Abstract
The interactions of polycyclic aromatic hydrocarbons (PAH) and cytochromes P450 (CYP) are complex; PAHs are enzyme inducers, substrates, and inhibitors. In T-47D breast cancer cells, exposure to 0.1 to 1 μM benzo(k)fluoranthene (BKF) induced CYP1A1/1B1-catalyzed 17β-estradiol (E2) metabolism, whereas BKF levels greater than 1 μM inhibited E2 metabolism. Time-course studies showed that induction of CYP1-catalyzed E2 metabolism persisted after the disappearance of BKF or co-exposed benzo(a)pyrene, suggesting that BKF metabolites retaining Ah receptor agonist activity were responsible for prolonged CYP1 induction. BKF metabolites were shown, through the use of ethoxyresorufin O-deethylase and CYP1A1-promoter-luciferase reporter assays, to induce CYP1A1/1B1 in T-47D cells. Metabolites formed by oxidation at the C-2/C-3 region of BKF had potencies for CYP1 induction exceeding those of BKF, whereas C-8/C-9 oxidative metabolites were somewhat less potent than BKF. The activities of expressed human CYP1A1 and 1B1 with BKF as substrate were investigated by use of HPLC with fluorescence detection, and by GC/MS. The results showed that both enzymes efficiently catalyzed the formation of 3-, 8-, and 9-OHBKF from BKF. These studies indicate that the inductive effects of PAH metabolites as potent CYP1 inducers are likely to be additional important factors in PAH-CYP interactions that affect metabolism and bioactivation of other PAHs, ultimately modulating PAH toxicity and carcinogenicity.
Keywords: benzo(k)fluoranthene, benzo(a)pyrene, PAH metabolism, CYP1 induction, aryl hydrocarbon receptor
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are widespread environmental contaminants that arise from biogenic, petrogenic, and pyrogenic sources (Page et al., 1999). They are generally found as complex mixtures in the environment in matrices such as coal tars, vehicle exhaust, soot, and smoke (Thorsen et al., 2004; Mahadevan et al., 2004). A number of PAHs are converted to carcinogens through metabolism (Wood et al., 1976; Deutsch-Wenzel, 1983; Denissenko et al., 1996; Shimada and Fujii-Kuriyama, 2004; Xue and Warshawsky, 2005). The formation of the most potent ultimate PAH mutagens, the bay- and Fjord-region dihydrodiol epoxides, is catalyzed by cytochromes P450 (CYP or P450) together with epoxide hydroxylase (Shimada et al., 1999). Additional pathways of PAH metabolism associated with toxic consequences involve (1) the formation of orthoquinones that participate in redox cycling, the generation of reactive oxygen species, and the formation of depurinating adducts (Penning, 2004; Park et al., 2005; 2006); (2) the one-electron oxidation of PAHs to adductive intermediates (Chen et al., 1996); and (3) the formation of reactive PAH sulfates arising from metabolism of methyl-substituted PAHs (Suhr and Miller, 1994; Glatt, 2000). In the human liver, several P450s are capable of catalyzing the metabolic activation of PAHs (Shou et al., 1994), whereas in extrahepatic tissues, CYP1A1 and CYP1B1 appear to play the major role in the metabolic activation of PAHs (Spivack et al., 1997; Einolf et al., 1997; Shimizu et al., 2000; Shimada and Fujii-Kurimaya, 2004).
In both the dihydrodiol epoxide and the orthoquinone pathways, the initial PAH oxidations are catalyzed by P450s of the CYP1 gene family. The expression of CYP1A1, CYP1A2, and CYP1B1 is under the regulatory control of the Ah receptor (AhR). Individual PAHs can, to varying degrees, induce their own metabolism by induction of these CYP1 family enzymes (Schmoldt et al., 1981; Willet et al., 1997) through binding to and activation of the AhR (Cherng et al., 2001). These inductions, and the differences in AhR agonist activities among the individual PAHs, form the basis of the toxic equivalency factor system used to estimate toxic effects of environmental compounds (Willet et al., 1997; Jones and Anderson, 1999; Bosveld et al., 2002). The toxicity and carcinogenicity of a PAH mixture are influenced by a complex set of interactions, involving not only the properties of the individual PAH components as P450 inducers through AhR activation, but also their roles as substrates (Shimada et al., 1996; Kim et al., 1998) and as inhibitors of P450-catalyzed reactions (Willet et al., 1998; Arcaro et al., 1999; Spink et al., 2002; Shimada and Guengerich, 2006). Through such interactions, a non-carcinogenic PAH may either enhance or suppress the metabolic activation of a carcinogenic PAH to a mutagenic form.
Studies in our laboratory have investigated interactions of PAHs with other environmental toxicants in human hepatocytes (Vakharia et al., 2001a; Liu et al., 2001), HepG2 hepatoma cells (Vakharia et al., 2001b; Bessette et al., 2005), and T-47D human breast cancer cells (Spink et al., 2002; Wu et al., 2003). In the present study, we focused on the CYP1-inductive effects of benzo(k)fluoranthene (BKF), alone and in combination with benzo(a)pyrene (BAP). CYP1 metabolic activities were assessed in assays using the probe substrates 17β-estradiol (E2) and ethoxyresorufin; BKF metabolites were identified and quantified by high-performance liquid chromatography (HPLC) with fluorescence detection and by gas chromatography/mass spectrometry (GC/MS); and the efficacy for CYP1 induction was determined in ethoxyresorufin O-deethylase (EROD) and CYP1A1-promoter-reporter luciferase assays. The results revealed that several metabolites of BKF are potent inducers of CYP1A1 and 1B1, suggesting that the properties of PAH metabolites must also be considered in PAH-CYP interactions.
Materials and Methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM without phenol red, and LipofectAMINE 2000 were from Invitrogen (Carlsbad, CA). Fetal bovine serum (characterized) and bovine calf serum (Cosmic calf serum) were obtained from Hyclone (Logan, UT). Dimethylsulfoxide (DMSO) and β-glucuronidase/sulfatase (Type H-2) were obtained from Sigma (St. Louis, MO). 2,3,7,8-Tetrachlorodibenzo-p-dionxin (TCDD) was from Cambridge Isotope Laboratories (Woburn, MA). Benz[a]anthracene (BAA), BAP, and BKF were obtained from AccuStandard (New Haven, CT). The following PAH metabolites were obtained from the NCI Chemical Carcinogen Reference Standard Repository distributed by the Midwest Research Institute (Kansas City, MO): benzo[k]fluoranthene-trans−8,9-dihydrodiol (BKF-8,9-diol); benzo[k]fluoranthene-trans−2,3-dihydrodiol (BKF-2,3-diol); benz[a]anthracene-trans−3,4-dihydrodiol (BAA-3,4-diol); dibenz[a,h]anthracene-cis−5,6-dihydrodiol (DBAHA-5,6-diol); 3-, 8-, and 9-hydroxybenzo-[k]fluoranthene (3-, 8-, and 9- OHBKF). N,O-bis(trimethylsilyl)-trifluoroacetamide, trimethylchlorosilane, and the bicinchoninic acid protein assay were from Pierce (Rockford, IL). HPLC-grade solvents were from J. T. Baker (Phillipsburg, NJ). Human CYP1A1 and CYP1B1 expressed in Sf9 insect cells (Supersomes) were from BD Gentest (Bedford MA). The Dual-Luciferase Reporter 1000 Assay System was from Promega (Madison, WI).
T-47D cell culture
T-47D human breast cancer cells were obtained from the American Type Culture Collection (Rockville, MD). Stock T-47D cultures were maintained in DF5 medium as described (Wu et al., 2003). For experimental protocols, DC5 medium was used (Spink et al., 2002). BKF, BAP, and TCDD were added to culture medium with DMSO as the vehicle; DMSO did not exceed a final concentration of 0.2% (v/v) in the culture medium.
Determination of BKF and BAP cellular uptake
Uptake of BKF and BAP into T-47D cells was determined as described by Vakharia et al. (2001a).
Assay of cellular E2 metabolism
E2 metabolism assays were performed as described (Spink et al., 1994; 1998) in 6-well plates with 2 mL medium per well. After conjugate hydrolysis with β-glucuronidase/sulfatase and solid-phase extraction, E2 metabolites were analyzed as their trimethysilyl (TMS) derivatives by GC/MS with quantitation by stable isotope dilution (Spink et al., 1990; 1994). Rates of 2- and 4-methoxyestradiol (2- and 4-MeOE2) formation were normalized to total protein content as determined by using the bicinchoninic acid protein assay.
Analysis of BKF metabolites by HPLC
T-47D cells were plated in 6-well plates with 2 mL medium per well. At approximately 80% confluence, cultures received DC5 medium containing 3 μM BKF or the solvent vehicle, DMSO (0.1% v/v). At the indicated times, the media were removed, DBAHA-5,6-diol and BAA were added as internal standards, and BKF metabolites were subjected to conjugate hydrolysis by incubation with β-glucuronidase/sulfatase, or were subjected to a combined acid hydrolysis/extraction procedure by means of a modification of the method of Tang and Crone (1989). When β-glucuronidase/sulfatase was used, recovered media samples were adjusted to pH 5 by addition of 10% acetic acid, and metabolite conjugates were hydrolyzed by incubation with 3800 U β-glucuronidase and 340 U aryl sulfatase for 18 h at 37 °C. Protein was precipitated from the hydrolyzed samples by addition of 3 volumes of methanol, samples were centrifuged at 1000 × g for 5 min, and the supernatant was then recovered, spiked with the recovery standard (BAA 3,4-diol) and analyzed by HPLC. For combined acid hydrolysis/extraction, a 0.5-mL aliquot of the medium with internal standards, a saturating amount (0.125 g) of NaCl, and sulfuric acid (0.5 M final concentration) were added to a glass, screw-cap tube with a Teflon-lined cap. Ethyl acetate (3 mL) was added and the contents were mixed on a rotator for 15 min. The tubes were then centrifuged for 10 min at 1000 × g. The organic phase was recovered, evaporated to dryness under nitrogen, and the metabolites were re-dissolved in 100 μL ethanol containing the recovery standard, BAA 3,4-diol.
The metabolites of BKF were resolved by HPLC essentially as reported by Weyand et al. (1988). Portions of the sample extracts (50 μL) were analyzed by reversed-phase HPLC using a Waters 2690 Alliance System with a NovaPak C18 column (0.8 × 10 cm; 4 μm particle size). For chromatography of the extracts of cell culture medium, an initial condition of 50% water: 50 % methanol for 15 min was followed by a linear gradient to 100% methanol over 60 min, a hold at 100% methanol for 10 min, and a return to 50% water: 50% methanol over 1 min, with a constant flow rate of 1 mL/min. A compressed methanol gradient was used for the analysis of BKF metabolites produced in microsomal reactions (described below); it consisted of an initial condition of 32% water:68% methanol for 7 minutes at a flow rate of 1.5 mL/min followed by a linear increase to 15% water:85% methanol over 8 min. Upon attainment of 85% methanol, the flow rate was decreased to 0.65 mL/min over 1 min. The elution continued with a linear increase to 100% methanol over 3 min, together with a linear increase in flow, finally returning to 1.5 mL/min. The column was then washed with 100% methanol for 10 min at 1.5 mL/min. Fluorescence at 460 nm from excitation at 300 nm was recorded using a Waters 474 scanning fluorescence detector.
Assay of microsomal BKF metabolism
For in vitro experiments with human CYP1A1 and CYP1B1, Supersomes (2 pmol of P450) were pre-incubated in 100 mM sodium phosphate buffer, pH 7.4, with 5 mM MgCl2 and 0.1−10 μM BKF for 5 min at 37°C. The reactions were initiated by addition of NADPH (1.4 mM final concentration), incubated at 37°C for 10 min, and terminated by addition of sulfuric acid (0.5 M final concentration). Internal standard (200 pmol BAA) was added along with 0.125 g of NaCl. The reactions were extracted twice with 3-mL portions of ethyl acetate. The pooled organic phases were evaporated to dryness under nitrogen, and the metabolites were resuspended in 100 μL of methanol and analyzed by HPLC as described above.
GC/MS of BKF metabolites
Microsomal reactions with human CYP1A1 and CYP1B1, and BKF as substrate, were incubated, and the metabolites were extracted as described above. The ethyl acetate extracts were evaporated to dryness under nitrogen, and TMS derivatives of the metabolites were prepared by reaction with 50 μL of N,O-bis(trimethylsilyl)-trifluoroacetamide containing 1% trimethylchlorosilane at 60 °C for 30 min. The metabolite derivatives were analyzed on a Hewlett Packard 5890 gas chromatograph interfaced with a MicroMass (Waters) Quattro mass spectrometer. BKF metabolite-TMS derivatives were resolved on a DB-1 column (30 m × 0.25 mm I.D, 0.25 μm film thickness; J&W Scientific, Folsum, CA) using the following temperature program: initial temperature, 150 °C for 2 min; ramp at 20 °C/min to 240 °C; ramp at 2 °C per min to 300 °C, hold 5 min. The injection port was maintained at 250 °C, and the GC interface was maintained at 280 °C. Full-scan mass spectrometry was performed over the m/z 50 to 550 range with 1-s scans. Selected-ion monitoring of m/z 325, 340, and 430 was performed with 100-ms dwell times.
EROD assays
EROD assays were performed by the 96-well plate method of Donato et al. (1993) as described earlier (Spink et al., 2003).
CYP1A1 promoter activity
The construct used for analysis of CYP1A1 promoter activity was the human CYP1A1-promoter-luciferase reporter construct, pHu-1A1-FL, described by Bessette et al. (2005); it contains approximately 1450 bp of the human CYP1A1 5′ upstream promoter region inserted upstream of the firefly luciferase gene. T-47D cells were seeded in 24-well plates at 6 × 105 cells/well in DC5 medium. After incubation for 24 h, the cells were co-transfected with pHu-1A1-FL (180 ng/well) and pRL-CMV encoding Renilla luciferase (20 ng/well), using pBluescript SK vector (Stratagene) as carrier DNA (300 ng/well) plus LipofectAMINE 2000 (Invitrogen; 1.6 μL/well) in DMEM. The cells were incubated at 37 °C for 24 h followed by exposure to varying concentrations of BAP, BKF, BKF metabolites, or TCDD in DC5 medium for 18 h. The cultures were then assayed for luciferase activities by means of the Dual-Luciferase Reporter 1000 Assay System.
Results
Effects of BKF and BAP on E2 metabolism in T-47D cells
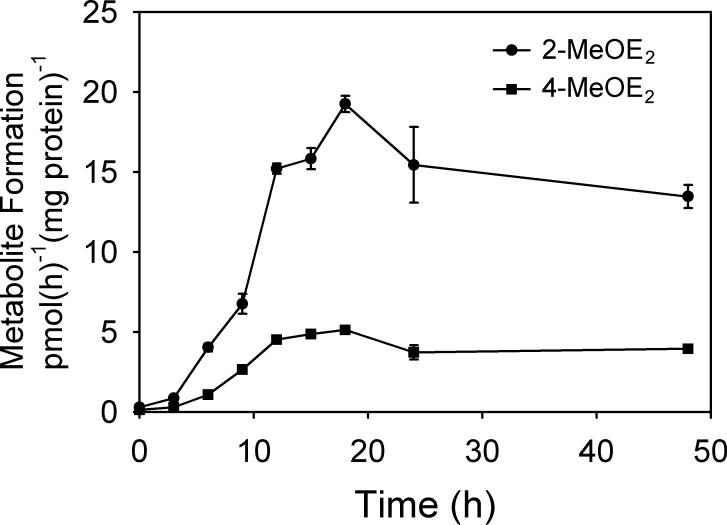
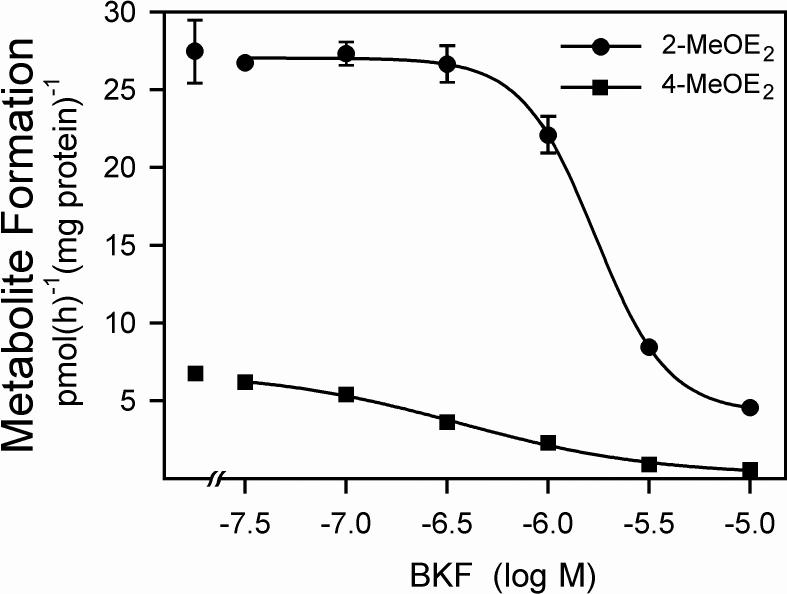
Exposure to BKF markedly increased the rate of E2 metabolism in T-47D cells. The time course of 2- and 4-MeOE2 formation after exposure of T-47D cells to 3 μM BKF is shown in Fig. 1. The rate of formation of 2-MeOE2, reflective of the CYP1A1-catalyzed 2-hydroxylation pathway, was elevated 22-fold at 18 h, and remained elevated for at least 48 h. Similarly, the induction of 4-MeOE2 formation, reflective of the CYP1B1-catalyzed E2 4-hydroxylation, was greatly elevated, 17-fold by 18 h, and remained elevated for at least 48 h. The potential of BKF to inhibit CYP1A1- and CYP1B1-catalyzed E2 metabolism was investigated through induction of the P450s with 10 nM TCDD, and then determination of the rates of E2 metabolism in the presence of varying concentrations of BKF. In T-47D cultures, BKF inhibited both 2- and 4-MeOE2 formation, with respective EC50 values of 1.71 ± 0.06 and 0.37 ± 0.14 μM (Fig. 2), indicating the inhibition of both the CYP1A1- and CYP1B1-catalyzed E2 hydroxylase activities.
Fig. 1.
Time course of the inductive effects of BKF exposure on E2 metabolism in T-47 D cells. Confluent cultures of T-47D cells in 6-well plates were exposed to a single application of medium containing 3 μM BKF (2 ml/well). At the indicated times, the medium was replaced with medium containing 1 μM E2 with 0.1% DMSO as the vehicle. After a 6-h assay period, media were recovered, conjugates were hydrolyzed, and rates of 2- and 4-MeOE2 formation were determined relative to cellular protein content, as described in Materials and Methods. Data are the mean ± SE of triplicate determinations. Rates of 2- and 4-MeOE2 formation for control cultures (without BKF exposure) were 0.30 + 0.02 and 0.14 + 0.02 pmol(h)−1(mg protein)−1, respectively.
Fig. 2.
Inhibition of TCDD-induced E2 metabolism in T-47D cells by BKF. Cultures were exposed to 10 nM TCDD or 0.1% (v/v) DMSO for 72 h. Media were then replaced with media containing 1 μM E2 and the indicated concentration of BKF. After an additional 6 h, media were recovered, conjugates were hydrolyzed, and the levels of 2- and 4-MeOE2 formed in the absence of BKF addition (leftmost data points), and the levels of 2- and 4-MeOE2 formed in the presence of the indicated levels of BKF were determined. Rates of metabolite formation are expressed relative to cellular protein content. Values shown are the means of triplicate determinations, ±SE.
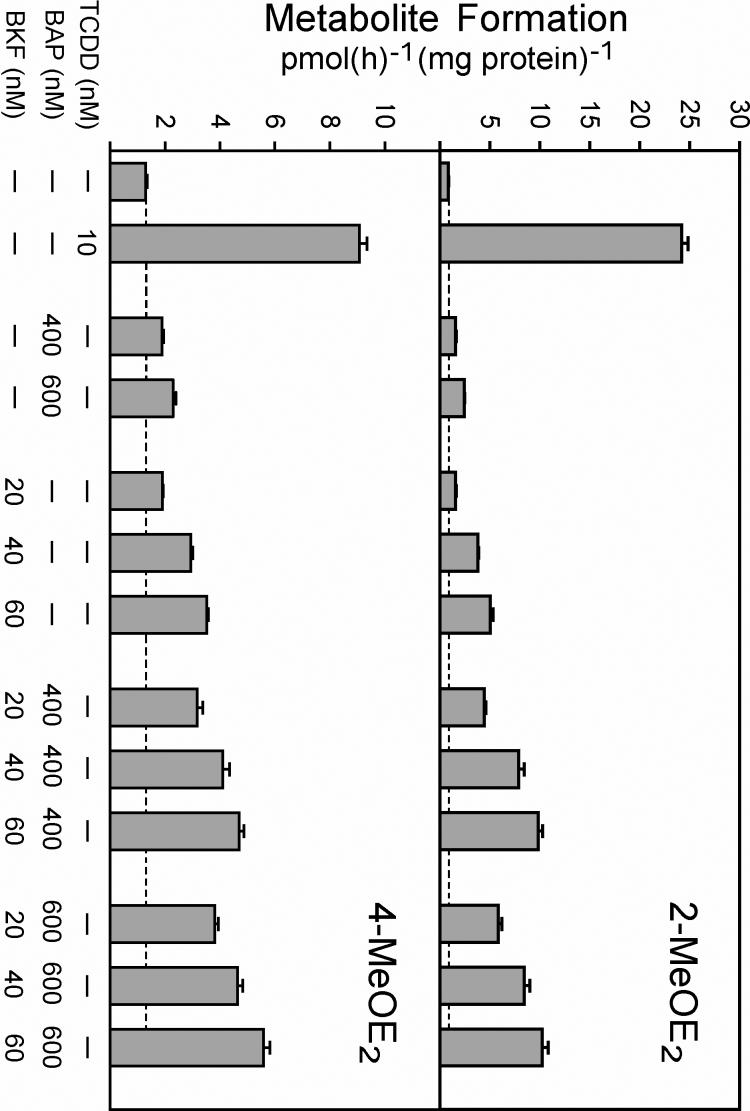
A goal of these studies was to investigate potential interactive effects of two PAHs having AhR agonist activity, namely, BKF and BAP. The effects of low levels of BKF and BAP, both singly and in combination, on the induction of E2 metabolism in T-47D cells are shown in Fig. 3. Synergistic effects on induction of the 2-MeOE2 pathway were observed when 20 to 600 nM levels of BKF and BAP were applied to T-47D cultures in combination. For example, 2-MeOE2 formation was induced 1.55- and 1.57-fold by 400 nM BAP and 20 nM BKF, respectively, when the two PAHs were added to the cultures individually (Fig. 3, upper panel). However, when 400 nM BAP was added in combination with 20 nM BKF, 2-MeOE2 formation was induced 4.43-fold. Essentially additive effects on the 4-MeOE2 pathway were observed when 20 to 600 nM levels of BKF and BAP were applied in combination in these same experiments (Fig. 3, lower panel). When cultures were exposed to higher levels of BKF and BAP in combination, 1 to 10 μM of total PAH (experiments not shown), reduced rates of 2- and 4-MeOE2 formation were observed relative to the rates observed after exposure to the sub-micromolar levels of PAH used in Fig. 3, presumably due to P450 inhibition by BAP (Spink et al., 2002) and BKF (Fig. 2).
Fig. 3.
Effects of mixtures of BKF and BAP on E2 metabolism in T-47D cells. Cultures were exposed to 0.1% (v/v) DMSO, 10 nM TCDD, or the indicated concentrations of BAP and BKF, alone or in combination, for 9 h. The medium was then replaced with medium containing 1 μM E2. After a further 6 h, media were recovered, conjugates were hydrolyzed with β-glucuronidase/sulfatase, and levels of 2-MeOE2 (upper panel) and 4-MeOE2 (lower panel) were determined as in Figure 1. Rates of metabolite formation expressed relative to cellular protein content are the means of triplicate determinations ± SE. Rates of 2- and 4-MeOE2 formation for the control cultures (without TCDD or PAH exposure) were 0.85 + 0.05 and 1.30 ± 0.06 pmol(h)−1(mg protein)−1, respectively.
Uptake of BKF and BAP by T-47D cells
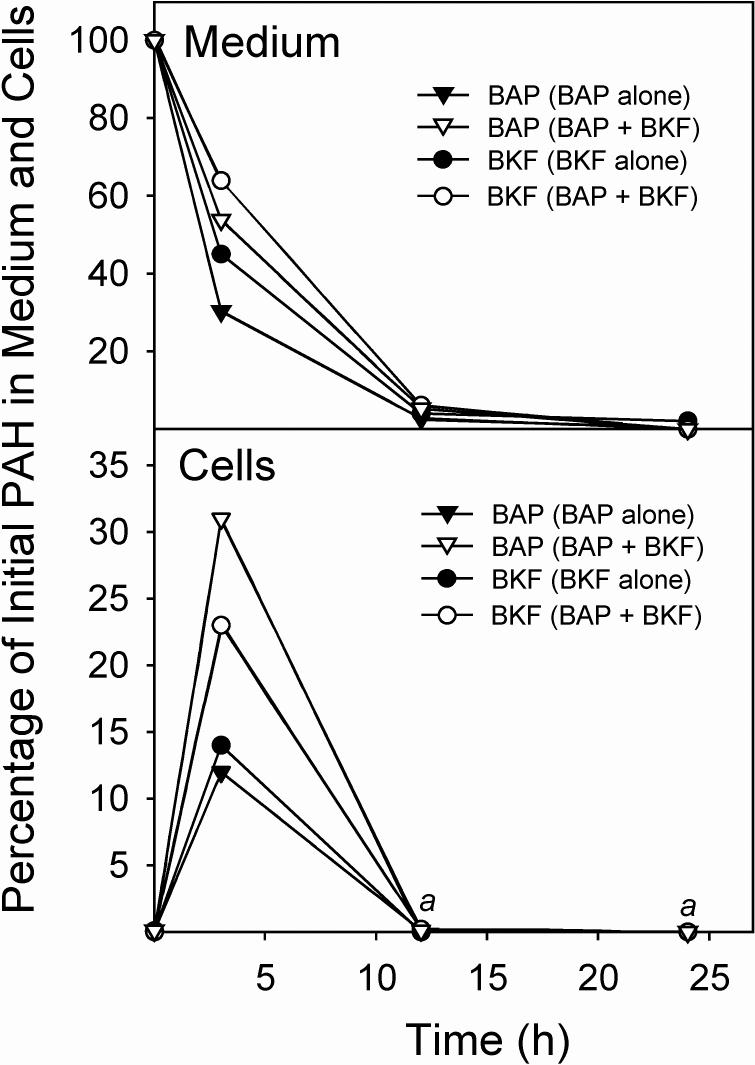
To investigate whether the persistence of the induction of E2 metabolism by BKF compared with the more transient induction caused by BAP (Spink et al., 2002) was due to differences in the persistence of the PAH inducers in the cultures, we determined the rates of disappearance of BKF and BAP from the medium. As shown in Fig. 4, the rates were comparable. Levels of both BKF and BAP in the medium fell to less than 4% of the initial level at 24 h. In co-exposure experiments, the presence of BKF caused a decrease in the rate of BAP disappearance from the medium over the first 4 h; the presence of BAP likewise caused a decrease in the rate of BKF disappearance from the medium. Cellular uptake studies also showed differences in PAH levels associated with the cellular fractions when binary PAH mixtures were applied to the cultures versus when BAP and BKF were added individually. Here, we define cellular uptake as the amount of PAH that was not removed by washing the cell monolayer with culture medium, but that could be extracted from the cell pellets that were recovered after trypsinization and centrifugation (i.e., the cellular fractions). The levels of BAP and BKF associated with the cellular fractions showed transient elevations at 4 h after exposure, but by 12 h they fell to nondetectable levels (<0.3% of the initial amount of BKF or BAP) when exposed to the cells singly or as a binary mixture. However, in the experiments with binary mixtures, the presence of BKF caused a 2.6-fold increase in the amount of BAP that was recovered from the cellular fraction at 4 h in comparison with the level when BAP was administered alone. Similarly, the presence of BAP caused a 1.8-fold increase in the BKF that was recovered from the cellular fraction at 4 h (Fig. 4).
Fig. 4.
Uptake and metabolism of BAP and BKF, individually and in a binary mixture, in T-47D cells. Confluent cultures of T-47D cells in 6-well plates were exposed to medium (2 mL/well) containing BAP, BKF, or BAP plus BKF. At the indicated times, the media and cell monolayers were harvested, extracted, and analyzed for BAP and BKF by HPLC with fluorescence detection. The percentages of the initial BAP or BKF remaining in the medium (upper panel) and cells (lower panel) when 1.5 μM BAP was added alone (BAP alone), when 1.5 μM BKF was added alone (BKF alone), or when 1.5 μM BAP and 1.5 μM BKF were added together (BAP + BKF) at the time points, are shown. aIndicates that neither BKF nor BAP was detectable (<0.3% of the initial PAH amount) in any of the cell extracts at these time points.
Metabolism of BKF in T-47D cells
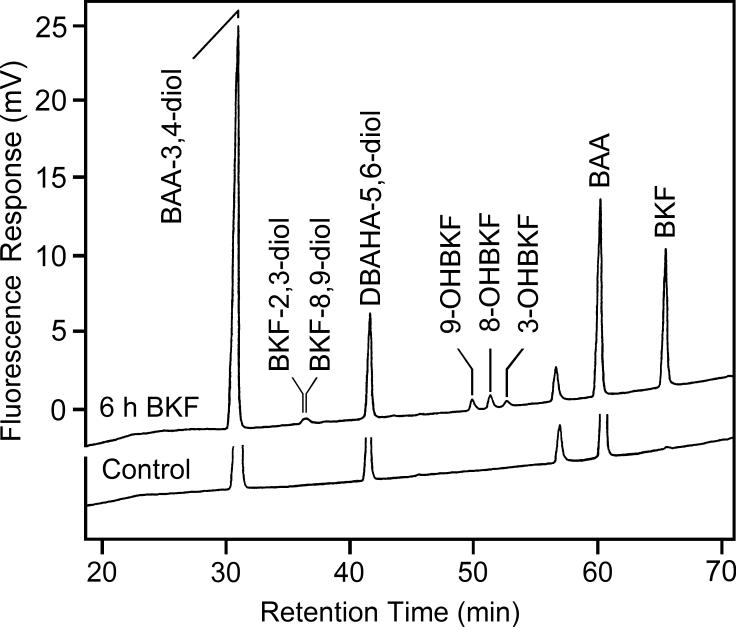
Since the BKF-induced increase in E2 metabolism persisted long after the disappearance of the BKF from the medium and the cells, we investigated the metabolism of BKF in order to determine what metabolites were formed, and whether metabolites of BKF were responsible for the prolonged induction. Medium recovered from BKF-exposed cultures was hydrolyzed, extracted, and analyzed by HPLC for the presence of BKF metabolites. We found hydroxylation of BKF at the 3, 8, and 9 positions to occur in T-47D cultures, as determined from detection of fluorescent products with retention times identical to those of 3-, 8-, and 9-OHBKF in the extracts of culture medium (Fig. 5). Similar results were obtained using conjugate hydrolysis with β-glucuronidase/sulfatase or acid treatment. Time-course studies indicated that nanomolar concentrations of these metabolites persisted for at least 72 h. Comparison of the levels with and without conjugate hydrolysis (Table 1) indicated that the metabolites existed mainly in conjugated form.
Fig. 5.
Analysis of cellular BKF metabolism by HPLC with fluorescence detection. T-47D cells were exposed for 6 h to medium without (Control, lower chromatogram) or with 3 μM BKF (upper chromatogram). Media were then recovered, internal standards added, conjugates were hydrolyzed with β-glucuronidase/sulfatase, and extracts were analyzed by HPLC with fluorescence detection. Retention times of BKF, the BKF metabolite standards, BKF-2,3-diol, BKF-8,9-diol, and 3-, 8-, and 9-OHBKF, the internal standards (DBAHA-5,6-diol and BAA), and the recovery standard (BAA-3,4-diol) are indicated.
Table 1.
Concentrations of BKF metabolites in medium of T47-D cultures after exposure to 3 μM BKF
| Time point | ||||
|---|---|---|---|---|
| Metabolite | Conjugate hydrolysisa | 6 h | 24 h | 48 h |
| 3-OHBKF | − | NDb | ND | ND |
| + | 18.0 + 1.3c | 14.4 ± 2.3 | 18.6 ± 0.4 | |
| 8-OHBKF | − | 30.5 ± 6.9 | 2.4 ± 0.3 | 3.1 + 3.3 |
| + | 54.1 ± 4.6 | 37 ± 14 | 50.7 ± 0.6 | |
| 9-OHBKF | − | 3.4 ± 0.6 | ND | ND |
| + | 9.2 ± 0.9 | 10.6 ± 2.3 | 11.4 ± 0.7 | |
Metabolite levels were determined with or without conjugate hydrolysis by acid treatment.
ND, not detected (< 1.5 nM).
Concentrations are in nM, mean ± SD, n = 3.
BKF metabolism catalyzed by human CYP1A1 and CYP1B1
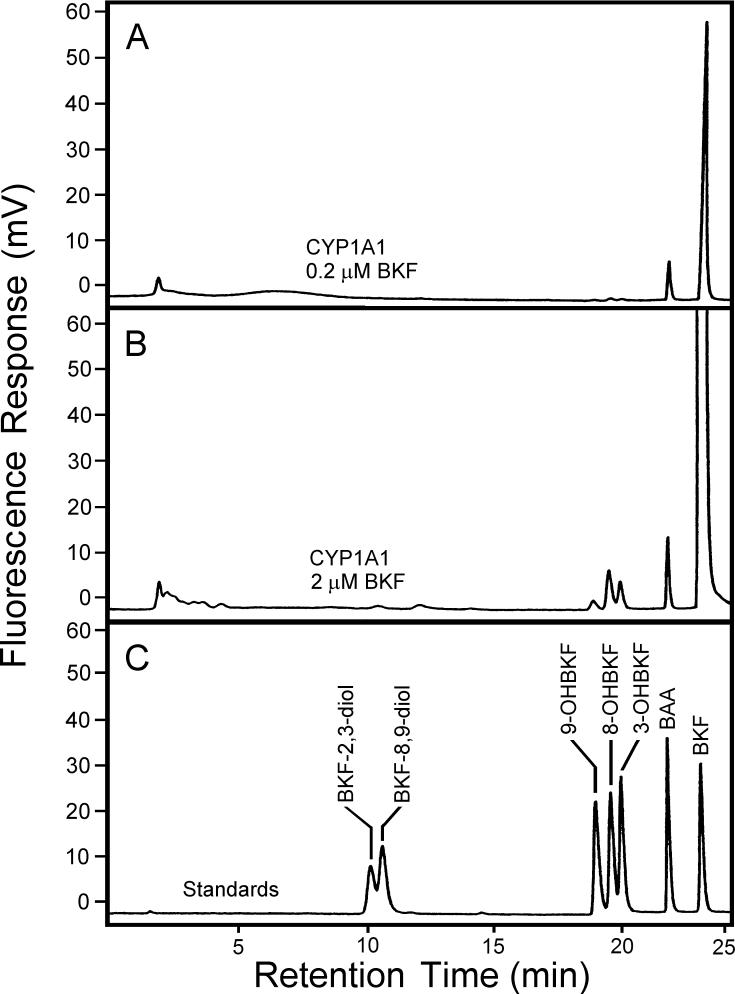
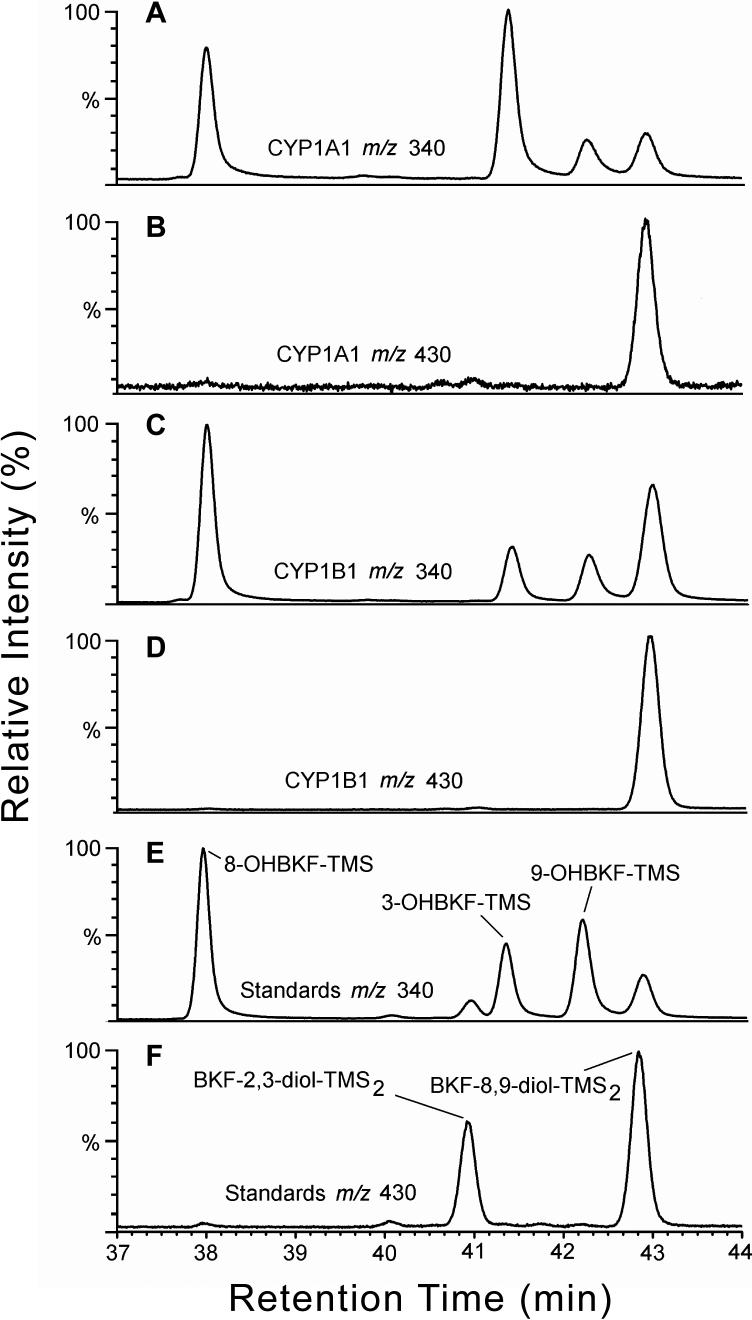
Both CYP1A1 and CYP1B1 are inducible by TCDD and PAHs in T-47D cells (Spink et al., 1998; 2002) and could therefore be responsible for the observed metabolism of BKF. To investigate this possibility, we assessed the activities of cDNA-expressed human CYP1A1 and CYP1B1 with BKF as substrate. Analysis of microsomal incubations by HPLC with fluorescence detection indicated the production of 3-, 8- and 9-OHBKF in both CYP1A1- and CYP1B1-containing microsomal reactions. Representative HPLC analyses of extracts of CYP1A1 reaction mixtures with 0.2 and 2 μM BKF as substrate are shown in Fig. 6. Peaks corresponding to 3-, 8-, and 9-OHBKF were observed in both chromatograms. Analyses by GC/MS confirmed that 3-, 8-, and 9-OHBKF are products of BKF metabolism catalyzed by human CYP1A1 and CYP1B1 (Fig. 7). We developed a GC temperature program in which 3-, 8-, and 9-OHBKF, BKF-2,3-diol, and BKF-8,9-diol were resolved. We then used this program for the GC/MS analysis of derivatized extracts of microsomal reaction mixtures with CYP1A1 and CYP1B1 with BKF as substrate. The selected-ion chromatograms of m/z 340, i.e., the value of the M+. ions of the OHBKF-TMS derivatives, showed peaks with retention times of 38.0, 41.4, and 42.3 min, corresponding respectively to the TMS derivates of 3-, 8- and 9-OHBKF, (Figs. 7A and C).
Fig. 6.
Analysis of CYP1A1-catalyzed BKF metabolism by HPLC with fluorescence detection. Microsomal incubations with 2 pmol CYP1A1 and 0.2 μM (panel A) or 2 μM (panel B) BKF as substrate were extracted and analyzed by HPLC with fluorescence detection. Shown are the chromatograms from the analysis of the CYP1A1 microsomal extract and the BKF metabolite standard mixture (panel C).
Fig. 7.
Analysis of microsomal BKF metabolism by GC/MS. After microsomal incubations (0.5-mL) with 10 pmol P450 and 10 μM BKF as substrate were extracted, TMS derivatives of the metabolites were prepared, and were analyzed by GC/MS together with BKF metabolite standards. Shown are selected-ion chromatograms for m/z 340 (A, C, and E), i.e., the value for the M+. ions of the TMS derivatives of OHBKFs, and for m/z 430 (B, D, and F) i.e., the value for the M+. ions of the TMS derivatives of BKF dihydrodiol metabolites, from the analysis of derivatized extracts of incubations with expressed CYP1A1 (A and B), CYP1B1 (C and D), and a derivatized standard mixture containing 3-, 8-, and 9-OHBKF-TMS, BKF-2,3-diol-TMS2 and BKF-8,9-diol-TMS2 (E and F).
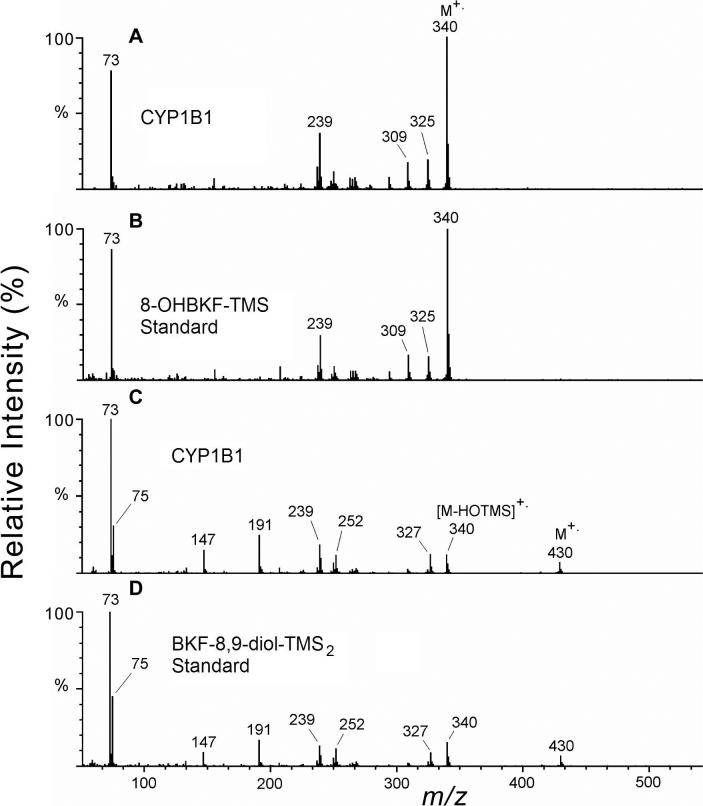
Additional peaks at retention time 42.9 min in these m/z 340 ion chromatograms, corresponding to the TMS derivative of BKF 8,9-diol, were observed in the analysis of the CYP1A1 and CYP1B1 microsomal extracts. Analysis of the TMS derivatives of a standard mixture containing 3-, 8-, and 9-OHBKF, BKF-2,3-diol, and BKF 8,9-diol (Figs. 7E and F) revealed that the TMS derivatives of BKF-2,3-diol and BKF 8,9-diol produced responses in both the m/z 340 and 430 selected-ion chromatograms. Peaks at retentions times 40.9 and 42.9 min in the selected-ion chromatograms of m/z 430 (Fig. 7F) represent the M+. ions of BKF-2,3-diol-TMS2 and BKF-8,9-diol-TMS2, respectively. Peaks in the m/z 340 ion chromatograms at these same retention times (Fig. 7E) are attributed to neutral losses of HOSi(CH3)3 (90 Da) from the M+. ions. Significant peaks at 42.9 min, but not at 40.9 min, in the m/z 340 and 430 ion chromatograms (Figs. 7A-D) indicate considerable formation of BKF 8,9-diol, but not of BKF-2,3-diol, in the microsomal reactions with expressed CYP1A1 and CYP1B1. Representative full-scan mass spectra are shown in Fig. 8.
Fig. 8.
Mass spectra of the TMS derivatives of BKF metabolites. The electron ionization mass spectrum recorded at 38.0 min from the CYP1B1 microsomal extract (A) is shown in comparison with that of the standard 8-OHBKF-TMS derivative (B), and the mass spectrum recorded at retention time 42.9 min from analysis of the CYP1B1 microsomal extract (C) is shown in comparison with that of the standard BKF-8,9-diol-TMS2 derivative (D).
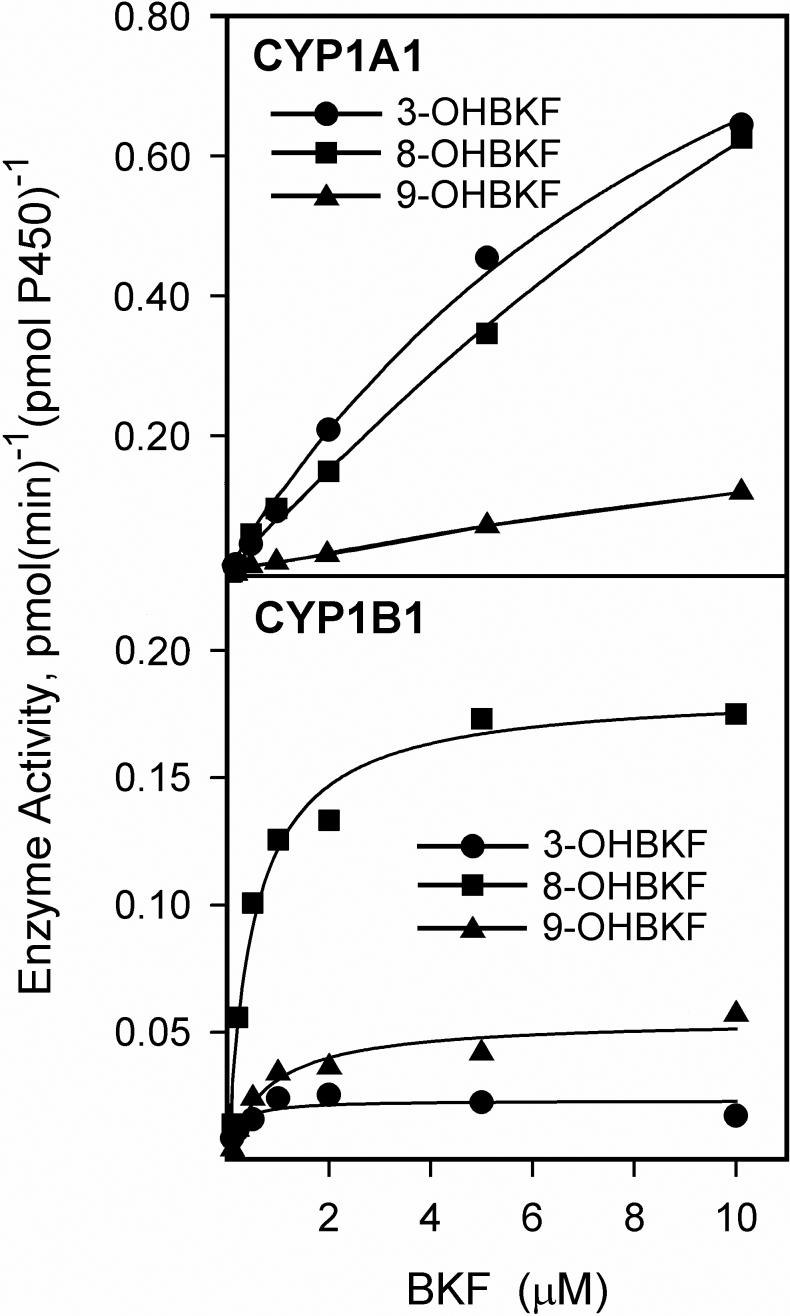
The kinetics of BKF hydroxylation catalyzed by CYP1A1 and CYP1B1 were investigated with a substrate concentration range of 0.1 to 10 μM (Fig. 9); the upper limit of this range was dictated by the solubility of BKF in the reaction buffer. We could not determine kinetic parameters for CYP1A1, because saturation of BKF hydroxylation at each of the three positions was not reached within this concentration range. CYP1B1 catalyzed 3-, 8-, and 9-hydroxylation of BKF with KM values below 1 μM (Table 2). While both CYP1A1 and CYP1B1 catalyzed 3-, 8-, and 9-hydroxylation of BKF, the prevalence of the individual metabolites produced differed between the two enzymes. CYP1A1 preferentially catalyzed 3-and 8- hydroxylation of BKF, with a lesser rate of 9-hydroxylation, whereas CYP1B1 preferentially catalyzed 8-hydroxylation, with lesser rates of 9- and 3-hydroxylation.
Fig. 9.
Kinetics of BKF hydroxylation catalyzed by human CYP1A1 and CYP1B1. Reaction mixtures containing 2 pmol of cDNA-expressed human CYP1A1 or CYP1B1 were incubated with 0.1, 0.5, 1, 2, 5 or 10 μM BKF. The metabolites produced were analyzed by HPLC with fluorescence detection as described in Materials and Methods. Values shown are the average of duplicate determinations for rate formation of 3-, 8-, and 9-OHBKF. Curves represent best fits to the Michaelis-Menten equation.
Table 2.
Kinetic parameters of BKF hydroxylation catalyzed by human CYP1B1 and CYP1A1
| CYP1B1 | CYP1A1 | |||
|---|---|---|---|---|
| KM, μMa | Vmax, nmol(min)−1(nmol P450)−1a | Vmax/KM | V, nmol(min)−1(nmol P450)−1b | |
| Product | ||||
| 3-OHBKF | 0.165 ± 0.085 | 0.0231 ± 0.0023 | 0.14 | 0.645 |
| 8-OHBKF | 0.51 ± 0.10 | 0.184 ± 0.009 | 0.36 | 0.626 |
| 9-OHBKF | 0.759 ± 0.055 | 0.055 ± 0.004 | 0.072 | 0.119 |
Kinetic parameters determined with 2 pmol of CYP1B1 and substrate concentrations of 0.1, 0.5, 1, 2, 5 and 10 μM BKF.
Enzymatic velocity determined with 2 pmol CYP1A1 and 10 μM BKF as substrate. Values are the mean of duplicate determinations.
Effects of BKF metabolites on EROD activity in T-47D cells
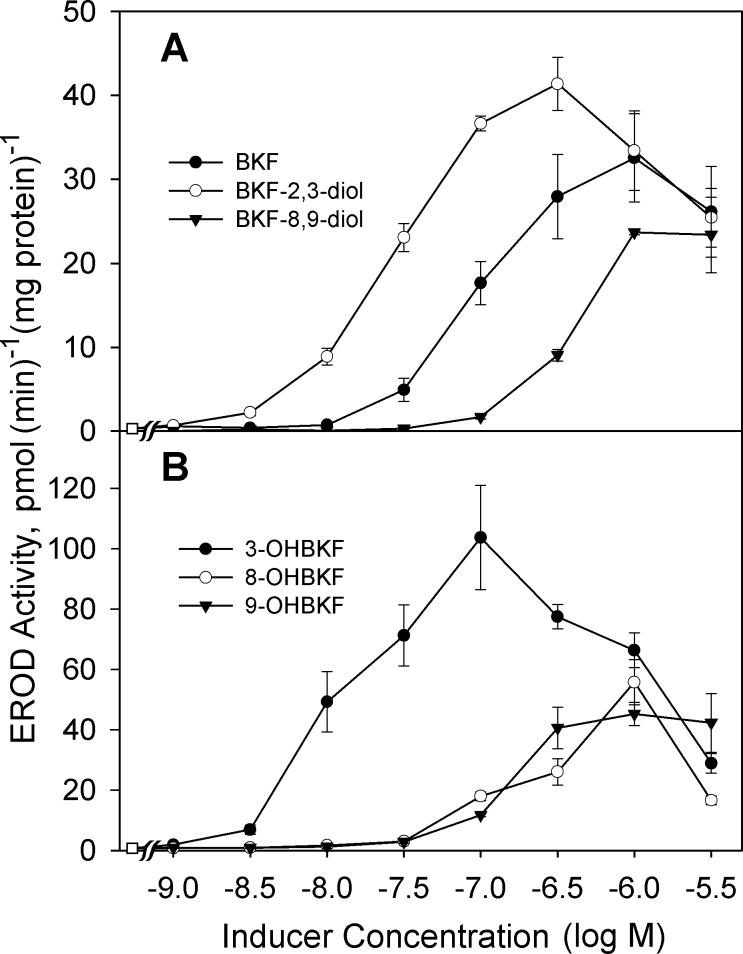
Having established that 3-, 8-, and 9-OHBKF are metabolites of BKF produced by the action of CYP1A1 and CYP1B1 and are formed in T-47D cells, we next investigated the ability of these compounds to induce CYP1 enzymes in T-47D cells. As shown in Fig. 10, each of 3-, 8-, and 9-OHBKF was an inducer of EROD activity in T-47D cells. Of the three OHBKF isomers, the most potent EROD inducer was 3-OHBKF; it caused significant elevation of EROD activity at 10 nM, whereas the 8- and 9-OHBKF metabolites induced EROD activity only at higher concentrations (100 nM to 1 μM). Concentrations of 3-OHBKF above 100 nM exhibited levels of induced EROD activity that were lower than for the 100 nM concentration, likely due to enzyme inhibition. The dihydrodiol metabolites also showed regiospecificity for EROD induction. BKF 2,3-diol induced significant EROD activity at 10 nM, whereas a concentration of 300 nM was required for BKF 8,9-diol to give a comparable level of EROD induction. These results indicated that oxidation of BKF at the C-2/C-3 region gives rise to more potent EROD inducers than does oxidation of BKF at the C-8/C-9 region. At 3 μM, all of the BKF metabolites showed signs of CYP1 inhibition; reduced levels of induced EROD activity were observed in response to the BKF metabolites at 3 μM compared with those observed when lower concentrations of the BKF metabolites were used as enzyme inducers.
Fig. 10.
Effects of BKF and metabolites on EROD activity in T-47D cells. In (A) the effects of the indicated concentrations of BKF, BKF-2,3-diol, and BKF-8,9-diol are shown, and in (B) the effects of 3-, 8-, and 9-OHBKF on EROD activity after a 18-h induction period are shown. Values are the mean ± SE of quadruplicate determinations. Control EROD activity (without BKF or metabolite exposure) was 1.25 ± 0.15 pmol(min)−1(mg protein)−1.
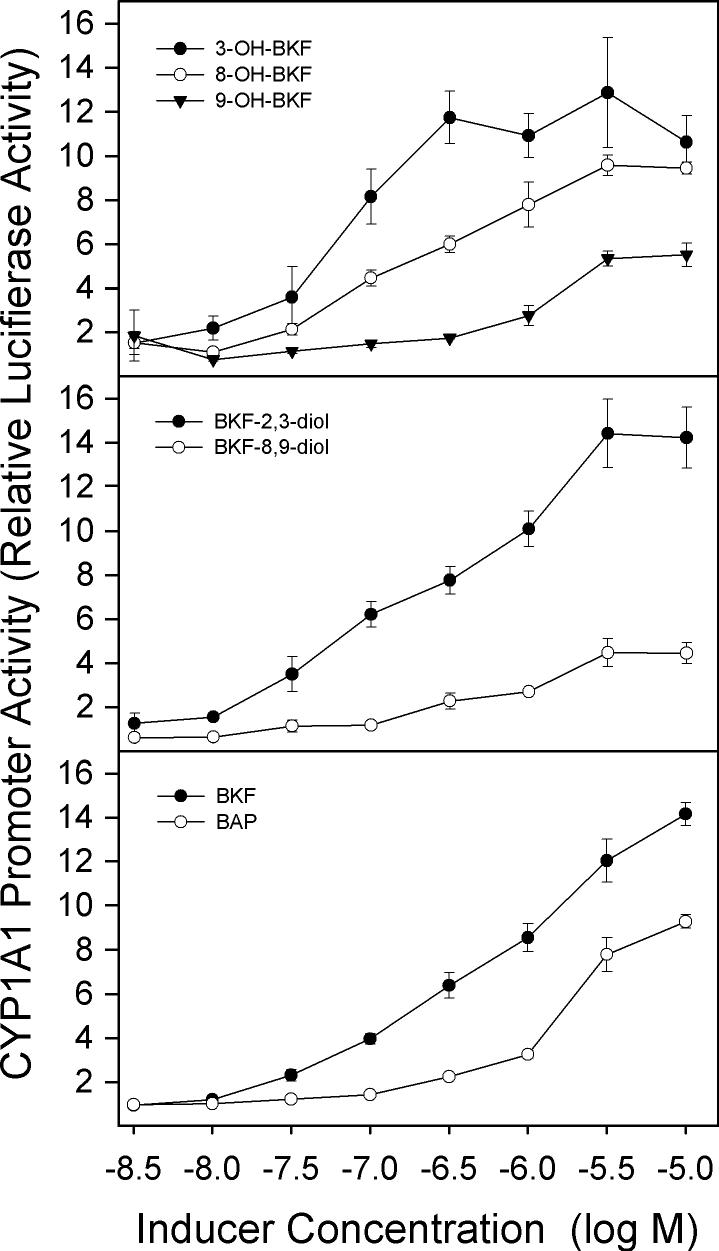
Effects of BKF metabolites on CYP1A1 promoter activity in T-47D cells
Analyses of CYP1A1 promoter activity also indicated that the 3-, 8- and 9-hydroxy metabolites of BKF are inducers of CYP1A1, as are the dihydrodiol metabolites, BKF-2,3-diol and BKF-8,9-diol (Fig. 11). The relative potencies of the BKF metabolites for induction of CYP1A1 promoter activity showed the same general trend as was observed for the induction of EROD activity by these compounds: 3-OHBKF was a more potent inducer of promoter activity than 8- and 9-OHBKF, and BKF-2,3-diol was more potent than BKF-8,9-diol. However, in contrast to what was observed for the induction of EROD activity (Fig. 10), reduced promoter activity at the high concentrations (1−3 μM) of BKF or its metabolites was not observed.
Fig. 11.
Effects of BKF and metabolites on CYP1A1 promoter activity in T-47D cells. Cultures were co-transfected with the pHu-1A1-FL and pRL-CMV Renilla luciferase vectors for 24 h, followed by 18-h exposure to the indicated concentrations of the BKF metabolites, BKF, and BAP. Cells were lysed, firefly and Renilla luciferase activities were measured and the ratios of firefly to Renilla activities were determined as relative luciferase activity. Values shown are the mean ± SE of triplicate determinations.
Discussion
Investigations of the contributions of individual PAHs in complex mixtures to the toxicity and carcinogenicity of the mixture have identified 1) the ability of a given individual PAH to be metabolically activated, or converted to mutagenic forms through metabolism, and 2) the ability of PAHs to enhance this metabolic activation of themselves and other PAHs. The modulation can occur at the level of enzyme induction, given that individual PAHs can either be potent AhR agonists or they can entirely lack AhR agonist activity (Bosveld et al., 2002; Jones and Anderson, 1999). The rates of inactivating and activating metabolism of individual PAHs can be affected by other components in mixtures with differing CYP1 inductive capacity. The inhibition of CYP enzymes by individual PAHs, some acting as alternative substrates, may also influence the metabolic activation of PAH procarcinogens (Willet et al., 1998; Cherng et al., 2001; Shimada and Guengerich, 2006).
In this study, the addition of BKF elevated intra- and extra-cellular levels of co-administered BAP and vice versa; however, clearance from the medium and cellular fractions of T-47D cultures of both PAHs, either alone or in combination, was still quite rapid. The sustained induction of CYP1A1 and CYP1B1 in T47D cells after administration of BKF that was observed in the current study appears to be due to the continued presence of an AhR ligand(s). This inference is based on the rapid decay of induced CYP1A1 and CYP1B1 mRNAs and induced CYP1A1- and CYP1B1-catalyzed E2 metabolism that was observed in a previous study after a bolus of BAP was completely metabolized (Spink et al., 2002). In contrast to the earlier study of BAP-exposed T-47D cells, we here observed that, in BKF-exposed T-47D cells, elevated E2 metabolic activity persisted for at least 72 h after BKF administration; this time point was long after the BKF was completely metabolized. This finding is consistent with results of previous studies reporting sustained induction of CYP-catalyzed E2 metabolism by BKF in MCF-7 cells (Arcaro et al., 1999), and it suggests the involvement of BKF metabolites in the sustained CYP induction. Our studies showed that 3-, 8-, and 9-OHBKF are produced from BKF in T-47D cultures, and that their formation is catalyzed by both CYP1A1 and CYP1B1.
In order for BKF metabolites to be involved in the sustained CYP induction, the metabolites must persist in culture for several days and must have AhR agonist activity. We found that nanomolar levels of OHBKF metabolites remained in T-47D cultures for at least 72 h; however, they were present predominantly in conjugated forms that could be hydrolyzed by treatment with β-glucuronidase/sulfatase or strong acid. The conjugation is likely to comprise sulfates, analogous to what has been reported for the metabolism of BAP in T-47D cells (Merrick et al., 1985), given that T-47D cells express phenol sulfotransferase SULT1A1 (Spink et al., 2000), an enzyme that catalyzes sulfate conjugation of phenolic PAH metabolites (Wang et al., 2004). Although we found that all of the hydroxy and dihydrodiol metabolites of BKF tested were able to induce EROD and CYP1A1 promoter activities, it is unlikely that the BKF sulfates are high-affinity AhR ligands, given their polarity. Since T-47D cells are also known to express sulfatase activities (Chetrite et al., 1999), it is possible that a proportion of the PAH sulfates are converted back to hydroxy-PAHs, which then mediate CYP1 induction. This cycle of conjugation and hydrolysis would likely occur with administered hydroxy-PAHs as well as those formed from PAHs in the cells. Such a metabolic loop could possibly have in vivo implications, given that the PAH sulfates could be subjected to transport, re-absorption, and hydrolysis, and could either cause AhR activation or be converted to mutagenic phenol epoxides (Hecht, 2003) in other tissues.
To the best of our knowledge, this is the first report to indicate that metabolites of BKF are CYP1 inducers. Our finding is not without precedent, given that some metabolites of BAP, notably the phenolic metabolites, 3- and 9-hydroxyBAP (Almahmeed et al., 2004; Gozgit et al., 2004), and the BAP 3,6- and 7,8-diones (Burchiel et al., 2007; Jiang et al., 2006) were previously shown to cause CYP1 induction. Our results show regioselectivity of the OHBKF metabolites for CYP1 induction, with 3-OHBKF acting as a more potent inducer than 8- and 9-OHBKF. Analogous regioselectivity for CYP induction was observed for the dihydrodiol metabolites, and this regioselectivity of BKF metabolism appears to affect both AhR agonist activity and the mutagenicity of the resulting metabolites. BKF-8,9-diol is as mutagenic as BKF (Weyand et al., 1988), although we showed that its CYP1 inductive activity is reduced compared with that of BKF. The converse is true for the BKF-2,3-diol; we found this metabolite to be a potent CYP1 inducer, but it is only weakly mutagenic (Weyand et al., 1988).
The exact nature of the AhR ligand or ligands that mediate enzyme induction when metabolites of BKF and other PAHs are applied to the cell cultures is not known, since further metabolism of the applied compounds may occur within the cells. Jiang et al. (2006) found that BAP-7,8-diol was a much weaker CYP1B1 inducer in human bronchoaveolar H358 cells than was BAP-7,8-dione, and that expression of aldo-keto reductase 1A1, the enzyme that catalyzes the conversion of BAP-7,8-diol to BAP-7,8-dione, rendered the cells much more responsive to BAP-7,8-diol for induction of CYP1B1. Whether dihydrodiol metabolites of BKF are substrates for aldo-keto reductase 1A1, and whether this metabolism leads to formation of potent AhR agonists, are currently unknown.
We observed some apparent synergism between low levels of BAP and BKF, for induction of CYP1A1-catalyzed E2 metabolism. Synergistic interactions between two PAHs have been previously observed, notably between benzo(g,h,i)perylene, a PAH lacking CYP1 inductive capacity (Jones and Anderson 1999; Bosveld et al., 2005) and benzo(a)pyrene, an AhR agonist (Cherng et al., 2001). The latter synergism was hypothesized to involve metabolic interactions, possibly enzyme inhibition, in effects on CYP1A1 induction and adduct formation. A major difference between the benzo(g,h,i)perylene-BAP mixture used by Cherng et al. (2001) and the BKF-BAP binary mixture used in our study is that BKF, like BAP, is a potent CYP1 inducer (Schmolt et al., 1981; Vakharia et al., 2001). Both BAP and BKF are also CYP1 substrates. To fully characterize synergistic effects, detailed analysis of the interaction between two effectors requires information on the relative positions of the two inducer concentrations on their respective concentration-response curves (Kortenkamp and Altenburger, 1999). The efficacies of the PAHs as AhR ligands, CYP1 substrates and inhibitors and the rates of inactivating and bioactivating metabolism of the PAHs may influence the synergistic effects of PAHs.
One of the conclusions of this study, a conclusion consistent with findings of earlier investigations (Merrick et al, 1988; Spink et al., 2002) is that metabolism of PAHs in T-47D cell cultures is rapid, with complete metabolism of micromolar levels of BKF, BAP, or a combination of the two occurring within 24 h. In other E2-responsive human breast cancer cell lines that show comparable AhR-regulated P450 expression (MCF-7, ZR-75), effects attributed to parent PAHs could thus be mediated in part by PAH metabolites. Our studies indicate that the inductive affects of PAH metabolites as potent CYP1 inducers are likely to represent additional important factors in PAH-CYP interactions that affect metabolism and bioactivation of other PAHs, ultimately modulating PAH toxicity and carcinogenicity.
Acknowledgements
This research was supported by NIH grant CA81243 (to DCS) and by the U.S. Environmental Protection Agency's Science to Achieve Results (STAR) Program through grant R827180010 (to LSK). Although the research described in the article has been funded in part by the U.S. Environmental Protection Agency's STAR Program, it has not been subjected to the Agency's required peer and policy review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred. The authors gratefully acknowledge use of the Wadsworth Center Biochemistry Core Facility.
Abbreviations
- AhR
aryl hydrocarbon receptor
- BAP
benzo[a]pyrene
- BKF
benzo[k]fluoranthene
- BAA
benz[a]anthracene
- BAA-3,4-diol
benz[a]anthracene-trans−3,4-dihydrodiol
- BKF-2,3-diol
benzo[k]fluoranthene-trans−2,3-dihydrodiol
- BKF-8,9-diol
benzo[k]fluoranthene-trans−8,9-dihydrodiol
- CYP or P450
cytochrome P450
- DBAHA-5,6-diol
dibenz[a,h]anthracene-cis−5,6-dihydrodiol
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- E2
17β-estradiol
- EROD
ethoxyresorufin O-deethylase
- GC/MS
gas chromatography/mass spectrometry
- HPLC
high-performance liquid chromatography
- 3-, 8-, and 9-OHBKF
2- and 4-MeOE2, 2- and 4-methoxyestradiol
- 3-, 8-, and 9-
hydroxybenzo[k]fluoranthene
- PAH
polycyclic aromatic hydrocarbon
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TMS
trimethylsilyl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almahmeed T, Boyle JO, Cohen EG, Carew JF, Du B, Altorki NK, Kopelovich L, Fang JL, Lazarus P, Subbaramaiah K, Dannenberg AJ. Benzo[a]pyrene phenols are more potent inducers of CYPlAl, CYP1B1 and COX-2 than benzo[a]pyrene glucuronides in cell lines derived from the human aerodigestive tract. Carcinogenesis. 2004;25:793–799. doi: 10.1093/carcin/bgh078. [DOI] [PubMed] [Google Scholar]
- Arcaro KF, Yang Y, Gierthy JF. Benzo[k]fluoranthene enhancement and suppression of 17β-estradiol catabolism in MCF-7 breast cancer cells. J. Toxicol. Environ. Health A. 1999;58:413–426. doi: 10.1080/009841099157142. [DOI] [PubMed] [Google Scholar]
- Bessette EE, Fasco MJ, Pentecost BT, Kaminsky LS. Mechanisms of arsenite-mediated decreases in benzo[k]fluoranthene-induced human cytochrome P4501A1 levels in HepG2 cells. Drug Metab. Dispos. 2005;33:312–320. doi: 10.1124/dmd.104.002212. [DOI] [PubMed] [Google Scholar]
- Bosveld ATC, de Bie PAF, van den Brink NW, Jongepier H, Klomp AV. In vitro EROD induction equivalency factors for the 10 PAHs generally monitored in risk assessment studies in The Netherlands. Chemosphere. 2002;49:75–83. doi: 10.1016/s0045-6535(02)00161-3. [DOI] [PubMed] [Google Scholar]
- Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associated genes by benzo(a)pyrene 3,6-quinone and benzo(a)pyrene 1,6-quinone in MCF-10A human mammary epithelial cells. Toxicol. Appl. Pharmacol. 2007;221:203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Devanesan PD, Higginbotham S, Ariese F, Jankowiak R, Small GJ, Rogan EG, Cavalieri EL. Expanded analysis of benzo[a]pyrene-DNA adducts formed in vitro and in mouse skin: their significance in tumor initiation. Chem. Res. Toxicol. 1996;9:897–903. doi: 10.1021/tx960004a. [DOI] [PubMed] [Google Scholar]
- Cherng SH, Lin P, Yang JL, Hsu SL, Lee H. Benzo[g,h,i]perylene synergistically transactivates benzo[a]pyrene-induced CYP1A1 gene expression by aryl hydrocarbon receptor pathway. Toxicol. Appl. Pharmacol. 2001;170:63–68. doi: 10.1006/taap.2000.9082. [DOI] [PubMed] [Google Scholar]
- Chetrite GS, Ebert C, Wright F, Philippe AC, Pasqualini JR. Control of sulfatase and sulfotransferase activities by medrogestone in the hormone-dependent MCF-7 and T-47D human breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1999;70:39–45. doi: 10.1016/s0960-0760(99)00095-3. [DOI] [PubMed] [Google Scholar]
- Denissenko MF, Pao A, Tang MS, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in p53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- Deutsch-Wenzel RP, Brune H, Grimmer G, Dettbarn G, Misfeld J. Experimental studies in rat lungs on the carcinogenicity and dose-response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons. J. Natl. Cancer Inst. 1983;71:539–544. 1983. [PubMed] [Google Scholar]
- Donato MT, Gomez-Lechon MJ, Castell JV. A microassay for measuring cytochrome P450IA1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Anal. Biochem. 1993;213:29–33. doi: 10.1006/abio.1993.1381. [DOI] [PubMed] [Google Scholar]
- Einolf HJ, Story WT, Marcus CB, Larsen MC, Jefcoate CR, Greenlee WF, Yagi H, Jerina DM, Amin S, Park SS, Gelboin HV, Baird WM. Role of cytochrome P450 enzyme induction in the metabolic activation of benzo[c]phenanthrene in human cell lines and mouse epidermis. Chem. Res. Toxicol. 1997;10:609–617. doi: 10.1021/tx960174n. [DOI] [PubMed] [Google Scholar]
- Glatt H. Sulfotransferases in the bioactivation of xenobiotics. Chem.-Biol. Interact. 2000;129:141–170. doi: 10.1016/s0009-2797(00)00202-7. [DOI] [PubMed] [Google Scholar]
- Gozgit JM, Nestor KM, Fasco MJ, Pentecost BT, Arcaro KF. Differential action of polycyclic aromatic hydrocarbons on endogenous estrogen-responsive genes and on a transfected estrogen-responsive reporter in MCF-7 cells. Toxicol. Appl. Pharmacol. 2004;196:58–67. doi: 10.1016/j.taap.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. Erratum: Hecht, S.S., 2004. Nat. Rev. Cancer 4, 84. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vudathala DK, Blair IA, Penning TM. Competing roles of aldo-keto reductase 1A1 and cytochrome P4501B1 in benzo[a]pyrene-7,8-diol activation in human bronchoalveolar H358 cells: role of AKRs in P4501B1 induction. Chem. Res. Toxicol. 2006;19:68–78. doi: 10.1021/tx0502488. [DOI] [PubMed] [Google Scholar]
- Jones JM, Anderson JW. Relative potencies of PAHs and PCBs based on the response of human cells. Environ. Toxicol. Pharmacol. 1999;7:19–26. doi: 10.1016/s1382-6689(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stansbury KH, Walker NJ, Trush MA, Strickland PT, Sutter TR. Metabolism of benzo[a]pyrene and benzo[a]pyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis. 1998;19:1847–1853. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Altenburger R. Approaches to assessing combination effects of oestrogenic environmental pollutants. Sci. Total Environ. 1999;233:131–140. doi: 10.1016/s0048-9697(99)00228-4. [DOI] [PubMed] [Google Scholar]
- Liu N, Zhang QY, Vakharia D, Dunbar D, Kaminsky LS. Induction of CYP1A by benzo[k]fluoranthene in human hepatocytes: CYP1A1 or CYP1A2? Arch. Biochem. Biophys. 2001;389:130–134. doi: 10.1006/abbi.2001.2323. [DOI] [PubMed] [Google Scholar]
- Mahadevan B, Parsons H, Musafia T, Sharma AK, Amin S, Pereira C, Baird WM. Effect of artificial mixtures of environmental polycyclic aromatic hydrocarbons present in coal tar, urban dust, and diesel exhaust particulates on MCF-7 cells in culture. Environ. Mol. Mutagen. 2004;44:99–107. doi: 10.1002/em.20039. [DOI] [PubMed] [Google Scholar]
- Merrick BA, Mansfield BK, Nikbakht PA, Selkirk JK. Benzo[a]pyrene metabolism in human T 47D mammary tumor cells: evidence for sulfate conjugation and translocation of reactive metabolites across cell membranes. Cancer Lett. 1985;29:139–150. doi: 10.1016/0304-3835(85)90152-1. [DOI] [PubMed] [Google Scholar]
- Page D, Boehm PD, Douglas GS, Bence AE, Burns WA, Mankiewicz PJ. Pyrogenic polycyclic aromatic hydrocarbons in sediments record past human activity: a case study in Prince William Sound, Alaska. Mar. Pollut. Bul. 1999;38:247–260. [Google Scholar]
- Park JH, Gopishetty S, Szewczuk LM, Troxel AB, Harvey RG, Penning TM. Formation of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxo-dGuo) by PAH o-quinones: involvement of reactive oxygen species and copper(II)/copper(I) redox cycling. Chem. Res. Toxicol. 2005;18:1026–1037. doi: 10.1021/tx050001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Troxel AB, Harvey RG, Penning TM. Polycyclic aromatic hydrocarbon (PAH) o-quinones produced by the aldo-keto-reductases (AKRs) generate abasic sites, oxidized pyrimidines, and 8-oxo-dGuo via reactive oxygen species. Chem. Res. Toxicol. 2006;19:719–728. doi: 10.1021/tx0600245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM. Aldo-keto reductases and formation of polycyclic aromatic hydrocarbon o-quinones. Methods Enzymol. 2004;378:31–67. doi: 10.1016/S0076-6879(04)78003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoldt A, Jacob J, Grimmer G. Dose-dependent induction of rat liver microsomal aryl hydrocarbon monooxygenase by benzo[k]fluoranthene. Cancer Lett. 1981;13:249–257. doi: 10.1016/0304-3835(81)90025-2. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Shimada T, Gillamm EMJ, Oda Y, Tsumura F, Sutter TR, Guengerich FP, Inoue K. Metabolism of benzo[a]pyrene to trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by recombinant human cytochrome P4501B1 and purified liver epoxide hydrolase. Chem. Res. Toxicol. 1999;12:623–629. doi: 10.1021/tx990028s. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Guengerich FP. Inhibition of human cytochrome P450 1A1-, 1A2-, and 1B1-mediated activation of procarcinogens to genotoxic metabolites by polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2006;19:288–294. doi: 10.1021/tx050291v. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U.S.A. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou M, Korzekwa KR, Crespi CL, Gonzalez FJ, Gelboin HV. The role of 12 cDNA-expressed human, rodent, and rabbit cytochromes P450 in the metabolism of benzo[a]pyrene and benzo[a]pyrene trans-7,8-dihydrodiol. Mol. Carcinogen. 1994;10:159–168. doi: 10.1002/mc.2940100307. [DOI] [PubMed] [Google Scholar]
- Spink BC, Katz BH, Hussain MM, Pang S, Connor SP, Aldous KM, Gierthy JF, Spink DC. SULT1A1 catalyzes 2-methoxyestradiol sulfonation in MCF-7 breast cancer cells. Carcinogenesis. 2000;21:1947–1957. doi: 10.1093/carcin/21.11.1947. [DOI] [PubMed] [Google Scholar]
- Spink BC, Hussain MM, Katz BH, Eisele L, Spink DC. Transient induction of cytochromes P450 1A1 and 1B1 in MCF-7 human breast cancer cells by indirubin. Biochem. Pharmacol. 2003;66:2313–2321. doi: 10.1016/j.bcp.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Spink DC, Lincoln DW, 2nd, Dickerman HW, Gierthy JF. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17β-estradiol metabolism in MCF-7 breast tumor cells. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6917–6921. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink DC, Hayes CL, Young NR, Christou M, Sutter TR, Jefcoate CR, Gierthy JF. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17β-estradiol 4-hydroxylase. J. Steroid Biochem. Mol. Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Cao JQ, DePasquale JA, Pentecost BT, Fasco MJ, Li Y, Sutter TR. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- Spink DC, Katz BH, Hussain MM, Spink BC, Wu SJ, Liu N, Pause R, Kaminsky LS. Induction of CYP1A1 and CYP1B1 in T-47D human breast cancer cells by benzo[a]pyrene is diminished by arsenite. Drug Metab. Dispos. 2002;30:262–269. doi: 10.1124/dmd.30.3.262. [DOI] [PubMed] [Google Scholar]
- Spivack SD, Fasco MJ, Walker VE, Kaminsky LS. The molecular epidemiology of lung cancer. Crit. Rev. Toxicol. 1997;27:319–365. doi: 10.3109/10408449709089898. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Miller JA. Roles of electrophilic sulfuric acid ester metabolites in mutagenesis and carcinogenesis by some polynuclear aromatic hydrocarbons. Chem. Biol. Interact. 1994;92:351–362. doi: 10.1016/0009-2797(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Tang PW, Crone DL. A new method for hydrolyzing sulfate and glucuronyl conjugates of steroids. Anal. Biochem. 1989;182:289–294. doi: 10.1016/0003-2697(89)90596-4. [DOI] [PubMed] [Google Scholar]
- Thorsen WA, Cope WG, Shea D. Bioavailability of PAHs: effects of soot carbon and PAH source. Environ. Sci. Technol. 2004;38:2029–2037. doi: 10.1021/es0306056. [DOI] [PubMed] [Google Scholar]
- Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang QY, Kaminsky LS. Polycyclic aromatic hydrocarbon/metal mixtures: Effect on PAH induction of CYP1A1 in human HepG2 cells. Drug Metab. Dispos. 2001a;29:999–1006. [PubMed] [Google Scholar]
- Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang QY, Kaminsky LS. Effect of metals on polycyclic aromatic hydrocarbon induction of CYP1A1 and CYP1A2 in human hepatocyte cultures. Toxicol. Appl. Pharmacol. 2001b;170:93–103. doi: 10.1006/taap.2000.9087. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3'-phosphoadenosine 5'-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab. Dispos. 2004;32:1162–11699. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- Weyand EH, Geddie N, Rice JE, Czech A, Amin S, LaVoie EJ. Metabolism and mutagenic activity of benzo[k]fluoranthene and 3-, 8- and 9-fluorobenzo[k]fluoranthene. Carcinogenesis. 1988;9:1277–1281. doi: 10.1093/carcin/9.7.1277. [DOI] [PubMed] [Google Scholar]
- Willett KL, Gardinali PR, Sericano JL, Wade TL, Safe SH. Characterization of the H4IIE rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs). Arch. Environ. Contam. Toxicol. 1997;32:442–448. doi: 10.1007/s002449900211. [DOI] [PubMed] [Google Scholar]
- Willett KL, Randerath K, Zhou GD, Safe SH. Inhibition of CYP1A1-dependent activity by the polynuclear aromatic hydrocarbon (PAH) fluoranthene. Biochem. Pharmacol. 1998;55:831–839. doi: 10.1016/s0006-2952(97)00561-3. [DOI] [PubMed] [Google Scholar]
- Wood AW, Levin W, Lu AY, Yagi H, Hernandez O, Jerina DM, Conney AH. Metabolism of benzo(a)pyrene and benzo(a)pyrene derivatives to mutagenic products by highly purified hepatic microsomal enzymes. J. Biol. Chem. 1976;251:4882–4890. [PubMed] [Google Scholar]
- Wu SJ, Spink DC, Spink BC, Kaminsky LS. Quantitation of CYP1A1 and CYP1B1 mRNA in polycyclic aromatic hydrocarbon-treated human T-47D and HepG2 cells by a modified bDNA assay using fluorescence detection. Anal. Biochem. 2003;312:162–166. doi: 10.1016/s0003-2697(02)00444-x. [DOI] [PubMed] [Google Scholar]
- Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol. Appl. Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]