Abstract
Based on the protective effects of cooked dry bean consumption in a human intervention study, we evaluated which fraction of cooked dry beans is responsible for its cancer-preventive effects. Cooked navy beans (whole beans), the insoluble fraction (bean residue) or soluble fraction of the 60% (vol:vol) ethanol extract of cooked navy beans (bean extract), or a modified AIN-93G diet (16.6% fat including 12.9% lard) as control diet were fed to 160 male obese ob/ob mice after 2 azoxymethane injections. In comparison to control-fed mice, dysplasia, adenomas, or adenocarcinomas were detected in fewer mice on either bean fraction diet (percent reduction from control: whole beans 54%, P = 0.10; bean residue 81%, P = 0.003 ; bean extract 91%, P = 0.007) , and any type of colon lesions, including focal hyperplasia, were found in fewer mice on each of the 3 bean diets percent reduction from control: whole bean 56%, P = 0.04; bean residue 67%, P = 0.01; bean extract 87%, P = 0.0003. These results suggest that both the soluble and the insoluble fraction of the extract contribute to the cancer-protective effect of cooked navy beans.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed type of cancer and the fourth most common cause of cancer-related death worldwide (1). In the United States, CRC is the fourth leading cancer in incidence rates and the second leading cause of cancer-related mortality with a 5-yr survival rate of 64% (2). Despite the effectiveness of screening (3,4), there is limited impact for CRC prevention because of low screening rates (5). Nutrition remains critical for CRC prevention. It is estimated that nutrition could prevent 70−80% of all CRC cases (6). This is important, as the annual CRC treatment costs in the United States are estimated to be $6.5 billion (7). Two of the main risk factors for CRC, which are both diet related, are obesity and inflammation (8). Thus, ob/ob mice might provide a suitable animal model to study the link between diet and CRC because they have a mutation in the leptin gene, which results in hyperphagia, obesity, hyperinsulinemia, hyperglycemia, and increased inflammatory response to liposaccharides (9).
Dry beans (Phaseolus vulgaris L.), which belong to the Leguminosae family, are a dietary staple in many Latin American, Eastern, and South African countries that potentially could prevent CRC (10,11). Ecological analysis indicates a decreased risk of death associated with colon cancer in countries with higher consumption of beans (12). In studies that have examined the association between colon cancer and individual intakes of legumes, the results indicate a protective effect in populations with higher legume consumption (13–18). In the only study that examined the effect of dry bean consumption separately, male participants, who consumed at least 31 g of cooked dry beans daily, had reduced risk of advanced adenomatous polyp recurrence in a 4-yr nutrition intervention study (Polyp Prevention Trial (19); unpublished data). In animal models, dry beans commonly consumed in the United States, such as pinto, black, and navy beans, reduced azoxymethane(AOM)-induced colon adenocarcinomas in F344 rats (20,21); however, the cancer-preventive effect of dry bean fractions are not known. AOM is a carcinogen that is commonly used for animal models of human colon cancer and induces DNA mutations by alkylating DNA primarily at the O6-guanidine residues (22,23).
There are several bioactive food components of cooked dry beans that could be responsible for the cancer-preventive effect (11). These components include saponins (24–26); monosaccharides, disaccharides, and oligosaccharides (27); and the phenolic components ferulic acid (28,29), p-coumaric acid (30), kaempferol (31), phytate (32,33), and at smaller concentrations, catechins (34), anthocyanidins (35), and isoflavonoids (36). All of these components are primarily in aqueous-alcohol extracts of dry beans (37–40). Resistant starches and nonstarch polysaccharides are cancer-preventive components (41–43), which are primarily insoluble residues from aqueous-alcohol extracts of dry beans. Cancer-preventive components in dry beans could act either alone or in combination. Furthermore, their cancer-preventive effect could be either direct via their chemoprotective functions or indirect through their metabolites and changes in microflora and pH (11). The objective of this study was to examine the influence of cooked navy beans (white pea beans), the second most commonly consumed market class of dietary dry beans in the United States (44), and their fractions (aqueous-ethanol soluble fraction: bean extract; aqueous-ethanol insoluble fraction: bean residue) on colon carcinogenesis in male AOM-induced ob/ob mice in comparison to a modified AIN-93G control diet. Our hypothesis was that dietary navy beans as a whole and 1 of the fractions of the whole beans (e.g., bean residue, bean extract) reduce AOM-induced colon carcinogen-esis in ob/ob mice.
MATERIALS AND METHODS
Animals and Housing
This research was conducted with approval from the Michigan State University All-University Committee on Animal Use and Care. Male ob/ob mice (n = 160; B6.V-Lepob/J, stock number 000632) were received at 4 to 5 wk of age from Jackson Laboratory (Bar Harbor, ME) and were housed 4 mice per cage in animal research facilities overseen by the Michigan State University Laboratory Animal Resources Unit in the Food Science and Human Nutrition Department. Mice were housed in plastic cages in temperature (24 ± 2°C) and humidity (40−60%) controlled rooms with a 12 h light–dark cycle. All mice had continual access to food and water and were observed daily for health status.
Preparation of Diets
To prepare navy beans for the diets, 682 kg of raw navy beans, grown in Michigan, were soaked overnight in distilled water at 4°C and then cooked at 98°C for 1 h in open top steam kettles (Models: N 30 SP, D 10 SP, and D 5 SP; Groen M.F.G. Co., Chicago, IL). For preparation of whole beans, approximately 250 kg of the 682 kg were dried in a large convection oven overnight at 75 ± 5°C (Machine number K 12395; Proctor and Schwartz, Inc., Philadelphia, PA). After drying, the beans were ground to pass through a screen with 1.6 mm holes using a Fitzmill (Model D Comminuting Machine, The W.J. Fitzpatrick Co., Chicago, IL).
The remaining 432 kg (dry weight = 379.7 kg) of the 682 kg were used for preparation of the bean fractions. The cooked navy beans were ground without drying and then mixed with 95% (vol:vol) ethanol so that the final concentration was close to 60% (vol:vol) ethanol. The mixture of beans and ethanol was continuously mixed and extracted overnight. The following day, the mixture was allowed to settle, and the soluble fraction (liquid bean extract) was removed. The semisolid residue that settled out of the aqueous-ethanol mix was placed in burlap bags and pressed at 20 kg/cm2 to collect as much liquid bean extract as possible (Bar N.A., Inc.; Seymour, IL). The solid 60% ethanol insoluble fraction was then dried in a convection oven overnight at 75 ± 5°C (Machine number K 12395). After drying, the bean residue (final weight = 332.2 kg) was ground to pass through a screen with 1.6 mm holes using a Fitzmill (Model D Comminuting Machine).
The liquid 60% ethanol soluble navy bean fraction (approximately 3,123 L) was concentrated for 9.5 h in a Marriot Walker Falling Film Evaporator (Marriot Walker, Birmingham, MI) to approximately 132 L (wet weight: 116.4 kg, 27% solids; dry weight: 31.4 kg). The concentrated bean extract was mixed with cellulose, casein, and cornstarch in the proportions of 33.7:5.1:6.3:13.0 (wt:wt:wt:wt), respectively. The mixture was dried overnight in a convection oven at 75 ± 5°C (Machine number K 12395) and then ground to pass through a screen with 1.6 mm holes using a Fitzmill (Model D Comminuting Machine).
The nutrient composition of the cooked whole beans and the bean residue fraction were similar in protein and fiber content, whereas the bean extract was similar in oligosaccharides and phenolic components to the whole beans. The total phenol content per kg of diet for the whole beans, the bean residue fraction, and the bean extract fraction, as measured and calculated using the Folin-Ciocalteu assay (45) (Folin-Ciocalteu phenol reagent; Sigma, St. Louis, MO), was 8.36, 3.55, and 11.56 mg of (+)-catechin equivalents per kg of diet, respectively. The crude protein content of the whole beans, the bean residue, and the bean extract was 22.7%, 22.4%, and 4.9%, respectively.
The control diet was a modified AIN-93G diet (Table 1). To better reflect the caloric contribution of dietary components in Western diets, the fat content was increased to 16.6% in comparison to the standard AIN-93G diet (47) by adding lard (12.9%; wt/wt) and corn oil (1.1%; wt/wt) and decreasing soybean oil from 7% (wt/wt) to 2.6% (wt/wt). The diets were formulated to be isocaloric and isonitrogenous. Tryptophan, the second limiting amino acid in bean protein, was added to the whole bean and the bean residue diet to equal that in casein to prevent slower growth rates of mice on the whole bean and the bean residue diet as we had previously reported in rats fed beans as the sole protein source (20). To compare the efficacy of the 3 bean diets, we calculated whole-bean equivalents, which is the amount of whole beans consumed on the bean fraction diets relative to the amount consumed on the whole bean diet. Given that there were no differences in food disappearance and therefore assuming equal food intake in the 3 bean groups, mice on the bean residue diet consumed 14% [(379.7 kg whole beans/332.2 kg bean residue)/(740 g whole beans/kg diet/740 g bean residue/kg diet)] more whole bean equivalents. Mice on the bean extract diet consumed 47% [(379.7 kg whole bean/31.4 kg bean extract)/(740 g whole beans/kg diet/90 g bean extract/kg diet)] more whole bean equivalents. A Hobart Mixer (Model H600; Hobart, Troy, OH) was used to mix the diets.
TABLE 1.
Composition of Diets
| Composition | Control | Whole Beans | Bean Residue | Bean Extract |
|---|---|---|---|---|
| Ingredients (g/kg diet) | ||||
| Whole beansa | — | 740 | — | — |
| Bean extract residuea | — | — | 740 | — |
| Bean extractb | — | — | — | 90 |
| Casein | 180 | — | — | 180 |
| Methionine | 3 | 3 | 3 | 3 |
| Tryptophan | — | 0.05 | 0.05 | — |
| Corn oil | 11 | — | — | 11 |
| Lard | 129 | 129 | 129 | 129 |
| Soybean oil | 26 | 26 | 26 | 26 |
| Cornstarch | 444 | 40 | 40 | 354 |
| Fiber (cellulose) | 144 | — | — | 144 |
| Sucrose | 10 | 10 | 10 | 10 |
| Calcium carbonate | 3 | 3 | 3 | 3 |
| Mineral mixc | 36 | 36 | 36 | 36 |
| Vitamin mix3 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 3 | 3 | 3 | 3 |
| Tertiary butyl hydroquinone | 0.014 | 0.014 | 0.014 | 0.014 |
| Nutrients | ||||
| Energy (J/kg) | 17.2 | 17.5 | 17.5 | 17.2 |
| Fat | 166 | 166 | 166 | 166 |
| Fiber | 144 | 144 | 144 | 144 |
| Protein | 171 | 171 | 171 | 171 |
Composition of 740 g whole navy beans and navy bean residue was 144 g fiber, 168 g protein, and estimated to be 243 g starch, 11 g fat, and 29 g total sugar (46). The bean residue diet was formulated to be equal to the whole bean diet in everything except for having lower concentrations of phenolics and oligosaccharides.
The bean extract diet was formulated to be equal to the whole bean diet except for having higher concentrations of phenolics and oligosaccharides.
Composition of AIN 93-MX mineral mix and AIN 93-VX vitamin as described in Reeves et al. (47).
Administration of Diets and Carcinogens
Once received, mice were provided access to the control diet, which was a powdered, modified, AIN-93G diet. After 3 wk of acclimatization, mice were injected subcutaneously 2 times 1 wk apart: each dose was 7 mg AOM/kg of body weight (Ash-Stevens, Detroit, MI; AOM dissolved in 0.15 M NaCl). Then 1 wk following the last injection, the 160 mice were randomly assigned by body weight to 4 experimental diets: (a) control, (b) whole bean, (c) bean residue, and (d) bean extract (Table 1). Body weights of mice were measured on Day 1, 6, 16, 28, 43, 74, 111, 139, and 167 after introduction of the experimental diet (Fig. 1). The average body weight per cage was obtained by weighing all mice per cage and then dividing the weight by the number of mice per cage. Between Days 131 and 179 on the experimental diets, a total of 9 mice (control: 0; whole bean: 6; bean residue: 2; bean extract: 1) died spontaneously. The colon and liver tissues of these mice could not be used for further analysis. Two mice (1 on Day 181 of being on the whole bean diet and 1 on Day 148 of being on the bean residue diet) were sacrificed shortly before dying. The colon, liver, and kidneys of the moribund mice appeared normal; however, their bladder and seminal vesicles appeared enlarged. Between Days 139 and 141 on the experimental diets, the first group of mice (n 8 for control, whole bean, and bean extract, respectively, and = n = 7 for bean residue) were sacrificed after the first mouse in the experiment died. Because the tumor incidence rate was low (4 of 31 mice) at the initial sacrifice, the remaining mice were sacrificed after consuming the experimental diets for 181 to 194 days, when more mice became moribund.
FIG. 1.
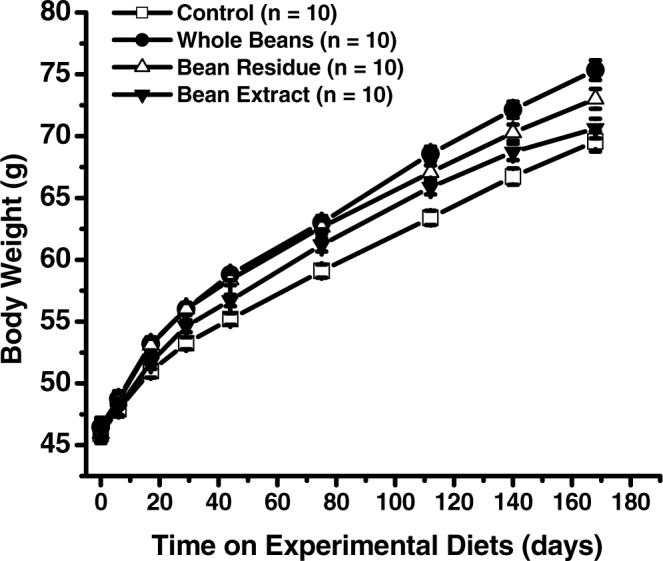
No decrease in body weight of azoxymethane-induced obese (ob/ob) mice fed cooked navy beans or their fractions (bean residue, bean extract) in comparison to mice fed the control diet. To get the average body weight, all mice per cage were weighed and divided by the number of mice per cage (n = 10 cages). Data represent least squares mean ± SEM. Mice fed whole beans or bean residue had significantly (P < 0.05) higher body weights than mice fed the control diet starting at Day 17 on diet. Mice fed bean extract had significantly (P < 0.05) higher body weights than mice fed the control diet between Day 44 and 140 on diet.
Necropsy and Histopathology
At the end of the dietary treatment period, mice were sacrificed using CO2 inhalation and exsanguination. For necropsy, the colons were cut longitudinally, rinsed with lukewarm tap water to remove intestinal contents, palpated for raised lesions, flattened between filter paper, and then fixed in 10% (vol:vol) neutral buffered formalin (pH 7.4). After 5 to 6 h, the fixed colons were examined macroscopically by 2 observers independently for colon lesions. Lesions considered abnormal were opaque in appearance and rose slightly above the lumen surface. Their opaque appearance distinguished them from the nearby translucent lymphoid tissue. The colons were then cut into 4 sections: proximal section (5 cm), midsection 1 (2 cm), midsection 2 (2 cm), and anal/distal section (3 cm). The location of any suspected colon lesions was recorded. Then the colon sections were cut transversally, processed, embedded in paraffin, and then hematoxylin and eosin stained. The sections with suspected lesions were cut as close as possible to the lesion location before they were embedded into paraffin blocks. Other internal organs were also examined for tumors. Liver tissue with lesions as well as representative samples of visually normal liver tissue were fixed in 10% (vol:vol) neutral-buffered formalin (pH 7.4), paraffin embedded, and then hematoxylin and eosin stained.
Histological examination of colon and liver tissue was done by a pathologist. The pathologist examined slides of 89 sites of macroscopically abnormal colon tissue (control: 44 sites of 22 mice; whole bean: 20 sites of 16 mice; bean residue: 16 sites of 11 mice; bean extract: 9 sites of 8 mice). Abnormal growth of colon tissue was categorized into focal hyperplasia, dysplasia, adenoma, and adenocarcinoma (Table 2) based on standard histological grading criteria (48). The pathologist examined 20 sites of macroscopically normal liver tissue (5 sites for each diet) and 10 sites of macroscopically abnormal liver tissue (control: 1 site of 1 mouse; whole bean: 1 site of 1 mouse; bean residue: 2 sites of 2 mice; bean extract: 6 sites of 6 mice).
TABLE 2.
Incidence of Colon Lesions (Focal Hyperplasia, Dysplasia, Adenoma, Adenocarcinoma) in Azoxymethane-Induced Obese (ob/ob) Mice Fed Cooked Navy Beans or Their Fractions (Bean Residue, Bean Extract)
| Type of Colon Lesion |
|||||
|---|---|---|---|---|---|
| Number of Miceb | Total at Sacrificea | Focal Hyperplasia | Dysplasia | Adenoma | Adenocarcinoma |
| Control | 40 | 9a | 4 | 5 | 4ab |
| Whole Bean | 34 | 1b | 0 | 0 | 5a |
| Bean Residue | 38 | 3ab | 0 | 1 | 1ab |
| Bean Extract | 39 | 1b | 1 | 0 | 0b |
40 mice were assigned to each diet. Starting at Day 131 on the experimental diets, a total of 9 mice (control: 0; whole bean: 6; bean residue: 2; bean extract: 1) died spontaneously.
Within each column, incidences followed by different subscript letters differ significantly at P < 0.05. We observed only in the control group multiple lesions of the same type within 1 mouse (11 hyperplastic sites in 9 mice, 5 dysplastic sites in 4 mice, and 5 adenocarcinomas in 4 mice).
Statistical Analysis
The data were analyzed using SAS version 8.2 (49). We used linear mixed models (PROC MIXED) to compare longitudinal profiles of the average mouse weight per cage across diets. The fixed covariates were diet (i.e., control, whole bean, bean residue, bean extract) and day on diet (i.e., Day 1, 6, 16, 28, 43, 74, 111, 139, and 167) using a dummy variable at each time point. An unrestricted variance-covariance matrix was used to allow for random effects for each time point as well as a random intercept. Overall, body weights significantly differed between diets depending on days on diet (P < 0.0001). Thus, we examined differences in average body weights per cage between diets separately at each time point. Significant differences in body weights across diets were observed at each time point except Day 1 and 6. To estimate the effect of whole beans or their fractions on body weight, the average body weight of mice receiving each diet was compared by using a t-test in the ESTIMATE statement of PROC MIXED. Least square means ± standard error of the mean (SEM) are presented in Fig. 1.
For the incidence data of various colon lesions, a likelihood ratio test for binomial data (PROC GENMOD) and Fisher's exact test (PROC FREQ) were used. To test for global differences in incidence rates across diets, logistic regression with a linear effect of diet was fit in PROC GENMOD. The linear effect was significant for incidence rates of focal hyperplasia (P = 0.01); focal hyperplasia or dysplasia (P = 0.008); adenoma or adenocarcinoma (P = 0.007); dysplasia, adenoma, or adenocarcinoma (P = 0.001); and any type of lesions (P = 0.0002) in the colon. We proceeded with pairwise comparisons using Fisher's exact test and compared the number of mice with and without the various colon lesions receiving each diet. Means ± SEM are presented in Figs. 2–4. All statistical tests were 2-sided. Significance was declared at P < 0.05, and trends toward significance were declared at P < 0.10.
FIG. 2.
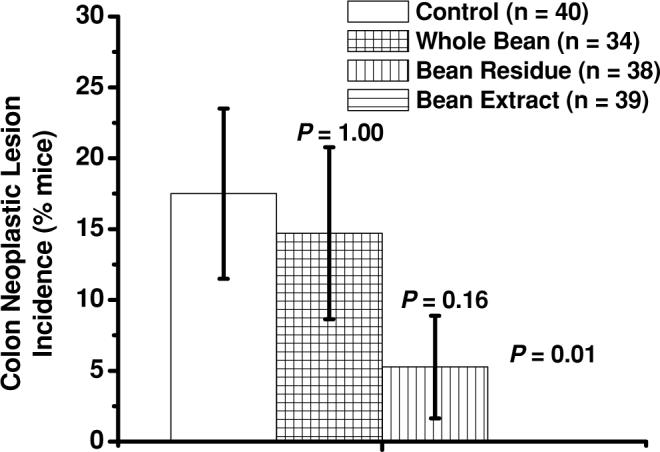
The 60% ethanol-soluble fraction of dietary cooked navy beans significantly decreased the incidence of neoplastic colon lesions (adenoma and adenocarcinoma) to zero out of 39 in azoxymethane-induced obese (ob/ob) mice. Bars represent means ± SEM. P values above bars represent comparisons with control-fed group of mice.
FIG. 4.
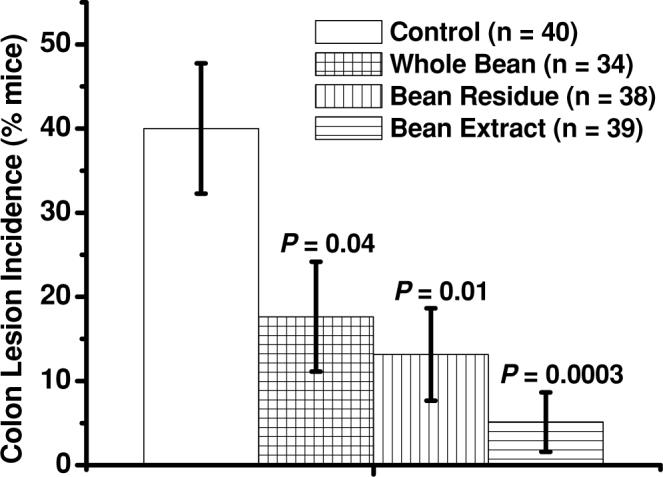
Dietary cooked navy beans and their fractions (bean residue, bean extract) significantly decreased the incidence of colon lesions (focal hyperplasia, dysplasia, adenoma, and adenocarcinoma) in azoxymethane-induced obese (ob/ob) mice. Bars represent means ± SEM. P values above bars represent comparisons with control-fed group of mice.
RESULTS
Male AOM-induced ob/ob mice fed the diets containing cooked navy beans (whole beans) or their fractions (bean residue, bean extract) had higher body weights than mice on the modified AIN-93G control diet (Fig. 1). Colon lesion types are listed to mirror the progression of colon carcinogenesis. The incidence of observed focal hyperplasia was lower in mice fed whole beans (1 of 34 mice; P = 0.02) or bean extract (1 of 39 mice; P = 0.01) in comparison to mice fed the control diet (9 of 40 mice; Table 2). The incidence of adenocarcinomas was lower in mice fed bean extract (0 of 39 mice; P = 0.02) than in mice fed the whole bean diet (5 of 34 mice; Table 2). We observed only in the control group multiple lesions of the same type within 1 mouse and different types of lesions within 1 mouse. All lesions were in the vicinity of lymphatic nodules (results not shown). All lesions were in the middle or distal colon except for 1 adenoma and 1 adenocarcinoma in the control group, which were located in the proximal colon.
Less mice on the bean extract diet (0 of 39 mice) developed neoplastic lesions (adenoma or adenocarcinoma) than mice fed the control diet (7 of 40 mice; P = 0.01) or the whole bean diet (5 of 34 mice; P = 0.02; Fig. 2). When combining incidence data of dysplastic and neoplastic lesions, fewer mice on both fraction diets, bean residue (2 of 38 mice; P = 0.003) and bean extract (1 of 39 mice; P = 0.0007), had dysplastic or neoplastic lesions than mice fed the control diet (13 of 40 mice; Fig. 3). When combining all 4 types of colon lesions (focal hyperplasia, dysplasia, adenoma, adenocarcinoma), fewer mice on each of the 3 bean diets—whole beans (6 of 34 mice; P = 0.04), bean residue (5 of 38 mice; P = 0.01), and bean extract (2 of 39 mice; P = 0.0003)—had colon lesions than mice fed the control diet (16 of 40 mice; Fig. 4). We observed fewer mice on each of the 3 bean diets—whole beans (1 of 34 mice; P = 0.004), bean residue (3 of 38 mice; P = 0.04), and bean extract (2 of 39 mice; P = 0.01)—with focal hyperplasia or dysplasia than on the control diet (11 of 40 mice). Among the dry bean groups, mice fed bean residue or bean extract and mice fed the bean residue or whole beans did not significantly differ in any type of colon lesion grouping. No significant differences in hepatomas were observed across diets (control: 2 hepatomas in 1 mouse; whole bean: 0 hepatomas; bean residue: 2 hepatomas in 1 mouse; bean extract: 4 hepatomas in 3 mice).
FIG. 3.
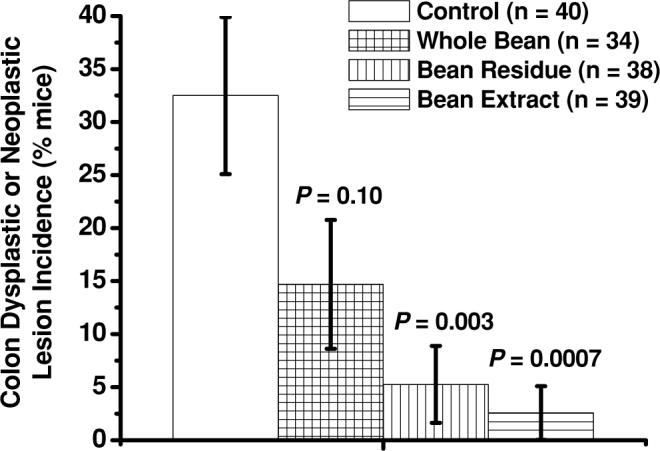
Fractions of dietary cooked navy beans significantly decreased the incidence of dysplastic or neoplastic (adenoma and adenocarcinoma) colon lesions in azoxymethane-induced obese (ob/ob) mice. Bars represent means ± SEM. P values above bars represent comparisons with control-fed group of mice.
DISCUSSION
Our results indicate that whole cooked navy beans provide protection against colon carcinogenesis in male AOM-induced ob/ob mice, specifically against earlier stages of colon carcino-genesis. This is evidenced by the lower incidence of focal hyperplasia (Table 2), the lack of lesion multiplicity, and the lower incidence of any type of lesions (55.9% of inhibition from control; Fig. 4) compared to mice fed the control diet. However, the incidence of adenocarcinomas was not affected by feeding whole dry beans (Table 2) as it was in studies by Hangen and Bennink (20) and Hughes et al. (21). Hughes et al. (21) reported that diets rich in cooked pinto beans were associated with significantly lower incidence rates of any adenocarcinomas (50%), neoplastic lesions (52.4%), and any type of lesions (49.2%) in colons of AOM-induced F344 rats. Similarly, Hangen and Bennink (20) reported that AOM-induced F344 rats fed diets rich in cooked black beans or cooked navy beans had significantly lower incidence rates of adenocarcinomas (75.0% or 61.1%, respectively) and neoplastic colon lesions (53.7% or 58.8%, respectively). Furthermore, Murillo et al. (50) reported that a diet containing 10% flour of garbanzo beans, another commonly consumed dry bean market class (44), was associated with a significant 64% decrease in colonic aberrant crypt foci, a surrogate marker for early stages of colon carcinogenesis, in AOM-induced CF-1 mice. In addition, all 3 bean diets (whole bean: 55.9%; bean residue: 67.1%; bean extract: 87.2%) significantly lowered the incidence of any colon lesions compared to mice on the control diet (Fig. 4). Therefore, although we did not observe a protective effect of the whole bean diet against formation of adenocarcinomas, results of this and other animal studies suggest a protective effect of dry bean consumption against colon carcinogenesis.
It is unclear to us why the number of adenocarcinomas was not affected by feeding whole cooked navy beans. One potential explanation is that the whole bean diet inhibited early but not invasive steps of colon carcinogenesis. However, this explanation is difficult to reconcile with the protective effect of whole bean diets against adenocarcinomas in other animal studies (20,21). Furthermore, both bean fractions nearly completely inhibited formation of adenocarcinomas in this study (bean residue: 1 adenocarcinoma; bean extract: 0 adenocarcinomas; Table 2). One potential explanation that could account for the differences in results is that the whole navy bean diet had a lower bioavail-ability of cancer-preventive compounds than previous studies and that the 60% ethanol extraction procedure improved the bioavailability of these compounds. However, statistically significant differences in incidence rates were observed only for incidence rates of adenocarcinomas between the whole bean and bean extract diet (Table 2). One has to also consider the amount of whole beans consumed on the bean fraction diets relative to the amount consumed on the whole bean diet. Assuming equal food intake in the 3 bean groups, mice on the bean residue diet consumed 14% more whole bean equivalents, whereas mice on the bean extract diet consumed 47% more whole bean equivalents.
A potential limitation of other AOM-induced rat studies was that the bean diets might have been limiting in tryptophan content, which could have caused the lower body weights of the bean-fed rats compared to the rats fed the control diet (20,21). Therefore, we adjusted the diets for tryptophan content, which is the second limiting amino acid in dry beans. We did not observe decreased body weights in mice receiving cooked navy beans or its fractions, but rather, we observed the opposite, that is, increased body weights (whole beans: 8.3%, bean residue: 5.0%, bean extract: 1.5%; Fig. 1). We conclude that even high concentrations of cooked navy beans or their fractions do not have detrimental effects on growth.
Several components of dry beans, such as phenolic compounds, saponins, phytic acid, residual activity of protease inhibitors, resistant starches, and nonstarch polysaccharides, have been proposed to account for the colon cancer-protective effect of dry beans (11,19–21). Most of the cancer-protective compounds, except for resistant starches and nonstarch polysaccha-rides, are soluble in aqueous alcohol (37–40). Therefore, our hypothesis was that the diet containing the bean extract might be more efficacious than the diet containing the bean residue. Consistent with our hypothesis, the lowest incidence rates of neoplastic lesions, neoplastic or preneoplastic lesions, or any type of lesions in colons were observed in mice fed the bean extract diet (Figs. 2–4). However, we did not observe significant differences in incidence rates of various types of colon lesions between mice fed diets containing bean residue or bean extract (Table 2; Figs. 2–4). We conclude that both the 60% ethanol-soluble fraction and the insoluble fraction of dry beans are efficacious in inhibiting colon carcinogenesis and contribute to the cancer-preventive effect of cooked dry beans.
The human relevance of our results is suggested by results from human intervention and epidemiological studies (13–19). Results of a human intervention study suggest that daily consumption of at least 31 g of cooked dry beans can prevent recurrence of advanced adenomatous polyps (19). Therefore, we chose for this study diets with large differences in dry bean contents. We are aware that diets containing 74% of dry beans (Table 1) are not feasible in humans; however, results by Murillo et al. (50) in AOM-induced CF-1 mice suggest that diets with lower dry bean contents might be also effective in preventing at least early steps of colon carcinogenesis. Furthermore, the bean extract might provide an opportunity to receive the same health benefits as from high dry bean intakes at much lower consumption levels. Dose-response studies of the bean extract are required to determine the lowest efficacious dose for cancer prevention.
Azoxymethane-induced ob/ob mice have not been previously used as a colon cancer model. The ob/ob mouse has a mutation in the leptin gene resulting in hyperphagia, obesity, hyperinsulinemia, hyperglycemia, and increased inflammatory response to liposaccharides (9). We chose the ob/ob mouse as the animal model to examine the protective effects of dry beans against CRC in humans because subjects of the Polyp Prevention Trial in which increased dry bean consumption was associated with decreased advanced adenoma recurrence were on average over-weight (19). In comparison to well-established models of colon cancer (20,21), the AOM-induced ob/ob mice in our study had a much lower incidence of neoplastic lesions (Fig. 2). We used a comparatively mild AOM induction protocol (2 dosages of 7 mg AOM/kg of body weight 1 wk apart) to achieve human-relevant low incidence rates, as the lifetime risk in humans to develop adenocarcinomas is low, with 5.8% for men and 5.4% for women (2). However, the low incidence rates create difficulties to reach statistical significances for dietary colon cancer prevention studies. Further studies that use higher AOM dosages, more AOM cycles, or both are warranted to evaluate whether higher tumor incidences, similar to that of other colon cancer models, can be achieved in AOM-induced ob/ob mice.
In conclusion, our study confirms the protective effect of cooked dry bean consumption against colon carcinogenesis in a new colon cancer animal model, the AOM-induced ob/ob mouse. Furthermore, we demonstrated that both the 60% ethanol-soluble and the insoluble fraction of cooked navy beans are efficacious in inhibiting colon carcinogenesis. This suggests that components in the soluble fraction as well as the insoluble fraction of the ethanol extract have preventive properties against colon carcinogenesis. Further studies are warranted to determine which specific components in each fraction contribute to the cancer-protective effect so that even more potent dietary components for colon cancer prevention can be developed.
ACKNOWLEDGMENTS
G. Bobe and K. G. Barrett contributed equally to the work. We thank the Michigan Bean Commission for the donation of navy beans. This research was supported financially in part by the Intramural Research Program of the National Institute of Health and National Cancer Institute and in part by a grant from the United States Agency for International Development through the Bean/Cowpea Collaborative Research Support Program (CRSP) and by the Michigan Agricultural Experiment Station.
Contributor Information
Gerd Bobe, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute—Frederick, Frederick, Maryland, USA; and the Cancer Prevention Fellowship Program, Office of Preventive Oncology, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Kathleen G. Barrett, Department of Food Science and Human Nutrition, Michigan State University, East Lansing, Michigan, USA
Roycelynn A. Mentor-Marcel, Laboratory of Cancer Prevention, Center for Cancer Research, National Cancer Institute—Frederick, Frederick, Maryland, USA; and the Cancer Prevention Fellowship Program, Office of Preventive Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
Umberto Saffiotti, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Paul S. Albert, Biometric Research Branch, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
Maurice R. Bennink, Department of Food Science and Human Nutrition, Michigan State University, East Lansing, Michigan, USA
Elaine Lanza, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
REFERENCES
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5. version 2.0. IARCPress; Lyon, France: 2004. [April 10, 2008]. Available: http://wwwdep.iarc.fr/. [Google Scholar]
- 2.American Cancer Society . Cancer Facts and Figures 2007. American Cancer Society; Atlanta, GA: 2007. [Google Scholar]
- 3.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Cancer. 2006;107:1624–1633. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society . Colorectal Cancer Facts & Figures Special Edition 2005. American Cancer Society; Atlanta, GA: 2005. [Google Scholar]
- 6.World Cancer Research Fund/American Institute for Cancer Research . Food, Nutrition and the Prevention of Cancer: A Global Perspective. AICR; Washington, DC: 1997. pp. 213–251. [Google Scholar]
- 7.Gill S, Sinicrope FA. Colorectal cancer prevention: is an ounce of prevention worth a pound of cure? Semin Oncol. 2005;32:24–34. doi: 10.1053/j.seminoncol.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1271–1279. [PubMed] [Google Scholar]
- 9.Harkins JM, Moustaid-Moussa N, Chung YJ, Penner KM, Pestka JJ, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal VS, Pastor-Corrales MA, Chirwa RM, Buruchara RA. Andean bean (Phaseolus vulgaris L.) with resistance to angular leaf spot pathogen (Phaeoisariopsis griseola) in southern and eastern Africa. Euphytica. 2004;136:201–210. [Google Scholar]
- 11.Serrano J, Goni I. Role of black bean Phaseolus vulgaris on the nutritional status of Guatemalan population. Arch Latinoam Nutr. 2004;54:36–44. [PubMed] [Google Scholar]
- 12.Correa P. Epidemiological correlations between diet and cancer frequency. Cancer Res. 1981;41:3685–3689. [PubMed] [Google Scholar]
- 13.Benito E, Stiggelbout A, Bosch FX, Obrador A, Kaldor J, et al. Nutritional factors in colorectal cancer risk: a case-control study in Majorca. Int J Cancer. 1991;49:161–167. doi: 10.1002/ijc.2910490202. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi S. Nutrient and food groups and large bowel cancer in Europe. Eur J Cancer Prev. 1999;8:S49–S52. [PubMed] [Google Scholar]
- 15.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-Day Adventists. Am J Clin Nutr. 1999;70:532S–538S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 16.Michels KB, Giovannucci E, Chan AT, Singhania R, Fuchs CS, et al. Fruit and vegetable consumption and colorectal adenomas in the Nurses’ Health Study. Cancer Res. 2006;66:3942–3953. doi: 10.1158/0008-5472.CAN-05-3637. [DOI] [PubMed] [Google Scholar]
- 17.Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidem. 1998;148:761–774. doi: 10.1093/oxfordjournals.aje.a009697. [DOI] [PubMed] [Google Scholar]
- 18.Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study: I. vegetables and fruit. Int J Cancer. 1993;53:711–719. doi: 10.1002/ijc.2910530502. [DOI] [PubMed] [Google Scholar]
- 19.Lanza E, Hartman TJ, Albert PS, Shields R, Slattery M, et al. High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the Polyp Prevention Trial. J Nutr. 2006;136:1896–1903. doi: 10.1093/jn/136.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hangen L, Bennink MR. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr Cancer. 2002;44:60–65. doi: 10.1207/S15327914NC441_8. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JS, Ganthavorn C, Wilson-Sanders S. Dry beans inhibit azoxymethane-induced colon carcinogenesis in F344 rats. J Nutr. 1997;127:2328–2333. doi: 10.1093/jn/127.12.2328. [DOI] [PubMed] [Google Scholar]
- 22.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? a systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–1922. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Pegg AE. Methylation of the O6 position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Invest. 1984;2:223–231. doi: 10.3109/07357908409104376. [DOI] [PubMed] [Google Scholar]
- 24.Kerwin SM. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr Med Chem Anti-Cancer Agents. 2004;4:263–272. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 25.Rao AV, Sung MK. Saponins as anticarcinogens. J Nutr. 1995;125:717S–724S. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, et al. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7:67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- 27.Pierre F, Perrin P, Champ M, Bornet F, Mefiah K, et al. Short-chain fructooligosaccharides reduce the occurrence of colon tumors and develop gut-associated lymphoid tissue in Min mice. Cancer Res. 1997;57:225–228. [PubMed] [Google Scholar]
- 28.Jayaprakasam BL, Vanisree M, Zhang Y, Dewitt DL, Nair MG. Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. J Agric Food Chem. 2006;54:5375–5381. doi: 10.1021/jf060899p. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, et al. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 30.Janicke B, Önning G, Oredsson SM. Differential effects of ferulic acid and p-coumaric acid on S phase distribution and length of S phase in the human colonic cell line Caco-2. J Agric Food Chem. 2005;53:6658–6665. doi: 10.1021/jf050489l. [DOI] [PubMed] [Google Scholar]
- 31.Mutoh M, Takahashi M, Fukuda K, Matsushima-Hibiya Y, Mutoh H, et al. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis. 2000;21:959–963. doi: 10.1093/carcin/21.5.959. [DOI] [PubMed] [Google Scholar]
- 32.Graf E, Eaton JW. Suppression of colonic cancer by dietary phytic acid. Nutr Cancer. 1993;19:11–19. doi: 10.1080/01635589309514232. [DOI] [PubMed] [Google Scholar]
- 33.Vucenik E, Shamsuddin AM. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: from laboratory to clinic. J Nutr. 2003;133:3778S–3784S. doi: 10.1093/jn/133.11.3778S. [DOI] [PubMed] [Google Scholar]
- 34.Franke AA, Custer LJ, Cooney RV, Tanaka Y, Xu M, et al. Inhibition of colonic aberrant crypt formation by the dietary flavonoids (+)-catechin and hesperidin. Adv Exp Med Biol. 2002;505:123–133. doi: 10.1007/978-1-4757-5235-9_11. [DOI] [PubMed] [Google Scholar]
- 35.Cooke D, Schwarz M, Boocock D, Winterhalter P, Steward WP, et al. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the Apc Min mouse model of intestinal carcinogenesis— relationship with tissue anthocyanin levels. Int J Cancer. 2006;119:2213–2220. doi: 10.1002/ijc.22090. [DOI] [PubMed] [Google Scholar]
- 36.Bises G, Bajna E, Manhardt T, Gerdenitsch W, Kallay E, et al. Gender-specific modulation of markers for premalignancy by nutritional soy and calcium in the mouse colon. J Nutr. 2007;137:211S–215S. doi: 10.1093/jn/137.1.211S. [DOI] [PubMed] [Google Scholar]
- 37.Drumm TD, Gray JI, Hosfield GL, Uebersax MA. Lipid, saccharide, protein, phenolic acid and saponin contents of four market classes of edible dry beans as influenced by soaking and canning. J Sci Food Agric. 1990;51:425–435. [Google Scholar]
- 38.Luthria DL, Pastor-Corrales MA. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J Food Comp Anal. 2006;19:205–211. [Google Scholar]
- 39.Matella NH, Dolan KD, Stoeckle AW, Bennink MR, Lee YS, et al. Use of hydration, germination, and α-galactosidase treatments to reduce oligosaccharides in dry beans. J Food Sci. 2005;70:C203–C207. [Google Scholar]
- 40.Oomah BD, Cardador-Martinez A, Loarca-Piña G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). J Sci Food Agric. 2005;85:935–942. [Google Scholar]
- 41.Bauer-Marinovic M, Florian S, Müller-Schmehl K, Glatt H, Jacobasch G. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis. 2006;27:1849–1859. doi: 10.1093/carcin/bgl025. [DOI] [PubMed] [Google Scholar]
- 42.Pool-Zobel BL. Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br J Nutr. 2005;93:S73–S90. doi: 10.1079/bjn20041349. [DOI] [PubMed] [Google Scholar]
- 43.Reddy BS. Role of dietary fiber in colon cancer: an overview. Am J Med. 1999;106:16S–19S. doi: 10.1016/s0002-9343(98)00341-6. [DOI] [PubMed] [Google Scholar]
- 44.Lucier G, Lin BH, Allshouse J, Kantor LS. Factors Affecting Dry Bean Consumption in the United States. Economic Research Service/USDA; 2000. [April 10, 2008]. Available: http://www.ers.usda.gov/Briefing/Consumption/gallery/DryBeanConsumption.pdf. [Google Scholar]
- 45.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- 46.USDA National Nutrient Database for Standard Reference, Release 18 [April 10, 2008];Beans, navy, mature seeds, raw. NDB No. 16037. 2005 Available: http://grande.nal.usda.gov/NDL/cgi-bin/list_nut_edit.pl.
- 47.Reeves P, Nielsen F, Fahey G., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 48.Krutovskikh VA, Turusov VS. Tumors of the intestines. IARC Sci Publ. 1994;111:195–221. [PubMed] [Google Scholar]
- 49.SAS . SAS®User's Guide: Statistics, version 9.1. SAS Inst., Inc.; Cary, NC: 2002. [Google Scholar]
- 50.Murillo G, Choi JK, Pan O, Constantinou AI, Mehta RG. Efficacy of garbanzo and soybean flour in suppression of aberrant crypt foci in the colons of CF-1 mice. Anticancer Res. 2004;24:3049–3056. [PubMed] [Google Scholar]


