Abstract
S-(2-succinyl)cysteine (2SC) is a chemical modification of proteins formed by a Michael addition reaction between the Krebs cycle intermediate, fumarate, and thiol groups in protein—a process known as succination of protein. Succination causes irreversible inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in vitro. GAPDH was immunoprecipitated from muscle of diabetic rats, then analyzed by ultra-performance liquid chromatography–electrospray ionization–mass spectroscopy. Succination of GAPDH was increased in muscle of diabetic rats, and the extent of succination correlated strongly with the decrease in specific activity of the enzyme. We propose that 2SC is a biomarker of mitochondrial and oxidative stress in diabetes and that succination of GAPDH and other thiol proteins may provide the chemical link between glucotoxicity and the pathogenesis of diabetic complications.
Keywords: protein, chemical modification, cysteine, diabetes, fumarate, glyceraldehyde-3-phosphate dehydrogenase, oxidative stress, mitochondrial stress, succination
Introduction
In a Unifying Hypothesis on the origin of diabetic complications, Brownlee and colleagues proposed that inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a critical, early step in the metabolic derangements that lead to the development of diabetic complications.1,2 Inactivation of this enzyme causes an increase in intracellular concentrations of glucose and glycolytic intermediates, leading to enhanced activity of the polyol pathway, the hexosamine pathway, and the protein kinase C pathways and accelerated formation of advanced glycation end products (AGE). The original proposal, that GAPDH was inactivated by reactive oxygen species, such as super-oxide,1 was followed by a later study indicating that GAPDH was inactivated by poly-ADP-ribosylation.3 However, the Krebs cycle intermediate, fumarate, reacts with cysteine residues in proteins, producing S-(2-succinyl)cysteine (2SC)—a process known as succination of protein (Fig. 1)4; succination of GAPDH in vitro causes inactivation of this enzyme. When GAPDH was immunoprecipitated from skeletal muscle of control and streptozotocin-induced (type 1) diabetic rats and tryptic peptides analyzed by matrix-assisted laser desorption/ionization (MALDI)–time-of-flight (TOF) mass spectroscopy (MS), we observed that succination of GAPDH was significantly increased in muscle of diabetic rats.5 While MALDI–TOF is not considered a quantitative technique, we concluded that the extent of succination was consistent with the decrease in specific activity of GAPDH in muscle of diabetic rats. In the present study, we provide additional evidence that succination contributes to inactivation of GAPDH in muscle of diabetic rats.
FIGURE 1.
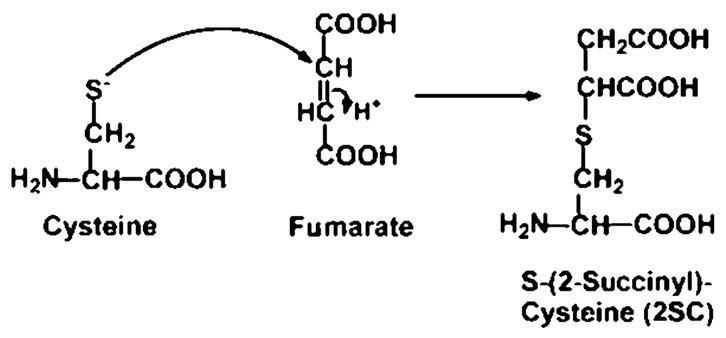
Mechanism of formation of S-(2-succinyl)cysteine (2SC). Nucleophilic addition of cysteine to fumarate yields 2SC by a Michael addition reaction.
Results
To obtain additional information regarding the extent of modification of GAPDH, we isolated the enzyme by immunoprecipitation from muscle of control and diabetic rats, then applied ultra-performance liquid chromatography (UPLC)–electrospray ionization (ESI+)–MS for analysis of the native (vinylpyridine [VP]-modified) and 2SC peptides. GAPDH has four cysteine residues, of which only Cys-149 and Cys-244, in peptides #17 and #26, respectively, are subject to succination; Cys-149 is the active site cysteine, and Cys-244 is a peripheral, nucleophilic, cysteine residue. Chemical modification of either of these thiol groups is known to cause inactivation of the enzyme.5
As shown in Figure 2, only traces of 2SC peptides are detected in muscle from control rats (Fig. 2A & C), while the 2SC peptides are readily detected in GAPDH from diabetic rats (Fig. 2B & D). As reported previously,5 the ratio of 2SC:VP peptide was significantly increased in muscle of diabetic compared to control rats (P < 0.01 for both peptides), and there was a strong correlation between the ratio of 2SC:VP peptides and the decrease in specific activity of GAPDH in muscle of diabetic rats (P < 0.01 for peptide #17, and P < 0.05 for peptide #26). As a more quantitative approach to estimating the extent of succination of peptides, we used the relative area (RA) technique of Brock et al.,6 in which the sum of the area units for the several charge states of the native (VP) peptide is compared to the sum of the area units of a reference peptide in native enzyme. A decrease in the RA of the VP peptide versus reference peptide is indicative of modification of the peptide. Peptide #2 (VGVNGFGR) was chosen as the reference peptide because it lacks a cysteine residue and yields a strong, well-resolved signal on ESI+–liquid chromatography (LC)/MS. While the RA method does not establish that all of the loss of native peptides is caused by succination, the mean RA value decreased significantly in diabetic compared to control rats (P < 0.01 for peptide #17; P < 0.05 for peptide #26). In addition, there was a strong correlation between the RA of the native peptides and the specific activity of GAPDH (Fig. 3)—as the RA decreased, there was a corresponding decrease in the specific activity of GAPDH.
FIGURE 2.
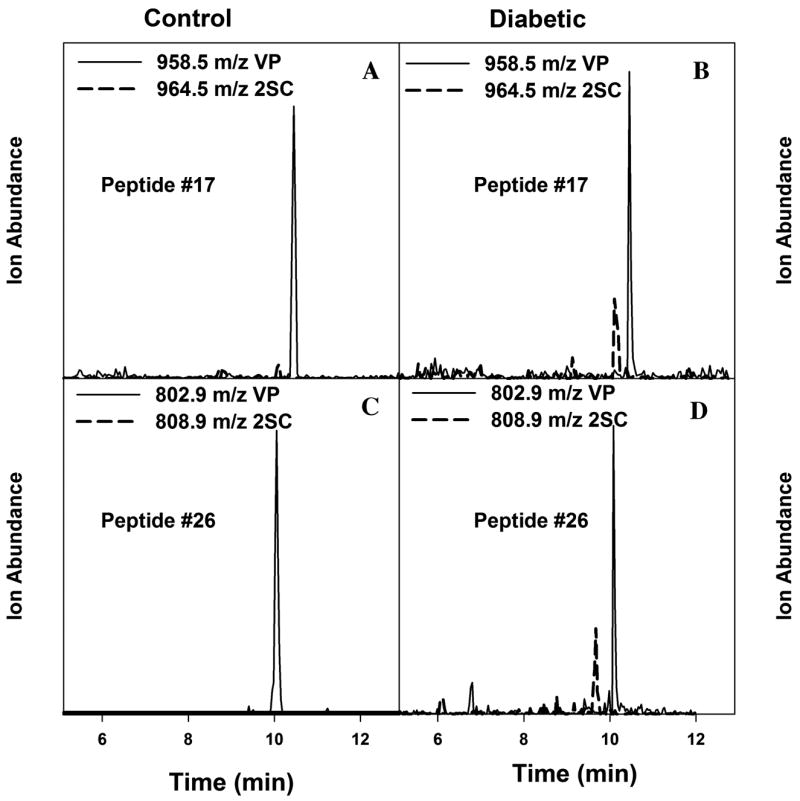
Increased 2SC modification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) peptides isolated from gastrocnemius muscle of diabetic versus control rats. GAPDH immunoprecipitates were reduced with vinylpyridine (VP), fractionated by 1D-SDS-PAGE, stained with Coomassie blue, digested in-gel with trypsin, then analyzed by ultra-performance liquid chromatography–electrospray ionization–mass spectroscopy; details of methods are presented elsewhere.5 (A) Peptide #17 from control rat; (B) peptide #17 from diabetic rat; (C) peptide #26 from control rat; (D) peptide #26 from diabetic rat. Solid lines, VP peptide; dotted lines, 2SC peptide.
FIGURE 3.
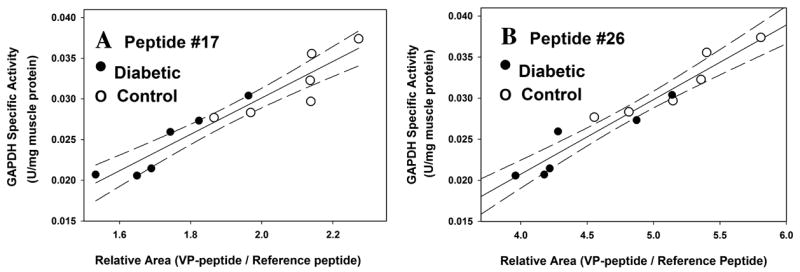
Correlation between extent of modification of peptides and specific activity of GAPDH in control and diabetic rat gastrocnemius muscle. (A) Correlation between relative area (RA) of 2SC peptide #17 and specific activity of GAPDH (P <0.0001; r2 = 0.90). (B) Correlation between RA of 2SC peptide #26 and specific activity of GAPDH (P <0.0001; r2 = 0.92).
Discussion
The studies described here with UPLC–ESI+–MS complement the results of MALDI–TOF analysis of tryptic peptides from GAPDH isolated from muscle of control and diabetic rats.5 Based on correlations between the extent of modification and the specific activity of the enzyme (Fig. 3), we conclude that succination is a major cause of the decrease in specific activity of GAPDH in diabetic muscle. In other work, we have shown that succination of proteins is increased in adipocytes grown in cell culture media at high (30 mmol/L) glucose concentration7 and in adipose tissue of db/db diabetic mice, a model of type 2 diabetes (unpublished observations). Overall, it appears that succination of proteins is increased in the presence of higher-than-normal glucose concentration, both in vitro and in vivo, and that succination may be an indication of mitochondrial stress resulting from glucotoxicity in both type 1 and type 2 diabetes.
Mechanistically, we propose that succination is an early step in the response to excess glucose concentration in cell culture or in vivo in diabetes. The abundant fuel supply and accumulation of ATP would lead to an increase in mitochondrial nicotinamide adenine dinucleotide (NADH) and then to hyperpolarization of the inner mitochondrial membrane, as seen in adipocytes.8 The increase in NADH would then lead to accumulation of Krebs cycle intermediates, including fumarate, causing an increase in succination of proteins. The accumulation of NADH may also trigger the production of reactive oxygen species.9 Eventually, damage to the integrity of the mitochondrial membranes would open the mitochondrial permeability transition pore, precipitating a series of events culminating in apoptosis.10 Studies on succination of protein may permit early detection of mitochondrial stress, perhaps even in obesity and metabolic syndrome, and trigger effective clinical intervention at the earliest stages in development of diabetes and its complications.
Acknowledgments
This work was supported by the United States Public Health Service Research Grant DK-19971 from the National Institutes of Diabetes, and Kidney and Digestive Diseases.
Footnotes
Conflict of Interest:
The authors declare no conflicts of interest.
References
- 1.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T, Edelstein D, DU XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 3.Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderson NL, Wang Y, Blatnik M, et al. S-(2-succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Blatnik M, Frizzell N, Thorpe SR, Baynes JW. Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine: a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes. 2008;57:41–49. doi: 10.2337/db07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock JWC, Hinton DJS, Cotham WE, et al. Proteomic analysis of the site specificity of glycation and carboxymethylation of ribonuclease. J Proteome Res. 2003;2:506–513. doi: 10.1021/pr0340173. [DOI] [PubMed] [Google Scholar]
- 7.Nagai R, Brock JWC, Blatnik M, et al. Succination of proteins in adipocytes: S-(2-succinyl)cysteine is a biomarker of oxidative stress during maturation of adipocytes. J Biol Chem. 2007;282:34219–34228. doi: 10.1074/jbc.M703551200. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Berg AH, Iyengar P, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 9.Kohanski MA, Dwyer DJ, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Belizario JE, Alves J, Occhiucci JM, et al. A mechanistic view of mitochondrial death decision pores. Braz J Med Biol Res. 2007;40:1011–1024. doi: 10.1590/s0100-879x2006005000109. [DOI] [PubMed] [Google Scholar]


