Abstract
There are few detailed descriptions of the coronary arterial patterns in the mouse. Some recent reports on coronary anomalies in mutant mouse models have uncovered the importance of several genes (i.e. iv and connexin43) in coronary morphogenesis. These mutations spontaneously appeared (iv) or were generated (connexin43) in a C57BL/6 background, which is widely used for the development of mutant mice. We have studied the origin and course of the main coronary arteries of two C57BL/6 mouse strains. Unusual anatomical coronary arterial patterns were found, including: solitary ostium in aorta, accessory ostium, high take-off, aortic intramural course, slit-like ostium, sinus-like ostium and origin of a septal artery from the left coronary artery. In humans, some of these conditions are clinically relevant. Most of these patterns, which differ from those observed in wild mice and Swiss albino mice, coincide with those previously found in iv/iv and connexin43 knockout mice. The results indicate that there is variability in the coronary arterial arrangement of the laboratory mouse. Care should be taken when analysing coronary phenotypes of mutant mouse models.
Keywords: connexin43, coronary arteries, iv, mouse, mutant, strain
Introduction
The use of the mouse (Mus musculus) as an animal model for cardiovascular research involves anatomical (Durán et al. 1992; Icardo & Colvee 2001), embryological (González-Iriarte et al. 2003; Ratajska et al. 2005), functional (van den Boos et al. 2005; Kumar et al. 2005; Takagawa et al. 2007) and genetic (Fernández et al. 2000; Li et al. 2002; Walker et al. 2005; Clauss et al. 2006; Liu et al. 2006) studies of the coronary vascular tree. It is clear that any coherent interpretation of the results obtained from the different approaches requires accurate knowledge of the murine coronary arteries. This is especially important when surgical coronary arterial occlusions are performed, or when the coronary pattern is studied in the setting of genetically modified mice.
Detailed descriptions of the normal arrangement of the coronary arteries (CAs) in the mouse are scarce. Icardo and Colvee (2001) reported on the coronary arterial anatomy of adult mice belonging to the Swiss albino strain. Their study comprised a comparative analysis with the iv/iv mutant mouse. A wide variety of anomalies in the origin and course of the CAs was found within the mutant mice; these included single CA, accessory coronary ostium, high take-off, slit-like ostium, sinus-like ostium and origin of the septal artery from the left CA. All these conditions appeared in normal adult hearts, were independent of the heart situs, did not compromise mouse survival and were considered to be a side-effect of the iv mutation (Icardo & Colvee 2001). Curiously, connexin43α1 (Cx43) knockout mice show a similar set of coronary pattern anomalies (Li et al. 2002; Clauss et al. 2006).
In an ongoing study on the anatomy and embryology of the murine CAs, we detected several coronary anomalies in mice belonging to the C57BL/6J mouse strain, which is widely used for the generation of genetically modified mouse models. This prompted us to investigate the coronary phenotype in this strain and in the closely related C57BL/6N. Put simply, the entire set of coronary pattern anomalies occurring in iv/iv and Cx43 mice was detected in C57BL/6 mice as well. Our aim here was to report our findings, and to stress the importance of proper selection of the mouse strain in studies dealing with the coronary system.
Material and methods
C57BL/6J mice (n = 108) were purchased from Charles River Laboratories, France. C57BL/6N mice (n = 17) were purchased from Taconic, Denmark. The animals were handled in accordance with European and Spanish guidelines for animal welfare. They were housed in standard cages and fed water and chow ad libitum. The animals were killed via an anaesthetic overdose (ketamin/xylazine) or by cervical dislocation.
The origin and course of the CAs of neonatal and adult specimens were analysed by means of scanning electron microscopy (SEM) (n = 61), histological methods for light microscopy (n = 54) and a corrosion-cast technique (n = 10). For SEM, the ascending aorta, together with the valve, and part of the left outflow tract were dissected out, and the coronary ostia were carefully exposed. The specimens were then fixed, dehydrated, gold-coated and observed under a JeolJMS scanning electron microscope operated at 10 or 15 kV (Sans-Coma et al. 1996). For histological techniques, adult and neonatal hearts were fixed, dehydrated and embedded in Paraplast, and serially cut from the ascending aorta to the apex or middle of the heart. Five-, 7- or 10-µm thick transversal or sagittal sections were stained with haematoxylin-eosin, resorcin-fuchsin for the detection of elastin, as well as Mallory's and Masson-Goldner's trichrome stains (Sans-Coma et al. 1993). For the corrosion-cast technique, Rhodopas AX85/15 was used as previously described (Durán et al. 1992). The resin was infused through the apex of the left ventricle. After resin polymerization and tissue maceration, the casts of the CAs and the left ventricle were observed under a binocular microscope. Images were acquired using a Nikon DXM1200 camera.
The nomenclature used is that of Sans-Coma et al. (1993) and Durán et al. (2005, 2007).
Results
The coronary arterial pattern was similar in C57BL/6J and C57BL/6N mice. As in other rodents species, the heart of the mouse has no interventricular grooves and two CAs, right and left, are usually present; they arise from the right- and left-facing aortic sinuses, respectively (Fig. 1a), and become intramyocardial shortly after their origin.
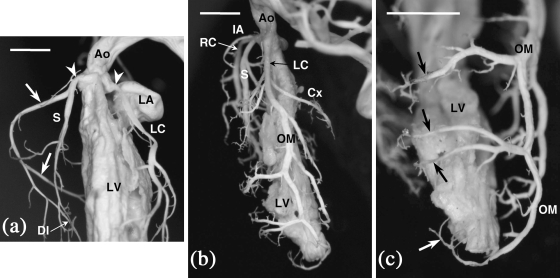
Fig. 1.
Internal casts of the left ventricle and the CAs of C57BL/6J mice. (a) Frontal view. The right and left CAs arise from the right- and left-facing aortic sinuses (arrowheads), respectively. The right CA (arrows) surrounds the tricuspid valve, and gives rise to the dorsal interventricular (DI) branch. The septal artery (S) originates from the right CA, soon after its origin from the aorta. Ao = aorta; LA = left atrium; LC = left CA; LV = left ventricle. (b) Left view. The left main coronary trunk (LC) divides into the left circumflex (Cx) and the obtuse marginal (OM) arteries. Note that an infundibular artery (IA) arises from the right CA (RC), very close to its origin from the aorta. Ao = aorta; LV = left ventricle; S = septal artery. (c) Frontal view. Perforating vessels (arrows) arise from the distal segment of the obtuse marginal artery (OM) and irrigate the distal portion of the ventricular septum. LV = left ventricle. Scale bars = 100 µm.
The right CA (Fig. 1a) supplies the right side of the heart. It runs more or less parallel to the right atrioventricular sulcus, reaching usually the dorsal interventricular boundary. Having surpassed the acute margin of the heart, the vessel gives off a branch that crosses the right ventricle dorsal wall more or less obliquely. Thereafter, this branch turns towards the apex of the heart as a dorsal interventricular branch, which can be considered equivalent to the posterior descending artery of humans. At the level of the acute margin, the right CA often gives rise to an acute marginal branch of a somewhat variable course.
The left CA (Fig. 1b), which irrigates the left side of the heart, consists of a left main coronary arterial trunk that divides into the left circumflex and the obtuse marginal arteries. The left circumflex artery courses more or less parallel to the left atrioventricular groove, terminating close to the crux cordis. The obtuse marginal artery runs along the obtuse margin down to the apex of the heart, giving rise to branches that irrigate the ventral and the dorsal walls of the left ventricle. This artery can be considered equivalent to the left anterior descending artery of humans. A short ventral interventricular branch arising from the left main coronary arterial trunk seldom exists in the mouse.
In all C57BL/6 mice examined, the interventricular septum is supplied by one or two septal arteries (Figs 1a,b and 2), connected to the CAs or directly to the aortic sinuses. Moreover, the distal portion of the septum is frequently supplied by perforating vessels, coming from the right and/or the left CAs (Fig. 1c). In this regard, it should be noted that in the specimens examined, the coronary arterial pattern shows individual variation with regard to the presence and size of the coronary branches. Nevertheless, as in other rodents with intramyocardial coronary arteries, in C57BL/6 mice the presence of a septal artery is a constant feature. In the present study, particular attention was paid to the number and origin of the septal arteries, the proximal course of the coronary arterial trunks, and the number, shape and position of the coronary ostia in the aortic root (Table 1).
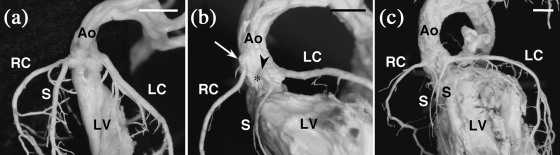
Fig. 2.
Internal casts of the left ventricle and the CAs of C57BL/6J mice. Frontal views. (a) The septal artery and the right CA arise from a common ostium at the right-facing aortic sinus. (b) The septal artery originates from a separate ostium at the left aortic sinus (arrowhead). The ostium of the septal artery is located close to the ventral commissure (asterisk), whereas the right coronary ostium displays a high take-off (arrow). (c) One septal artery originates from the right CA and another from the left CA. In both cases, the septal artery and the right or left CA arise from a common ostium at the right- and left-facing aortic sinuses. Ao = aorta; LC = left CA; LV = left ventricle; RC = right CA; S = septal artery. Scale bars = 100 µm.
Table 1.
Variation (%) in the coronary arterial arrangement found in 108 C57BL/6J and 17 C57BL/6N mice
| Septal artery | Accessory ostium | High take-off | Intramural course | Single CA | ||
|---|---|---|---|---|---|---|
| Right | Left | Both | ||||
| C57BL/6J | ||||||
| n = 47 (C + H) | n = 107 (C + H + S) | n = 108 (C + H + S) | n = 37 (H) | n = 108 (C + H + S) | ||
| 34% | 26% | 40% | 31% | 58% | 62% | 5% |
| C57BL/6N | ||||||
| n = 17 (H) | n = 17 (H) | n = 17 (H) | n = 17 (H) | n = 17 (H) | ||
| 35% | 24% | 41% | 24% | 59% | 65% | 6% |
C = corrosion-cast technique; H = light microscopy; n = number of specimens examined; S = scanning electron microscopy. See text for further explanation.
Sixteen (34%) of 47 C57BL/6J and six (35%) of 17 C57BL/6N mice examined by corrosion-cast or histological techniques showed a septal artery originating from the right CA (Fig. 2a) or directly from the right aortic sinus. In other 12 (26%) C57BL/6J and four (24%) C57BL/6N specimens, the septal artery arose from the left CA or sinus (Fig. 2b). The remaining 19 (40%) C57BL/6J and seven (41%) C57BL/6N mice possessed two septal arteries of variable size, one originating from the right and the other from the left CA (Fig. 2c) or sinus. Each septal artery supplied a different portion of the septum.
Two or more coronary ostia in the same sinus (Figs 2b and 3a,c) were found in 33 (31%) of 107 C57BL/6J specimens and four (24%) of 17 C57BL/6N specimens examined by corrosion-cast, histological or SEM techniques. The accessory ostium corresponded to either a septal artery (Fig. 2b), or a small artery that irrigated the right ventricular infundibulum or the atrium. Accessory ostia of the infundibular or atrial arteries were smaller than those of the septal artery and the main trunks of the CAs (Fig. 3c).
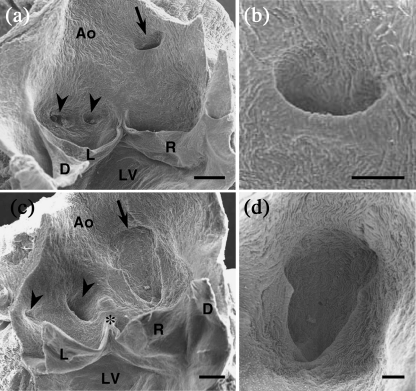
Fig. 3.
Scanning electron micrographs of the aortic valves of C57BL/6J mice. Frontal views from the luminal side. (a) In this specimen, two ostia with similar sizes and rounded openings (arrowheads) are present in the left aortic sinus, whereas the right coronary ostium (arrow) is located above the aortic sinus and shows a slit-like opening. (b) High magnification of a slit-like ostium corresponding to a CA with a high take-off. Note that the stem of the CA forms an acute angle with respect to the longitudinal axis of the aorta. As a consequence, the ostium shows a slit-like shape. (c) In this specimen, the left aortic sinus holds two different sized ostia (arrowheads). The small one corresponds to an atrial artery. The large one corresponds to the left CA and is displaced to the right, close to the ventral commissure (asterisk). The right coronary ostium (arrow) is extremely large, is located above the aortic sinuses and has a sinus- or keyhole-like shape. (d) High magnification of a sinus-like ostium. Note the wide opening and keyhole-like shape. Ao = aorta; D = dorsal leaflet; L = left leaflet; R = right leaflet; LV = left ventricle. Scale bars: a,c, 200 µm; b,d, 100 µm.
The position of the coronary ostia at the aortic root was highly variable among the 125 C57BL/6 mice studied. In 45 (42%) C57BL/6J and seven (41%) C57BL/6N mice, the ostia were located in the aortic sinuses, considering the sinotubular junction as the distal limit of the sinuses. The remaining 63 (58%) C57BL/6J and ten (59%) C57BL/6N specimens exhibited one or two CAs, which originated above the sinuses, a condition named high take-off (Figs 2b and 3a). High take-offs were usually much higher and more frequent in the right CA than in the left CA. In some cases with aortic sinuses holding accessory ostia, both ostia showed a high take-off. Often, one ostium of the left aortic sinus was displaced towards the right, locating close to the ventral (anterior in man) commissure (Figs 2b and 3c).
We observed three different ostium morphologies in both C57BL/6J and C57BL/6N strains. Most of the ostia located in the aortic sinuses showed a rounded opening (Fig. 3a). The ostia corresponding to high take-offs often displayed a slit-like shape (Fig. 3a,b). In several cases with a high take-off, the ostia presented a wide, sinus-like or keyhole-like shape (Fig. 3c,d). In all cases with a high take-off and slit-like ostium, the CA arose from the aortic root, forming an acute angle with regard to the longitudinal axis of the ascending aorta (Fig. 3a,b). In addition, the proximal segment (stem) of these coronary arterial trunks run parallel to the aorta, both sharing the outer adventitia and part of the media, a condition called intramural course (Fig. 4a,c,d). An aortic intramural course was assessed in 23 (62%) of 37 C57BL/6J and 11 (65%) of 17 C57BL/6N specimens examined by histological procedures. This condition was found to be the most frequent, but not unique, in CAs with a high take-off.
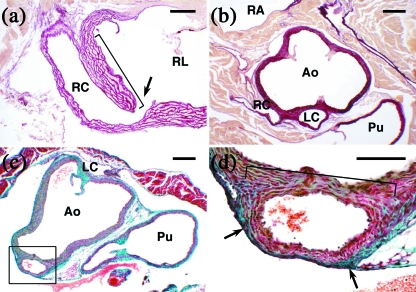
Fig. 4.
Photomicrographs of longitudinal (a), transversal (b) and oblique (c,d) histological sections of the aortic valves and ascending aortas of C57BL/6J mice, stained with resorcin-fuchsin (a,b) and Masson-Goldner's trichrome (c,d). (a) The right CA (RC) of this specimen shows a high take-off (arrow) and an aortic intramural course (bracket). Note that the sinus wall shears part of the media with the CA. RL = right leaflet. (b) This specimen has a solitary, high take-off, coronary ostium in aorta. The section corresponds to the level where the single coronary trunk divides into a right (RC) and a left (LC) CA. The right CA courses the wall of the right ventricle. The left CA runs between the aorta (Ao) and the pulmonary artery (Pu). RA = right atrium. (c,d) In this specimen, the right CA (square) shows a high take-off. Note that part of the aortic and coronary walls shear the media (bracket) and adventitia (arrows). (d) is a close-up of the square outlined in (c). Ao = aorta; LC = left commissure; Pu = pulmonary artery. Scale bars: a,d, 100 µm; b,c, 200 µm.
In five (5%) C57BL/6J specimens and one (6%) C57BL/6N specimen examined by histological techniques and SEM, the aortic root showed only one ostium located in or above the right aortic sinus, whereas the left aortic sinus was devoid of any ostium, a condition named solitary coronary ostium in aorta (Fig. 4b). Study of serial sections of the specimens examined by histological techniques revealed that the left ventricle was irrigated by an anatomically normal left CA, which was connected to the right CA, instead of being connected to the left aortic sinus. The left main coronary trunk branched from the right CA soon after its origin from the aorta, and passed between the aorta and the pulmonary artery (Fig. 4b) towards the left ventricle, giving off the left coronary arterial components. In two cases, the septal artery originated from the left main coronary trunk, when it passed between the right and left outflow tracts. In the remaining three specimens, the septal artery arose from the right CA.
Discussion
In mammals, two CAs normally carry blood to the heart. They originate from the aortic root and follow subepicardial or intramyocardial courses, depending on each species (Hadziselimovic et al. 1974). In those species with intramyocardial CAs, the interventricular septum is mainly irrigated by a distinct artery, not equivalent to the anterior descending artery in humans, named the septal artery, the anatomical origin of which varies among species (Durán et al. 1991; for a review, see Durán et al. 1992). In the wild-living house mouse (Durán et al. 1992), the CAs are intramyocardial, and the septal artery originates from the right CA or, less frequently, directly from the right aortic sinus. The same pattern has been described for the Swiss albino strain of the laboratory mouse (Icardo & Colvee 2001). However, in the present study we have found that in the C57BL/6J and C57BL/6N strains, the septal artery may originate from the right CA, from the left CA, or two septal arteries may coexist, each originating from a different CA or sinus. The presence of left and double septal arteries in C57BL/6J mice has been previously reported (Salto-Tellez et al. 2004), and constitutes a frequent arrangement in other rodent species, such as the Syrian hamster (Mesocricetus auratus) (for a review, see Durán et al. 1992). Therefore, the anatomical origin of the septal artery in the mouse appears to be a variable condition that depends on the strain or population analysed.
The most striking feature observed in this study is that C57BL/6 mice exhibit, in addition to left and double septal arteries, a pool of anomalies in the origin and course of the main coronary trunks. This includes accessory ostium, high take-off, slit- and keyhole-like ostium, aortic intramural course and solitary coronary ostium in aorta. In this regard, it is important to emphasize that the specimens studied here belonged to two different C57BL/6 strains (‘J’ and ‘N’), which were purchased from different companies and maintained in different laboratories. The C57BL/6J strain was developed by the Jackson Laboratories (USA) in 1948, and the C57BL/6N strain derives from several C57BL/6J animals that were transferred to the National Institutes of Health (USA) in 1951. Therefore, the anomalous coronary arterial arrangements described here can be considered distinguishing traits of the C57BL/6 strains. These strains are the most widely used in the generation of genetically manipulated mice.
All the anomalies detected in C57BL/6 mice, including the origin of the septal artery from the left CA, have been previously described in two mutant mouse models: the iv/iv mouse (Icardo & Colvee 2001) and the Cx43 knockout mouse (Li et al. 2002; Clauss et al. 2006). Hearts of iv/iv mice with normal situs (concordant ventriculoarterial and atrioventricular connections) show the entire set of coronary arterial anomalies reported herein, except for the aortic intramural course, which was not mentioned in the study of Icardo & Colvee (2001). Moreover, the proportion of anomalous patterns found in the present C57BL/6 mice is very similar to that reported by these authors in the iv/iv mutants. The iv/iv mice studied by Icardo & Colvee (2001) were produced in the C57BL/6J background strain, whereas the comparative analyses were performed using mice from the Swiss albino strain. The present results indicate that the coronary arterial anomalies recorded in iv/iv mice should be ascribed to the C57BL/6 genetic background, and not to the iv gene mutation. Cx43 knockout mice show concordant heart connections and outflow tract obstruction due to the abnormal formation of infundibular pouches (Reaume et al. 1995). In addition, several defects in the formation of the CAs have been detected, including reduction of vascular smooth muscle myosin in the media (Li et al. 2002), aneurisms (Liu et al. 2006), reduced branching complexity and persistence of subepicardial vascular plexuses (Walker et al. 2005). Coronary pattern anomalies have been described in two different papers (Li et al. 2002; Clauss et al. 2006). These pattern anomalies include all the arrangements described in the present paper, except for slit- and sinus-like ostia. The shape of the ostium was properly observed here by SEM, a method not employed in the two studies previously cited. In both former studies the anomalies occurred in a high and similar proportion of homozygous and heterozygous mutant specimens, but not in wild-type mice. Although the strain background to which the wild-type animals belonged was not specifically indicated in the papers, it was stated that, in order to obtain the experimental animals, Cx43 knockout parental mice in a C57BL/6 background were outcrossed once or twice to animals belonging to three independent strains (129sv, CD1, FVB/n) (Li et al. 2002; Clauss et al. 2006). Obviously, it is difficult to ascertain the background of the wild and mutant mice from which comparisons were performed. Therefore, similar to the case of iv/iv mice, it is possible that the anomalies in the coronary arterial pattern detected by these authors are not the consequence of the Connexin mutation, but the result of the C57BL/6 genetic background. Alternatively, the C57BL/6 vascular phenotype described in the present study might be the consequence of (a) spontaneous mutation(s) in the Cx43 gene or its pathway, occurring earlier than 1951. It should be noted, however, that we have not found any of the cardiovascular defects associated with the Cx43 deletion cited above, which are most probably the consequence of Cx43 deficiency during embryogenesis. Following the most parsimonious hypothesis, we propose that the anomalous patterns described here constitute a C57BL/6-associated vascular phenotype, which was added to the Cx43 and iv mutant phenotypes when these mutant strains were initially generated.
The different arrangements of the origin of the CAs reported in the present study are similar to those found in humans. For instance, the presence of accessory or supernumerary ostia occurs in a significant proportion of the human population (Becker 1981; Teplitsky et al. 1987), causing no deficiency in coronary perfusion. Likewise, the abnormal position of one coronary ostium above the level of the sinus of Valsalva (high take-off) does not cause any physiological constraint per se. However, this condition appears usually to be associated with a slit-like opening of the CA. Patients with a slit-like ostium show a high risk to severe myocardial ischaemia and even sudden death (Mahowald et al. 1986; García Rinaldi et al. 1994; Basso et al. 2002). This is probably due to the closure of the slit or flap, caused by an enhanced left diastolic pressure during exercise. Similarly, exercise may induce a compression of the wall of the CA, when it possesses an aortic intramural course, resulting in severe ischaemia (Sacks et al. 1977; Mahowald et al. 1986). However, these anomalies do not seem to cause any disadvantage in C57BL/6 mice, because affected animals have a normal life span, and show no signs of myocardial ischaemia. Nevertheless, we are not aware of experiments involving endurance exercise in C57BL/6 mice.
Patients with a solitary coronary ostium in aorta are at a high risk of sudden death, when the CA irrigating the left heart originates anomalously and passes between the aortic and pulmonary trunks (Cheitlin et al. 1974; Liberthson et al. 1979; Barth et al. 1986; Basso et al. 2000). This artery may be compressed during the cardiac cycle, causing a drastic perfusion defect. In the mouse specimens presented here, the left CA coursed between the aorta and the pulmonary artery. We have previously reported on the occurrence of a similar anomalous pattern in a relatively high proportion of laboratory hamsters belonging to an inbred colony (Durán et al. 2005), as well as in wild house mice (Arqué et al. 1986). Thus, it can be concluded that, although the existence of two CAs is probably the most efficient irrigation pattern for the mammalian heart, the presence of a solitary coronary ostium in aorta does not cause any disadvantage in animals with intramyocardial CAs.
Concluding remarks
We have found a pool of distinct coronary arterial arrangements in C57BL/6 mice, including the left origin of the septal artery, accessory coronary ostium, high take-off, slit- and sinus-like ostium, aortic intramural course, and solitary coronary ostium in aorta. These patterns can appear in different combinations, and affect a high proportion of specimens belonging to C57BL/6 strains. The significance of these findings should be underlined. C57BL/6 mice may provide us with a unique opportunity to unveil many of the factors implicated in normal and abnormal coronary development.
The coronary arterial patterns presented here are very similar to those found in iv/iv and Cx43 mutant mice, both of which were developed in a C57BL/6 background. We conclude that, in all probability, the mutations of these genes do not cause the cited alterations in the coronary pattern, which are apparently a result of the C57BL/6 genetic background. Crossbreeding of the different mutant mice with specimens from unrelated strains, having no coronary anomalies, should be performed, in order to ensure the function of these genes in coronary arterial development. Further studies should also address whether these coronary phenotypes are exclusive to the C57BL/6 strain, or may also appear in other mouse strains. Nevertheless, researchers dealing with coronary arterial anatomy, embryology or physiology, using the mouse as an animal model, should be aware of the existence of differences in the coronary arterial pattern among strains, which might cause misinterpretation of their results.
Acknowledgments
We thank J. Moncayo for his help with histological methods, G. Martín for assistance with electron microscopy and L. Vida for his technical assistance. This study was supported by grants SAF2006-01548 and CGL2004-06306-C02-01/BOS from the Ministerio de Educación y Ciencia. T.F.-G. is the recipient of a fellowship from the Spanish Society of Cardiology.
References
- Arqué JM, Cruz V, Rosado LM, Sans-Coma V. Congenital anomalies of coronary arteries in rodents. Am J Cardiol. 1986;57:498–499. doi: 10.1016/0002-9149(86)90789-7. [DOI] [PubMed] [Google Scholar]
- Barth III CW, Roberts WC. Left main coronary artery originating from the right sinus of Valsalva and coursing between the aorta and the pulmonary trunk. J Am Coll Cardiol. 1986;7:366–373. doi: 10.1016/s0735-1097(86)80507-1. [DOI] [PubMed] [Google Scholar]
- Basso C, Maron B, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- Basso C, Corrado D, Thiene G. Coronary artery anomalies and sudden death. Cardiac Electrophysiol Rev. 2002;6:107–111. doi: 10.1023/a:1017907810269. [DOI] [PubMed] [Google Scholar]
- Becker AE. Variations of the main coronary arteries. In: Becker AE, Losekoot G, Marcelletti C, Anderson RH, editors. Paediatric Cardiology. Edinbourgh: Churchill Livingstone; 1981. pp. 263–277. [Google Scholar]
- van den Boos EJ, Mees BME, de Waard MC, de Crom R, Duncker DJ. A novel model of crioinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol. 2005;289:1291–1300. doi: 10.1152/ajpheart.00111.2005. [DOI] [PubMed] [Google Scholar]
- Cheitlin MD, De Castro CM, Mcallister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva. Circulation. 1974;50:780–787. doi: 10.1161/01.cir.50.4.780. [DOI] [PubMed] [Google Scholar]
- Clauss SB, Walker DL, Kirby ML, Schimel D, Lo CW. Patterning of coronary arteries in wildtype and connexin43 knockout mice. Dev Dyn. 2006;235:2786–2794. doi: 10.1002/dvdy.20887. [DOI] [PubMed] [Google Scholar]
- Durán AC, Sans-Coma V, Cardo M, Arqué JM. The blood supply to the interventricular septum of the heart in Soricoidea (Mammalia) Zool Anz. 1991;227:279–285. [Google Scholar]
- Durán AC, Sans-Coma V, Arqué JM, Cardo M, Fernández B, Franco D. Blood supply to the interventricular septum of the heart in rodents with intramyocardial coronary arteries. Acta Zool. 1992;73:223–229. [Google Scholar]
- Durán AC, Fernández-Gallego T, Fernández B, Fernández MC, Arqué JM, Sans-Coma V. Solitary coronary ostium in the aorta of Syrian hamsters. A morphological study of 130 cases. Cardiovasc Pathol. 2005;14:303–311. doi: 10.1016/j.carpath.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Durán AC, Arqué JM, Fernández B, Fernández MC, Fernández-Gallego T, Sans-Coma V. Separate origin of the main components of the left coronary artery in Syrian hamsters (Mesocricetus auratus. J Vet Med A Physiol Pathol Clin Med. 2007;54:297–301. doi: 10.1111/j.1439-0442.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Fernández B, Buehler A, Wolfram S, et al. Transgenic myocardial overexpression of fibroblast growth factor-1 increases coronary density and branching. Circ Res. 2000;87:207–213. doi: 10.1161/01.res.87.3.207. [DOI] [PubMed] [Google Scholar]
- García Rinaldi R, Cabardillo J, Giles R, Del Toro E, Porro R. Right coronary artery with anomalous origin and slit ostium. Ann Thorac Surg. 1994;58:828–832. doi: 10.1016/0003-4975(94)90760-9. [DOI] [PubMed] [Google Scholar]
- González-Iriarte M, Carmona R, Pérez-Pomares JM, Macías D, Costell M, Muñoz-Chápuli R. Development of the coronary arteries in a murine model of transposition of the great arteries. J Mol Cell Cardiol. 2003;35:795–802. doi: 10.1016/s0022-2828(03)00134-2. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic H, Secerov D, Gmaz-Nikulin E. Comparative anatomical investigations on coronary arteries in wild and domestic animals. Acta Anat. 1974;90:16–35. [PubMed] [Google Scholar]
- Icardo JM, Colvee E. Origin and course of the coronary arteries in normal mice and in iv/ivmice. J Anat. 2001;199:473–482. doi: 10.1046/j.1469-7580.2001.19940473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Hacker TA, Buck J, et al. Distinct mouse coronary anatomy and myocardial infarction consequent to ligation. Coron Artery Dis. 2005;16:41–44. doi: 10.1097/00019501-200502000-00008. [DOI] [PubMed] [Google Scholar]
- Li W, Waldo K, Linask KL, et al. An essential role for connexin43 gap junctions in mouse coronary artery development. Development. 2002;129:2031–2042. doi: 10.1242/dev.129.8.2031. [DOI] [PubMed] [Google Scholar]
- Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin from the aorta. Report of 18 patients, review of the literature and delineation of natural history and management. Circulation. 1979;59:748–754. doi: 10.1161/01.cir.59.4.748. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu F, Schneider AE, St Amand T, Epstein JA, Gutsein DE. Distinct cardiac malformations caused by absence of connexin43 in the neural crest and in the non-crest neural tube. Development. 2006;133:2063–2073. doi: 10.1242/dev.02374. [DOI] [PubMed] [Google Scholar]
- Mahowald JM, Blieden LC, Coe JI, Edwards JE. Ectopic origin of a coronary artery from the aorta. Sudden death in 3 of 23 patients. Chest. 1986;89:669–672. doi: 10.1378/chest.89.5.668. [DOI] [PubMed] [Google Scholar]
- Ratajska A, Zlotorowicz R, Blazejczyk M, Wasiutyñski A. Coronary artery embryogenesis in cardiac defects induced by retinoic acid in mice. Birth Defects Res (Part A) 2005;73:966–979. doi: 10.1002/bdra.20200. [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, et al. Cardiac malformations in neonatal mice lacking connexin 43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Sacks JH, Londe SP, Rosenbluth A, Zalis EG. Left main coronary bypass for aberrant (aortic) intramural left coronary artery. J Thorac Cardiovasc Surg. 1977;73:733–737. [PubMed] [Google Scholar]
- Salto-Tellez M, Lim SY, El Oakley RM, Tang TPL, Almsherqi ZAM, Lim S-K. Myocardial infarction in the C57BL/6J mouse. A quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- Sans-Coma V, Arqué JM, Durán AC, Cardo M, Fernández B, Franco D. The coronary arteries of the Syrian hamster, Mesocricetus auratus(Waterhouse, 1839) Ann Anat. 1993;175:53–57. doi: 10.1016/s0940-9602(11)80239-6. [DOI] [PubMed] [Google Scholar]
- Sans-Coma V, Fernández B, Durán AC, et al. Fusion of valve cushions as a key factor in the formation of congenital bicuspid aortic valves in Syrian hamsters. Anat Rec. 1996;244:490–498. doi: 10.1002/(SICI)1097-0185(199604)244:4<490::AID-AR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Takagawa J, Zhang Y, Wong ML, et al. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102(6):2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitsky I, Wurzel M, Melamed R, Aygen M. Anomalous origin of the coronary arteries. Angiology. 1987;38:128–132. doi: 10.1177/000331978703800206. [DOI] [PubMed] [Google Scholar]
- Walker DL, Vacha SJ, Kirby ML, Lo CW. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Dev Biol. 2005;284:479–498. doi: 10.1016/j.ydbio.2005.06.004. [DOI] [PubMed] [Google Scholar]