Abstract
During embryo implantation, invasive trophoblast cells mediate embryo invasion into the decidualized stroma, forming a rich network of lacunae that connect the embryonic tissues to the maternal blood vessels. Placentation is probably guided by the composition and organization of the endometrial extracellular matrix. Certain pathological conditions that occur during pregnancy, including diabetes, have been linked to abnormal placental morphology and consequent fetal morbidity. We used immunoperoxidase techniques to identify members of the collagen, proteoglycan and glycoprotein families in the various compartments of the rat placenta and to determine whether experimentally induced diabetes affects placental morphology and alters the distribution of these molecules during pregnancy. Single injections of alloxan (40 mg kg−1 i.v.) were used to induce diabetes on day 2 of pregnancy in Wistar rats. Placentas were collected on days 14, 17, and 20. Type I and III collagen, as well as the proteoglycans decorin and biglycan, were found to be distributed throughout the placentas of control and diabetic rats. In both groups, laminin expression decreased at the end of pregnancy. In contrast, fibronectin was detected in the labyrinth region of diabetic rats at all gestational stages studied, whereas it was detected only at term pregnancy in the placentas of control rats. These results show for the first time that some extracellular matrix molecules are modulated during placental development. However, as diabetic rats presented increased fibronectin deposition exclusively in the labyrinth region, we speculate that diabetes alters the microenvironment at the maternal–fetal interface, leading to developmental abnormalities in the offspring.
Keywords: extracellular matrix, fibronectin, placenta, pregnancy, rats, trophoblast
Introduction
The placenta plays an essential role in the maintenance of pregnancy and fetal growth. Placental morphogenesis and development require a coordinated process of proliferation and differentiation of trophoblast cells. In this context, adequate trophoblast invasion is essential for embryo implantation and placentation in mammals (Paria et al. 2002; Van den Brule et al. 2005). Reproductive disorders such as infertility, miscarriage, and pre-eclampsia appear to be related to abnormal implantation and to defects occurring during placental development (Lessey, 2000; Jauniaux & Burton, 2005; Norwitz, 2006).
The basic phases of trophoblast differentiation and placenta development are common among species that have haemochorial placentation, such as rodents, non-human primates, and humans (Enders & Welsh, 1993). In rodents, placentation begins with trophoblast cell proliferation, which forms the ectoplacental cone. The cone-derived cells then differentiate to form the labyrinth, the spongiotrophoblast layer, and the giant cell compartment. These structures constitute the fetal interface with the maternal decidua (Muntener & Hsu, 1977; Pijnenborg et al. 1981; Enders & Welsh, 1993; Cross, 2000). To implant the blastocyst, trophoblast cells have to invade the endometrial stroma and migrate through the maternal extracellular matrix (ECM). Therefore, we can assume that the composition of the ECM plays a role during embryo implantation and placentation.
The ECM comprises a variety of versatile proteins and polysaccharides arranged in a cell surface-associated network. The ECM is required for many specialized cell functions and consists of various combinations of molecules, such as collagens, elastin, glycosaminoglycans, and proteoglycans, which form either long fibres or porous sheets. Multi-adhesive proteins are also important ECM constituents, binding to cell surface receptors and to other ECM components (Alberts et al. 1994).
High levels of plasma glucose alter the gene expression of ECM molecules in renal cells (Ziyadeh et al. 1990; Ayo et al. 1991) and endothelial cells (Cagliero et al. 1988). In addition, the following alterations in the expression of ECM components have been detected in the kidneys of diabetic rats: increased collagen synthesis (Taylor et al. 1980; Hasslacher et al. 1982); decreased heparan sulfate-rich proteoglycans synthesis (Rohrbach et al. 1983; Shimomura & Spiro, 1987); increased protein and mRNA laminin expression (Poulsom et al. 1988). In diabetic individuals, ECM components are frequently affected in various tissues, suggesting that changes in the expression and distribution of these molecules are common characteristics of this pathology (Fukui et al. 1992). Surprisingly, despite the potential importance of ECM molecules for the structure and function of the placenta, very few studies have addressed the composition and distribution of ECM molecules in the haemochorial placenta of diabetic animals. Forsberg et al. (1998) described changes in type IV collagen, laminin and fibronectin in the placentas of diabetic rats. However, that study focused exclusively on term placentas, and there have been no in situ studies of the distribution of ECM molecules in the various compartments of normal and diabetic placentas in the early stages of pregnancy. In view of the structural and functional heterogeneity of the placenta, such in situ studies are necessary to determine which such molecules correlate with placental structure and function. With the objective of contributing to the body of knowledge in this field, the aim of the present study was to analyze the distribution of various ECM molecules (types I and III collagen; the proteoglycans decorin and biglycan; and the glycoproteins fibronectin and laminin) in the placentas of rats with experimentally induced diabetes and in the early stages of pregnancy.
Materials and methods
Animals
All experiments were conducted in accordance with the ethical principles of animal research adopted by the Brazilian College of Animal Experimentation. The study design was approved by the joint Ethics in Animal Research Committee of the Institute of Biomedical Sciences and the University of Sao Paulo (authorization no. 115/2000–107/2000).
Female Wistar rats (n = 36) were obtained at 14 weeks of age from colonies maintained at the Animal Facilities of the Institute of Biomedical Sciences. The rats were housed in a temperature controlled environment (21 ± 1 °C), maintained on a 12-h light/dark cycle, and given free access to tap water and standard rat chow. Each female was housed with a male for the purpose of copulation. Vaginal smears were taken daily, and the day on which spermatozoa were found in the vaginal smear was considered day 1 of pregnancy (term, 20 days).
On day 2 of pregnancy, diabetes was induced by intravenous injection of 40 mg kg−1 of alloxan (Sigma, St. Louis, MO, USA) freshly prepared in saline solution (pH 7.0) as previously described (Fortes et al. 1989). Diabetes (defined as glycaemia > 200 mg dL−1) was confirmed 48 h after the induction, by photometric estimation of blood sugar, using a digital glycosimeter, Accu-Chek Active® (Roche Ltd, Basel, Switzerland), using peripheral blood. The study rats also received subcutaneous injections of 1 IU of NPH insulin (Iolin; Biobras, Montes Carlos, Brazil) every 48 h. Control rats received injections of vehicle (physiological saline solution).
Control and diabetic pregnant rats were weighed and killed by decapitation on days 14, 17, or 20 of pregnancy. Fetuses and placentas were quickly extracted from the uterus and weighed. Fetuses were counted.
Tissue collection
The placentas were removed, fixed in methacarn solution (absolute methanol, chloroform, glacial acetic acid; 6 : 3 : 1) for 3 h at 4 °C, and embedded in Paraplast (Oxford, St Louis, MO, USA) at 60 °C. Sections of 5 µm in thickness were cut and adhered to glass slides using 0.1% poly-l-Lysine (Sigma) and then dried at room temperature (25 °C). Prior to the immunoreaction, some samples were stained with haematoxylin and eosin for the morphological and morphometric studies.
Morphometric analysis
Sections (5 µm thick) were cut from near the midline of placentas harvested on days 14, 17, and 20 (four placentas harvested on each day). The sections were embedded in paraffin as described above, after which they were stained with haematoxylin and eosin. The spongiotrophoblast layer of each placenta was viewed using a Zeiss Axioskop 2 microscope (Carl Zeiss, Jena, Germany) with a 5× objective. Images of this region were captured using a digital camera Canon Power Shot G5 (Canon USA, Lake Success, NY, USA) and ks 1003.0 software (Carl Zeiss). The entire area of the spongiotrophoblast layer was measured (in mm2) using the image-pro plus software (Media Cybernetics, Silver Spring, MD, USA).
Statistical analysis
Calculations and statistics were performed using the graphpad prism 4 software. All data are presented as means ± standard error of the mean (SEM).
Analysis of the areas was performed by means of each experimental group. Statistical significance was accepted when P < 0.05, using the one-way anova test followed by the Tukey-Kramer multiple comparisons test.
Antibodies
Table 1 lists the antibodies and enzymes used in the present study. All antibodies were polyclonal antibodies raised in rabbit. The antibodies against types I and III collagen recognize the entire collagen molecule (Chemicon International, Temecula, CA, USA). The antibodies to the proteoglycans decorin and biglycan (antibodies LF-113 and LF-106, respectively) recognize the core protein of each macromolecule (Fisher et al. 1995). The anti-fibronectin and anti-laminin antibodies recognize the entire molecule (Chemicon International).
Table 1.
Primary antibodies and enzymes used in this study
| Primary antibody | Specificity | Enzymatic pretreatment | Working dilution |
|---|---|---|---|
| Polyclonal* | Anti-type I collagen (whole molecule) | – | 1:500 |
| Polyclonal* | Anti-type III collagen (whole molecule) | – | 1:500 |
| Polyclonal LF-113† | Anti-decorin (IIPYDPDNPLISMC-[LPH]) | 0.2U Chondroitinase ABC‡ | 1:1000 |
| Polyclonal LF-106† | Anti-biglycan (VPDLDSVTPTFSAMS-[LPH]) | 0.2 U Chondroitinase ABC‡ | 1:250 |
| Polyclonal* | Anti-fibronectin (whole molecule) | – | 1:250 |
| Polyclonal* | Anti-laminin (whole molecule) | 0.002% Trypsin§ | 1:250 |
Chemicon International, Temecula, CA, USA.
Dr Larry Fisher (National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA).
Seikagaku Corp., Tokyo, Japan.
Merck, Darmstadt, Germany.
Immunoperoxidase procedures
Sections were treated with 3% (v/v) H2O2 in phosphate-buffered saline (PBS) for 30 min to block endogenous peroxidase activity. Each of the succeeding steps was followed by a thorough rinse with PBS. All steps were performed in a humid chamber, and care was taken to avoid the desiccation of the sections. Nonspecific staining was blocked by immersion in Cas-Block solution (Zymed Laboratories, South San Francisco, CA, USA) for 10 min. As shown in Table 1, the samples were pretreated with specific enzymes according to the antibodies employed.
Sections were incubated with the primary antibodies diluted in PBS containing 0.3% (v/v) Tween 20, overnight at 4 °C. After extensive rinsing in PBS, all sections were incubated for 1 h at room temperature with biotin-conjugated goat anti-rabbit IgG (Rockland, Gilbertsville, PA, USA), diluted 1 : 1000 in PBS. After rinsing in PBS, sections were incubated with Vectastain ABC kit (Vector) for 1 h at room temperature. Peroxidase reaction was visualized using NovaRED kit (Vector, Burlingame, CA, USA). After immunostaining, sections were lightly stained with Mayer's haematoxylin (Merck, Darmstadt, Germany). For each immunohistochemical reaction, controls were performed by incubating the sections with normal rabbit serum or by omitting the primary antibody. Sections were examined in a Zeiss Axioskop 2 microscope, and the images were captured using a digital camera (Canon) and ks100 3.0 software (Zeiss).
Results
Characteristics of the groups studied
Table 2 shows that blood glucose concentrations were sharply elevated (consistently above 500 mg dL−1). Although insulin treatment (1 IU of NPH insulin every 48 h) allowed the pregnancies to proceed to term, it did not normalize the blood glucose levels. Compared with controls, pregnant rats rendered diabetic by the injection of alloxan exhibited significantly less weight gain. In the diabetic group, there were fewer fetuses, fetal weights were lower, and the mean placental wet weight was greater (Table 2).
Table 2.
General characteristics of the groups on days of pregnancy (DOP) 14, 17, and 20
| DOP 14 | DOP 17 | DOP 20 | ||||
|---|---|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | Control | Diabetic | |
| Blood glucose concentration (mg dL−1) | 111 ± 7 | 617 ± 45* | 92 ± 4 | 590 ± 30* | 94 ± 2 | 582 ± 47* |
| Maternal body weight (g) | 264 ± 19 | 247 ± 12 | 312 ± 11 | 270 ± 15* | 343 ± 16 | 280 ± 19* |
| Offspring number | 12.8 ± 1.4 | 8.2 ± 1.6* | 12 ± 1.5 | 7.6 ± 1.7* | 13.5 ± 1.3 | 9.2 ± 1.8* |
| Offspring body weight (mg) | 55 ± 3 | 57 ± 4 | 540 ± 8 | 550 ± 7 | 2006 ± 4 | 1740 ± 4* |
| Placenta weight (mg) | 140 ± 8 | 160 ± 7 | 210 ± 16 | 220 ± 13 | 390 ± 21 | 690 ± 24* |
Values are presented as means ± SEM for n animals in each group.
P < 0.001 vs. control group.
Placental morphology
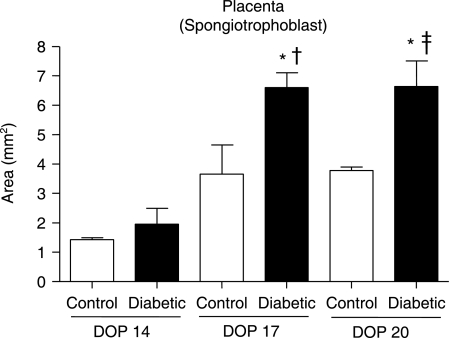
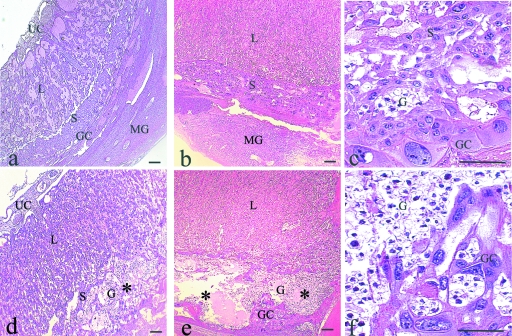
Placentas harvested from diabetic rats presented various morphological alterations over the course of pregnancy, chief among which was an increase in the area of the spongiotrophoblast layer (Fig. 1). In addition, the classical pattern of trophoblast giant cell distribution was not observed in these placentas (Fig. 2f).
Fig. 1.
Morphometric analysis of the spongiotrophoblast layer of placentas obtained from control and diabetic rats on days of pregnancy (DOP) 14, 17, and 20. Spongiotrophoblast area (mm2) of placentas from control (white bars) and diabetic (black bars) with 14th, 17th and 20th DOP. The bars represent means ± SEM of each group. *P < 0.05 vs. diabetic group on DOP 14; †P < 0.05 vs. control group on DOP 17; ‡P < 0.05 vs. control group on DOP 20.
Fig. 2.
Morphology of placentas with H&E staining: Placentas from control rats (a–c). Day 14 of pregnancy (a): overall view of the labyrinth (L) region, spongiotrophoblast (S) layer, and trophoblast giant cell (GC) compartment in contact with the metrial gland; (b) Day 20: Note the growth of the L and S regions; (c) high magnification of (b) showing the S. Placentas from diabetic rats (d–f) Day 14 (d): Note the presence of glycogen-rich (G) cells in the S layer (*); Day 20 (e): The S layer is expanded and shows vacuolization (*) and spatial disorganization (*); (f) High magnification of (e) showing a decrease in the number of S cells, an increase in the number of G cells and disorganization of the GC compartment. UC, umbilical cord; MG, metrial gland. Scale bar = 100 µm.
Immunoperoxidase staining
Type I collagen
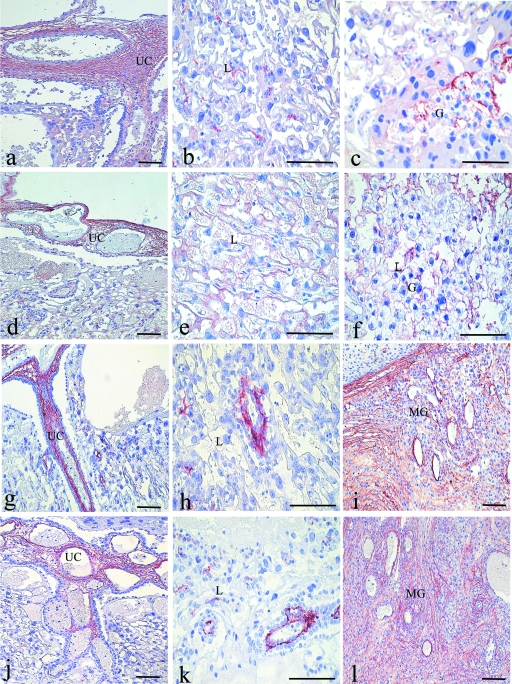
In placentas obtained from control rats on day 14 of pregnancy, type I collagen was detected in the mesenchyme of the umbilical cord, as well as in the spongiotrophoblast layer. Type I collagen was immunodetected primarily in the blood vessels located in the stroma of the metrial gland. Diabetes did not modify the distribution of type I collagen in any of these structures. By day 17, the immunoreaction for type I collagen had spread into the mesenchyme surrounding the blood vessels of the labyrinth and the spongiotrophoblast layer of control and diabetic group placentas. In term placentas, immunoreaction for type I collagen was observed in all placental regions, (Fig. 3a–c), as well as in the stroma of the metrial gland. Similar to what was observed for the previous stages, there were no differences between the two groups (Fig. 3d–f).
Fig. 3.
Immunoperoxidase staining for type I collagen. Placenta of a control rat on day 20 of pregnancy (a–c). Immunoreaction is strong in the mesenchyme of the umbilical cord (UC), labyrinth (L) region, and surrounding glycogen-rich (G) cells. Placenta of a diabetic rat on day 20 (d–f): Note that the immunoreaction is similar to that observed in control samples. Immunoperoxidase staining for type III collagen. Placenta of control rat on day 20 (g–i): Immunoreaction is observed in the mesenchyme of the UC, L region and the stroma of the metrial gland (MG); Placenta of a diabetic rat on day 20 (j–l): The immunoreaction is similar to that observed in control samples. Scale bar = 100 µm.
Type III collagen
Type III collagen was detected surrounding maternal blood vessels in the mesenchyme of the umbilical cord and in the stroma of the metrial gland on days 14 and 17 in the placentas of control and diabetic rats. On day 20, this pattern of distribution remained similar in the control and diabetic groups (Fig. 3g–i and 3j–l, respectively), and the immunoreactive areas broadened only as the placenta grew. No immunostaining for type III collagen was observed in the spongiotrophoblast layer.
Decorin
From day 14 to day 17, immunoreactivity for decorin was observed in the mesenchyme of umbilical cord and fetal blood vessels of the labyrinth region. The immunoreactivity was present in the stroma of the metrial gland near decidual cells and surrounding the maternal blood vessels. The immunoreactive areas broadened only as the placenta grew.
Throughout pregnancy, the presence and distribution of decorin immunoreactivity in control group placentas (Fig. 4a–c) was similar to that observed in the diabetic group placentas (Fig. 4d–f).
Fig. 4.
Immunoperoxidase staining for decorin. Placenta of a control rat on day 20 of pregnancy (a–c): Immunoreaction is observed in the mesenchyme of the umbilical cord (UC), labyrinth (L) region and the stroma of the metrial gland (MG) near decidual cells (*); Placenta of a diabetic rat on day 20 (d–f): The immunoreaction is similar to that observed in control samples. Immunoperoxidase staining for biglycan. Placenta of a control rat on day 20 (g–h): Immunoreaction is observed in the mesenchyme of the UC and the L region. Placenta of a diabetic rat on day 20 (i–j). The immunoreaction is similar to that observed in control samples. Scale bar = 100 µm.
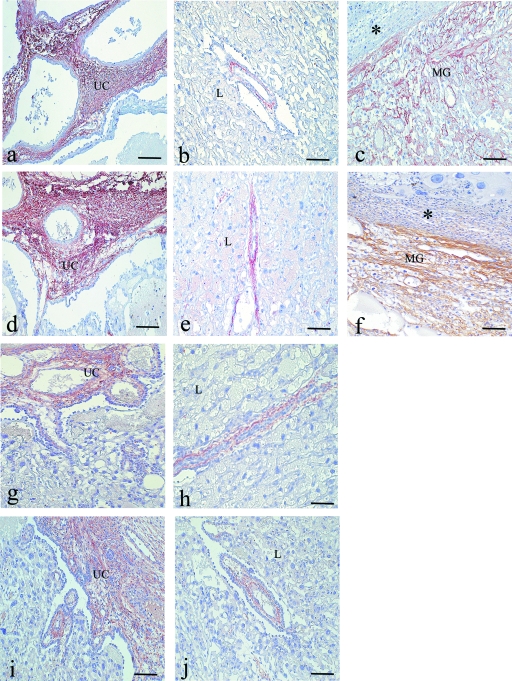
Biglycan
Biglycan was detected in the mesenchyme of the umbilical cord on day 14. By day 17, the immunoreactivity had also spread to the mesenchyme of the fetal blood vessels in the distal region of the labyrinth. In contrast to what was observed for decorin, no biglycan immunoreactivity was detected in the basal decidua or in the stroma of the metrial gland. By day 20, the immunoreactivity for biglycan had increased in the mesenchyme of the umbilical cord (Fig. 4g) and surrounding the fetal blood vessels of labyrinth (Fig. 4h) but remained absent from the basal decidua and the stroma of the metrial gland. The immunoreactivity for biglycan was not affected by the diabetic condition (Fig. 4i,j).
Fibronectin
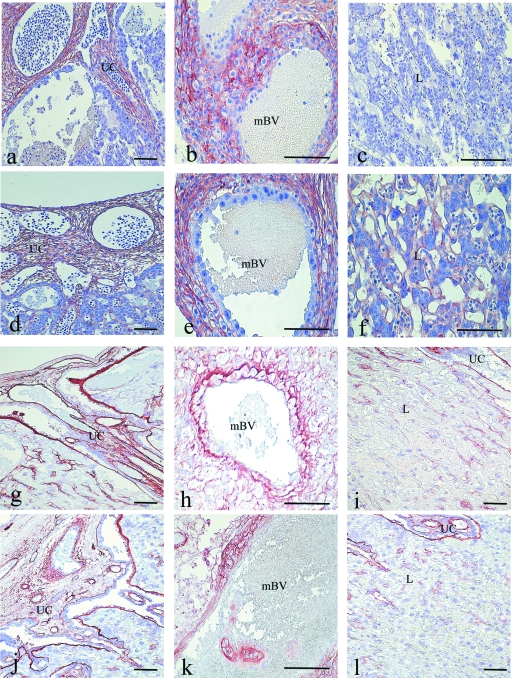
In placentas obtained from control rats on day 14, fibronectin was observed in the mesenchyme of the umbilical cord (Fig. 5a) and in the spongiotrophoblast layer. In the stroma of the metrial gland, immunoreactivity for fibronectin was primarily observed in the basal decidua and surrounding the maternal blood vessels (Fig. 5b). By day 17, the fibronectin immunoreactivity had spread to the area between the spongiotrophoblast layer and the giant cell compartment. On days 14 and 17, no immunoreaction for fibronectin was detected in the labyrinth region (Fig. 5c). In term placentas, the distribution of fibronectin remained as on the previous days. In these placentas, a weak reaction for fibronectin was observed in the labyrinth region.
Fig. 5.
Immunoperoxidase staining for fibronectin. Placenta of a control rat on day 14 of pregnancy (a–c): Strong immunoreaction is observed in the mesenchyme of the umbilical cord (UC) and surrounding the trophoblast cells near the maternal blood vessels (mBV) in the metrial gland (MG). No immunoreaction for fibronectin is observed in the mesenchyme of the labyrinth region. Placenta of a diabetic rat on day 14 (d–f): The immunoreaction is similar to that observed in control placentas in the mesenchyme of the UC and the stroma of the MG. However, the immunoreaction is strong in the mesenchyme of the labyrinth (L) region. Immunoperoxidase staining for laminin. Placenta of a control rat on day 20 (g–i). Immunoreaction is observed in the mesenchyme of the UC and surrounding the mBV of the MG (g–h). In the mesenchyme of labyrinth near to umbilical cord (i) the immunoreaction is diminished compared with the previous days. Placenta of diabetic rat on day 20 (j–l): The immunoreaction is similar to that observed in the control samples. Scale bar = 100 µm.
In placentas obtained from diabetic rats, fibronectin was observed in all regions previously described for control rats (Fig. 5d,e). However, in contrast to the control placentas, diabetic placentas presented fibronectin expression in the labyrinth region during all stages of pregnancy (Fig. 5f).
Laminin
On day 14 of pregnancy, control and diabetic placentas both presented immunoreactivity for laminin in the mesenchyme of the umbilical cord, surrounding the fetal blood vessels. In the labyrinth region, the immunoreactivity was observed in the basement membrane of fetal vessels and was intense in the area surrounding the spongiotrophoblast cells. Weak immunoreactivity was observed on the surface of the trophoblast giant cells. In the stroma of the metrial gland, the immunoreactivity was observed surrounding the maternal blood vessels. No laminin was detected in the basal decidua. By day 17, in control and diabetic placentas, the immunoreactivity had increased in the basement membrane of fetal vessels of labyrinth and on the surface of the trophoblast giant cells. In term placentas, laminin immunoreactivity persisted in the basement membrane of fetal vessels in the labyrinth near the spongiotrophoblast layer. However, immunoreactivity for laminin disappeared from the area of the labyrinth near the umbilical cord (Fig. 5i,l). This peculiar distribution of laminin in the labyrinth region was found in the placentas of both groups. In the other regions of the placenta, the immunoreactivity was similar to that observed on the previous days (Fig. 5g–k).
Discussion
The present study showed that ECM molecules are modulated during placental development. In addition we showed that diabetes has a direct effect on the distribution and expression of some ECM molecules studied herein. Whereas types I and III collagen, as well as the proteoglycans decorin and biglycan, are distributed throughout the placentas at different stages, laminin expression decreased at the end of pregnancy and fibronectin is detected only at term placentas. In addition, fibronectin is detected in the labyrinth region of the placentas from diabetic rats at all gestational stages, suggesting that by altering the microenvironment at the maternal–fetal interface, diabetes may lead to developmental abnormalities in the offspring.
Normal fetal growth and development depends on normal placental function. Some forms of intrauterine growth retardation in human and animal fetuses have been related to disturbances in placental blood flow or to the transfer of nutrients from the mother to the fetus (Cassady, 1981).
According to classical criteria, diabetic fetopathia in man is characterized by increased birth weight, hyperinsulinaemia, increased pancreatic insulin and general signs of fetal distress (Eriksson et al. 1980). Rigorous control of the maternal blood sugar concentrations during pregnancy has been shown to dramatically decrease the incidence of manifest diabetic fetopathia. Although diabetes could be responsible for the increased body weight in human fetuses (Freinkel, 1980), we observed low body weight in the offspring of diabetic rats. However, there are studies showing that fetal growth may be retarded early in diabetic pregnancy, which in a minority group of cases leads to a lowered birth weight (Pedersen & Molsted-Pedersen, 1979). It remains to be seen whether this reflects poor diabetes control both before and during early gestation.
In addition, it was shown that the infant birth weight correlates with the duration of pregnancy and/or with carbohydrate intake. In one study, infant birth weight was positively associated with gestational duration and negatively with carbohydrate intake. There were no large-for-gestational age infants among women whose carbohydrate intake exceeded 210 g day−1. Therefore the authors concluded that for women with gestational diabetes undergoing intensive management, higher carbohydrate intake is associated with decreased incidence of macrosomia (Romon et al. 2001). In another study, it was concluded that gravidas with a low dietary glycaemic index have reduced infant birth weight and approximately a twofold increased risk of a small-for-gestational age birth (Scholl et al. 2004). In rats, Eriksson and colleagues concluded that, in addition to the delayed skeletal maturation, the slow gain in birth weight of diabetes suggests a general retardation of development (Eriksson et al. 1980). Whether this is reflection of a relative lack of insulin in the fetus, intrauterine nutritional deficiencies, or other factors related to the diabetic state of the mother cannot be decided easily. Furthermore, the resulting relative lack of insulin, together with the comparatively short period of gestation in the rat, may prevent the development of macrosomia despite the plethora of carbohydrate substrates available to the fetus of the diabetic mother.
Diabetes induced by cytotoxic chemicals in rats is a suitable model of human type I diabetes and is useful for clarifying some of its outstanding problems (Kalter, 1996). The results of the present study show that the diabetes model employed promotes morphological changes in the size and organization of the spongiotrophoblast layer. Accumulation of cystic vesicles in the spongiotrophoblast layer has been described in rat models of streptozotocin-induced diabetes (Prager et al. 1974; Gewolb et al. 1986; Néeman et al. 1987). Together with the results of the present study, this suggests that among these, the spongiotrophoblast region is the placental compartment that is the most sensitive to diabetes conditions.
In the present study, immunolocalization was used in conjunction with a comprehensive morphological analysis. This combination allowed us to observe that ECM molecules are differentially expressed in the various compartments of the placenta, suggesting that specific microenvironments are created within the placenta.
This study demonstrates, for the first time, that types I and III collagen, as well as biglycan, are present in normal and diabetic rat placentas. As in the majority of adult connective tissues, type I collagen, type III collagen, biglycan, and decorin were found to be colocalized in the mesenchyme of the umbilical cord and fetal blood vessels of the labyrinth region. This indicates that these molecules participate in the organization of these tissues from the early stages of development. Interestingly, type III collagen, decorin, and biglycan were not detected in the spongiotrophoblast layer, demonstrating that this region has a peculiar molecular organization, and that these types of molecules are not required for spongiotrophoblast function.
An important observation of the present study was that diabetes affects the expression of fibronectin. In placentas from diabetic animals, fibronectin was present in the mesenchyme of umbilical cord, labyrinth and in the extracellular matrix of the metrial gland. Whereas high levels of immunoreactivity for fibronectin were observed in the mesenchyme of the labyrinth region, i.e. in the extracellular tissue excluding trophoblasts and endothelial cells, in the diabetic group at all stages of pregnancy analyzed, a weak fibronectin immunoreactivity in this region was detected exclusively in the term placentas from control rats. Fibronectin has been associated with the degree of tissue maturation. According to Yamaguchi et al. (1985), high levels of fibronectin characterize immature chorionic villi in human placentas. Despite these previous findings, we found no morphological alterations suggestive of inadequate maturation of the diabetic placentas. Using a streptozotocin diabetic rat model, Forsberg et al. (1998) also showed increased fibronectin expression in various regions of term placentas. Therefore, in terms of fibronectin distribution in the placenta, alloxan-induced diabetes promotes alterations that are similar to those seen in diabetes induced by streptozotocin. However, our findings extend those of Forsberg et al. (1998) by showing that fibronectin is present in the labyrinth region of diabetic rat placentas up to day 14 of pregnancy. In fact, as we have shown, high immunoreactivity for fibronectin was detected from the earliest stages of placentation. This abnormal deposition of fibronectin might disturb the organization of the ECM, thereby affecting essential exchanges between mother and fetus, as previous suggested by Forsberg et al. (1998). The relationship between immaturity and increased molecular thickness in the labyrinth region may be related with a decrease of maternal–fetal interchanges and may contribute to retarding of the fetal weight.
The source of placental fibronectin remains unknown, and the mechanism by which fibronectin distribution increases in the placentas of diabetic animals is not well understood. The deposition of ECM molecules in the tissues of diabetic patients has been associated with increased expression of transforming growth factor beta, an inductor of fibronectin production in the glomeruli of diabetic rats (Yamamoto et al. 1993). Further studies of transforming growth factor beta expression in the placentas of normal and diabetic rats could further the understanding of this issue.
In rodents, the labyrinth region of the placenta is a complex structure, containing vascular channels, located at the maternal–fetal interface, which are responsible for efficient nourishment of the fetus. In the present study, immunoreactivity for laminin in the term placentas of normal and diabetic rats was found to be lower in the labyrinth region near the umbilical cord, whereas in the labyrinth close to the spongiotrophoblast, laminin expression remained unchanged throughout the pregnancy. In contrast to what was observed for fibronectin, diabetes did not affect the distribution of laminin in the placenta. However, diabetes has been shown to increase laminin expression in the glomeruli during diabetic nephropathy and has been implicated in reduced glomerular filtration rates in the basement membrane (Nerlich & Schleicher, 1989, 1991). Interestingly, although the glomeruli and the labyrinth region of the rodent placenta are structures containing similarly rich networks of blood vessels, they were differently affected by the diabetes condition, which had a tissue-specific effect on the ECM molecules.
In summary, we have shown that, during placental development, specific ECM molecules are secreted in the various regions of the placenta. In addition, the deposition of some of these molecules appears to be modulated by the pregnancy itself according to the degree of placental maturity. However, we have shown that the deposition of fibronectin, different from that of the other molecules studied, is specifically affected by diabetes.
Altered deposition of ECM molecules and changes in the morphological organization of the spongiotrophoblast layer might alter the microenvironment of the placenta, leading to developmental abnormalities in the offspring of diabetic rats. Further studies are needed to evaluate the true consequences of these placental alterations for the descendants of such animals.
Acknowledgments
This project was supported by grants from DIPUV (Universidad de Valparaiso, Valparaiso, Chile; grant no. 24/2004) and FAPESP (Sao Paulo, Brazil; grant nos. 04/05472-9 and 05/58074-3). This study was carried out as partial fulfilment of a M.Sc. degree by F. Giachini (advisor: R. Tostes) and was supported by a fellowship from CNPq (Brazil).
The authors are grateful to Dr Larry Fisher (National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA) for kindly supplying the antibodies to decorin and biglycan. We gratefully acknowledge the technical assistance provided by Makarena Gonzalez.
S. San Martin is a member of CREAS.
References
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J. Molecular Biology of the Cell. 3. New York & London: Garland; 1994. pp. 973–978. [Google Scholar]
- Ayo SH, Radnik RA, Glass WE, II, Garoni JA, Rampt ER, Appling DR, et al. Increased extracellular matrix synthesis and mRNA in mesangial cells grown in high-glucose medium. Am J Physiol. 1991;260:F185–191. doi: 10.1152/ajprenal.1991.260.2.F185. [DOI] [PubMed] [Google Scholar]
- Cagliero E, Maiello M, Boeri D, Roy S, Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988;82:735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady G. The small-for-date infant. In: Avery GB, editor. Neonatology: Pathophysiology and Management of the Newborn. Philadelphia: Lippincott; 1981. pp. 262–286. [Google Scholar]
- Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool. 1993;266:578–587. doi: 10.1002/jez.1402660608. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ, Andersson A, Efendic S, Elde R, Hellerström C. Diabetes in pregnancy: effects on the fetal and newborn rat with particular regard to body weight, serum insulin concentration and pancreatic contents of insulin, glucagon and somatostatin. Acta Endocrinol (Copenh) 1980;94:354–364. doi: 10.1530/acta.0.0940354. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Stubb JT, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–65. [PubMed] [Google Scholar]
- Forsberg H, Wentzel P, Eriksson UJ. Maternal diabetes alters extracellular matrix protein level in rat placentas. Am J Obstet Gynecol. 1998;179:772–778. doi: 10.1016/s0002-9378(98)70081-x. [DOI] [PubMed] [Google Scholar]
- Fortes ZB, Scivoletto R, Garcia-Leme J. Functional changes in the microcirculation of alloxan-induced diabetic rats. Gen Pharmacol. 1989;20:615–620. doi: 10.1016/0306-3623(89)90096-7. [DOI] [PubMed] [Google Scholar]
- Freinkel N. Banting Lecture. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- Fukui M, Nakamura T, Ebihara I, Shirato I, Tomino Y, Koide H. ECM gene expression and its modulation by insulin in diabetic rats. Diabetes. 1992;41:1520–1527. doi: 10.2337/diab.41.12.1520. [DOI] [PubMed] [Google Scholar]
- Gewolb IH, Merdian W, Warshaw JB, Enders AC. Fine structural abnormalities of the placenta in diabetic rats. Diabetes. 1986;35:1254–1261. doi: 10.2337/diab.35.11.1254. [DOI] [PubMed] [Google Scholar]
- Hasslacher C, Kopeschke HG, Burklin E, Gechter F, Reichenbacher R. In vivo studies on biosynthesis in diabetic and nondiabetic rats. Res Exp Med. 1982;181:245–251. doi: 10.1007/BF01851197. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. Pathophysiology of histological changes in early pregnancy loss. Placenta. 2005;26:114–123. doi: 10.1016/j.placenta.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Kalter H. Reproductive toxicology in animals with induced and spontaneous diabetes. Reprod Toxicol. 1996;10:417–438. doi: 10.1016/s0890-6238(96)00129-3. [DOI] [PubMed] [Google Scholar]
- Lessey BA. The role of the endometrium during embryo implantation. Hum Reprod. 2000;15:39–50. [PubMed] [Google Scholar]
- Muntener M, Hsu YC. Development of trophoblast and placenta of the mouse. A reinvestigation with regard to the in vitro culture of mouse trophoblast and placenta. Acta Anat (Basel) 1977;98:241–252. [PubMed] [Google Scholar]
- Néeman Z, Barash V, Rosenmann E, Shafrir E. Localization of glycogen in the placenta of diabetic rats: a light and electron microscopic study. Placenta. 1987;8:201–208. doi: 10.1016/0143-4004(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Nerlich A, Schleicher E. Immunohistochemical localization of various components of the basal membrane and interstitial collagen in diabetic nephropathy. Verh Dtsch Ges Pathol. 1989;73:133–138. [PubMed] [Google Scholar]
- Nerlich A, Schleicher E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am J Pathol. 1991;139:889–899. [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- Pedersen JF, Mølsted-Pedersen L. Early growth retardation in diabetic pregnancy. Br Med J. 1979;1:18–19. doi: 10.1136/bmj.1.6155.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenborg R, Robertson WB, Brosens I, Dixon G. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2:71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Poulsom R, Kurkinen M, Prockop D, Boot-Handford R. Increased steady-state levels of laminin B1 mRNA in kidneys of long-term streptozotocin-diabetic rats. No effect of an aldose reductase inhibitor. J Biol Chem. 1988;263:10072–10076. [PubMed] [Google Scholar]
- Prager R, Abramovici A, Liban E, Laron Z. Histopathological changes in the placenta of streptozotocin induced diabetic rats. Diabetologia. 1974;10:89–91. doi: 10.1007/BF00421419. [DOI] [PubMed] [Google Scholar]
- Rohrbach DH, Wagner CW, Star VL, Martin GR, Brown KS, Yoon JW. Reduced synthesis of basement membrane heparan sulfate proteoglycan in streptozocin-induced diabetic mice. J Biol Chem. 1983;258:11672–11677. [PubMed] [Google Scholar]
- Romon M, Nuttens MC, Vambergue A, et al. Higher carbohydrate intake is associated with decreased incidence of newborn macrosomia in women with gestational diabetes. J Am Diet Assoc. 2001;101:897–902. doi: 10.1016/S0002-8223(01)00220-6. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Chen X, Khoo CS, Lenders C. The dietary glycemic index during pregnancy: influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am J Epidemiol. 2004;159:467–474. doi: 10.1093/aje/kwh068. [DOI] [PubMed] [Google Scholar]
- Shimomura H, Spiro RG. Studies on macromolecular components of human glomerular basement membrane and alterations in diabetes: decreased levels of heparan sulfate proteoglycan and laminin. Diabetes. 1987;36:374–381. doi: 10.2337/diab.36.3.374. [DOI] [PubMed] [Google Scholar]
- Taylor SA, Price RG, Kang SS, Yudkin J. Modification of the glomerular basement membrane in sucrose-fed and streptozocin diabetic rats. Diabetologia. 1980;19:364–372. doi: 10.1007/BF00280522. [DOI] [PubMed] [Google Scholar]
- Van den Brule F, Berndt S, Simon N, et al. Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163–180. doi: 10.1159/000087833. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Isemura M, Yosizawa Z, et al. Changes in the distribution of fibronectin in the placenta during normal human pregnancy. Am J Obstet Gynecol. 1985;152:715–718. doi: 10.1016/s0002-9378(85)80055-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN, Snipes ER, Watanabe M, Alvarez RJ, Goldfarb S, Haverty TP. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol. 1990;259:F704–714. doi: 10.1152/ajprenal.1990.259.4.F704. [DOI] [PubMed] [Google Scholar]