Abstract
Cells migrating from the neural crest are known to septate the outflow tract of the developing heart, and to contribute to the formation of the arterial valves, their supporting sinuses, the coronary arteries and cardiac neural ganglia. Neural crest cells have also been suggested to contribute to development of the venous pole of the heart, but the extent and fate of such cells remains unclear. In this study, in the mouse, it is shown that cells from the neural crest contribute to the parasympathetic and, to a lesser extent, the sympathetic innervation of the venous pole of the heart. Nerves within the venous pole of the heart are shown to be of mixed origin, with some being derived from the neural crest, while others have an alternative origin, presumably placodal. The neurons innervating the nodal tissue, which can exert chronotropic effects on cardiac conduction, are shown not to be derived from the neural crest. In particular, no evidence was found to support previous suggestions that cells from the neural crest make a direct contribution to the myocardial atrioventricular conduction axis, although a small subset of these cells do co-localize with the developing left bundle branch. We have therefore confirmed that cells from the neural crest migrate to the venous pole of the heart, and that their major role is in the development of the parasympathetic innervation. In addition, in some embryos, a population of cells derived from the neural crest persist in the leaflets of the atrioventricular valves, but their role in subsequent development remains unknown.
Keywords: cardiac development, innervation, nerves, parasympathetic, Wnt1-cre
Introduction
Cells derived from the neural crest are known to play crucial roles during cardiovascular development, although their precise roles and ultimate fates still remain to be determined. Absence, or reduced numbers, of neural crest cells entering the outflow tract of the heart are associated with a range of cardiovascular defects, which include common arterial trunk (persistent truncus arteriosus), and abnormalities in the remodelling of the arteries running through the pharyngeal arches (Kirby et al. 1985; Besson et al. 1986; Bockman et al. 1989; Conway et al. 1997). Lineage tracing of such cells in developing chicks and mice has shown that they also contribute to the cardiac ganglia, the proximal regions of the coronary arteries and the arterial valves (Poelmann et al. 1998; Jiang et al. 2000; Pietri et al. 2003), whilst a more recent work shows that they also contribute to the atrioventricular valves (Nakamura et al. 2006).
Retroviral labelling studies in developing chicken embryos have shown that cells from the neural crest also enter the venous pole of the heart, where the majority were reported to co-localize with the developing conduction tissues, with some labelled cells also found within the atrioventricular cushions (Poelmann & Gittenberger-de Groot, 1999). These retroviral labelled cells at the venous pole were shown to be programmed for apoptosis, as are the majority of the neural crest-derived cells found populating the proximal outflow tract. As the cells entering through the venous pole of the heart did not seem to make any permanent contribution to cardiac structures, Poelmann & Gittenberger-de Groot (1999) proposed an inductive role for them. More recently, the same authors showed that neural crest-derived cells migrate into the venous pole of the developing mouse (Poelmann et al. 2004), with these cells again seeming to co-localize with the developing cardiac conduction system. They did not, however, report any association between the cells from the neural crest entering the venous pole of the mouse heart and cardiac innervation, although this has been widely described in the chicken embryo. In the chick, it is suggested that cells of neural crest origin follow the placodally derived vagal nerve to the venous pole of the heart, where they form the ganglia of the posterior cardiac plexus, along with the parasympathetic ganglia within the atria (Verberne et al. 1988, 1999, 2000).
Cardiac ganglia are well known to be associated with the sinus node, the roots of the caval (venae cavae) and pulmonary veins, and the interatrial septum, and to be found in proximity to the atrioventricular node. With the recent innovations in Cre-lox technology, the lineage tracing of cells originating from the neural crest has become almost routine. This has been achieved using the Wnt1-Cre, HtPA-Cre or PO-Cre mouse lines coupled with an appropriate reporter strain. Several such studies have reported the presence of cardiac ganglia derived from the neural crest at the arterial pole of the heart (Yamauchi et al. 1999; Jiang et al. 2000; Pietri et al. 2003). In contrast, a detailed analysis of the development of the innervation of the venous pole of the mouse heart has not been reported. In this study we have used the Wnt1-Cre line, crossed with the ROSA 26R reporter strain, to trace the lineage of cells migrating from the neural crest to the venous pole of the heart. Our studies show that, as in the chicken embryo, most of the cells migrating to the atrial chambers through the dorsal mesocardial connection are destined to become autonomic nerves and ganglia. We find no evidence to show that the cells contribute materially to the myocardial components of the atrioventricular conduction axis. It this respect, we are unable to endorse the recent study by Nakamura et al. (2006).
Materials and methods
Mouse strains and embryo collection
Male and female mice were mated overnight to generate timed pregnancies. The middle of the day in which a copulation plug had been identified in the morning was designated E0.5. Wnt1-Cre (Danielian et al. 1998), ROSA 26R (Soriano, 1999) and Sp2H (Epstein et al. 1991) mice were intercrossed to produce litters in which the cells derived from the neural crest stained blue following a reaction with X-gal (Jiang et al. 2000). Embryos were dissected, fixed and prepared either for X-gal staining or for embedding in paraffin wax. At least three embryos of each genotype were examined at each gestational age.
β-Galactosidase staining of Wnt1-Cre/ROSA 26R embryos
Wnt1-Cre/R26R embryos were dissected into ice-cold 1% phosphate-buffered saline, and then fixed at 4 °C in a solution containing 0.1 m phosphate buffer, 2% paraformaldehyde (PFA), 5 mm EGTA, 0.2% glutaraldehyde and 2 mm MgCl2. E9.5 embryos were fixed for 5 min, E10.5–E12.5 embryos for 15 min and older embryos for 20 min. The heads and tails of embryos at age E11.5 and older were removed, and the left ventricle was punctured to allow penetration of the staining solution. Following fixation, the embryos were washed at room temperature in wash buffer (0.1 m phosphate buffer, 0.01% Na-deoxycholate, 0.02% Nonidet-P40 and 2 mm MgCl2) at the stages and times described above. After washing, the embryos were placed in staining solution containing wash buffer supplemented with 1 mg mL−1 X-gal in dimethyl formamide, 10 mm K-ferricyanide and 10 mm K-ferrocyanide. Embryos were left to stain in darkness at 37 °C overnight. After staining, embryos were washed in wash buffer, then fixed in 4% PFA at 4 °C (E9.5 and E10.5 embryos for 24 h, older embryos for 48 h). Embryos were embedded in paraffin wax, sectioned and counterstained with a 1% aqueous eosin solution. X-gal staining of cryoembedded sections of Wnt1-Cre/ROSA 26R fetuses confirmed that the stain had penetrated the heart in the samples stained as wholemounts. At least three embryos were studied at each gestational age.
Immunohistochemistry
All immunohistochemistry was performed on PFA-fixed, paraffin-embedded, tissue sections using standard protocols. In some cases, antibody staining was carried out subsequent to X-gal staining. In this case, stained embryos were post-fixed in PFA overnight, and processed for wax embedding as normal. Tissue sections were de-waxed in Histoclear (National Diagnostics) and hydrated through a graded ethanol series before treatment with 0.6% H2O2. Sections were blocked in a 10% solution of fetal bovine serum (Sigma). Primary antibodies were diluted in 2% fetal bovine serum and incubated with the sections, overnight at 4 °C. Secondary antibodies, conjugated to biotin, were also diluted in 2% fetal bovine serum, overlaid on the sections and incubated for 1 h at room temperature. Antibodies were visualized by treating sections with avidin–biotin complex conjugated to horseradish peroxidase (ABComplex, Dako), then staining with diaminobenzidine (Sigma). The p75 nerve growth factor receptor antibody (Chemicon International) required the standard peroxidase treatment described above, while the neurofilament 160D antibody (clone NN18, Sigma), tyrosine hydroxylase antibody (clone TH-2, Sigma) and the myocardial marker Cardiac Troponin (Hytest) required additional antigen retrieval by microwaving in Declere (Cell Marque). Goat anti-mouse IgG FITC (Sigma) was used as the secondary antibody for the myocardial marker MF20 (Developmental Studies Hybridoma Bank, Iowa city). The Islet1 antibody (40.2D6, Developmental Studies Hybridoma Bank) required antigen retreival by microwaving in citrate buffer (pH 6). Experiments were performed at least three times at each stage with each antibody and included a negative control in which the secondary antibody was omitted. Sections were counterstained in 0.5% aqueous methyl green solution unless previously X-gal-stained, in which case 1% aqueous eosin was used.
Results
Neural crest cells enter the venous pole of mouse embryos later than the arterial pole
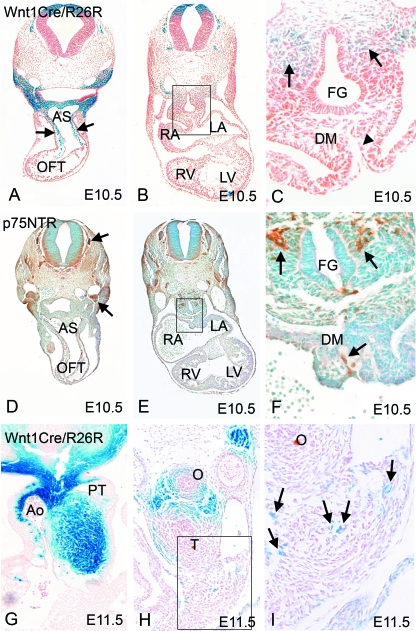
The Wnt1-Cre mouse line, when intercrossed with ROSA 26R mice, produces animals in which the cells derived from the neural crest, and all their derivatives, are marked permanently by their expression of β-galactosidase (β-gal; Jiang et al. 2000). These cells, which stain blue, were first seen in the pharyngeal mesenchyme. They were then seen colonizing the distal region of the outflow tract at E9.5 (data not shown). By E10.5, the blue cells were abundant in the distal outflow tract, and were also seen more proximally within the outflow tract (arrows in Fig. 1A). At this stage, however, no blue cells were found within, or close by, the dorsal mesocardial connection (indicated by the arrowhead in Fig. 1C), this being the connection between the body wall and the atria at the venous pole of the heart. Neural crest cells, nonetheless, were present at this stage in the mesenchyme surrounding the foregut (arrows in Fig. 1C). Matched sections to those shown in Fig. 1C were stained with p75 NTR (Fig. 1D–F), which identifies cells committed to the autonomic lineage, many of which are neural crest-derived. This staining, in brown, revealed that, although there was labelling of the neural crest-derived dorsal root and cranial ganglia (arrows in Fig. 1D), the cells migrating from the neural crest close to the arterial pole of the heart did not express p75 NTR (data not shown). This confirms, as has been shown in other studies, that there is only limited neurogenic commitment for the neural crest cells migrating to the arterial pole. However, p75 NTR staining was abundant in the foregut mesenchyme close to the venous pole of the heart, in the same domain as was stained by β-gal (Fig. 1E,F), thus demonstrating the neurogenic commitment of these particular cells derived from the neural crest.
Fig. 1.
Neural crest cells arrive at the arterial pole before the venous pole. (A–C) Neural crest cells labelled blue by expression of β-gal using the Wnt1-cre/ROSA 26R system, are abundant in the outflow tract by E10.5 (arrows in A), but are absent from the dorsal mesocardium at this stage (arrowhead in C). Neural crest cells can be seen in the mesenchyme surrounding the foregut at this stage (arrows in C). (D–F) p75 NTR staining (brown) is absent from the branchial arches, aortic sac and outflow tract cushions at E10.5, but is found in the dorsal root and cranial ganglia (arrows in D). p75 NTR is also found in the dorsal mesocardium and in the mesenchyme surrounding the foregut (arrows in F). AS, aortic sac; DM, dorsal mesocardium; FG, foregut; LA, left atrium; LV, left ventricle; O, oesophagus; RA, right atrium; RV, right ventricle; T, trachea. (G) Neural crest cells are abundant in the outflow region at E11.5. (H,I) Only a few isolated neural crest cells (arrows in I) can be seen in the dorsal mesocardial connection at E11.5.
Although we did not find cells derived from the neural crest within the dorsal mesocardial connection before E11.5 (Fig. 1H, arrows in Fig. 1I), p75 NTR staining had already been observed in this area at E10.5 (Fig. 1F, arrow). As p75 NTR marks developing autonomic neurons of all lineages, this indicates that neuronal cells from sources other than the neural crest are already present within the dorsal mesocardial connection prior to the arrival of any cells derived from the neural crest.
Cells derived from the neural crest contribute to the posterior cardiac plexus and ganglia
In chicken embryos, neural crest-derived cells have been reported to migrate towards the venous pole of the heart via the vagal nerve, and to form ganglion cells in the posterior cardiac plexus (Verberne et al. 1988, 2000). With this in mind, we used the neurofilament marker NF160D to determine, in the mouse, whether the cells known to have originated from the neural crest also expressed markers of differentiated neurons. No such neurofilamentous staining was seen at E9.5, either in the outflow tract or in the region of the dorsal mesocardial connection (data not shown). By E10.5, however, NF160D staining was found in the dorsal mesocardial connection (data not shown), similar to that seen for p75 NTR (Fig. 1F), so confirming the presence of neurons in the dorsal mesocardium at this early stage.
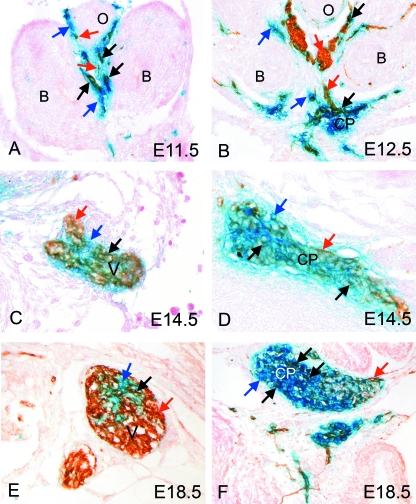
By E11.5, when cells from the neural crest were first observed within the dorsal mesocardial connection (Fig. 1H, and marked by arrows in enlargement Fig. 1I), double staining for β-gal and NF160D in the region of the forming vagal nerve revealed NF160D-positive neuronal cells within the domain of the cells known to have originated from the neural crest (Fig. 2A, red and black arrows). At this stage, there was more extensive blue than brown staining (Fig. 2A, blue arrows), the brown staining specifically identifying the neuronal component. By E12.5, the vagal nerve stained positively for NF160D (Fig. 2B). At this stage, the blue cells from the neural crest were found together with the NF160D-positive cells, although their relative numbers were reduced compared with 1 day earlier (Fig. 2B, arrows). At this and later stages, the cells within the developing vagus nerve were shown to have a mixed origin. The majority of cells located centrally within the neuronal trunks were stained only brown, showing them to be neurons derived from a source other than the neural crest, presumably the vagal placode (Fig. 2B,C,E, red arrows). Some cell bodies located at the periphery of the nerves were exclusively coloured blue, thus indicating that they were not neuronal, even though they are derived from the neural crest (Fig. 2B,C,E, blue arrows). Yet a third population was double stained, with this last population also localizing to the periphery of the vagal nerve, showing them to be neurons derived from the neural crest (Fig. 2B,C,E, black arrows).
Fig. 2.
Neural crest cells contribute to the vagal nerve and the posterior cardiac plexus at the venous pole of the heart. In each case, blue arrows mark solely β-gal-stained cells (blue), red arrows mark solely NF160D-stained cells (brown) and black arrows marked double labelled cells (blue/brown). (A) By E11.5, double labelling for β-gal (blue; neural crest-derived cells) and NF160D (brown; neurons) shows that the vagal nerve is forming in the foregut mesenchyme and that this contains a mixture of brown (red arrows), blue (blue arrows) and double labelled (black arrows) cells. (B) At E12.5, the vagal nerve has differentiated further and can be seen to comprise mainly neural cells of non-neural crest origin (brown cells; red arrows). There is a smaller contribution of neural crest-derived neural cells (double labelled; black arrows) and neural crest cells of non-neural fate (blue cells; blue arrows). By this stage the cardiac plexus can also be seen. This also comprises cells of mixed origin (see above), although in this case the majority of cells derive from the neural crest (stained blue). (C,E) The vagus nerve continues to have neurons of neural crest and non-neural origin at E14.5 and at E18.5, with the majority being of non-neural crest origin (brown). (D,F) In contrast, the majority of neuronal cell bodies in the posterior cardiac plexus are of neural crest origin (blue). B, bronchus; CP, cardiac plexus; O, oesophagus; V, vagal nerve.
By E12.5, the neuronal posterior cardiac plexus could readily be identified at the venous pole of the heart (Fig. 2B). In contrast to the vagal nerves, cells of the cardiac plexus were predominantly blue, indicating their origin from the neural crest, although some cells were present that stain solely with the neurofilament marker NF160D. Again, many cell bodies, particularly those in the central region of the posterior cardiac plexus, were positive for both markers (black arrows in Fig. 2B), showing that cells derived from the neural crest had differentiated into neurons. This mixture of cell types in the posterior cardiac plexus was maintained throughout gestation (Fig. 2D,F, red arrows). The cells that are stained exclusively with NF160D, and hence brown, must be neuronal cells that are not derived from the neural crest. Like the vagal nerve, they are presumably of placodal origin. The cells that are only stained blue are either undifferentiated neural crest cells or else non-neuronal cells of neural crest origin, for example satellite cells or glia.
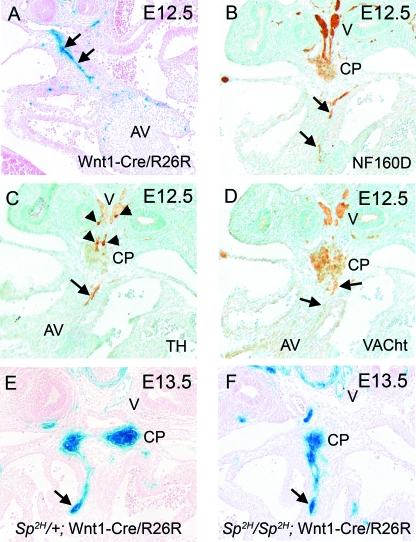
Analysis of Wnt1-Cre/R26R embryos at E12.5 revealed blue cells arranged in lines, reminiscent of nerve tracts within the venous pole of the heart (Fig. 3A, arrows). These tracts of cells did not stain with either of the myocardial markers MF20 or Cardiac Troponin 1 (data not shown), but did stain with the neuronal marker NF160D (Fig. 3B). In order to determine the specificity of the various neuronal elements, we used markers that differentiate sympathetic from parasympathetic neurons. Staining for tyrosine hydroxylase, a marker of sympathetic neurons, revealed little expression in the vagal trunks, and only minimal expression in the dorsal region of the posterior cardiac plexus (arrowheads in Fig. 3C). Staining was also observed, however, in the nerves found within the dorsal mesocardial connection (arrow in Fig. 3C). In contrast, staining for the vesicular acetylcholine transporter (VAChT), which specifically stains parasympathetic neurons, revealed robust staining in the vagal trunks, the posterior cardiac plexus and also within the neuronal cells migrating into the venous pole (Fig. 3D). This demonstrates that the major neuronal input into the venous pole of the heart is parasympathetic. Interestingly, analysis of the neural crest cell-derived nerves in the neural crest-deficient mouse mutant Splotch2H (Sp2H) showed that the posterior cardiac plexus, the vagal nerve and the nerves entering the heart via the dorsal mesocardium all formed normally in Sp2H/Sp2H fetuses (Fig. 3E,F), despite dramatic reductions in the number of neural crest cells at the arterial pole of the heart (data not shown; see also Epstein et al. 2000). This supports the idea that the neural crest cells at the arterial and venous poles of the heart have distinct origins and fates.
Fig. 3.
At E12.5, the majority of cells in the vagal nerve and posterior cardiac plexus are cholinergic parasympathetic neurons. (A) β-Gal staining reveals a stream of neural crest-derived cells (blue) within the dorsal mesocardial connection, which is reminiscent of nerve tracts (arrows). (B) NF160D staining marks the vagal nerve, the posterior cardiac plexus and confirms the presence of neuronal staining within the dorsal mesocardium (arrows). (C) The sympathetic neuron marker tyrosine hydroxylase marks only a subset of the cells in the vagal nerve and cardiac plexus (arrowheads), but does stain the nerve within the dorsal mesocardium. (D) In contrast, VAChT appears to stain the majority of the vagal nerve and posterior cardiac plexus and also localizes to the nerve in the dorsal mesocardium (arrows). (E) In Sp2H/+ embryos, which display a phenotype comparable with wild-type embryos, the stream of neural crest-derived cells resembling nerve tracts can be seen entering the heart through the dorsal mesocardium at E13.5 (arrow). Neural crest-derived cells are also observed in the vagal branches and cardiac plexus. (F) In Sp2H/Sp2H embryos at E13.5, similar patterns of β-gal staining are observed, indicating that the vagus nerve and cardiac plexus are formed normally and that cardiac innervation through the dorsal mesocardium occurs (arrow). AV, atrioventricular cushions; CP, cardiac plexus; V, vagal nerve.
Cells derived from the neural crest co-localize with cardiac nerves
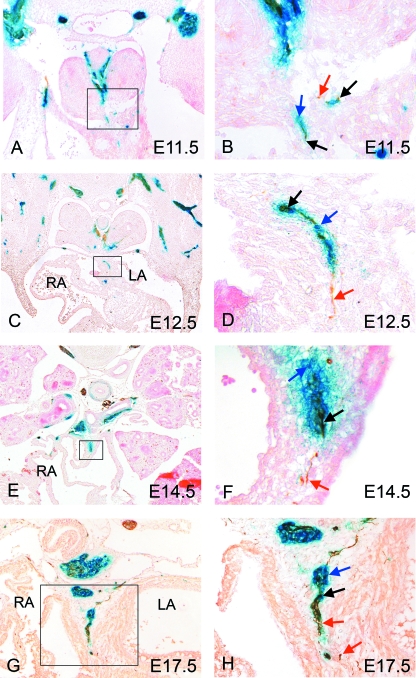
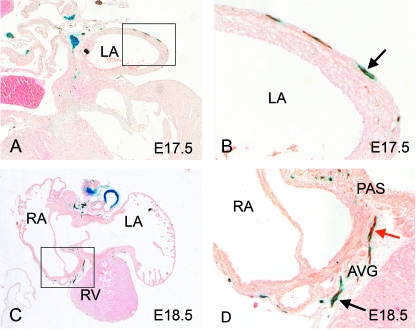
Double staining for β-gal and NF160D at E11.5 revealed some co-localization in the dorsal mesocardium, suggesting that these nerves may have at least a partial origin from the neural crest (Fig. 4A,B, black arrows). There were also, however, some NF160D-positive neurofilaments that were not β-gal positive (Fig. 4B, red arrow), similar to those observed in the main body of the vagal nerves and within the cardiac plexus. Overall, β-gal staining was more abundant and surrounded the nerve, similar to the pattern observed in the early stages of development of the vagal nerve (Fig. 4B, blue arrow). Staining at later stages revealed that the in-growing nerves seen proximally were β-gal negative (Fig. 4C–F, red arrows). More distally, the close association with β-gal-positive staining was maintained, again with a large amount of β-gal staining surrounding the nerve (Fig. 4C–F, black and blue arrows). These observations are consistent with cells derived from the neural crest travelling along developed cardiac nerves to form some neurons, but also supporting cells and glia, as reported in the chick (Kirby et al. 1983; Verberne et al. 1988). At E17.5, just prior to birth, co-localization of both NF160D and β-gal was observed along the developing nerves, although some peripheral neurofilaments remained β-gal negative (Fig. 4G,H, red arrows), and non-neuronal cells derived from the neural crest were still evident (Fig. 4H, blue arrow). A close association between β-gal-positive cells and NF160D-positive cells was also evident at E17.5 and E18.5 in the atrial wall, the primary atrial septum and the atrioventricular grooves (Fig. 5A–D).
Fig. 4.
Nerves growing into the venous pole of the heart are a mixture of neural crest- and non-neural crest-derived neurons. In each case, blue arrows mark solely β-gal-stained cells (blue), red arrows mark solely NF160D-stained cells (brown) and black arrows marked double labelled cells (blue/brown). (A,B) Neural crest cells expressing β-gal (blue arrows) can first be seen in the dorsal mesocardium at E11.5, co-localizing with neuronal staining (red arrows). (C–H) Nerves can be identified within the venous pole of the heart throughout gestation, in each case comprising a mixed population of neural crest-derived neurons (black arrows), neural crest-derived non-neural cells (blue arrows) and more proximally, non-neural-crest derived neurons (red arrows). LA, left atrium; RA, right atrium.
Fig. 5.
At E17.5 (A,B) and E18.5 (C,D) nerves of both neural crest and non-neural crest derivation can be seen in the walls of the atria and in the atrio-ventricular sulcus. Blue arrows mark blue-stained neural crest-derived cells, while black arrows mark cells of neural crest origin (blue-stained) that also stain with the neuronal marker NF160D (brown). AVG, atrioventricular groove; LA, left atrium; PAS, primary atrial septum; RA, right atrium; RV, right ventricle.
Innervation of the conduction tissues
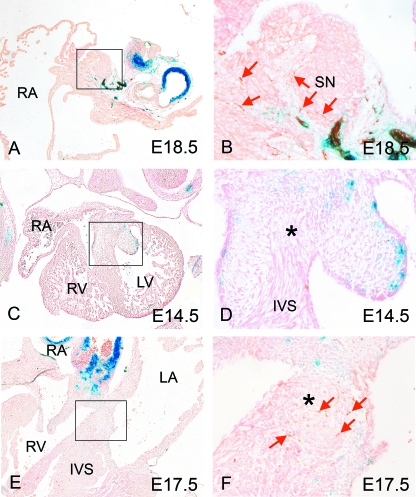
At E12.5, before the nodal tissue can be identified, close examination of the regions at the anticipated sites of formation of the sinus and atrioventricular nodes failed to reveal any blue staining (data not shown). This situation was maintained throughout E13.5 and E14.5, by which time the nodal tissues and the atrioventricular conduction axis, by now recognizable on the basis of their anatomy, had become distinct, with the sinus node also being identified by its expression of Islet 1 (data not shown) (Sun et al. 2007). The sinus node itself did not contain any cells of neural-crest origin (Fig. 6A,B). By E17.5 and E18.5, staining for NF160D was found in the environs of both the sinus node and the atrioventricular conduction axis, indicating that they were now both innervated; however, these neurons are not derived from the neural crest (Fig. 6A,B,E,F). Significantly, at no stage did we identify any part of the myocardial atrioventricular conduction axis as being derived from the neural crest.
Fig. 6.
Neural crest cell (NCC)-derived neurons do not directly innervate the nodal tissue. (A,B) At E18.5, NF160D staining shows that the sinus node is innervated, although only directly by cells of non-neural crest origin (brown; red arrows). (C,D) There are no neurons in close proximity to the developing atrioventricular conduction axis (asterisk) at E14.5. (E,F) By E17.5, Neuronal cell bodies of non-neural crest origin (red arrows) can be detected at the periphery of the atrioventricular conduction axis (asterisk). No blue, neural crest-derived cells, were detected. Ao, aorta; AVN, atrioventricular node; IVS, interventricular septum; LA, left atrium; LV, left ventricle; PAS, primary atrial septum; RA, right atrium; RV, right ventricle; SN, sinus node; SCV, superior caval vein.
Cells derived from the neural crest enter the atrioventricular cushions
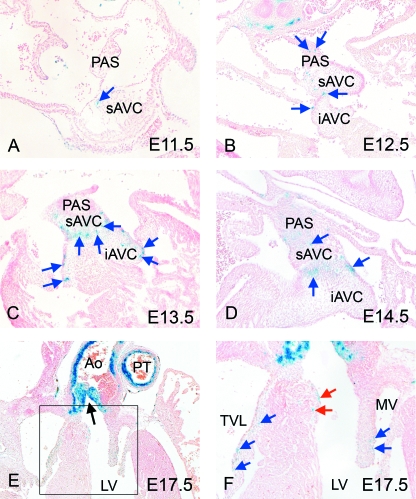
Blue-stained, β-galactosidase-positive, cells were seen within the atrioventricular cushions of some, but not all, of the embryos examined at E11.5 (Fig. 7A, arrow), and again at E12.5 (Fig. 7B). Isolated blue cells were also seen within the dorsal mesocardial connection, and in the primary atrial septum. Those within the atrioventricular cushions became more abundant by E13.5 and E14.5, localizing to the periphery, where they were seen subendocardially, within and close to the sites of fusion of the cushions. The number of cells within the atrioventricular cushions was sparse when compared with those in the outflow tract (compare Fig. 7C,D with Fig. 1G). By E17.5, the marked cells were localized to the leaflets of the tricuspid and mitral valves (Fig. 7E,F, blue arrows), while others were seen in close proximity to the left bundle branch of the developing ventricular conduction system (Fig. 7F, red arrows). Our analysis suggests that these cells derived from the neural crest enter via the venous rather than the arterial pole of the heart, although we cannot be certain in the light of the continuity between the endocardial cushions of the atrioventricular canal and those of the proximal outflow tract in the region of the inner heart curve.
Fig. 7.
Neural crest cells are found in the atrioventricular cushions. (A,B) Neural crest cells are found as isolated cells in the atrioventricular cushions at E11.5 (arrow) and are more abundant by E12.5 (arrows). (C,D) Neural crest cells are more abundant in the atrioventricular cushions by E13.5 and E14.5, with many appearing in the endocardial region of the developing valves and the fusion seams between the cushions. (E,F) At E17.5, neural crest cells are seen within the leaflets of the atrioventricular valves (blue arrows) as well as some cells being seen within the interventricular septum, close to the septal crest (red arrows). The aortic outflow is marked by an arrow in E. Ao, aorta; AVC, atrioventricular cushion (i = inferior, s = superior); IVS, interventricular septum; LV, left ventricle; MV, mitral valve; PAS, primary atrial septum; PT, pulmonary trunk; TVL, tricuspid valve leaflet.
Discussion
Cells derived from the neural crest are known to invade the venous pole of the developing heart (Poelmann et al. 1998; Jiang et al. 2000; Pietri et al. 2003). Their role in this region is less well understood than that at the arterial pole, where they are known to play an essential role in septation, as well as making contributions to the arterial roots and cardiac ganglia (Kirby et al. 1985; Besson et al. 1986; Bockman et al. 1989). In this study, we have demonstrated that, in the mouse, cells derived from the neural crest enter the venous pole and contribute to the posterior cardiac plexus and ganglia. We also show that not all the nerves entering the heart through the venous pole take their origin from the neural crest, or even have a contribution from this source. Importantly, we have also shown that cells derived from the neural crest have no direct role in the innervation of the sinus and atrioventricular nodes. More significantly, they do not contribute to the conduction axis itself, although at later stages a few cells derived from the neural crest are found in close proximity to the left bundle branch. Neural crest cells are also seen scattered sparsely within the mesenchyme of the atrioventricular cushions of some, but not all, of the embryos examined.
Migration to the venous pole
The cells migrating from the embryonic neural crest reach the heart through the venous pole at day 11.5, whereas they enter the arterial pole a full day earlier. We are confident that this temporal lag is real, and not an artefact of our model, given that the Cre-recombination event takes place before the migratory cells leave the neural crest (Jiang et al. 2000). Wnt1 itself is not expressed in the developing heart, so it can be assumed that any cells within the heart that express β-gal have migrated there from the neural crest. Many of these β-gal-expressing cells show neuronal activity, or co-localize with neuronal elements, although some neuronal cells are already found in the dorsal mesocardium at the venous pole prior to the appearance of this migrating population. It is striking that the posterior cardiac plexus in the mouse, rather than being derived exclusively from the neural crest, merely has a contribution from this source, with many other ganglionic cells also being present, presumably derived from the neurogenic placodes, as is the vagus nerve itself.
In the outflow tract, many of the cells derived from the crest either disappear (Jiang et al. 2000) or become restricted to a subendothelial layer within the newly separated aortic and pulmonary components of the outflow tract. A further small subset of neural crest cells is known to contribute to innervation of the arterial pole (Jiang et al. 2000; Pietri et al. 2003). In contrast, although fewer neural crest cells enter the venous pole of the heart via the dorsal mesocardial connection, we show that a significant number make a permanent contribution to the innervation of this part of the heart. This distinction between the neural crest cells that contribute to the arterial and venous poles of the heart is supported by our analysis of the neural crest cell-deficient Sp2H mouse. In Sp2H/Sp2H fetuses, there was normal formation of the cardiac plexus, vagal nerve and the nerves entering the heart via the dorsal mesocardium, despite the dramatic and well-reported reduction in the number of neural crest cells entering the arterial pole of the heart (Conway et al. 1997; Epstein et al. 2000). Thus, cells from the neural crest entering at opposing poles of the heart are likely to have different origins and fates, with those entering the arterial pole originating in the occipital region of the hindbrain neural tube, and those entering the venous pole probably arising slightly more posteriorly, within the so-called ‘vagal’ neural crest region, in the anterior region of the trunk.
Poelmann et al. (2004) have argued that, in the mouse, neural crest-derived cells co-localize with the developing ventricular conduction system. They suggested that these cells may play some form of inductive role. In this respect, they reported, but did not show, cells derived from the neural crest arriving in the heart at embryonic day 8.5. We, however, were unable to find cells derived from the neural crest within the heart at this early stage. Although the development of the conduction system was not the focus of our study, we did find a small subpopulation of neural crest-derived cells that were seen to co-localize with the ventricular conduction system, particularly those of the left bundle branch, by day 17.5 of gestation.
We found no evidence to suggest that neural crest-derived cells contributed materially to the penetrating bundle of His, as was suggested by Nakamura et al. (2006). Our data presented here are in keeping with those of Nakamura et al. (2006), our findings using the ROSA 26R marker being virtually identical to their data using green fluorescent protein. We cannot, however, support their interpretation. This is because the tracts of neural crest cells that enter the venous pole of the heart contain neurofilamentous material, and can be traced to the cardiac plexus. Coupled with the fact that cells within this tract also express vesicular acetylcholine transporter and tyrosine hydroxylase, specific markers of parasympathetic and sympathetic neurons, respectively, our findings demonstrate that the marked cells are neurogenic rather than conductive in nature. These cells, therefore, are contributing to the innervation of the heart. Indeed, the use of solely neural markers (NF160, TuJ1 and BLBP) by Nakamura et al. (2006) may call into question their interpretation of the fate of the neural crest-derived structures they observe as being components of the conduction system.
We have doubts as to whether the structure identified by Nakamura and colleagues as the ‘bundle of His’ truly represents the penetrating component of the atrioventricular conduction axis. The gold standard for recognition of the bundle of His, the only muscular structure crossing the developing plane of atrioventricular insulation, remains anatomical (Tawara, 1906). Although it was, at one time, believed that the conduction system originated from neural crest-derived cells (Gorza et al. 1988), it is now known that the cells forming the atrioventricular conduction axis are myocardial rather than neural in origin (Gourdie et al. 1995, 2003; Cheng et al. 1999). The tracts of neural crest cells that we saw entering the heart through the dorsal mesocardial connection, which were seemingly identified by Nakamura et al. as the Bundle of His, did not stain with our myocardial markers.
We were, however, able to trace neurons from the cardiac plexus to the venous pole of the heart, showing that some of these structures came into close proximity to the sites of development of the atrioventricular and sinus nodes. These neural elements may influence the speed of conduction, but are unlikely to be involved in the act of conduction itself. This interpretation is supported by the observation that tissue-specific knockout of HF-1b/Sp4 in neural crest cells results in conduction defects, but with these being related to a decrease in innervation of the atrioventricular node (St Amand et al. 2006). This should not be unexpected, as it is well recognized that the heart is innervated through the arterial and venous poles. The arterial pole provides the major input for sympathetic nerves, whilst the parasympathetic nerves enter the heart almost exclusively, if not exclusively, through the venous pole. Poelmann et al. (2004) have proposed an inductive role for the cells in the mouse derived from the neural crest. We have shown, however, that the cells are not observed in the proximity of the nodal tissue until after the nodes have formed. Thus, it is too late for these cells to play any role in induction of the conduction tissues themselves.
Cells from the neural crest populate the atrioventricular cushions
We were surprised to find, in some of our samples, cells derived from the neural crest within the atrioventricular cushions. These cells were scattered in individual fashion throughout the cushions, but were more abundant alongside the sites of fusion between the cushions and, subsequently, along the subendocardial surfaces of the valvar leaflets. We can only presume that the cells play some role in fusion of the cushions, or else are involved in the moulding of the cushions into the leaflets of the atrioventricular valves. They do remain identifiable in the atrioventricular valvar leaflets of postnatal mice, as was also demonstrated by Nakamura et al. (2006). Their presence seems to be dependent on genetic background (Nakamura et al. 2006), as they are not seen in every embryo examined. No relationship was found between this particular subset of neural crest-derived cells and the developing cardiac plexus or the developing atrioventricular conduction axis, although more complex epigenetic interactions cannot be ruled out.
Acknowledgments
We thank the British Heart Foundation, which funded this project in its entirety.
References
- Besson WT 3rd, Kirby ML, Van Mierop LH, Teabeaut JR 2nd. Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiovascular defects. Circulation. 1986;73:360–364. doi: 10.1161/01.cir.73.2.360. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Kirby ML. Alteration of early vascular development after ablation of cranial neural crest. Anat Rec. 1989;255:209–217. doi: 10.1002/ar.1092250306. [DOI] [PubMed] [Google Scholar]
- Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development. 1997;124:505–514. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Epstein DJ, Vekemans M, Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Li J, Lang D, et al. Migration of cardiac neural crest cells in Splotch embryos. Development. 2000;127:1869–1878. doi: 10.1242/dev.127.9.1869. [DOI] [PubMed] [Google Scholar]
- Gorza L, Schiaffino S, Vitadello M. Heart conduction system: a neural crest derivative? Brain Res. 1988;457:360–366. doi: 10.1016/0006-8993(88)90707-x. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, et al. His-Purkinje lineages and development. Novartis Found Symp. 2003;250:110–122. discussion 122–1244, 276–279, review. [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Steward DE. Neural crest origin of cardiac ganglion cells in the chick embryo. Identification and extirpation. Dev Biol. 1983;97:433–443. doi: 10.1016/0012-1606(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KL 3rd, Hays BM. Characterization of conotruncal malformations following ablation of ‘cardiac’ neural crest. Anat Rec. 1985;231:87–93. doi: 10.1002/ar.1092130112. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Mikawa T, Gittenberger-de Groot AC. Neural crest cells in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Dev Dyn. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC. A sub-population of apoptosis prone cardiac neural crest cells targets the venous pole: multiple functions in heart development? Dev Biol. 1999;207:271–286. doi: 10.1006/dbio.1998.9166. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Jongbloed MR, Molin DG, et al. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: a role in induction? Anat Embryol. 2004;208:389–393. doi: 10.1007/s00429-004-0401-6. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St Amand TR, Lu JT, Zamora M, et al. Distinct roles of HF-1b/Sp4 in ventricular and neural crest cells lineages affect cardiac conduction system development. Dev Biol. 2006;291:208–217. doi: 10.1016/j.ydbio.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liang X, Najafi N, et al. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara S. Das Reizleitungssystem des Säugetierherzens. Eine anatomische Studie über das atrioventrikuläre Bündel und die Purkinjeschen Fäden. Mit einem Vorwort von L. Aschoff. Jena: 1906. G. Fischer. [Google Scholar]
- Verberne ME, Gittenberger-De Groot AC, Poelmann RE. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat Embryol. 1988;189:171–184. doi: 10.1007/s004290050175. [DOI] [PubMed] [Google Scholar]
- Verberne ME, Gittenberger-De Groot AC, Van Iperen L, Poelmann RE. Contribution of the cervical sympathetic ganglia to the innervation of the pharyngeal arch arteries and the heart in the chick embryo. Anat Rec. 1999;255:407–419. doi: 10.1002/(SICI)1097-0185(19990801)255:4<407::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Verberne ME, Gittenberger-De Groot AC, van Iperen L, Poelmann RE. Distribution of different regions of cardiac neural crest in the extrinsic and intrinsic cardiac nervous system. Dev Dynam. 2000;217:191–204. doi: 10.1002/(SICI)1097-0177(200002)217:2<191::AID-DVDY6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Abe K, Mantani A, et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev Biol. 1999;212:191–203. doi: 10.1006/dbio.1999.9323. [DOI] [PubMed] [Google Scholar]