requires less development time and has the capability to explore new reactions on fluorous support directly.7 As a “beadless” synthetic technology, fluorous synthesis has been applied to parallel and mixture synthesis8 of small molecules, peptides,9 and oligosaccharides.10
We have recently developed several methods for synthesis of heterocyclic systems by using an orchestrated sequence of microwave-assisted fluorous multicomponent reaction (F-MCR) and fluorous-solid phase extraction (F-SPE) to speed up reactions and simplify purifications.6,11 Reported in this paper are approaches to three novel triaza tricyclic and tetracyclic ring systems 2–4 (Scheme 1). Proline derivatives 1 generated from one-pot, three-component [3+2] cycloaddition12 of azomethine ylides are further converted to hydantoin-, piperazinedione-, and benzodiazepinedione-fused compounds 2–4, respectively. Each of these three heterocyclic scaffolds has four stereocenters on the central pyrrolidine ring and up to four points of diversity (R1 to R4). Compound 2 has a similar ring skeleton as tricyclic thrombin inhibitors.13 The structure of compound 3 is partially related to diketopiperazine-based inhibitors of human hormone-sensitive lipase.14,15 Compound 4 contains a privileged benzodiazepine moiety which has a wide range of pharmaceutical utilities.16
Scheme 1.

Fluorous Synthesis of Heterocyclics 2–4
Results and Discussion
Preparations of fluorous amino esters 5 and one-pot, three-component 1,3-dipolar cycloaddition reactions were conducted by following established procedures.6a,b Thus a mixture of 1.0 equiv of a fluorous aminoester, 1.2 equiv of a benzaldehyde, 1.5 equiv of an N-alkylmaleimide, and 3 equiv of Et3N in DMF was heated under microwave at 130 °C for 20 min to afford proline derivative 1 (Scheme 2).17,18 Since the fluorous amino ester 5 was used as the limiting agent, only the desired product 1 was expected to be fluorous. The crude product was loaded on a FluoroFlash cartridge. The non-fluorous components such as unreacted aldehyde, N-alkylmaleimide, and Et3N salt were eluted out with a fluorophobic solvent (80:20 MeOH-H2O). Fluorous compound 1 was collected by eluting with MeOH, a more fluorophilic solvent. After F-SPE purification, the purity of the product is usually greater than 90% by 1H NMR analysis (Figure 1). Bicyclic prolins 1 with different R1–R3 substitution groups were synthesized in 75–90% yields. The stereochemistry of compound 1a was established based on the literature information17c,17g and confirmed by single-crystal X-ray diffraction (Figure 2, left). No evidence shows the racemization of the amino acid 5 during the cycloaddition.
Scheme 2.
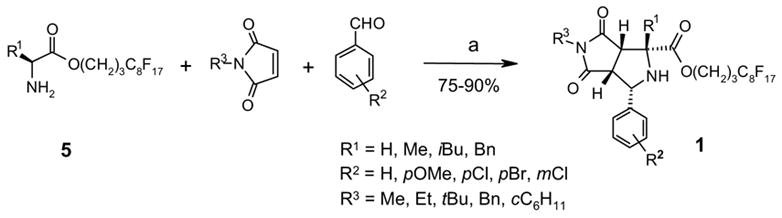
Synthesis of fluorous proline derivatives by one-pot [3+2] cycloaddition of azomethine ylides. a) 5 (1 equiv), aldehyde (1.2 equiv), maleimide (1.5 equiv), Et3N (3 equiv), DMF, μw (130 °C, 20 min), F-SPE.
Figure 1.
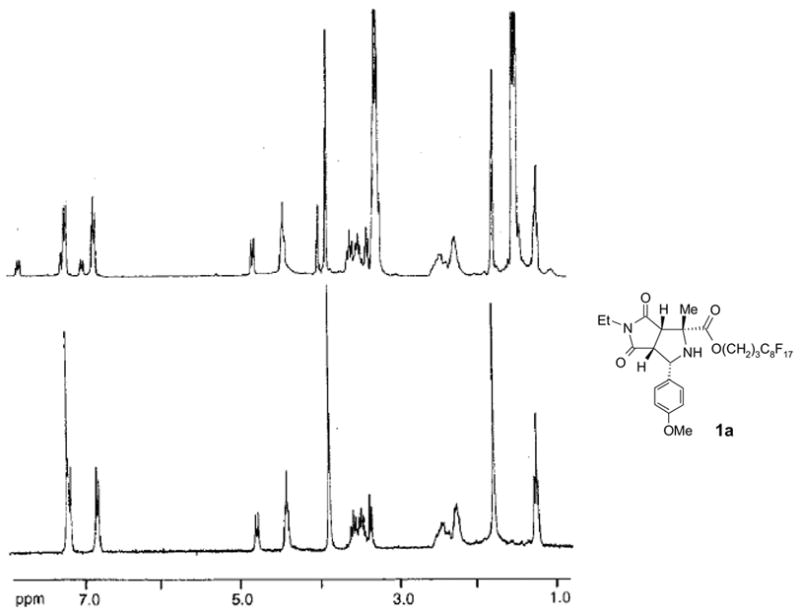
1H NMR (in CDCl3) analysis of compound 1a, before (top) and after (bottom) F-SPE.
Figure 2.
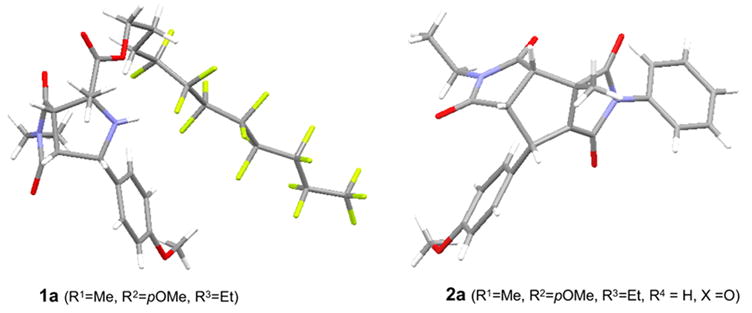
Single-crystal X-ray structures of compounds 1a and 2a
With the key intermediates 1 in hands, we then performed post-condensation reactions to generate different heterocyclic ring systems. The reaction of 1 with 5 equiv of a phenylisocyanate or a phenylthioisocyanate in the presence of catalytic amount of N,N-4-dimethylaminopyridine (DMAP) in toluene gave urea or thiourea 6. After F-SPE purification, compound 6 was mixed with K2CO3 and heated under microwave at 100 °C for 5 min. Fluorous tag cleavage and hydantoin ring formation produced tricyclic compound 2 (Scheme 3). Four analogs of 2 were produced in 75–85% yields. After F-SPE followed by HPLC purifications, the products had greater than 95% purities. The stererochemistry of compound 2a was confirmed by single-crystal X-ray diffraction (Figure 2, right).
Scheme 3.
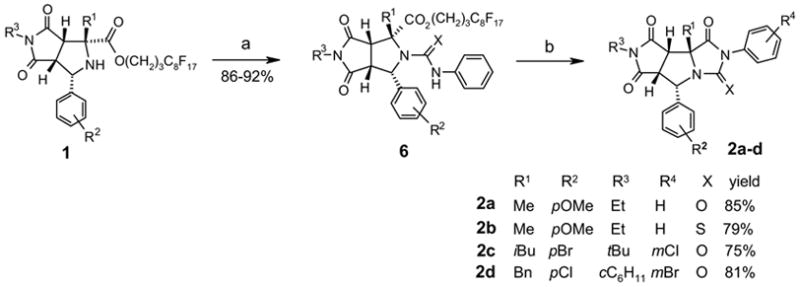
Synthesis of hydantoin-fused tricyclic compounds 2a–d. a) R4-PhNCX (5.0 equiv), DMAP (0.5 equiv), toluene, μw (130 °C, 10 min), F-SPE. b) K2CO3 (2 equiv), DMF, μw (100 °C, 5 min), F-SPE, HPLC.
In the synthesis of piperazinedione-fused tricyclic compounds 3a and 3b (Scheme 4), direct N-acylations of 1a with α-aminoacids or α-aminoacid chlorides were attempted, but reactions gave products in very low yields (10–25%). Acylation of 1a with chloroacetyl chloride followed by chlorine displacement with BuNH2 or 3,5-dimethylaniline gave compounds 8a and 8b in 92% and 90% yields, respectively. The detag/cyclization reactions were promoted by 1,8-diazabicyclo[4.3.0]non-5-ene (DBU) under microwave irradiation at 180 °C for 15 min to give product 3a in 45% yield. However, under the same conditions, only a very small amount of 3b (<5%) was detected from the reaction mixture by LCMS.
Scheme 4.

Synthesis of piperazinedione-fused tricyclic compounds 3a–d. a) ClCH2COCl (1.5 equiv), Et3N (2.5 equiv), CH2Cl2, 25 °C, 30 min, F-SPE. b) R4NH2 (2.5 equiv), MeOH, μw (120°C, 10 min), F-SPE. c) DBU (2 equiv), MeOH-DMF, μw (180 °C, 15 min), F-SPE, HPLC.
Synthesis of benzodiazepine-fused tricyclic compounds 4a–c were accomplished by a three-step reaction sequence (Scheme 5). N-acylation of 1 with 2-nitrobenzoyl chloride gave acylation product 9. We have found that the N-acylation reaction was sensitive to the R1 substitution; only small R1 groups such as H and Me gave products in good yields. Compounds 9 were then reacted with zinc dust in acetic acid under sonication to reduce the nitro group and form 10. The cyclative tag cleavage of compounds 10 with DBU produced tricyclic compound 4a–c in 45–58% yields.
Scheme 5.
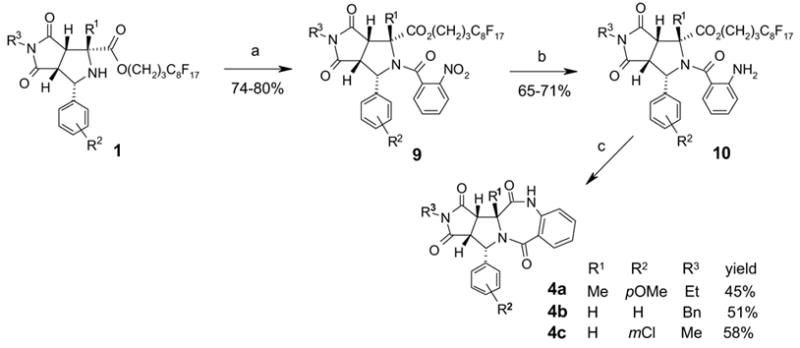
Synthesis of benzodiazepinedione-fused tetracyclic compounds 3a–d.a) 2-nitrobenzoylchloride (3 equiv), Et3N (2 equiv), DMF, 80 °C, 2 h, F-SPE. b) Zn dust (10 equiv), AcOH, sonication, 25 °C, 2 h, F-SPE, 65–71%. c) DBU (2 equiv), dioxane, μw (130 °C, 5 min), F-SPE, HPLC.
Conclusion
In summary, we have developed synthetic routes to three triaza tricyclic and tetracyclic rings systems using the common intermediates generated by [3+2] cycloaddition of azomethine ylides. Microwave-assisted fluorous synthesis speeds up reactions and simplifies product purifications. These heterocyclic compounds with ring skeleton, stereochemistry, and substitution variations are good candidates for diversity-oriented synthesis.
Supplementary materials
Acknowledgments
This work was supported by the National Institute of General Medical Sciences SBIR Grants (2R44GM062717-02 and 2R44GM067326-02). We thank Professor Peter Wipf and Dr. John Hodges for helpful suggestions and discussions.
Footnotes
Supporting Information General experimental procedures and analytical data for representative intermediates and all final products are provided.
References
- 1.Curran DP. Angew Chem Int Ed Eng. 1998;37:1175–1196. [Google Scholar]
- 2.Selected reviews and monographs on fluorous synthesis: Gladysz JA, Curran DP, Horvath IT, editors. Handbook of Fluorous Chemistry. Wiley-VCH: Weinheim; 2004. ; b) Zhang W. Chem Rev. 2004;104:2531–2556. doi: 10.1021/cr030600r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang W. Curr Opin Drug Disc Dev. 2004;7:784–797. [PMC free article] [PubMed] [Google Scholar]; d) Pozzi G, Shepperson I. Coord Chem Rev. 2003;242:115–124. [Google Scholar]; e) Zhang W. Tetrahedron. 2003;59:4475–4489. [Google Scholar]; f) Dobbs AP, Kimberley MR. J Fluorine Chem. 2002;118:3–17. [Google Scholar]; g) Tzschucke CC, Markert C, Bannwarth W, Roller S, Hebel A, Haag R. Angew Chem Int Ed. 2002;41:3964–4000. doi: 10.1002/1521-3773(20021104)41:21<3964::AID-ANIE3964>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; h) Barthel-Rosa LP, Gladysz JA. Coordination Chem Rev. 1999;190–192:587. [Google Scholar]; i) Horvath IT. Acc Chem Res. 1998;31:641–650. [Google Scholar]; j) Studer A, Hadida S, Ferritto SY, Kim PY, Jeger P, Wipf P, Curran DP. Science. 1997;275:823–826. doi: 10.1126/science.275.5301.823. [DOI] [PubMed] [Google Scholar]; k) Horvath IT, Rabai T. Science. 1994;266:72–76. doi: 10.1126/science.266.5182.72. [DOI] [PubMed] [Google Scholar]
- 3.Chen CHT, Zhang W. Mol Diversity. 2005;9:353–359. doi: 10.1007/s11030-005-8104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Curran DP. Synlett. 2001:1488–1496. [Google Scholar]; b) Curran DP. In: Handbook of Fluorous Chemistry. Gladysz JA, Curran DP, Horvath IT, editors. Wiley-VCH; Weinheim: 2004. p. 101. [Google Scholar]
- 5.a) Zhang W, Nagashima T, Lu Y, Chen CHT. Tetrahedron Lett. 2004;45:4611–4613. [Google Scholar]; b) Zhang W, Chen CHT, Lu Y, Nagashima T. Org Lett. 2004;6:1473–1476. doi: 10.1021/ol0496428. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang W, Lu Y, Chen CHT. Mol Diversity. 2003;7:199–202. doi: 10.1023/b:modi.0000006825.12186.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Olofeeson K, Kim SY, Larhed M, Curran DP, Hallberg A. J Org Chem. 1999;64:4539–4541. [Google Scholar]; e) Larhed M, Hoshino M, Hadida S, Curran DP, Hallberg A. J Org Chem. 1997;62:5583–5587. [Google Scholar]
- 6.a) Lu Y, Zhang W. Mol Diversity. 2005;9:91–98. doi: 10.1007/s11030-005-1293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang W, Chen CHT.Tetrahedron Lett 2005461807–1810.18079977 [Google Scholar]; c) Lu Y, Zhang W. QSAR Comb Sci. 2004;23:827–835. doi: 10.1901/jaba.2004.23-827. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang W, Tempest P. Tetrahedron Lett. 2004;45:6757–6760. [Google Scholar]
- 7.Zhang W, Lu Y, Geib S. Org Lett. 2005;7:2269–2272. doi: 10.1021/ol0507773. [DOI] [PubMed] [Google Scholar]
- 8.Selected papers on fluorous mixture synthesis: Zhang Q, Curran DP. Chem Eur J. 2005;11:4866–4880. doi: 10.1002/chem.200500076.; b) Zhang W. Arkivoc. 2004:101–109. [PMC free article] [PubMed] [Google Scholar]; c) Zhang W, Luo Z, Chen CHT, Curran DP. J Am Chem Soc. 2002;124:10443–10450. doi: 10.1021/ja026947d. [DOI] [PubMed] [Google Scholar]; d) Luo Z, Zhang Q, Oderaotoshi Y, Curran DP. Science. 2001;291:1766–1769. doi: 10.1126/science.1057567. [DOI] [PubMed] [Google Scholar]
- 9.a) Goto K, Miura T, Hosaka D, Matsumoto H, Mizuno M, Ishida H-k, Inazu T. Tetrahedron. 2004;60:8845. [Google Scholar]; b) Montanari V, Kumar K. J Am Chem Soc. 2004;126:9528–9529. doi: 10.1021/ja0479033. [DOI] [PubMed] [Google Scholar]; c) Mizuno M, Goto K, Miura T, Hosaka D, Inazu T. Chem Commun. 2003:972. doi: 10.1039/b300682d. [DOI] [PubMed] [Google Scholar]; d) de Visser PC, van Helden M, Filippov DV, van der Marel GA, Drijfhout JW, van Boom JH, Noortc D, Overkleeft HS. Tetrahedron Lett. 2003;44:9013–9016. [Google Scholar]; e) Filippov DV, van Zoelen DJ, Oldfield SP, van der Marel GA, Overkleeft HS, Drijfhout JW, van Boom JH. Tetrahedron Lett. 2002;43:7809–7812. [Google Scholar]
- 10.a) Manzoni L, Castelli R. Org Lett. 2004;6:4195–4198. doi: 10.1021/ol048474g. [DOI] [PubMed] [Google Scholar]; b) Jing Y, Huang X. Tetrahedron Lett. 2004;45:4615–4618. [Google Scholar]; c) Miura T, Goto K, Hosaka D, Inazu T. Angew Chem Int Ed. 2003;42:2047–2051. doi: 10.1002/anie.200250531. [DOI] [PubMed] [Google Scholar]; d) Manzoni L. Chem Commun. 2003:2930–2931. doi: 10.1039/b311448a. [DOI] [PubMed] [Google Scholar]; e) Palmacci ER, Hewitt MC, Seeberger PH. Angew Chem Int Ed. 2001;40:4433–4437. doi: 10.1002/1521-3773(20011203)40:23<4433::aid-anie4433>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]; f) Curran DP, Ferritto R, Hua Y. Tetrahedron Lett. 1998;39:4937–4940. [Google Scholar]
- 11.Nagashima T, Zhang W. J Comb Chem. 2004;6:942–949. doi: 10.1021/cc049885r. [DOI] [PubMed] [Google Scholar]
- 12.Recent reviews and monographs on [3+2] cycloadditions of azomethine ylides: Coldham I, Hufton R. Chem Rev. 2005;105:2765–2809. doi: 10.1021/cr040004c.; b) Ruck-Braun K, Freysoldt THE, Wierschem F. Chem Soc Rev. 2005;34:507–516. doi: 10.1039/b311200b. [DOI] [PubMed] [Google Scholar]; c) Harju K, Yli-Kauhaluoma J. Mol Diversity. 2005;9:187–207. doi: 10.1007/s11030-005-1339-1. [DOI] [PubMed] [Google Scholar]; d) Padwa A, Pearson WH, editors. Synthetic Application of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products. Wiley; Hoboken: 2003. [Google Scholar]; e) Najera C, Sansano JM. Curr Org Chem. 2003;7:1105–1150. [Google Scholar]; f) Albers M, Meyer T. In: Handbook of Combinatorial Chemistry. Nicolaou KC, Hartwig W, editors. Vol. 1. Wiley-VCH; Weinheim: 2002. p. 453. [Google Scholar]; f) Kantorowski EJ, Kurt MJ. Mol Diversity. 1996;2:207–216. doi: 10.1007/BF01715636. [DOI] [PubMed] [Google Scholar]
- 13.Olsen J, Seiler P, Wagner B, Fisher H, Tschopp T, Obst-Sander U, Banner DW, Kansy M, Muller K, Diederrich F. Org Biomol Chem. 2004;2:1339–1352. doi: 10.1039/b402515f. [DOI] [PubMed] [Google Scholar]
- 14.Slee DH, Bhat AS, Nguyen TN, Kish M, Lundeen K, Newman MJ, McConnell SJ. J Med Chem. 2003;46:1120–1122. doi: 10.1021/jm020460y. [DOI] [PubMed] [Google Scholar]
- 15.For related tricyclic piperazinedione ring systems, see Furutsuka K, Hayashi H, Shiono Y. J Nat Prod. 1999;62:315–317. doi: 10.1021/np9802623.; b) Roe JM, Webster RAB, Ganesan A. Org Lett. 2003;5:2825–2827. doi: 10.1021/ol034822n. [DOI] [PubMed] [Google Scholar]; c) Ley SV, Cleator E, Hewitt PR. Org Biomol Chem. 2003;1:3492–3494. doi: 10.1039/b308288a. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 16.a) Kamal A, Reddy KL, Devaiah V, Shankaraiah N, Reddy DR. Mini-Rev Med Chem. 2006;6:53–68. doi: 10.2174/138955706775197875. [DOI] [PubMed] [Google Scholar]; b) Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; c) Grieder A, Thomas AW. Synthesis. 2003:1707–1711. [Google Scholar]; d) Boojamra C, Burow KM, Thompson LA, Ellman JA. J Org Chem. 1997;62:1240–1256. and references cited there in. [Google Scholar]
- 17.Solid-supported [3+2] cycloaddition reactions of azomethine ylides: Komatsu M, Okada H, Akaki T, Oderaotashi Y, Minakata S. Org Lett. 2002;4:3505–3508. doi: 10.1021/ol026634n.; b) Ganguly AK, Seah N, Popov V, Wang CH, Kuang R, Saksena AK, Pramanik BN, Chan TM, McPhail AT. Tetrahedron Lett. 2002;43:8981–8983. [Google Scholar]; c) Hoveyda HR, Hall DG. Org Lett. 2001;3:3491–3494. doi: 10.1021/ol016533+. [DOI] [PubMed] [Google Scholar]; d) Barrett AGM, Boffey RJ, Frederiksen MU, Newton CG, Roberts RS. Tetrahedron Lett. 2001;42:5579–5581. [Google Scholar]; e) Dondas HA, Grigg R, MacLachlan WS, MacPherson DT, Markandu J, Sridharan V, Suganthan S. Tetrahedron Lett. 2000;41:967–970. [Google Scholar]; f) Peng G, Sohn A, Gallop MA. J Org Chem. 1999;64:8342–8349. doi: 10.1021/jo990969+. [DOI] [PubMed] [Google Scholar]; g) Bicknell A, Hird NW. Bioorg Med Chem Lett. 1996;6:2441–2444. [Google Scholar]; h) Hamper BC, Dukesherer DR, South MS. Tetrahedron Lett. 1996;37:3671–3674. [Google Scholar]; i) Hollinshead SP. Tetrahedron Lett. 1996;37:9157–9160. [Google Scholar]
- 18.Fluorous silane-assisted [3+2] cycloaddition of azomethine ylides Komatsu M, Okada H, Yokoi S, Minakata S. Tetrahedron Lett. 2003;44:1603–1606.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


