Abstract
Calretinin is a calcium-binding protein found widely distributed in the central nervous system and chemosensory cells of the teleosts, but its presence in the peripheral nervous system of fishes is unknown. In this study we used Western blot analysis and immunohistochemistry to investigate the occurrence and distribution of calretinin in the cranial nerve ganglia, dorsal root ganglia, sympathetic ganglia, and enteric nervous system of the adult zebrafish. By Western blotting a unique and specific protein band with an estimated molecular weight of around 30 kDa was detected, and it was identified as calretinin. Immunohistochemistry revealed that calretinin is selectively present in the cytoplasm of the neurons and never in the satellite glial cells. In both sensory and sympathetic ganglia the density of neurons that were immunolabelled, their size and morphology, as well as the intensity of immunostaining developed within the cytoplasm, were heterogeneous. In the enteric nervous system calretinin immunoreactivity was detected in a subset of enteric neurons as well as in a nerve fibre plexus localized inside the muscular layers. The present results demonstrate that in addition to the central nervous system, calretinin is also present in the peripheral nervous system of zebrafish, and contribute to completing the map of the distribution of this protein in the nervous system of teleosts.
Keywords: calcium-binding proteins, calretinin, peripheral nervous system, zebrafish
Introduction
Calcium ions (Ca2+) play a critical role in neuronal physiology, and the maintenance of Ca2+ intracellular homeostasis is necessary to generate the nerve impulse (Hactkney et al. 2005). Among the mechanisms regulating the intracytoplasmic concentrations of Ca2+ are the Ca2+-binding proteins (Ca2+BP; for a review see Grabarek, 2006). Calretinin is a Ca2+BP which contains six helix–loop–helix EF-hand motifs, and has predominant neuronal localization. It interacts with cytoskeletal components in a Ca2+-dependent manner (Marilley & Schwaller, 2000) and acts as a Ca2+-buffer (Billing-Marczak & Kuznicki, 1999), although its biological role may vary from one cell to another (for a review see Palczewska et al. 2005). In fish, calretinin has been found widely distributed in the central nervous system (Castro et al. 2006a,b) as well in some chemosensory cells (Porteros et al. 1997; Pombal et al. 2002; Díaz-Regueira et al. 2005; Germanà et al. 2007). Nevertheless, the occurrence and distribution of calretinin in the peripheral nervous system of teleosts is completely unknown.
Over recent decades, the zebrafish (Danio rerio) has been used as a model in experimental embryology and cell biology studies (Bang et al. 2001), and it also serves as a model for the study of several human diseases (see Lieschke & Currie, 2007). Here we used Western blot and immunohistochemistry to investigate the occurrence and distribution of calretinin in the peripheral nervous system of adult zebrafish, including the cranial nerve ganglia, dorsal root ganglia (DRG), sympathetic ganglia, and enteric nervous system (ENS) at the stomach and intestinal levels.
Materials and methods
Materials and tissue preparation
Adult zebrafish (Danio rerio; n = 8), 6–8 months old, were obtained from CISS (Centro di Ittiopatologia Sperimentale per la Sicilia), University of Messina, Italy. The specimens were anaesthetized with MS222 (ethyl-m-amino benzoate; 0.4 g L−1) and decapitated. The heads and the bodies were fixed in Bouin's fixative for 24 h and then routinely processed for paraffin embedding.
Western blot
Two whole frozen zebrafish were processed for Western blotting. Experiments were carried out in triplicate as follows: the animals were divided into small blocks, rinsed in cold saline, and then pooled and homogenized (1 : 2, w/v) with a Potter homogenizer in Tris-HCl buffered saline (TBS; 0.1 m, pH 7.5) containing 1 µm leupeptin, 10 µm pepstatin and 2 mm phenylmethylsulfonyl fluoride. The homogenates were afterwards centrifuged at 25 000 g for 15 min at 4 °C, and the resulting pellet dissolved in 10 mm Tris HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 100 mm dithiothreitol, and 10% glycerol at 4 °C. The pellets were thawed and analyzed by electrophoresis in 15% discontinuous polyacrylamide SDS gels. After electrophoresis, proteins were transferred onto a nitrocellulose membrane and blocked by immersion for 3 h in phosphate-buffered saline containing 5% dry milk, and 0.1% Tween-20. The membranes were then incubated at 4 °C for 2 h with a primary antibody to calretinin. This antibody was raised in goat directed against a peptide mapping at the N-terminus of human calretinin (Santa Cruz Biotechnology, Santa Cruz, CA; calretinin N-18, code sc-11644; diluted 1 : 1000). After incubation, the membranes were washed with Tris-buffered saline (pH 7.6) containing 20% Tween-20, and incubated again for 1 h with donkey anti-goat IgG (Santa Cruz Biotechnology) diluted 1 : 1000, at room temperature. Membranes were washed one more time and incubated with the PAP complex diluted 1 : 100 for 1 h at room temperature. Finally, the reaction was developed using a chemiluminescent reagent (ECL, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) and exposed to Hyperfilm. Marker proteins were visualized by staining with Brilliant Blue.
Immunohistochemistry
The pieces were cut 10 µm thick in serial frontal, horizontal and sagittal sections, and collected on gelatine-coated microscope slides. The sections were processed for indirect peroxidase immunohistochemistry as follows: deparaffinized and rehydrated sections were rinsed in Tris-HCl buffer (0.05 m, pH 7.5) containing 0.1% bovine serum albumin and 0.2% Triton-X 100. The endogenous peroxidase activity and nonspecific binding were blocked (3% H2O2 and 25% fetal calf serum, respectively) and sections were incubated overnight at 4 °C with the same primary antibody described above, used diluted 1 : 1000. After incubation with the primary antibody, sections were rinsed in the same buffer, and incubated with peroxidase-labelled donkey anti-goat IgG (Santa Cruz Biotechnology) diluted 1 : 1000, for 1 h at room temperature. Finally, sections were washed and the immunoreaction visualized using 3-3'DAB as a chromogen. The specificity of the immunoreactivity developed was tested substituting the primary antibody by a non-immune serum, omitting the primary antibody, and incubating the sections with specifically pre-absorbed sera (5 µg of calretinin-blocking peptide –sc 11644P- in 1 mL of anti-calretinin working solution).
Quantitative study
The percentages and sizes of the total neurons, as well as of the calretinin-positive neurons in each type ganglia, were determined using an automatic image analysis system (Quantimet 550, Leica, QWIN Program, Servicio de Analisis de Imagenes, University of Oviedo). Measurements were made on three sections, 30 µm apart, per specimen evaluating all nerve cell profiles present in the entire section. The total number (100%) of neurons was determined by counting cells in serial sections stained with Cresyl violet, since current neuronal markers, like neurofilaments, PGP 9.5 or Hu, failed to immunolabel neurons or did not immunolabel all neurons. Neurons displaying calretinin immunoreactivity were also counted and measured on three sections, 30 µm apart, per specimen. The neurons within each ganglion were divided according to cell soma mean diameter into three size groups, referred as small (mean diameter < 5 µm), intermediate (mean diameter = 5–10 µm), and large (mean diameter > 10 µm).
Results
Western blot
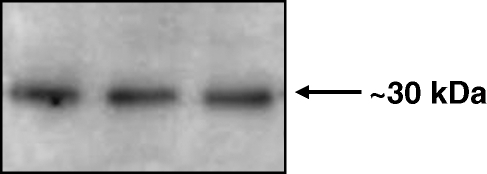
A unique and specific protein band was detected by Western blotting in homogenates of whole adult zebrafish. The band labelled with the antibody to calretinin showed an estimated molecular weight of 30 kDa (Fig. 1), which was identical to that found earlier in our laboratory (Germana et al. 2007).
Fig. 1.
Western blot detection of calretinin in homogenates of whole adult Danio rerio. The antibody used recognizes a unique protein band with an estimated molecular weight of about 30 kDa.
Immunohistochemistry
The following peripheral ganglia were examined: dorsal root ganglia (DRG), trigeminal and anterodorsal ganglia (V/AD g), facial and anteroventral ganglia (VII/AV g), statoacoustic ganglion (VIII g), glossopharyngeal ganglion (IX), vagal ganglion (X), posterolateral line ganglion (PL g), and sympathetic ganglia. AD, AV, and PL ganglia innervate the superficial and canal neuromasts of the lateral line system (Raible & Kruse, 2000). In addition, ENS was examined at the stomach and intestine levels.
The antibody used for calretinin selectively labelled neurons in all the examined ganglia as well as in the gastric and intestinal walls. The pattern of immunostaining was cytoplasmic, the nucleus being devoid of immunoreaction. In the control sections processed as described above no positive immunoreactivity was detected (data not shown).
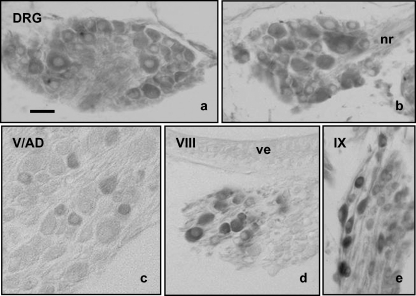
DRG in adult zebrafish, as in all vertebrates, contains neurons of different sizes. Apparently, all neurons, independently of their size, displayed calretinin immunoreactivity without regional variations in the pattern of expression (Fig. 2a, corresponding to a cranial DRG, and Fig. 2b, corresponding to a caudal DRG). The immunoreactivity was restricted to the neuronal somata, whereas the axons emerging from them were unreactive (Fig. 2).
Fig. 2.
Immunohistochemical localization of calretinin in cephalic (a) and caudal (b) dorsal root ganglia (DRG), V/AD ganglia (c), VIII ganglion (d), and IX ganglion (e). Calretinin immunoreactivity was detected in the cytoplasm of a neuronal subpopulation within each sensory ganglia. The neuronal size and the intensity of immunostaining varied widely among ganglia. nr = nerve root in a dorsal root ganglion; ve = vestibular sensory epithelium in the inner ear. Scale bar = 20 µm.
Regarding the sensory ganglia of the cranial nerves, the pattern of immunostaining varied greatly. In the V/AD ganglia a small neuronal subpopulation of small and intermediate sized neurons displayed calretinin immunoreactivity (Fig. 2c), the percentage of the immunolabelled neurons being about 10–15% (Table 1). The immunoreactive neurons were not concentered in a segment of the ganglia but were scattered throughout the entire section. Similar findings, in both the size and pattern of distribution of the calretinin immunoreactive neurons, were observed in the VII/AV ganglia (Table 1). In the VIII ganglion, only the intermediate and large sized neurons were immunoreactive (Fig. 2d; Table 1), and they were primarily placed along the external border and the cephalic pole of the ganglion; they represented about 15% of the total number of neurons. Data from PL ganglion paralleled those from the VIII ganglion (Table 1). Finally, in the IX and X ganglia most neurons were calretinin positive (Fig. 2e; Table 1), being the peripheral ones with a more intense immunostaining. Interestingly, the processes of these neurons also were immunoreactive.
Table 1.
Neuron-size distribution of neurons in the ganglia of the peripheral nervous system in the adult zebrafish. Values are mean ± SEM and represent the value of the mean diameter. The percentage of calretinin (CR) immunoreactive neurons within each pre-established size classes is also indicated
| % of size classes | % of CR immunoreactive Neurons in each size classes | |||||
|---|---|---|---|---|---|---|
| < 5 µm | 5–10 µm | > 10 µm | < 5 µm | 5–10 µm | > 10 µm | |
| Cranial ganglia | ||||||
| V/AD g | 31 | 58 | 11 | 12 | 3 | |
| VII/AV g | 26 | 66 | 9 | 9 | 4 | |
| VIII g | 20 | 62 | 18 | 5 | 10 | |
| IX g | 34 | 66 | 6 | 26 | 60 | 6 |
| X g | 38 | 63 | 9 | 31 | 62 | 9 |
| PL g | 23 | 58 | 19 | 4 | 12 | |
| Dorsal root ganglia | 19 | 59 | 22 | 19 | 59 | 22 |
| Sympathetic ganglia | 31 | 48 | 21 | 12 | 35 | 21 |
V/AD g = trigeminal and anterodorsal ganglia; VII/AV g = facial and anteroventral ganglia, VIII = statoacoustic ganglion, IX g = glossopharyngeal ganglio, X g = vagal ganglion, PL g: posterolateral line ganglion.
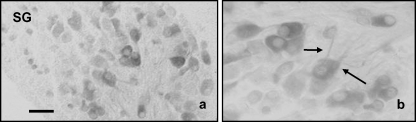
Regarding the sympathetic ganglia, the size range of the immunoreactive neurons was extremely variable, as well as the intensity of immunolabelling (Fig. 3a; Table 1). In the smallest neurons only the cytoplasm was immunolabelled, whereas in the largest sized ones the initial segment of the neuronal processes also displayed calretinin immunoreactivity (Fig. 3b).
Fig. 3.
Immunohistochemical localization of calretinin in the sympathetic ganglia. Neurons displaying immunoreactivity were very heterogeneous in size and shape, and in the large sized ones the neuronal processes were also immunolabelled. Scale bar = 20 µm.
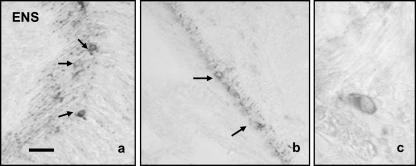
The pattern of distribution of calretinin immunoreactivity in ENS was rather homogeneous along the gastrointestinal tract, and it was found in the neuronal somata and the myenteric plexus (Fig. 4a,b,c). Because the general neuronal markers assessed (PGP 9.5, neurofilaments, Hu antigen) were unable to immunolabel enteric neurons, the neuronal density in the gastrointestinal tract as well as the percentage of these neurons displaying calretinin immunoreactivity cannot be calculated.
Fig. 4.
In the enteric nervous system the immunoreactivity for calretinin was observed in scattered neurons (arrows) in the stomach (a) and the intestine (b), as well as in a dense nerve plexus placed between the inner and outer muscular layers. The pattern of cell immunostaining was cytoplasmic (c). Scale bar = 20 µm.
In no case were images resembling satellite glial cells in peripheral ganglia or enteric glial cells in ENS obtained in sections processed for calretinin immunodetection. Moreover, commercially polyclonal antibodies to S100 protein and glial fibrillary acidic protein failed to label these cells. S100 protein immunoreactivity was regularly detected in discrete neuronal populations, and glial fibrillary acidic protein failed to immunolabel any cell. Interestingly, satellite glial cells displayed S100 protein immunoreactivity in cranial sensory ganglia of Salmo salarand Salmo trutta(data not shown; Catania et al. 2007).
Discussion
Recently, two papers have described in detail the distribution of the calretinin-positive cells in the central nervous system of the zebrafish (Castro et al. 2006a,b). It is also known that chemosensory cells in taste buds and olfactory epithelium in this teleost express calretinin (Germanà et al. 2007). But surprisingly the distribution of this Ca2+BP in the peripheral nervous system of the zebrafish is unknown, and the same applies for teleosts. In the present study we attempted to complete the map of the distribution of the calretinin in the zebrafish, analyzing its expression at the protein level. By Western blotting we have detected a protein with an estimated molecular weight of 30 kDa similar to that estimated for calretinin in higher vertebrates (see Huerta et al. 1996), and identical to that estimated for this protein in adult zebrafish (Germana et al. 2007). Furthermore, the polyclonal antibody we have used specifically immunolabels neuronal populations in both sensory and sympathetic ganglia, as well as in ENS.
In higher vertebrates, including man, calretinin immunoreactivity was detected in a neuronal subpopulation of sensory neurons of dorsal root and cranial ganglia, occasionally co-localized with other Ca2+BP (Ichikawa et al. 1992, 1993, 2005); in the sympathetic ganglia (Huerta et al. 1996; Papka et al. 1999; Burden & Zary, 2002); and in ENS (McConalogue et al. 1994; Belai & Burnstock, 1999; Brehmer et al. 2004). Therefore our results about the distribution of calretinin immunoreactivity in the peripheral nervous system of the adult zebrafish roughly matches that in higher vertebrates. Nevertheless, further studies are necessary to fully characterize the calretinin-containing neurons in this teleost, especially by analyzing the occurrence of this protein in the nerve fibres which innervate the putative peripheral targets of the calretinin-positive neurons. Based on morphological criteria, the satellite glial cells lack calretinin immunoreactivity, confirming previous data from our laboratory in mammals (Huerta et al. 1996) and zebrafish (Germana et al. 2007b).
The possible role of calretinin in the peripheral nervous system of the adult zebrafish remains to be elucidated. Calretinin is able to interact with cytoskeletal components in a calcium-dependent manner (Marilley & Schwaller, 2000) and acts as a calcium buffer (Billing-Marczak & Kuznicki, 1999), although its biological role may vary from one cell to another (for review see Palczewska et al. 2005). Because calcium signalling is crucial for development, regulation of gene expression, secretion and cell movement (see Ashworth & Brennan, 2005), it could be speculated that calretinin participates in the functions indirectly by controlling intracellular Ca2+ homeostasis. The use of zebrafish could therefore be of particular interest in the study of aging and neurodegenerative diseases in which changes in the intracellular Ca2+levels have been involved (Foster, 2007; Mattson, 2007).
Acknowledgments
This study was supported by a grant PRA 2004 (Research Project of the University of Messina) to RL, and a grant form Instituto Asturiano de Odontología (Oviedo, Spain) to TC and JAV. The authors thank Mr V. Sidoti for technical support.
References
- Ashworth R, Brennan C. Use of transgenic zebrafish reporter lines to study calcium signalling in development. Brief Funct Genomic Proteomic. 2005;4:186–193. doi: 10.1093/bfgp/4.2.186. [DOI] [PubMed] [Google Scholar]
- Bang PI, Sewel WF, Malick JJ. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio) J Comp Neurol. 2001;438:173–190. doi: 10.1002/cne.1308. [DOI] [PubMed] [Google Scholar]
- Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn's disease human small intestine. Dig Dis Sci. 1999;44:1579–1587. doi: 10.1023/a:1026658826010. [DOI] [PubMed] [Google Scholar]
- Billing-Marczak K, Kuznicki J. Calretinin-sensor or buffer-function still unclear. Pol J Pharmacol. 1999;51:173–178. [PubMed] [Google Scholar]
- Brehmer A, Croner R, Dimmler A, Papadopoulos T, Schrödl F, Neuhuber W. Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Auton Neurosci. 2004;31:49–59. doi: 10.1016/j.autneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Burden HW, Zary JT. Localization of calretinin in the rat ovary and in relation to nerve cell bodies in dorsal root and paravertebral ganglia projecting to the ovary. Microsc Res Tech. 2002;59:490–494. doi: 10.1002/jemt.10226. [DOI] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadón R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J Comp Neurol. 2006a;494:435–459. doi: 10.1002/cne.20782. [DOI] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadón R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. II. Midbrain, hindbrain, and rostral spinal cord. J Comp Neurol. 2006b;494:792–814. doi: 10.1002/cne.20843. [DOI] [PubMed] [Google Scholar]
- Catania S, Germanà A, Cabo R, Ochoa-Erena FJ, Guerrera MC, Hannestad J, et al. Neurotrophin and Trk neurotrophin receptors in the inner ear of Salmo salar and Salmo trutta. J Anat. 2007;210:78–88. doi: 10.1111/j.1469-7580.2006.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Regueira SM, Lamas I, Anadón R. Calretinin immunoreactivity in taste buds and afferent fibers of the grey mullet Chelon labrosus. Brain Res. 2005;1031:297–301. doi: 10.1016/j.brainres.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Germanà A, Paruta S, Germana PG, Ochoa-Erena FJ, Montalbano G, Cobo J, et al. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio. Brain Res. 2007;1162:48–55. doi: 10.1016/j.brainres.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Grabarek Z. Structural basis for diversity of the EF-hand calcium binding proteins. J Mol Biol. 2006;359:509–525. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- Hactkney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentration of calcium buffering proteins in mammalian cochlear cells. J Neurosci. 2005;25:7867–7886. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta JJ, Nori S, Llamosas MM, Vazquez MT, Bronzetti E, Vega JA. Calretinin immunoreactivity in human sympathetic ganglia. Anat Embryol. 1996;194:373–378. doi: 10.1007/BF00198539. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jacobowitz DM, Sugimoto T. Calretinin-immunoreactivity in the oro-facial and pharyngeal regions of the rat. Neurosci Lett. 1992;146:155–158. doi: 10.1016/0304-3940(92)90066-g. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jacobowitz DM, Sugimoto T. Calretinin-immunoreactive neurons in the trigeminal and dorsal root ganglia of the rat. Brain Res. 1993;617:96–102. doi: 10.1016/0006-8993(93)90618-w. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jin HW, Terayama R, Yamaai T, Jacobowitz DM, Sugimoto T. Calretinin-containing neurons which co-express parvalbumin and calbindin D-28k in the rat spinal and cranial sensory ganglia; triple immunofluorescence study. Brain Res. 2005;1061:118–123. doi: 10.1016/j.brainres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Marilley D, Schwaller B. Association between the calcium-binding protein calretinin and cytoskeletal components in the human colon adenocarcinoma cell line WiDr. Exp Cell Res. 2000;259:12–22. doi: 10.1006/excr.2000.4942. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Low AM, Williamson S, Bornstein JC, Furness JB. Calretinin-immunoreactive neurons and their projections in the guinea-pig colon. Cell Tissue Res. 1994;276:359–365. doi: 10.1007/BF00306121. [DOI] [PubMed] [Google Scholar]
- Palczewska M, Batta G, Groves P, Linse S, Kuznicki J. Characterization of calretinin I-II as an EF-hand, Ca2+, H+-sensing domain. Protein Sci. 2005;14:1879–1887. doi: 10.1110/ps.051369805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papka RE, Collins J, Copelin T, Wilson K. Calretinin-immunoreactive nerves in the uterus, pelvic autonomic ganglia, lumbosacral dorsal root ganglia and lumbosacral spinal cord. Cell Tissue Res. 1999;298:63–74. doi: 10.1007/s004419900071. [DOI] [PubMed] [Google Scholar]
- Pombal MA, de Arriba MC, Sampedro C, Álvarez R, Megías M. Immunocytochemical localization of calretinin in the olfactory system of the adult lamprey, Lampetra fluviatilis. Brain Res Bull. 2002;57:281–283. doi: 10.1016/s0361-9230(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Porteros A, Arévalo R, Weruaga E, Crespo C, Briñón JG, Alonso JR, et al. Calretinin immunoreactivity in the developing olfactory system of the rainbow trout. Brain Res Dev Brain Res. 1997;100:101–109. doi: 10.1016/s0165-3806(97)00037-0. [DOI] [PubMed] [Google Scholar]
- Raible DW, Kruse GJ. Organization of the lateral line system in embryonic zebrafish. J Comp Neurol. 2000;421:189–198. [PubMed] [Google Scholar]