Abstract
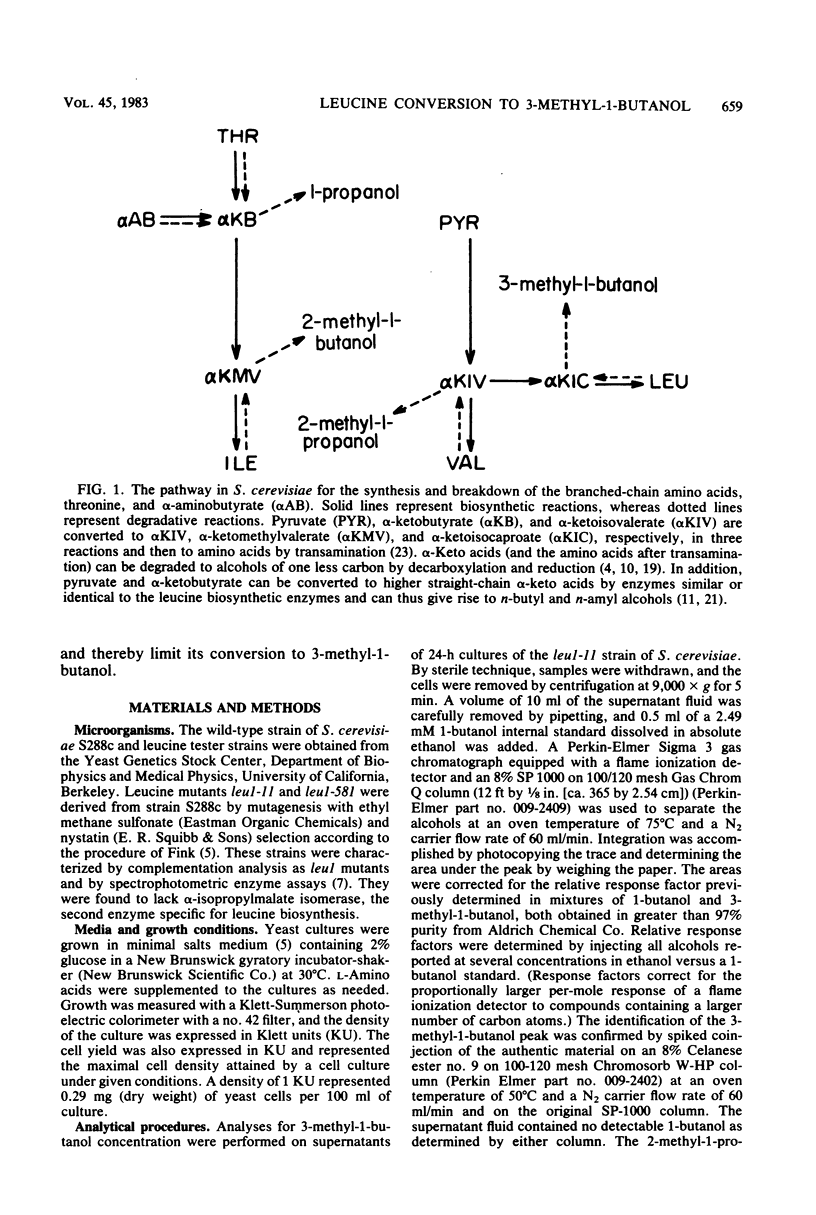
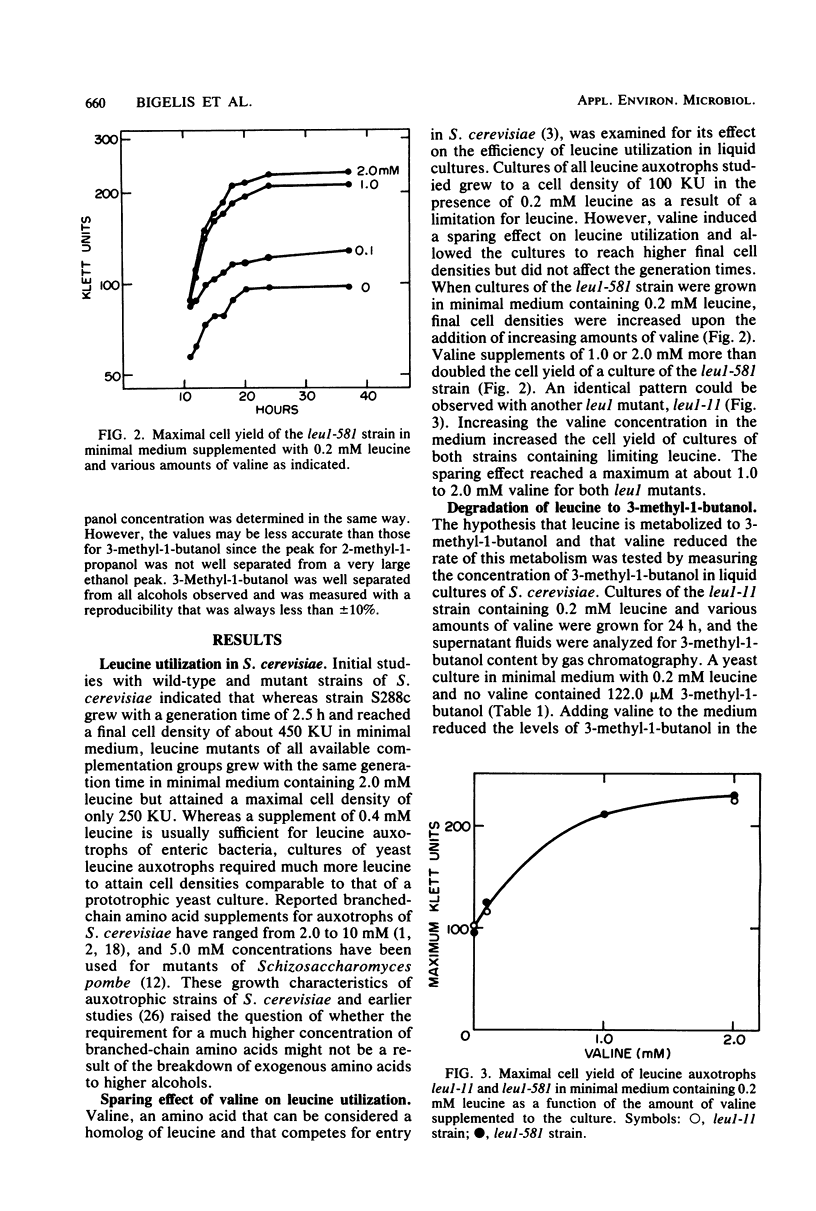
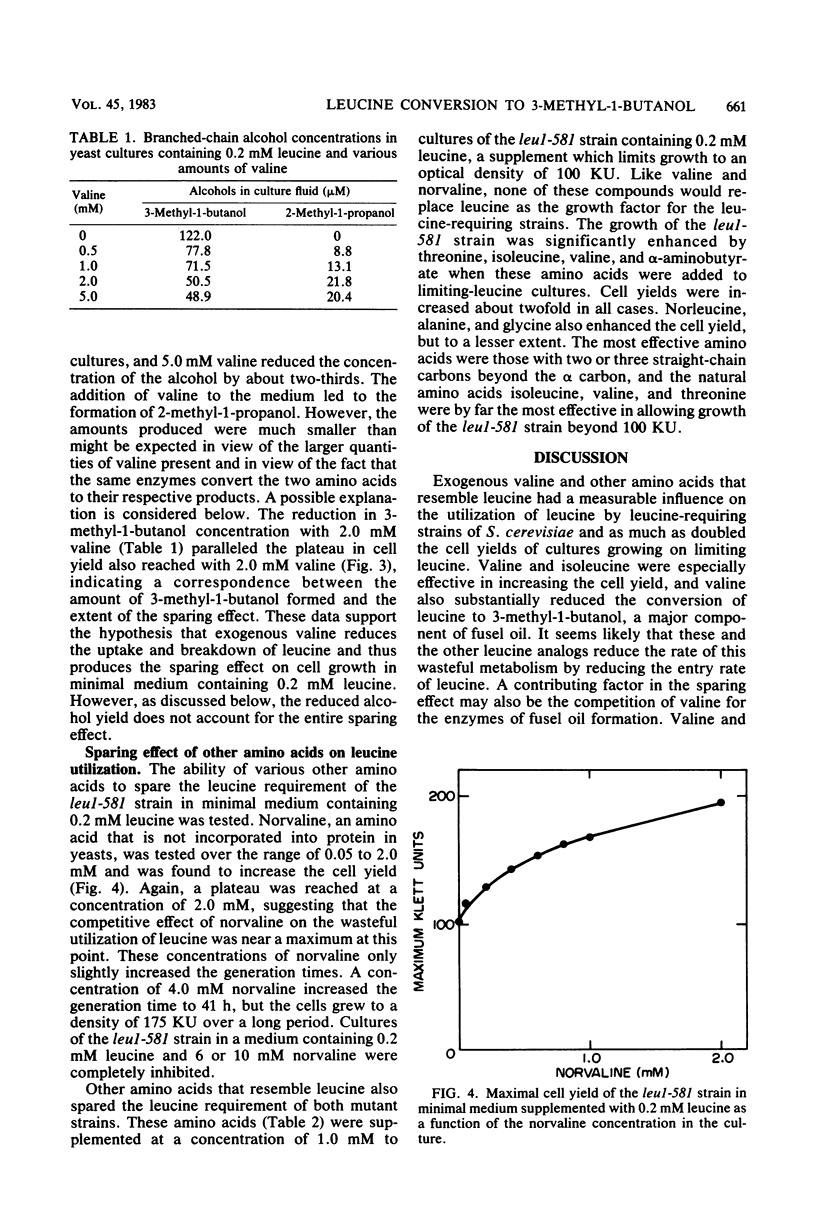
Mutant strains of the yeast Saccharomyces cerevisiae that require branched-chain amino acids must be supplemented with large concentrations (up to 10 mM) of these amino acids to satisfy their nutritional requirement. The utilization of one branched-chain amino acid, leucine, was examined in several leul strains of yeast grown aerobically in a glucose-ammonium salts minimal medium containing a limiting concentration (0.2 mM) of leucine. In this medium, the leucine requirement of the auxotrophic strains could be reduced by valine, another branched-chain amino acid. Increasing the valine concentration increased the cell yields of cultures and also reduced the levels of 3-methyl-1-butanol detected in the medium by gas chromatography. The concentration of 3-methyl-1-butanol was reduced from 122.0 to 48.9 μM when 5.0 mM valine was supplemented to limiting-leucine cultures. The amino acids isoleucine, threonine, norleucine, norvaline, α-amino-butyrate, alanine, and glycine also spared the leucine requirement of leucine auxotrophs, most likely because they resembled leucine and competed for its uptake. We propose that leucine analogs restrict the entry and degradation of leucine and thus reduce its conversion to 3-methyl-1-butanol, a major component of fusel oil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollon A. P., Magee P. T. Involvement of threonine deaminase in multivalent repression of the isoleucine-valine pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2169–2172. doi: 10.1073/pnas.68.9.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: regulation of synthesis of the enzymes of isoleucine and valine biosynthesis. J Bacteriol. 1969 May;98(2):623–628. doi: 10.1128/jb.98.2.623-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of the branched-chain amino acids in yeast: a leucine-binding component and regulation of leucine uptake. J Bacteriol. 1970 Aug;103(2):277–285. doi: 10.1128/jb.103.2.277-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUYMON J. F., INGRAHAM J. L., CROWELL E. A. The formation of n-propyl alcohol by Saccharomyces cerevisiae. Arch Biochem Biophys. 1961 Oct;95:163–168. doi: 10.1016/0003-9861(61)90122-9. [DOI] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):382–392. doi: 10.1128/jb.117.2.382-392.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R. A., Satyanarayana T., Kaplan J. G. Biosynthesis of branched-chain amino acids in Schizosaccharomyces pombe: regulation of the enzymes involved in isoleucine, valine, and leucine synthesis. Can J Biochem. 1974 Jan;52(1):51–59. doi: 10.1139/o74-009. [DOI] [PubMed] [Google Scholar]
- Pietruszko R., Crawford K., Lester D. Comparison of substrate specificity of alcohol dehydrogenases from human liver, horse liver, and yeast towards saturated and 2-enoic alcohols and aldehydes. Arch Biochem Biophys. 1973 Nov;159(1):50–60. doi: 10.1016/0003-9861(73)90428-1. [DOI] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. D., Kohlhaw G. B. Subcellular localization of isoleucine-valine biosynthetic enzymes in yeast. J Bacteriol. 1974 Nov;120(2):631–637. doi: 10.1128/jb.120.2.631-637.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E. D., Tracy J. W., Kohlhaw G. B. Subcellular localization of the leucine biosynthetic enzymes in yeast. J Bacteriol. 1973 Oct;116(1):222–225. doi: 10.1128/jb.116.1.222-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENTHESHANMUGANATHAN S., ELSDEN S. R. The mechanism of the formation of tyrosol by Saccharomyces cerevisiae. Biochem J. 1958 Jun;69(2):210–218. doi: 10.1042/bj0690210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENTHESHANUGANATHAN S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem J. 1960 Mar;74:568–576. doi: 10.1042/bj0740568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: correlation of biochemical blocks and genetic lesions in leucine auxotrophs. J Bacteriol. 1968 Dec;96(6):2012–2017. doi: 10.1128/jb.96.6.2012-2017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Kisumi M., Chibata I. Biosynthetic pathway of beta-methylnorleucine, an antimetabolite produced by Serratia marcescens. J Antibiot (Tokyo) 1981 Oct;34(10):1283–1289. doi: 10.7164/antibiotics.34.1283. [DOI] [PubMed] [Google Scholar]
- Templeton B. A., Savageau M. A. Transport of biosynthetic intermediates: regulation of homoserine and threonine uptake in Escherichia coli. J Bacteriol. 1974 Oct;120(1):114–120. doi: 10.1128/jb.120.1.114-120.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]


