Abstract
Adult male Sprague-Dawley rats were randomly assigned to two groups: control and anaemic. Anaemia was induced by periodical blood withdrawal. Extensor digitorum longus and soleus muscles were excised under pentobarbital sodium total anaesthesia and processed for transmission electron microscopy, histochemical and biochemical analyses. Mitochondrial volume was determined by transmission electron microscopy in three different regions of each muscle fibre: pericapillary, sarcolemmal and sarcoplasmatic. Muscle samples sections were also stained with histochemical methods (SDH and m-ATPase) to reveal the oxidative capacity and shortening velocity of each muscle fibre. Determinations of fibre and capillary densities and fibre type composition were made from micrographs of different fixed fields selected in the equatorial region of each rat muscle. Determination of metabolites (ATP, inorganic phosphate, creatine, creatine phosphate and lactate) was done using established enzymatic methods and spectrophotometric detection. Significant differences in mitochondrial volumes were found between pericapillary, sarcolemmal and sarcoplasmic regions when data from animal groups were tested independently. Moreover, it was verified that anaemic rats had significantly lower values than control animals in all the sampled regions of both muscles. These changes were associated with a significantly higher proportion of fast fibres in anaemic rat soleus muscles (slow oxidative group = 63.8%; fast glycolytic group = 8.2%; fast oxidative glycolytic group = 27.4%) than in the controls (slow oxidative group = 79.0%; fast glycolytic group = 3.9%; fast oxidative glycolytic group = 17.1%). No significant changes were detected in the extensor digitorum longus muscle. A significant increase was found in metabolite concentration in both the extensor digitorum longus and soleus muscles of the anaemic animals as compared to the control group. In conclusion, hypoxaemic hypoxia causes a reduction in mitochondrial volumes of pericapillary, sarcolemmal, and sarcoplasmic regions. However, a common proportional pattern of the zonal distribution of mitochondria was maintained within the fibres. A significant increment was found in the concentration of some metabolites and in the proportion of fast fibres in the more oxidative soleus muscle in contrast to the predominantly anaerobic extensor digitorum longus.
Keywords: anaemia, capillary density, fibre types, hypoxaemia, mitochondrial volume, oxidative metabolism, skeletal muscle
Introduction
Like any kind of cell, muscle fibres need a continuous supply of oxygen and nutrients to be able to carry out their vital functions. The muscle tissue has a well developed microvascular system made up of blood capillaries with an average diameter of 3–5 µm, depending on the muscle (Wiedeman, 1984; Mathieu-Costello, 1991).
August S. Krogh proved in a pioneering work that the number of capillaries was clearly higher when muscle had oxidative characteristics (Krogh, 1919). Muscles with evident glycolytic metabolism had less capillarization. More variables, such the dimensions of the muscle vascularization and its functional implications, are required to quantify the capillary network accurately (Egginton & Ross, 1992).
Physiological, biochemical and mechanical factors that play an important role in muscle capillarization are now widely known (Mathieu-Costello, 2001; Christov et al. 2007). Although muscles formed mainly by oxidative fibres have a high capillary density, some researchers have not found a direct correlation between the oxidative capacity of muscle and capillarity (Gray & Renkin, 1978; Maxwell et al. 1980). Despite their low mitochondrial content, glycolytic fibres apparently have a high number of capillaries (Weibel, 1984). This may be because of the dual role of muscle capillaries, which not only participate in respiratory gases exchange, but also supply energy substrates and eliminate metabolic waste substances such as lactate (Weibel, 1984; Hudlicka et al. 1987). This suggests that mitochondria act as a possible modulatory factor, supplying a higher volume of oxygen to muscle fibres (Ingjer, 1979).
In skeletal muscles, mitochondrial densities and volumes are a reliable oxygen supply indicator associated with capillary density (Londraville & Sidell, 1990). Many studies have shown that there was a higher mitochondrial volume close to capillaries and just under the sarcolemma than in other deeper regions of muscle fibre (Hoppeler et al. 1981; Kayar et al. 1986; Kayar & Banchero, 1987). These studies speculate whether the mitochondrial density can also reflect the local PO2. In other words, PO2 would be higher where the mitochondrial volume is higher and vice versa.
An important characteristic of the capillary network in skeletal muscle is its plasticity and its capacity to adapt to metabolic changes which is induced by different factors: training, hypoxia, electrical stimulation of muscle, low temperatures, etc. (Butler & Turner, 1988; Hudlicka & Price, 1990; Leon-Velarde et al. 1993). Chronic hypoxia has been considered a stimulus that elicits capillary growth (Hudlicka, 1982).
Capillary tortuosity seems to increase in skeletal muscle after chronic exposition to hypobaric hypoxia (Apell, 1980; Mathieu-Costello, 1987; Poole & Mathieu-Costello, 1989). Structural adaptations of skeletal muscle resulting from prolonged exposure to hypobaric hypoxia seem to be conducted by vascular endothelial growth factor (VEGF) signalling, which ultimately depends on the expression balance of the hypoxia inducible factor (HIF) system (Pugh & Ratcliffe, 2003).
Using different enzyme activities from biochemical determinations, Peter et al. (1972) classified mammalian muscle fibres into three different groups: SO (slow oxidative), FOG (fast oxidative glycolytic) and FG (fast glycolytic). Despite being an ‘academic’ simplification of the muscle reality, this nomenclature has been widely accepted by researchers in the field of muscle physiology because it attributes some metabolic characteristics to each type of fibre. These characteristics were subsequently shown to be related to the physiological properties of the different motor units.
Differences at histochemical and morphometrical level in capillary supply between the different types of fibres present in mammalian skeletal musculature were evaluated. Soleus (SOL) muscle and the extensor digitorum longus (EDL) of rat were taken as a model. Soleus muscle is a postural and slow-contraction (slow-twitch) muscle with strong oxidative metabolism. Extensor digitorum longus is a fast-contraction (fast-twitch) muscle that takes part in rapid activities and high intensity processes. Therefore, its metabolism is essentially glycolytic (Close, 1972). There are also differences in these two muscles in contractile properties (Close, 1964), types of fibres (Armstrong & Phelps, 1984), blood flow and blood supply (Armstrong & Laughlin, 1985).
Nowadays, a great amount of information is available in the literature on the effects of altitude, cold, training and other factors on capillarization and skeletal muscle morphofunctional parameters (Leon-Velarde et al. 1993). In contrast, there are few data about the effect of anaemia (Celsing et al. 1988; Sakkas et al. 2004). The aim of the present work was to study the different adaptations of morphofunctional levels in physiological responses to anaemia in rat skeletal muscle. The main goal was to describe the effect of the presumable tissue hypoxia induced by limited oxygen transport capacity of the blood on fibre composition, capillary density, metabolic oxidative capacity, mitochondrial density, mitochondrial distribution and the muscle concentration of different metabolites.
Materials and methods
Laboratory animals
Sprague-Dawley male rats weighing 329.6 ± 22.9 g (mean ± SE) were used. These animals were randomly assigned to two different groups of seven animals each. One group was used as a control (C). The animals in the other group were submitted to hypoxaemic hypoxia by anaemization (A). The anaemization process took 3 weeks and consisted of whole blood withdrawal from each animal (approximately 4 mL in each extraction) on alternate days. Haematocrit values fell (P < 0.0001) to 31.7 ± 2.0, which was significantly lower than the normal levels (48.5 ± 2.4) found in the control group.
Muscle sampling procedure
After the hypoxaemic hypoxic period, extensor digitorum longus and soleus muscles were extracted from both legs. These skeletal muscles have different metabolic and ergonomic characteristics. Soleus is a postural muscle with oxidative properties. It therefore has a homogeneous composition. Extensor digitorum longus is a mixed muscle. The heterolateral muscles were destined for morphofunctional and biochemical studies, respectively. Muscle samples from one leg were processed for transmission electronic microscopy (TEM) and histochemistry. Muscles samples from the other hind limb were used for biochemical studies of some of the metabolites involved in muscle function: ATP, inorganic phosphate (Pi), creatine (Cr), creatine phosphate (CrP) and lactate (Lac). Muscle samples were taken by autopsy. They were then frozen and stored in 2-methylbutane cooled to –160 °C with liquid nitrogen until further study, according to the recommendations of Dubowitz (1985).
TEM study: distribution and mitochondrial volume
Sample preparation
The equatorial regions of soleus and extensor digitorum longus muscles were used to study mitochondrial volume. Small cylinders (4 mm long × 1 mm × 1 mm) of tissue were prepared from each equatorial muscle section. These muscle tissue samples were fixed by immersion in vials which contained 1 mL of mixed solution glutaraldehyde 1.25% and paraformaldehyde 1% in a phosphate-buffered saline (PBS) buffer solution, at 4 °C for 48 h. Samples were washed with PBS 0.14 m (pH = 7.4) for 45 min. This process was repeated three times. Subsequent steps were carried out by the Serveis Cientifico-Tècnics (SCT) of the Universitat de Barcelona. In the SCT, samples were subjected to a post-fixation process in osmium tetraoxide (OsO4) 1% in PBS for 1 h. Samples were then dehydrated in acetone. They were left in different solutions with ascending acetone concentrations for 10 min. Dehydrated samples were mounted in Spurr's epoxide resin blocks. The resin blocks were cut and semithin sections (1.5 µm) were processed. These sections were then observed with an optic microscope at 40× to choose the regions of interest for processing and analysing using TEM. The chosen regions were cut and put into square and eyelet grids. They were contrasted so that they could be studied with TEM.
Determination of mitochondrial volume
Mitochondrial volume was studied using TEM in three different regions of each muscle fibre: pericapillary (pc), the fibre region close to a capillary; sarcolemmal (sl), the fibre region close to the sarcolema; and sarcoplasmatic (sp), the innermost part of the muscle fibre. To study all of these regions, we obtained photomicrographs from different randomly chosen fibres. Different magnifications were used to cover all studied areas. The images were digitally processed and the mitochondrial volume and density calculated. Each photomicrograph (×6600) obtained by TEM (Philips model 600A) was scanned to be used with SigmaScan image analysis software (Jandel Scientific, Erkrath, Germany). The stereological methods described by Weibel (1979) were used to determine the mitochondrial volume. These methods have been widely used in the study of different tissues. To ensure that no mitochondria were counted twice, we used the ‘point-counting’ method. A grid was drawn on the image to determine the number of intersection points that fell on mitochondria. The length of each square side of the grid was double the diameter of the mitochondria. Classic stereological techniques assume that mitochondria are randomly oriented and that the probability of finding mitochondria in a two-dimensional section of a tissue is related to the total mitochondria volume in a three-dimensional cell (Weibel, 1973). However, some authors (Eisenberg et al. 1974; Eisenberg, 1986) demonstrated that mitochondrial volume was not affected by its orientation in the fibre. Therefore, the geometrical probability theory can be used to calculate the average three-dimensional volumes from the study of two-dimensional samples without considering the orientation of mitochondria in the fibre. Mitochondrial volume measured by the ‘point-counting’ method was expressed as a percentage.
Histochemical procedures
Sample preparation
Histochemical studies were performed to determine the oxidative capacity, the contraction characteristics of each type of fibre, the capillary and fibre density, and the fibre composition. To carry out these studies, we used muscle samples that had not been processed for TEM. These samples were marked during the cryostat cuts to be able to identify the anatomic orientation of the muscle fragment and subsequent cuts. Greene's nomenclature for muscles was used (Greene, 1959). Serial transverse slices were obtained on the axis of the muscle fibres, with a thickness of 25 µm to SDH and mATPase stain, and 14 µm to the capillary mATPase stain. These cuts were done in a Frigocut Reichert-Jung (Heidelberg, Germany) cryostat between –22 °C and –20 °C. Samples were collected on gelatinized glass coverslips.
Sections were incubated for 5 min in a buffered fixative (Viscor et al. 1992) and stained for the following histochemical assays to identify the fibre types and capillaries: succinate dehydrogenase, SDH according to Nachlas et al. (1957) and myofibrillar adenosine triphosphatase, mATPase (Brooke & Kaiser, 1970). Endothelial ATPase was used to reveal muscle capillaries (Fouces et al. 1993; Torrella et al. 1993).
Capillarization and types of fibres in skeletal muscle
Once slices had been obtained in the cryostat, they were processed and mounted in a glycerine drop and photomicrographs were taken through an image capture system. This system enabled the images observed with the optical microscope to be digitalized directly. SigmaScan Image software was used to count the capillaries and fibres and to measure the following fibre dimensions: area, perimeter, Feret's diameter and the shape factor. Feret's diameter is the longest distance possible between any two points along the boundary of a region of interest. Here it is used as an estimation of the maximum diffusion distance between surrounding capillaries and the centre of the muscle fibre. The shape factor measures the shape of a geometrical figure. This unit less the parameter is defined as 4 ×π× the object's area divided by the perimeter squared (a perfect circle will have a shape factor of 1, whereas a line's shape factor will approach zero).
All photomicrographs were taken using 40× and 200× magnification in an optical microscope (Olympus, BX40, Japan) which incorporated a digital camera (Hitachi, KP-C550, Japan). Image calibration was done by taking a photo of a micrometric glass coverslip at each magnification at the beginning of each image capture session.
Capillary density (CD) and the percentage of each type of muscle fibre were counted in tissue fields of 2 × 105 µm2. The obtained number was multiplied by 5 to express the parameter value in mm2. The number of measurements oscillated between 20 and 100 fibres of each type in each field. However, all visible fibres were counted when the total number of fibres in the studied field was lower than this number.
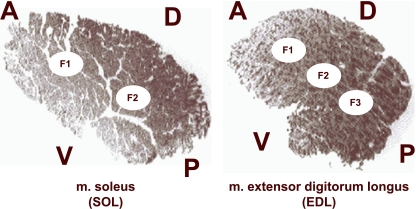
In all cases, we used the equatorial region of each muscle to obtain the data. By means of the sample procedure designed by Torrella et al. (1996), equatorial transverse sections from each muscle were divided into a grid-like structure from which some muscle fields were selected for measurement. As a result of this protocol, five fields were sampled in this study. Due to the highly homogeneous distribution pattern of the fibre types in soleus muscle, only two fields per equatorial section were studied. In contrast, three fields were analysed in extensor digitorum longus (Fig. 1). Non-significant differences between fields in the same muscle and for the same condition were detected. As a consequence, and for clearer presentation, mean values for all the sampled fields of each muscle are presented in Tables 1–3 and Figs 2–4.
Fig. 1.
Transverse sections in the equatorial region of soleus (SOL) and extensor digitorum longus (EDL) rat muscles. The images show the exact location of the studied fields (F1 to F3) used for fibre typing and capillarity measurements. Anatomical orientation: A, anterior (cranial); D, dorsal; P, posterior (caudal), V, ventral.
Table 1.
Mitochondrial volumes (%) in the pericapillary, sarcolemmal and sarcoplasmatic regions of soleus (SOL) and extensor digitorum longus (EDL) rat muscles. Results are expressed as mean ± SE. Significant differences between anaemic and control condition are indicated when observed
| SOL | EDL | |||
|---|---|---|---|---|
| Control | Hypoxaemic | Control | Hypoxaemic | |
| Pericapillary | 12.34 ± 1.26 | 9.98 ± 1.31*** | 16.98 ± 3.25 | 9.17 ± 1.64*** |
| Sarcolemmal | 9.68 ± 1.15 | 7.59 ± 1.39*** | 10.20 ± 2.06 | 6.60 ± 1.14*** |
| Sarcoplasmatic | 7.77 ± 0.85 | 6.00 ± 1.22*** | 5.47 ± 1.01 | 3.42 ± 1.06*** |
P < 0.001
Table 3.
Basal concentrations of some metabolites of the phosphate energy system in soleus (SOL) and extensor digitorum longus (EDL) muscles from control and hypoxemic animals. Metabolite concentrations are expressed as µmol per gram of tissue weight. Results are expressed as mean ± SE. Significant differences between control and hypoxemic muscles are indicated as
| SOL | EDL | |||
|---|---|---|---|---|
| Control | Hypoxaemic | Control | Hypoxaemic | |
| ATP | 2.4 ± 0.5 | 7.7 ± 0.5** | 2.84 ± 0.43 | 12.36 ± 7.3* |
| Creatine | 23.9 ± 4.5 | 32.5 ± 6.3* | 23.7 ± 3.13 | 49.3 ± 15.2* |
| Creatine phosphate | 1.2 ± 0.9 | 7.1 ± 0.9** | 2.37 ± 0.87 | 9.34 ± 5.8* |
| Inorganic phosphate | 15.2 ± 4.8 | 34.4 ± 7.8* | 15.6 ± 9.03 | 52.1 ± 15.9** |
| Lactate | 9.7 ± 4.7 | 14.36 ± 2.6 | 12.9 ± 1.7 | 42.3 ± 18.5* |
P < 0.05;
P < 0.01
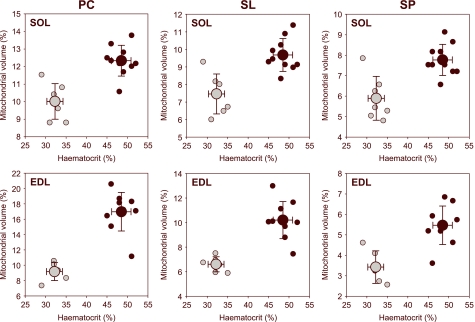
Fig. 2.
Relationship between the mitochondrial volume of extensor digitorum longus (EDL) and soleus (SOL) muscles and haematocrit. Plots are presented for the three fibrillary studied regions: pericapillary (PC), sarcolemmal (SL) and sarcoplasmatic (SP). Marked differences between hypoxemic animals and controls are evidenced. Black circles: normoxia; grey circles: hypoxia. Mean values are indicated by bigger symbols. Crosshair indicates the standard deviation of the mean.
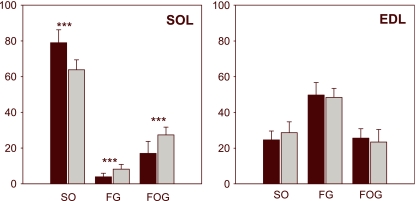
Fig. 4.
Changes in fibre type composition in soleus (SOL) and in extensor digitorum longus (EDL) muscles of animals submitted to hypoxemic hypoxia (grey bars) in comparison to controls (black bars). Hairlines indicate the standard deviation of the mean. Significant differences (control versus hypoxemic) are indicated: ***P < 0.001.
Biochemical determinations
Immediately after soleus and extensor digitorum longus muscles had been removed, metabolite extraction (ATP, PCr, Cr, Pi and lactate) was performed. Muscle tissue was crushed in a mortar which contained liquid nitrogen, and distributed to Eppendorf tubes containing 0.5 mL of PBS buffer. Each tube contained similar muscle tissue mass (between 15 and 20 mg). Samples were then homogenized using a Teflon tip on ice and low rotation velocity. Aliquots of 50 µL were obtained from the homogenate to determinate the different metabolites. In most cases, aliquots were diluted at 1/40. This entire process was carried out inside a cold chamber at 4 °C. Before proceeding to the assay for determining each different metabolite, aliquots were centrifuged and the supernatant was kept for further analysis. Metabolite concentrations, expressed in µmol g−1 tissue weight, were determined using spectrophotometric methods.
Statistics
The normality and homoscedasticity of data were studied for each parameter to apply the correct statistical methods (parametric or non-parametric). The Kolmogorov-Smirnov test (Lilliefors table) was used to verify the normal distribution of the data. The arcsine function was used to study the percentages of fibre types. Student's t-test for paired data was used in normal distributions to evaluate whether there were differences in metabolite concentration. The Wilcoxon sign-rank test of paired data was used in non-normal distributions. One-way and two-way analyses of variance (anova) were used to study the statistical differences between data groups. Morphometric differences between control and anaemic rats were calculated in each muscle. This method was also used to study the differences in distribution and mitochondrial volume between fibre regions (pericapillary, sarcolemmal and sarcoplasmatic) and the animals’ conditions (hypoxaemic hypoxia and controls). Unless otherwise indicated, results are expressed as sample mean ± SE.
Results
Mitochondrial volume and distribution
A statistically significant difference in the distribution (P < 0.001) of mitochondrial structures was found among the muscle fibres in the three studied regions: pericapillary, sarcolemmal and sarcoplasmatic (Table 1). When data from animal groups were tested independently, significant differences between control animals and those exposed to hypoxaemic hypoxia were detected for the same region. The anaemic rats had significantly lower values in all sampled regions than the control animals. Mitochondrial volume reduction was homogeneous in pericapillary, sarcolemmal and sarcoplasmatic regions. This decrease was directly related to the drop in haematocrit levels (Fig. 2).
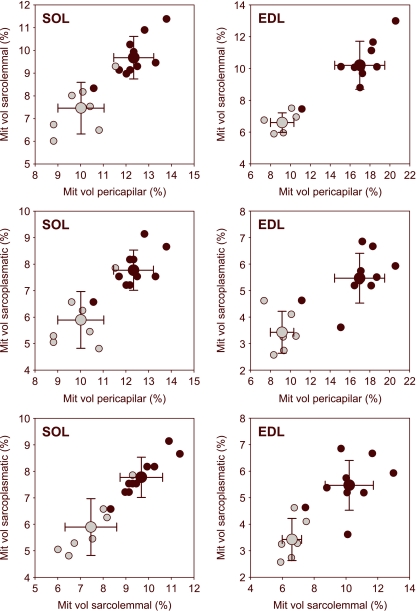
A diminishing gradient in mitochondrial volume inside the fibre was clearly apparent when the mitochondrial volume of the three studied regions was compared, regardless of the hypoxaemic status (pericapillary > sarcolemmal > sarcoplasmatic). This was in accordance with the oxygen diffusion cascade inside the cell (Fig. 3).
Fig. 3.
Mitochondrial distribution pattern in the three muscle fibre regions (pericapillary, sarcolemmal and sarcoplasmatic) of soleus (SOL) and extensor digitorum longus (EDL) muscles. A similar trend is observed for hypoxemic (grey circles) and control animals (black circles).
Types of fibres in soleus and extensor digitorum longus muscles
It has been shown that there is a certain degree of heterogeneity in the fibre composition along the longitudinal axis of the skeletal muscles (Torrella et al. 2000). Therefore, the present paper only focused on the study and exhaustive description of the morphofunctional parameters in the equatorial region of each muscle. Figure 1 shows representative transverse sections of soleus muscle (SOL, on the left) and muscle extensor digitorum longus (EDL, on the right). Each section displays the anatomical orientation and the location of the fields used for the fibre typification and capillarization measurements. Figure 4 shows the significant increase (P < 0.001) in the proportion of fast fibre in the soleus of the hypoxaemic animals (SO = 63.8%; FG = 8.2%; FOG = 27.4%), as compared to control rats (SO = 79.0%; FG = 3.9%; FOG = 17.1%). No significant changes were found in extensor digitorum longus muscle, although a slight increase in slow fibres was observed in hypoxic animals (SO = 28.7%; FG = 48.4%; FOG = 23.5%) when compared with the animals used as controls (SO = 24.7%; FG = 49.7%; FOG = 25.6%).
Muscle morphometry and capillarization
In soleus muscle, a significant increase (P < 0.01) in the cross-sectional area and Feret diameter of SO and FG type fibres was observed in hypoxaemic animals. In addition, a significant decrease in capillary density was observed. A slight reduction in fibre density and C/F ratio was also observed in the soleus muscle of anaemic rats. In extensor digitorum longus muscle, a significant decrease in capillary and fibre densities was found in anaemic animals. There was also a marked alteration in cross-sectional morphology of all the fibre types, as indicated by noticeable changes in the shape factor (Table 2).
Table 2.
Comparison of morphometric and capillarization parameters in extensor digitorum longus (EDL) and soleus (SOL) muscles between control and hypoxaemic animals. Results are expressed as mean ± SE. Significant differences between anaemic and control condition are indicated when observed
| SOL | ||||||
|---|---|---|---|---|---|---|
| Control | Anaemia | |||||
| Capillary density (cap mm−1) | 1073 ± 103 | 888 ± 87*** | ||||
| Fibre density (fib mm−1) | 302 ± 27 | 273 ± 46 | ||||
| Capillary to fibre ratio | 3.6 ± 0.5 | 3.3 ± 0.3 | ||||
| Fibre type | SO | FG | FOG | SO | FG | FOG |
| Area (µm) | 3003 ± 503 | 3810 ± 659 | 3399 ± 1340 | 3632 ± 520** | 4885 ± 931** | 3363 ± 480 |
| Perimeter (µm) | 235 ± 26 | 271 ± 41 | 243 ± 43 | 250 ± 21 | 297 ± 32 | 244 ± 25 |
| Feret diameter (µm) | 61.8 ± 4.4 | 69.4 ± 6.1 | 64.3 ± 13.7 | 67.5 ± 4.8** | 78.3 ± 7.4** | 65.3 ± 4.7 |
| Shape factor | 0.70 ± 0.08 | 0.67 ± 0.14 | 0.69 ± 0.10 | 0.72 ± 0.04 | 0.71 ± 0.05 | 0.70 ± 0.06 |
| EDL | ||||||
|---|---|---|---|---|---|---|
| Control | Anaemia | |||||
| Capillary density (cap mm−1) | 756 ± 71 | 670 ± 84** | ||||
| Fibre density (fib mm−1) | 643 ± 86 | 575 ± 67* | ||||
| Capillary to fibre ratio | 1.2 ± 0.1 | 1.2 ± 0.1 | ||||
| Fibre type | SO | FG | FOG | SO | FG | FOG |
| Area (µm) | 1519 ± 438 | 3659 ± 487 | 2239 ± 244 | 1577 ± 279 | 3761 ± 571 | 2463 ± 437 |
| Perimeter (µm) | 151 ± 14 | 244 ± 17 | 186 ± 14 | 155 ± 14 | 241 ± 18 | 195 ± 20 |
| Feret diameter (µm) | 42.2 ± 3.2 | 69.1 ± 2.5 | 53.6 ± 2.9 | 44.3 ± 4.0 | 68.6 ± 5.4 | 55.5 ± 5.1 |
| Shape factor | 0.79 ± 0.02 | 0.78 ± 0.02 | 0.78 ± 0.03 | 0.81 ± 0.02*** | 0.80 ± 0.02** | 0.80 ± 0.03* |
P < 0.05;
P < 0.01
P < 0.001
Biochemical data
In all the samples of the studied muscles, a significant increase (P < 0.05 or P < 0.01) in metabolite concentration (µmol·g−1 w/w) was observed in the anaemic animals as compared with the control group (Table 3).
Discussion and Conclusions
Hypoxaemic hypoxia is a stressful situation for most animals. To cope with the oxygen requirements, the organism is able to perform physiological and morphological adjustments to maintain a minimum level of aerobic metabolism under conditions of poor oxygen delivery to the tissues. The symmorphosis principle states that structural elements are formed to satisfy functional requirements without excess (Taylor & Weibel, 1981). Therefore, we would expect that when blood oxygen transport capacity is reduced, some adaptive responses must be elicited at muscle level to adjust the oxidative power of the muscle to this convective limitation.
In fact, in the present study we observed that the lower blood oxygen transport capacity associated with anaemia causes a change in the site of oxygen exchange at peripheral level. A clear diminution of soleus muscle capillarization was observed, accompanied by a similar, but less marked, trend in extensor digitorum longus muscle. This decrease in the number of capillaries per mm2 in both muscles does not agree with the findings of most studies on hypoxia effects (Valdivia, 1958; Banchero, 1975; Banchero et al. 1976), including studies previously undertaken in our laboratory (Panisello et al. 2007). However, the present results are in partial agreement with the few studies on anaemia and capillary density (Celsing et al. 1988). This trend could be explained by a simultaneous increase in fibre cross-sectional area whilst maintaining capillary numbers. Capillary density was quite different in the two studied muscles. It has also been shown that the number of capillaries is higher in soleus than in extensor digitorum longus (Hoppeler et al. 1981; Leon-Velarde et al. 1993) after hypoxia conditions. Therefore, our data indicate that hypoxaemic hypoxia leads to a marked reduction in the oxidative character of soleus muscle (which is a postural and slow-twitch muscle), whereas changes are less noticeable in a muscle such as extensor digitorum longus, which has fast-contraction activity and a marked anaerobic character. This is because the metabolism of extensor digitorum longus is essentially glycolytic (Itoh et al. 1990; Faucher et al. 2005). It can be concluded that hypoxemic hypoxia transforms the soleus muscle into a faster and less aerobic muscle, whereas extensor digitorum longus muscle is only modestly affected. The reduced response of extensor digitorum longus muscle to hypoxaemic hypoxia could result from its initial low capacity for O2 uptake.
In agreement with previous reports (Hoppeler et al. 1990), we have observed that a long period of exposure to hypoxaemic hypoxia conditions reduces the muscle oxidative capacity and the mitochondrial volume in all regions of muscle fibres of rat soleus muscle. As can be observed in Fig. 3, the decrease in mitochondrial volume was homogeneous when the three fibre regions (pericapillary, sarcolemmal and sarcoplasmatic) were compared. Thus, in spite of the fall in oxygen diffusion gradient, hypoxaemic animals showed the same arrangement as control animals in the relationship between mitochondrial volume and fibre regions depth (pericapillary > sarcolemmal > sarcoplasmatic). Under hypoxaemic conditions, extensor digitorum longus muscle also suffers a reduction in mitochondrial volume that is concomitant with the decrease in the capillary and fibre densities. This indicates that hypoxaemia can also affect the morphofunctional organization of predominantly anaerobic muscles. This finding can be considered an efficient response against anaemia. This interpretation is supported by studies showing preservation of mitochondrial function in young anaemic patients with chronic renal failure (Miro et al. 2002).
The increase in metabolite concentration found in the muscles of animals exposed to hypoxaemic hypoxia could be interpreted as a compensatory response to decreased perfusion and oxygen supply. This interpretation is in agreement with a clinical study using 31P magnetic resonance spectroscopy (MRS) and near-infrared spectroscopy (NIRS) in the calf muscle of patients with peripheral vascular disease. This study describes normal muscle cross-sectional area, ATP turnover and contractile efficiency, and higher phosphocreatine (PCr) changes during exercise (i.e. an increased shortfall of oxidative ATP synthesis). Slower PCr recovery and a decrease in functional capacity for oxidative ATP synthesis were also reported. These results indicate that a primary deficit in oxygen supply dominates muscle metabolism (Kemp et al. 2001). Thompson et al. (1993) described similar findings in chronically anaemic Wistar rats. The metabolic changes described were consistent with either reduction of the oxygen supply to the muscle or altered oxidative phosphorylation by mitochondria. Considering the possibility of mitochondrial function preservation, as proposed by Miro et al. (2002), reduced oxygen supply appears to be the main factor responsible for the increase in anaerobic character of skeletal muscle fibres manifested by anaemic animals in the present study. This interpretation is consistent with increased metabolite concentration in the muscles of the anaemic group.
In conclusion, under chronic oxygen delivery restrictions, skeletal muscle became more anaerobic and less dependent on blood flow. Considerable alterations in morphological organization are associated with these metabolic changes. Further studies are required to elucidate the functional significance of these changes and their possible limiting role in muscle work.
Acknowledgments
This study was supported in part by research grants PB96-0999 and BFI2003-03439 from Spain's Ministry of Science and from the High Sports Council of Spain 20/UNI21/97 and 09/UNI21/98. We acknowledge Robin Rycroft from the Language Advisory Service at the Universitat de Barcelona for his technical assistance in editing the manuscript.
References
- Apell HJ. Morphological studies on skeletal muscle capillaries under conditions of high altitude training. Int J Sports Med. 1980;1:103–109. [Google Scholar]
- Armstrong RB, Laughlin MH. Metabolic indicators of fiber recruitment in mammalian muscles during locomotion. J Exp Biol. 1985;115:201–213. doi: 10.1242/jeb.115.1.201. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle-fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Banchero N. Capillary density of skeletal muscle in dogs exposed to simulated altitude. Proc Soc Exp Biol Med. 1975;148:435–439. doi: 10.3181/00379727-148-38556. [DOI] [PubMed] [Google Scholar]
- Banchero N, Gimenez M, Rostami A, Eby SH. Effects of simulated altitude on O2transport in dogs. Respir Physiol. 1976;27:305–321. doi: 10.1016/0034-5687(76)90060-8. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types – how many and what kind. Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Turner DL. Effect of training on maximal oxygen-uptake and aerobic capacity of locomotory muscles in tufted ducks, aythya-fuligula. J Physiol (Lond) 1988;401:347–359. doi: 10.1113/jphysiol.1988.sp017166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celsing F, Ekblom B, Sylven C, Everett J, Astrand PO. Effects of chronic deficiency anaemia on myoglobin content, enzyme activity, and capillary density in the human skeletal muscle. Acta Med Scand. 1988;223:451–457. doi: 10.1111/j.0954-6820.1988.tb15897.x. [DOI] [PubMed] [Google Scholar]
- Christov C, Chrétien F, Abou-Khalil R, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of rat during development. J Physiol (Lond) 1964;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of mammalian skeletal-muscles. Physiol Rev. 1972;52:129–97. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Muscle Biopsy: A Practical Approach. London: Balliere Tindall; 1985. [Google Scholar]
- Egginton S, Ross HF. Planar analysis of tissue capillary supply. In: Egginton S, Ross HF, editors. Oxygen Transport in Biological Systems: Modelling of Pathways from Environment to Cell. Cambridge: Cambridge University Press.; 1992. pp. 165–195. [Google Scholar]
- Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, Adrian RH, editors. Handbook of Physiology. Skeletal Muscle. Bethesda, MD: American Physiological Society.; 1986. pp. 73–112. Sect. 10. [Google Scholar]
- Eisenberg BR, Kuda AM, Peter JB. Stereological analysis of mammalian skeletal muscle. 1. Soleus muscle of adult guinea-pig. J Cell Biol. 1974;60:732–754. doi: 10.1083/jcb.60.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher M, Guillot C, Marqueste T, et al. Matched adaptations of electrophysiological, and histological properties of skeletal muscle in response to chronic hypoxia. Eur J Physiol. 2005;450:45–52. doi: 10.1007/s00424-004-1370-6. [DOI] [PubMed] [Google Scholar]
- Fouces V, Torrella JR, Palomeque J, Viscor G. A histochemical ATPase method for the demonstration of the muscle capillary network. J Histochem Cytochem. 1993;41:283–289. doi: 10.1177/41.2.7678272. [DOI] [PubMed] [Google Scholar]
- Gray SD, Renkin EM. Micro-vascular supply in relation to fiber metabolic type in mixed skeletal muscles of rabbits. Microvasc Res. 1978;16:406–425. doi: 10.1016/0026-2862(78)90073-0. [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the Rat. New York: Hofner; 1959. Transactions of the American Philosophical Society, New series, vol XXVII. [Google Scholar]
- Hoppeler H, Kleinert E, Schlegel C, et al. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med. 1990;13(Suppl. 1):S166–S168. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Mathieu O, Krauer R, Claassen H, Armstrong RB, Weibel ER. Design of the mammalian respiratory system. 6. Distribution of mitochondria and capillaries in various muscles. Respir Physiol. 1981;44:87–111. doi: 10.1016/0034-5687(81)90078-5. [DOI] [PubMed] [Google Scholar]
- Hudlicka O. Growth of capillaries in skeletal and cardiac-muscle. Circ Res. 1982;50:451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Price S. The role of blood-flow and or muscle hypoxia in capillary growth in chronically stimulated fast muscles. Pflugers Arch. 1990;417:67–72. doi: 10.1007/BF00370770. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Hoppeler H, Uhlmann E. Relationship between the size of the capillary bed and oxidative capacity in various cat skeletal muscles. Pflugers Arch. 1987;410:369–375. doi: 10.1007/BF00586513. [DOI] [PubMed] [Google Scholar]
- Ingjer F. Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance-trained men – histochemical and ultrastructural study. Eur J Appl Physiol Occup Physiol. 1979;40:197–209. doi: 10.1007/BF00426942. [DOI] [PubMed] [Google Scholar]
- Itoh K, Moritani T, Ishida K, Hirofuji C, Taguchi S, Itoh M. Hypoxia-induced fibre type transformation in rat hindlimb muscles. Histochemical and electro-mechanical changes. Eur J Appl Physiol Occup Physiol. 1990;60:331–336. doi: 10.1007/BF00713495. [DOI] [PubMed] [Google Scholar]
- Kayar SR, Banchero N. Volume density and distribution of mitochondria in myocardial growth and hypertrophy. Respir Physiol. 1987;70:275–286. doi: 10.1016/0034-5687(87)90010-7. [DOI] [PubMed] [Google Scholar]
- Kayar SR, Claassen H, Hoppeler H, Weibel ER. Mitochondrial distribution in relation to changes in muscle metabolism in rat soleus. Respir Physiol. 1986;64:1–11. doi: 10.1016/0034-5687(86)90056-3. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roberts N, Bimson WE, et al. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. J Vasc Surg. 2001;34:1103–1110. doi: 10.1067/mva.2001.117152. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol-London. 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Velarde F, Sanchez J, Bigard AX, Brunet A, Lesty C, Monge C. High-altitude tissue adaptation in Andean coots – capillarity, fiber area, fiber type and enzymatic activities of skeletal muscle. J Comp Physiol B Biochem Syst Environ Physiol. 1993;163:52–58. doi: 10.1007/BF00309665. [DOI] [PubMed] [Google Scholar]
- Londraville RL, Sidell BD. Maximal diffusion distance within skeletal muscle can be estimated from mitochondrial distributions. Respir Physiol. 1990;81:291–302. doi: 10.1016/0034-5687(90)90110-k. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvas Res. 1987;33:98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Morphometric analysis of capillary geometry in pigeon pectoralis muscle. Am J Anat. 1991;191:74–84. doi: 10.1002/aja.1001910108. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Muscle adaptation to altitude: tissue capillarity and capacity for aerobic metabolism. High Alt Med Biol. 2001;2:413–425. doi: 10.1089/15270290152608598. [DOI] [PubMed] [Google Scholar]
- Maxwell LC, White TP, Faulkner JA. Oxidative capacity, blood-flow, and capillarity of skeletal muscles. J Appl Physiol. 1980;49:627–633. doi: 10.1152/jappl.1980.49.4.627. [DOI] [PubMed] [Google Scholar]
- Miro O, Marrades RM, Roca J, et al. Skeletal muscle mitochondrial function is preserved in young patients with chronic renal failure. Am J Kidney Dis. 2002;39:1025–1031. doi: 10.1053/ajkd.2002.32776. [DOI] [PubMed] [Google Scholar]
- Nachlas MM, Tsou KC, Desouza E, Cheng CS, Seligman AM. Cytochemical demonstration of succinyl dehydrogenase by the use of a new para-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957;5:420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Panisello P, Torrella JR, Pagés T, Viscor G. Capillary supply and fiber morphometry in rat myocardium after intermittent exposure to hypobaric hypoxia. High Alt Med Biol. 2007;8:322–30. doi: 10.1089/ham.2007.1030. [DOI] [PubMed] [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of 3 fiber types of skeletal muscle in guinea-pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Poole DC, Mathieu-Costello O. Skeletal muscle capillary geometry – adaptation to chronic hypoxia. Respir Physiol. 1989;77:21–29. doi: 10.1016/0034-5687(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol. 2003;13:83–89. doi: 10.1016/s1044-579x(02)00103-7. [DOI] [PubMed] [Google Scholar]
- Sakkas GK, Ball D, Sargeant AJ, Mercer TH, Koufaki P, Naish PF. Skeletal muscle morphology and capillarization of renal failure patients receiving different dialysis therapies. Clin Sci (Lond) 2004;107:617–23. doi: 10.1042/CS20030282. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Weibel ER. Design of the mammalian respiratory system. I. Problem and strategy. Respir Physiol. 1981;44:1–10. [PubMed] [Google Scholar]
- Thompson CH, Green YS, Ledingham JG, Radda GK, Rajagopalan B. The effect of iron deficiency on skeletal muscle metabolism of the rat. Acta Physiol Scand. 1993;147:85–90. doi: 10.1111/j.1748-1716.1993.tb09475.x. [DOI] [PubMed] [Google Scholar]
- Torrella JR, Fouces V, Palomeque J, Viscor G. Innervation distribution pattern, nerve ending structure, and fiber types in pigeon skeletal muscle. Anat Rec. 1993;237:178–186. doi: 10.1002/ar.1092370205. [DOI] [PubMed] [Google Scholar]
- Torrella JR, Fouces V, Palomeque J, Viscor G. Capillarity and fibre types in locomotory muscles of wild mallard ducks (Anas platyrhynchos) J Comp Physiol B Biochem Syst Environ Physiol. 1996;166:164–177. [Google Scholar]
- Torrella JR, Whitmore JM, Casas M, Fouces V, Viscor G. Capillarity, fibre types and fibre morphometry in different sampling sites across and along the tibialis anterior muscle of the rat. Cells Tissues Organs. 2000;167:153–162. doi: 10.1159/000016778. [DOI] [PubMed] [Google Scholar]
- Valdivia E. Total capillary bed in striated muscle of guinea pigs native to the Peruvian mountains. Am J Physiol. 1958;194:585–589. doi: 10.1152/ajplegacy.1958.194.3.585. [DOI] [PubMed] [Google Scholar]
- Viscor G, Torrella JR, Fouces V, Palomeque J. Skeletal muscle capillarization and fiber types in urban and homing pigeons (Columba livia) Comp Biochem Physiol Comp Physiol. 1992;101:751–757. doi: 10.1016/0300-9629(92)90354-s. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological techniques for electron microscopic morphometry. In: Hayat MA, editor. Principles and Techniques of Electron Microscopy. New York: Van Nostrand Reinhold.; 1973. pp. 237–296. [Google Scholar]
- Weibel ER. Practical Methods for Biological Morphometry. Vol. 1. London: Academic Press; 1979. Stereological Methods. [Google Scholar]
- Weibel ER. The Pathway for Oxygen. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- Wiedeman MP. Architecture. In: Renkin EM, Michel CC, editors. Handbook of Physiology. The Cardiovascular System. IV. Bethesda, MD: American Physiological Society.; 1984. pp. 11–40. Microcirculation. [Google Scholar]