Abstract
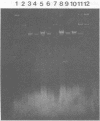
Escherichia coli (2492/pJB4JI) matings with Erwinia chrysanthemi produced kanamycin resistant (Kmr) transconjugants, a majority of which were gentamicin sensitive (Gms). A small proportion (about 0.8%) of the Kmr Gms clones were either auxotrophic or failed to catabolize galacturonate (Gtu−). The R plasmid (pJB4JI) DNA was detected in the parent E. coli strain and in a Kmr Gmr transconjugant, but not in Kmr GmsE. chrysanthemi strains carrying Tn5-induced mutations. In Hfr crosses, Kmr (Tn5) was found linked with most mutations. A majority (>95%) of prototrophic recombinants were Kms, except for Leu+ and Arg+ recombinants which were 30 to 50% Kms. Spontaneous revertants were obtained for all markers except car, gtu, lys, thr, and trp. Prototrophic revertants, with the exception of Met+, Leu+, or His+ clones, were Kms. We conclude from both genetic and physical data that Tn5 transposed from pJB4JI into different sites on the chromosome of E. chrysanthemi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K. Acceptance by Erwinia spp. of R plasmid R68.45 and its ability to mobilize the chromosome of Erwinia chrysanthemi. J Bacteriol. 1980 Apr;142(1):111–119. doi: 10.1128/jb.142.1.111-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Chrisope G. L., Fox C. W., Marshall R. T. Lecithin agar for detection of microbial phospholipases. Appl Environ Microbiol. 1976 May;31(5):784–786. doi: 10.1128/aem.31.5.784-786.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J Bacteriol. 1982 Jan;149(1):40–46. doi: 10.1128/jb.149.1.40-46.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilevich V. N., Stepanshin Y. G., Volozhanstev N. V., Golub E. I. Transposon-mediated insertion of R factor into bacterial chromosome. Mol Gen Genet. 1978 May 31;161(3):337–339. doi: 10.1007/BF00331010. [DOI] [PubMed] [Google Scholar]
- Ely B., Croft R. H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982 Feb;149(2):620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernalsteens J. P., De Greve H., Van Montagu M., Schell J. Mutagenesis by insertion of the drug resistance transposon Tn7 applied to the Ti plasmid of Agrobacterium tumefaciens. Plasmid. 1978 Feb;1(2):218–225. doi: 10.1016/0147-619x(78)90040-9. [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Transposon-facilitated recombination in Vibrio cholerae. Mol Gen Genet. 1979 Feb 16;170(1):93–101. doi: 10.1007/BF00268584. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., van Breukelen J., Korevaar K., Ooms G., Schilperoort R. A. Transposition of Tn904 encoding streptomycin resistance into the octopine Ti plasmid of Agrobacterium tumefaciens. J Bacteriol. 1980 Jan;141(1):129–136. doi: 10.1128/jb.141.1.129-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Steele D. A., Reichardt K., Botstein D. Specificity of insertion by the translocatable tetracycline-resistance element Tn10. Genetics. 1979 Aug;92(4):1023–1040. doi: 10.1093/genetics/92.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon TN10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1982 Apr;150(1):122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapillai V. DNA insertion mutagenesis in a Pseudomonas aeruginosa R plasmid. Plasmid. 1979 Apr;2(2):237–246. doi: 10.1016/0147-619x(79)90042-8. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Royle P., Lehrer J. Insertions of the transposon Tn1 into the Pseudomonas aeruginosa chromosome. Genetics. 1981 Mar-Apr;97(3-4):495–511. doi: 10.1093/genetics/97.3-4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M., Filser M., Dixon R., Elmerich C., Sibold L., Houmard J. The use of translocatable genetic elements to construct a fine-structure map of the Klebsiella pneumoniae nitrogen fixation (nif) gene cluster. J Gen Microbiol. 1980 Apr;117(2):509–520. doi: 10.1099/00221287-117-2-509. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Klapwijk P. M., Poulis J. A., Schilperoort R. A. Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol. 1980 Oct;144(1):82–91. doi: 10.1128/jb.144.1.82-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Staskawicz B. J., Panopoulos N. J., Peters S., Honma M. A host-dependent hybrid plasmid suitable as a suicidal carrier for transposable elements. Plasmid. 1981 Nov;6(3):325–331. doi: 10.1016/0147-619x(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M. Escherichia coli K-12 auxotrophs induced by insertion of the transposable element Tn5. Genetics. 1979 Jul;92(3):741–747. doi: 10.1093/genetics/92.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Hendson M., Magnes R. M. Mutagenesis by insertion of drug resistance transposon Tn7 into a vibrio species. J Bacteriol. 1981 Oct;148(1):374–378. doi: 10.1128/jb.148.1.374-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol. 1980 Mar;141(3):1134–1141. doi: 10.1128/jb.141.3.1134-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]