Abstract
Compared to the conventional linkage mapping, linkage disequilibrium (LD)-mapping, using the nonrandom associations of loci in haplotypes, is a powerful high-resolution mapping tool for complex quantitative traits. The recent advances in the development of unbiased association mapping approaches for plant population with their successful applications in dissecting a number of simple to complex traits in many crop species demonstrate a flourish of the approach as a “powerful gene tagging” tool for crops in the plant genomics era of 21st century. The goal of this review is to provide nonexpert readers of crop breeding community with (1) the basic concept, merits, and simple description of existing methodologies for an association mapping with the recent improvements for plant populations, and (2) the details of some of pioneer and recent studies on association mapping in various crop species to demonstrate the feasibility, success, problems, and future perspectives of the efforts in plants. This should be helpful for interested readers of international plant research community as a guideline for the basic understanding, choosing the appropriate methods, and its application.
1. INTRODUCTION
The level of the genetic diversity is pivotal for world food security and survival of human civilization on earth. Historically, humans exploited plant species for their livelihoods that resulted in domestication of many of them as improved cultivars to produce food for the better supply of the human diet [1]. Presently, out of 150 plant species cultivated in agriculture, twelve provide about 75% of human food and four produce 50% of human diet [2]. According to Food and Health Organization report, ~800 million people in the developing countries are suffering from food deficiency [3] that underlies an attention to improve agricultural production to eliminate or, at least, reduce the feeding problems.
The narrow genetic base of modern crop cultivars is the serious obstacle to sustain and improve crop productivity due to rapid vulnerability of genetically uniform cultivars by potentially new biotic and abiotic stresses [4]. However, plant germplasm resources worldwide, comprising of wild plant species, modern cultivars, and their crop wild relatives, are the important reservoirs of natural genetic variations, originated from a number of historical genetic events as a respond to environmental stresses and selection through crop domestication [1, 5]. The efficient exploiting these ex situ conserved genetic diversities is vital to overcome future problems associated with narrowness of genetic base of modern cultivars. However, many agriculturally important variations such as productivity and quality, tolerance to environmental stresses, and some of forms of disease resistance are controlled by polygenes and “multifactorial” that greatly depends on genetic × environmental (G × E) interactions [1, 6]. These complex traits are referred to as quantitative trait loci (QTLs), and it is challenging to identify QTLs based on only traditional phenotypic evaluation. Identification of QTLs of agronomic importance and its utilization in a crop improvement further requires mapping of these QTLs in a genome of crop species using molecular markers [1, 6]. This was the major breakthrough and accomplishment in many crops in “genomics era” since the end of the 20th century, and now extended to flourish in the 21st century.
In this review, we provide a brief description for the concept of genetic mapping; then, as a flourish of the crop genomics era, we thoroughly review one of the powerful genetic mapping tools for crops, linkage disequilibrium (LD)-based association study, as a high-resolution, broader allele coverage, and cost effective gene tagging approach in plant germplasm resources. This provides an opportunity to widely dissect and exploit existing natural variations for crop improvement.
2. GENETIC MAPPING OF CAUSATIVE VARIANTS
The main goal of genetic mapping is to detect neutrally inherited markers in close proximity to the genetic causatives or genes controlling the complex quantitative traits. Genetic mapping can be done mostly in two ways [1]: (1) using the experimental populations (also referred to as “biparental” mapping populations) that is called QTL-mapping as well as “genetic mapping” or “gene tagging,” and (2) using the diverse lines from the natural populations or germplasm collections that is called LD-mapping or “association mapping.” The details of the traditional QTL-mapping approach has recently been reviewed by Collard et al. [6], and further basic description of the approach here would be a redundant. For detailed concept, models and methodologies, problems, and perspectives of linkage analysis, readers are suggested refer to Liu [7] and Wu et al. [8]. Here, we briefly outline linkage mapping procedure for the sake of highlighting the merits of the alternative approach-association mapping.
So that such a linkage analysis can be done [6–8], firstly, the experimental populations such as F 2, back cross (BC), double haploid (DH), recombinant inbred line (RIL), and near isogenic line (NIL) populations, derived from the genetic hybridization of two parental genotypes with an alternative trait of interest, need to be developed. Secondly, these experimental populations including a large number of progenies or lines are measured for the segregation of a trait of interest in the different environmental conditions. Thirdly, a set of polymorphic DNA markers, differentiating the parental genotypes and segregating in a mapping population, need to be identified and genotyped. For that, usual practice is that, first, the parental genotypes are screened, and if markers are polymorphic over the parents, then, all individuals of a mapping population are genotyped with these polymorphic molecular markers. Once genotypic data of a mapping population is ready, marker data is used to construct the framework linkage maps, representing the order (position) and linkage (a relative genetic distance in cM) of used molecular markers along the linkage groups or segments of particular chromosomes. This is accomplished through assessing of recombination rates between the marker loci. Consequently, these markers ordered along the linkage map are statistically correlated with phenotypic characteristics of individuals of a mapping population, and QTL regions affecting a trait of interest, along with closely positioned marker tags to that QTL, are identified.
One can imagine these linkage marker maps as a “road map,” marker tags as the labels directing to specific places, and QTLs to a community/neighborhood (with specific function) on the map [6]. The precision of QTL-mapping largely depends on the genetic variation (or genetic background) covered by a mapping population, the size of a mapping population, and a number of marker loci used. Once QTLs affecting a trait of interest accurately tagged using above-outlined approach, marker tags are the most effective tools in a crop improvement that allows the mobilization of the genes of interest from donor lines to the breeding material through marker-assisted selection (MAS). Although traditional QTL-mapping will continue being an important tool in gene tagging of crops, it is a “now classical approach” and overall is very costly [1, 9], and has low resolution with simultaneous evaluation of only a few alleles [10] in a longer research time scale. In linkage mapping, the major limitation, hampering the fine mapping, is associated with the availability of only a few meiotic events to be used that occurred since experimental hybridization in a recent past [11].
3. ASSOCIATION MAPPING AS AN ALTERNATIVE APPROACH
These limitations, however, can be reduced with the use of “association mapping” [1]. Turning the gene-tagging efforts from biparental crosses to natural population of lines (or germplasm collections), and from traditional QTL-mapping to linkage disequilibrium (LD)-based association study became a powerful tool in mapping of the genes of interest [12]. This leads to the most effective utilization of ex situ conserved natural genetic diversity of worldwide crop germplasm resources. LD refers to a historically reduced (nonequilibrium) level of the recombination of specific alleles at different loci controlling particular genetic variations in a population. This LD can be detected statistically, and has been widely applied to map and eventually clone a number of genes underlying the complex genetic traits in humans [13–16].
The advantages of population-based association study, utilizing a sample of individuals from the germplasm collections or a natural population, over traditional QTL-mapping in biparental crosses primarily are due to (1) availability of broader genetic variations with wider background for marker-trait correlations (i.e., many alleles evaluated simultaneously), (2) likelihood for a higher resolution mapping because of the utilization of majority recombination events from a large number of meiosis throughout the germplasm development history, (3) possibility of exploiting historically measured trait data for association, and (4) no need for the development of expensive and tedious biparental populations that makes approach timesaving and cost-effective [17–19].
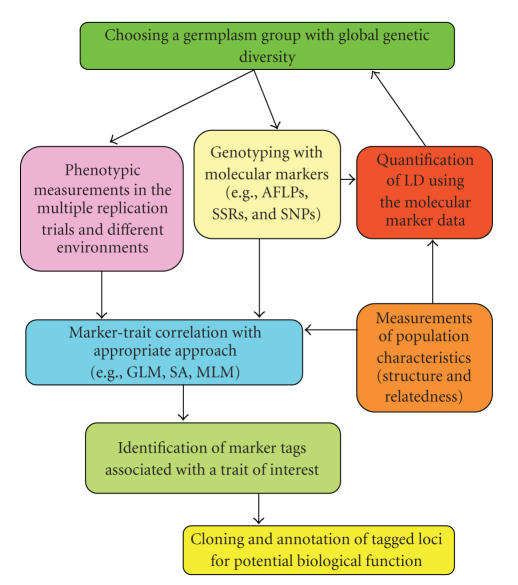
Although the overall approach of population-based association mapping in plants varies based on the methodology chosen (see below sections), assuming structured population samples, the performance of association mapping includes the following steps (see Figure 1): (1) selection of a group of individuals from a natural population or germplasm collection with wide coverage of genetic diversity; (2) recording or measuring the phenotypic characteristics (yield, quality, tolerance, or resistance) of selected population groups, preferably, in different environments and multiple replication/trial design; (3) genotyping a mapping population individuals with available molecular markers; (4) quantification of the extent of LD of a chosen population genome using a molecular marker data; (5) assessment of the population structure (the level of genetic differentiation among groups within a sampled population individuals) and kinship (coefficient of relatedness between pairs of each individuals within a sample); and (6) based on information gained through quantification of LD and population structure, correlation of phenotypic and genotypic/haplotypic data with the application of an appropriate statistical approach that reveals “marker tags” positioned within close proximity of targeted trait of interest. Consequently, a specific gene(s) controlling a QTL of interest can be cloned using the marker tags and annotated for an exact biological function (Figure 1). As a starting point for association mapping, it is important to gain knowledge of the patterns of LD for genomic regions of the “target” organisms and the specificity of the extent of LD among different populations or groups to design and conduct unbiased association mapping [20, 21]
Figure 1.
The scheme of association mapping for tagging a gene of interest using germplasm accessions. Note that the outlined scheme may vary based on population characteristics and methodology chosen for association study.
4. LINKAGE DISEQUILIBRIUM (LD)
4.1. Concept of LD
Genetic linkage generally refers to coinheritance of different loci within a genetic distance on the chromosome. There are two terms used in population genetics, linkage equilibrium (LE), and linkage disequilibrium (LD) to describe linkage relationships (co-occurrence) of alleles at different loci in a population. LE is a random association of alleles at different loci and equals the product of allele frequencies within haplotypes, meaning that at random combination of alleles at each locus its haplotypes (combination of alleles) frequency has equal value in a population. In contrast, LD is a nonrandom association of alleles at different loci, describing the condition with nonequal (increased or reduced) frequency of the haplotypes in a population at random combination of alleles at different loci. LD is not the same as linkage, although tight linkage may generate high levels of LD between alleles. Usually, there is significant LD between more distant sites or sites located in different chromosomes, caused by some specific genetic factors [9, 22–24] that will be discussed in below sections. Linkage disequilibrium also referred as “gametic phase disequilibrium” (GPD) or “gametic disequilibrium” (GLD) [11, 25] in the literature that describes the same nonrandom association of haplotypes within unrelated populations with a distantly shared ancestry, assuming Hardy-Weinberg equilibrium (HWE).
The concept of LD was first described by Jennings in 1917, and its quantification (D) was developed by Lewtonin in 1964. The simplified explanation of the commonly used LD measure, D or D′ (standardized version of D), is the difference between the observed gametic frequencies of haplotypes and the expected gametic haplotype frequencies under linkage equilibrium (D = PAB − PAPB = PABPab − PAbPaB) [26]. Besides D, a various different measures of LD (D′, r 2, D 2, D*, F, X (2), and δ) have been developed to quantify LD [25, 27–29]. The detail formulae and description of LD quantification was well explained by a number of review papers [10, 25, 26] with a number of hypothetical scenarios for LD and LE. The merits, sensitivity, comparison, appropriate statistical tests, and calculation methodology for these LD measures with the utilization of biallelic or multiallelic loci have been extensively described in the literature in detail [10, 26, 30, 31], and have recently been reviewed by Gupta et al. [25]. Hence here we highlight only some of key utility properties of LD measures to provide a brief understanding the merits of LD in association mapping.
Choosing the appropriate LD measures really depends on the objective of the study, and one performs better than other in particular situations and cases; however, D′ and r 2 is the most commonly used measures of LD [25, 26]. D′ is informative for the comparisons of different allele frequencies across loci and strongly inflated in a small sample size and low-allele frequencies; therefore, intermediate values of D′ is dangerous for comparative analyses of different LD studies and should be verified with the r 2 before using for quantification of the extent of LD [26]. The r 2, the square of the correlation coefficient between the two loci have more reliable sampling properties than D′ with the cases of low allele frequencies [26]. The r 2 is affected by both mutation and recombination while D′ is affected by more mutational histories (it might indicate minimal historic recombination when high D′ values used) [10, 25, 26, 31]. Considering the objective, the most appropriate LD quantification measure needed for association mapping is r 2 that is also an indicative of marker-trait correlations [25, 26, 32]. The r 2 value varies from 0 to 1, and it will be equal to 1 when only two haplotypes are present. The r 2 value of equal to 0.1 (10%) or above considered the significant threshold for the rough estimates of LD to reveal association between pairs of loci [33].
It is noteworthy to briefly mention here that the estimation of above described GLD (commonly used in association mapping) between different loci ordered within gametes assumes that a targeted population or sampled germplasm is randomly mating and under HWE. Nevertheless, many natural populations violate HWE due to different genetic events (bottleneck, mutation, admixture, artificial selection, population structure, etc.) occurred in history of a population, and are under Hardy-Weinberg disequilibrium (HWD). A concept of “zygotic disequilibrium (ZLD)” was introduced for such a nonequilibrium population [34] that measures LD between different loci of gametes. ZLD, being defined as a deviation of joint zygotic frequencies from the expected values of zero zygotic associations [35, 36], has a power to measure nonrandom associations at both gametic and zygotic level [34, 37]. It shares the most of statistical properties of GLD [36], and the results of GLD and ZLD are mostly in agreement, yet ZLD detects more extensive LD than determined by GLD [37]. The statistical models of ZLD measures for biallelic and multilocus data, its application for natural populations, and inference the genetic and demographic events from the comparisons of GLD and ZLD results as well as implication for whole genome association studies (WGAs) were excellently addressed and described by a number of studies [35–37].
4.2. Calculation and visualization of LD: LD triangle and decay plots
LD can be calculated using available haplotyping algorithms [26]. One of such efficient methodology is the maximum likelihood estimate (MLE) using an expectation maximization algorithm [38]. Several computer software packages are available and can be utilized for calculation of LD using variety type of molecular markers. These software packages were extensively listed and described in the review by Gupta et al. [25].
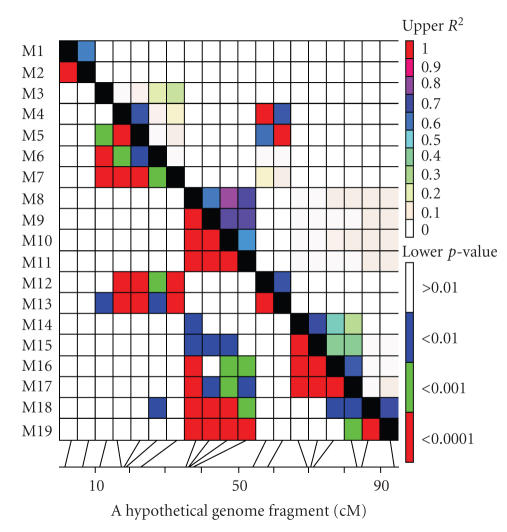
Graphical display of pairwise LD between two loci is very useful to estimate the LD patterns measured using a large number of molecular markers. Pairwise LD can be depicted as a color-code triangle plot (Figure 2) based on significant pairwise LD level (r 2, and p-value as well as D′) that helps to visualize the block of loci (red blocks) in significant LD. The large red blocks of haplotypes along the diagonal of the triangle plot indicate the high level of LD between the loci in the blocks, meaning that there has been a limited or no recombination since LD block formations. There is freely available specific computer software, “graphical overview of linkage disequilibrium” (GOLD) [39], to depict the structure and pattern of LD. Some other software packages measuring LD such as “Trait Analysis by aSSociation, Evolution and Linkage” (TASSEL) [33, 40] and PowerMarker [41] have also similar graphical display features. The strong block-like LD structures are of a great interest in association mapping which simplifies LD mapping efforts of complex traits [42]. LD blocks are very useful in association mapping when sizes are calculated, which suggest the needs for the minimum number of markers to efficiently cover the genome-wide haplotype blocks in association mapping.
Figure 2.
The TASSEL generated triangle plot for pairwise LD between marker sites in a hypothetical genome fragment, where pairwise LD values of polymorphic sites are plotted on both the X- and Y-axis; above the diagonal displays r 2 values and below the diagonal displays the corresponding p-values from rapid 1000 shuffle permutation test. Each cell represents the comparison of two pairs of marker sites with the color codes for the presence of significant LD. Colored bar code for the significance threshold levels in both diagonals is shown. The genetic distance scale for a hypothetical genome fragment was manually drawn. Note: this is for demonstration purposes only and does not have any real impact or correspond to any genomic fragment of an organism.
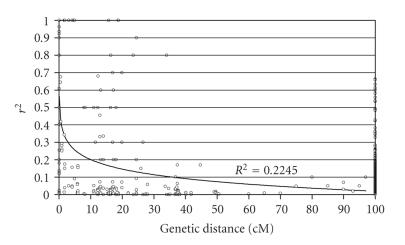
To estimate the size of these LD blocks, the r 2 values (alternatively, D′ can also be used) usually plotted against the genetic (cM) or weighted (bp) distance referred to as a “LD decay plot” (Figure 3). One can estimate an average genome-wide decay of LD by plotting LD values obtained from a data set covering an entire genome (i.e., with more or less evenly spaced markers across all chromosomes in a genome) against distance. Alternatively, the extent of LD for particular region (gene or chromosome) can be estimated from an LD decay plot generated using dataset obtained from a region of interest. When such a LD decay plot generated, usual practice is to look for distance point where LD value (r 2) decreases below 0.1 or half strength of D′ (D′ = 0.5) based on curve of nonlinear logarithmic trend line (see, e.g., [33, 43, 44]). This gives the rough estimates of the extent of LD for association study, but for more accurate estimates, highly significant threshold LD values (r 2 ≥ 0.2) are also used as a cutoff point. The decrease of the LD within the genetic distance indicates that the portion of LD is conserved with linkage and proportional to recombination [22, 25].
Figure 3.
Linkage disequilibrium (LD) decay plot depicted from the LD values of a hypothetical marker data to demonstrate a measure of an average genome-wide LD block sizes. A pairwise LD values (r 2) are plotted against a genetic distance. Inner fitted trend line is a nonlinear logarithmic regression curve of r 2 on genetic distance. LD decay is considered below r 2 = 0.1 threshold and based on trend line it is around 38–40 cM in above plot. A pairwise LD between unlinked marker loci is assigned to 100 cM distance point. Note: this is for demonstration purposes only and does not have any real impact or correspond to any genomic fragment of an organism.
4.3. Factors affecting LD and association mapping
There are many genetic and demographic factors that play a role in the shaping of the haplotypic LD blocks in a genome [9, 22, 23, 25, 26]. Although mutation and recombination are one of the strong impact factors influencing LD [24], generally, factors affecting LD can be grouped into two categories: (1) factors that increasing LD, and (2) factors that decreasing LD. The increase of LD is observed with new mutation, mating system (self-pollination), genetic isolation, population structure, relatedness (kinship), small founder population size or genetic drift, admixture, selection (natural, artificial, and balancing), epistasis, and genomic rearrangements [25, 26]. The decrease of LD is observed with high recombination and mutation rate, recurrent mutations, outcrossing, and gene conversions [25, 26].
LD conserved with linkage is very useful for association mapping. However, more often there is a significant LD between pairs of loci located far from each other or even in different chromosomes that might cause spurious correlations in association mapping. These long stretched LD or LD between unlinked loci indicate the existence of other LD generating factors than linkage itself in a genome [9, 22, 23]. One of those factors is selection that generate LD between unlinked loci through “a hitchhiking” effect (high-frequency sweeping and fixation of alleles flanking a favored variant) [45], and epistatic selection or the so-called coadapted genes [46] that is the result of coselection of loci during breeding for multiple traits [26], common in traditional crop breeding programs worldwide.
The population structure (existences of distinctly clustered subdivisions in a population) and population admixture are the main factors to create such an LD between unlinked loci. This primarily happens due to the occurrence of distinct allele frequencies with different ancestry in an admixed or structured population. Theoretically, relatedness generates LD between linked loci, yet it might also generate LD between unlinked loci pairs when predominant parents exist in germplasm groups. There is evidence that relatedness caused LD between linked and unlinked loci in an equal proportion in maize germplasm [22]. The high ratio value of linked to unlinked loci in LD is good indicative to draw conclusion about the role of LD generating factor(s) such as selection or population stratification (cryptic relatedness) [9, 22, 23]. The other factors such as genetic drift or bottlenecks might have also generated LD in a genome [22–24], which is evidenced by nonuniform distribution of LD in chromosomes [24].
Knowing these factors that are increasing or decreasing LD in a genome, obvious question one might ask is whether increased or decreased level of LD is favored in association mapping? Very extensive level of LD (means LD persists within a long distance), theoretically, reduces a number of markers needed for association mapping, but makes resolution lower (coarse mapping). In contrast, less extensive level of LD (means that LD quickly decays within a short distance) requires many markers to tag a gene of interest, but in high resolution (fine mapping). Hence, choosing a population with low or high level of LD depends on the objective of association mapping study. Furthermore, increased LD level due to LD between unlinked loci is not salutary in association mapping since it tends to cause spurious marker-trait associations. LD generated by selection, population structure, relatedness, and genetic drift might be theoretically useful for association mapping in specific situations and population groups that reduces number of markers needed for association mapping [9, 22], but requires serious attention to control factors affecting LD (e.g., population structure and relatedness) to perform unbiased population-based association mapping in plants [41, 47] (see next sections).
There are other factors affecting LD referred to as a whole “ascertainment bias” that are associated with an assayed sample and data characteristics. Some of these factors leading to inaccurate estimate of LD were well reviewed by Gupta et al. [25]. One of such factors largely affecting the LD and leading inaccurate estimates is the presence of minor alleles (also referred as to rare alleles that are present in only 5 to 10% individuals of the sample) in a dataset. Minor alleles are problematic in LD quantification as they largely inflate LD values (in particular the D′ and p-values) [43, 48–50]. The r 2 is also very sensitive and has a large variance with rare alleles [43, 51]. Hence in the quantification of LD and association mapping, markers with minor allele frequency of 5–10% (varied from study to study) are (1) removed before analysis (see, e.g., [17, 18, 43, 44, 51]), (2) pooled into common allelic class (see, e.g.,[44, 46]), and (3) replaced with missing values (see, e.g., [52, 53]).
4.4. Estimation of LD using dominant versus codominant markers
The quantification methodology of LD, perfectly suitable for biallelic codominant type of markers (majorly, single nucleotide polymorphisms (SNPs) and now largely extended to multiallelic simple sequence repeats-SSRs), has been well developed and used in human, animal, and plant populations (for reviews see [25, 27–30]). LD quantification using dominant markers (such as random amplified polymorphic DNAs-RAPD, and amplified fragment length polymorphisms-AFLPs) is poorly explored and usually subject to wrong perception and interpretation. However, many underrepresented plant species, like forest trees, or other crops with limited genomic information largely rely on dominant type of markers such as RAPDs and AFLPs [54]. Furthermore, even with codominant, and multiallelic SSR markers, there is a great challenge with assigning correct allelic relationships (identity by decent) of multiple band amplicons when diverse, reticulated, and polyploid germplasm resources, lacking historical pedigree information, are genotyped. Misassignment of allelic relationships of loci is the concern in association analysis [55]. To avoid such a challenging cases, (1) one might select only single band SSR loci and code a dataset as a codominant marker type, yet such a single band SSRs are usually not many in polyploid crop genomes and yield also multiple bands when very diverse germplasm resources are genotyped; (2) alternatively, multiple-band SSRs with unknown allelic relationship may be scored as a dominant marker taking each band as an independent marker locus (uniquely) with a clear size band separation (see, e.g., [52, 56]).
Could a dominant marker data be used for LD quantification? There are some reports where LD level of natural forest tree populations has been measured using dominant markers (AFLPs) and commonly used statistical approach (see, e.g., [57]). There are also a number of reports where dominantly coded (present versus absent) marker data of RAPD, RFLP, AFLP, “candidate gene” (CAPs), and SSRs were successfully used in genome-wide LD analyses and LD-based association mapping in plants (see, e.g., [17–19, 56, 58, 59, 60]), demonstrating the feasibility of dominantly coded molecular data in revealing of haplotypic associations. Although a dominant type of coding has limited statistical power compared to codominant markers in population-based analyses because of missing heterozygote information, previous studies suggested that it can be successfully applied to the clustering of individuals and grouping of populations using a Bayesian approach when a large number of loci are genotyped [61, 62]. Dominant-type markers can be a useful tool to estimate the kinship coefficients between individuals [63].
Recently, Li et al. [54] investigated the use of dominant markers in estimation of LD in diploid species and developed appropriate EM algorithm. Based on their conclusion from the comparative data simulation of dominant versus codominant markers, the dominant-type markers could effectively be used in LD analysis with preferentially large number of marker loci and population sample sizes of ≥200 for high heterozygous (proportion of alternative alleles (present versus absent) in a data set, i.e., 0.5 versus 0.5) marker data or with even larger sample size ≥400 for low-heterozygous (i.e., 0.9 versus 0.1) dominant markers. It is also recommended that a mixture of codominant and dominant markers should be used to better characterization of a genetic structure of a population [54].
4.5. LD quantification in plants
LD quantification and LD-based association mapping have been a research objective in plants beginning with the model organism as Arabidopsis, and now extended to crops as maize, barley, durum wheat, spring wheat, rice, sorghum, sugarcane, sugar beet, soybean, and grape, as well as in forest tree species, and forage grasses.
Nordborg et al. [20] sequenced 0.5–1 kb long 13 fragments from a 250 kb region surrounding the flowering time FRI gene in a 20 global sample of A. thaliana, highly selfing model plant species. They determined that LD decays within a 1 cM distance or 250 kb. Later, investigation of the same authors [21] with markers surrounding the disease resistance locus RPM1 in a globally-derived set of 96 Arabidopsis accessions revealed that a genome-wide LD extended up to 50–250 kb. LD blocks extended up to 50–100 cM in local Michigan Arabidopsis populations. These long-stretched LDs in local Arabidopsis population were explained as a genetic bottleneck or founder effect through introduction A. thaliana into North America in recent past (200 years ago). In contrast, in other study that targeted the region surrounding another disease resistance gene rps5, Tian et al. [64] reported much smaller LD block size, extended up to only 10 kb. Likewise, LD quickly decays within 10–50 kb distance around the CLAVATA 2 region of Arabidopsis [65]. Recently, Ehrenreich et al. [66] reported the LD decay within ~10 kb in extensive sequence analysis of 600-bp fragments of the regions MORE AXILLARY GROWTH 2 (MAX2) and MORE AXILLARY GROWTH 3 (MAX3) in a panel of 96 accessions from a restricted geographic range in Central Europe. In their genome-wide survey of 1347 fragments of 600-bp lengths, Plagnol et al. [67] reported that LD completely disappears after ~100 kb, which is comparable to that observed in human.
In maize (Zea mays L.), a highly outcrossing crop species, very rapid genome-wide LD decay was determined. Tenaillon et al. [68] first reported the extent of LD for maize, genotyping of 21 loci of chromosome 1 over the 25 individuals of the exotic landrace and United States maize germplasm. An average LD decay was determined to occur within 400 bp with r 2 = 0.2 and extended up to 1000 bp (~1 kb) in a group of US inbred lines. Later, Remington et al. [43] also reported a very rapid decline of LD in their survey of 6 genes (1.2–10 kb long) in 102 inbred lines, including tropical and semitropical lines with a wide genetic diversity. For these surveyed genes, LD declined generally within 200–2000 bp with r 2 = 0.1 except sugary1 (su1) loci, where LD remained significant (r 2 = 0.3 − 0.4) for 10 kb distance. This was explained by strong selective episodes in su1 gene. In the same study, Remington et al. [43] found higher level of LD with 47 SSR markers compared to those obtained from SNP data. This result was explained by different mutation rate of these two marker systems that tends to capture different historic information.
Long stretches of LD for maize also were reported. Thornsberry et al. [69] measured LD in and around the Dwarf8 locus. They found “localized LD” (i.e., restricted to particular regions, meaning that high LD stretches interspersed with regions of low LD) extended up to 3 kb. Jung et al. [70] reported the extent of LD within 500 kb in surveying adh1 locus. Stich et al. [22] examined the genetic diversity and LD in a cross section of 147 European and United States elite inbred material with 100 SSRs. They reported an average significant (P < .5) LD block size of 26 cM for flint group, or 41 cM for dent group with nonuniform distribution of LD among 10 chromosomes. They showed a very long stretched LD blocks up to 105 cM in chromosome 2 and up to 103 cM in chromosome 7 in flint and dent groups, respectively. Obtaining of different result from earlier studies [43] was explained due to using (1) much higher marker density, and (2) both related and unrelated inbred lines. In another study, the same authors [9] examined 72 European elite inbred lines with 452 AFLP and 93 SSR markers and reported much shorter average LD block sizes for AFLP (4 cM), but extensive LD for SSR (30-31 cM) in both flint and dent germplasm groups. This suggested a potential for exploiting both markers in association mapping, but with the favor of SSRs over AFLPs because of power of detecting LD. Recently, Andersen et al. [71] reported that LD is persisted over entire 3.5 kb phenylalanine ammonia lyase (PAL) gene with the r 2 > 0.2 in a survey of 32 European maize inbred lines.
In the selfing tetraploid wheat (Triticum durum Desf.), Maccaferri et al. [50] quantified LD in a 134 durum wheat accessions that extended up to 10 and 20 cM with D′ = 0.67 and 0.43, respectively. In hexaploid wheat (Triticum aestivum L), almost completely self-pollinating species, strong LD was determined to occur on average within <1 and ~5 cM for region on chromosome 2D and centromeric region 5A that was surveyed with 36 SSR markers in a 95 cultivars of winter wheat [52]. Recently, Chao et al. [72] investigated the genome-wide LD among 43 US wheat elite cultivars and breeding lines representing seven US wheat market classes using 242 SSRs distributed throughout the wheat genome. For this germplasm collection, a genome-wide LD estimates were generally less than 1 cM for the genetically linked loci pairs. Most of the LD regions observed were between loci less than 10 cM apart, suggesting LD is likely to vary widely among wheat populations [72]. Tommasini et al. [56] reported that LD on chromosome 3B extended up to 0.5 cM in 44 varieties or 30 cM in 240 RIL populations of winter wheat, surveyed with 91 SSR and STS markers. This suggested usefulness of cultivar germplasm over biparental mapping population in association mapping.
In rice (Oryza staiva L), a selfing species, Garris et al. [73] examined the LD surrounding disease resistance locus Xa5 using 21 SSRs in a survey of 114 rice accessions. They determined the strong LD within 100 kb with r 2 = 0.1. Agrama and Eizenga [74] investigated LD patterns in a worldwide collection of Oryza staiva, and its wild relatives using 176 SSR markers. Although it was not specifically indicated, LD decay plot suggests a long range LD declining ~50 cM with D′ = 0.5 in the “International” and “US” rice collections. Interestingly, LD persisted over an average of 225 cM distance with significant D′ > 0.5 in a wild accessions. In contrast, many other studies reported a less extent of LD in wild and landrace (broad-based) germplasm and high extent of LD in cultivar (narrow-based) germplasm resources in plants [9, 43]. There is evidence that the LD is remarkably different in other rice species. Rakshit et al. [75] reported that LD in O. rufipogon decays within 5 kb, while it declines at 50 kb in O. sativa ssp. indica accessions. Mather et al. [76] observed that the extent of LD is greatest in temperate japonica (>500 kb), followed by tropical japonica (~150 kb) and indica (~75 kb) that was revealed by using unlinked SNPs. LD extends over a shorter distance in O. rufipogon (≪40 kb) than in any of the O. sativa groups assayed in their study [76].
LD also has been extensively quantified another highly self-pollinated crop, barley (Hordeum vulgare L), where the extent of LD varied from 10 cM to 50 cM range depending on assayed set of a germplasm [17, 77]. Caldwell et al. [51] measured LD in four genes surrounding hardness locus (Ha) in three different gene pools and reported a long stretched LD extended up to at least 212 kb in inbred barley and 98 kb in landrace barley germplasm. In contrast to these long range LDs observed in barley germplasm, Morrell et al. [78] reported a rapid decay of LD detected within 300 bp in their study of 18 nuclear genes (average length of 1 361.1 bp) in 25 diverse wild barley accessions. In that, LD completely disappeared within a 1200 bp distance. This demonstrates another example of variability of LD quantification across germplasm resources, breeding material, and regions tested.
Furthermore, genome-wide LD has been quantified for many other plant species that extended up to 10 cM in sugar cane (Saccharum) [10], 10–50 cM in soybean (Glycine max) [79, 80], 3 cM in sugar beet (Beta vulgaris L) [81], 50 cM in sorghum (Sorghum bicolor) [44], 5–10 cM in grape (Vitis vinifera L) [53], 16–34 kb in poplar (Populus trichocarpa) [82], <500 bp in European aspen (Poplus termula) [83], 2000 bp in loblolly pine (Pinus taeda) [84], 1000 bp in Douglas-fir (Pseudostuga menziensii) [85], 100–200 bp in Norway Spruce (Picea abies) [86], 200-1, 200 bp in silage maize (Zea mays L) [87, 88], and 500–2000 bp in ryegrass (Lolium perenne) [89–91]. Also, LD quantification for other important crops, perhaps, is in progress. In this context, recently, we have quantified LD level for improved varieties and landrace stock germplasm of cotton (Gossypium hirsutum L) [92]. Survey of 200 microsatellite markers in 335 G. hirsutum variety germplasm demonstrated that a genome-wide averages of LD extended up to genetic distance of 25 cM with r 2 > 0.1. Likewise, our another companion study using 95 core set microsatellite markers in a total of 286 “exotic” G. hirsutum revealed that a genome-wide averages of LD decays within the genetic distance at <10 cM in the landrace stocks germplasm and >30 cM in photoperiodic variety germplasm, providing evidence of the potential for association mapping of agronomically important traits in cotton (Abdurakhmonov et al. unpublished, submitted elsewhere for publication).
4.6. Implications for association mapping gained from LD quantification studies in plants
Important information and implication for association mapping gained from above studies are that: (1) LD more quickly declines in outcrossing plant species than highly self-pollinating plants, enabling high resolution mapping of a trait of interest in outbreeder plant germplasm. At the same time, LD rapidly declines in crop variety groups (even in selfing species) compared to populations derived from biparental crosses, which provides an advantage of discovery more polymorphisms in the variety germplasms than biparental populations of self-pollinated crops [56]; (2) the extent of LD varies across the genomic regions, among population samples and between species with the examples of “localized LD”; (3) LD measures differ per marker systems used as a reflection of capturing of different historic information in a genome due to different mutation rate (e.g., SNP versus SSR or AFLP versus SSR); (4) an estimate of genome-wide averages for the extent of LD in plant germplasm may not adequately reflect LD patterns of specific regions or specific population groups. Each of these specific regions or population groups should additionally be explored for the extent of LD in order to conduct successful association mapping of variants within regions or populations of interest; (5) LD blocks in narrow-based germplasm groups are longer than broad-based germplasm groups in plants [9, 43]. This suggests an opportunity perform coarse mapping with less number of markers in narrow-based plant germplasm and then fine mapping in broad-based plant germplasm, assuming that genetic causations is sufficiently similar across germplasm groups [12]. This also suggest an opportunity develop a set of mapping populations with the required amount of LD and diversity for high-resolution mapping through directed crossing between selected broad- and narrow-based germplasm groups [86]; and (6) confounding population characteristics and biological behavior have serious impact on pattern and structure of LD in plant germplasm resources that need to be taken into consideration in conducting unbiased association mapping.
5. ASSOCIATION STUDIES IN PLANTS
5.1. The methodology overview
There are many types of different methodologies that have been developed and initially are widely used for association mapping studies in human (comprehensively reviewed by Schulze and McMahon [93]), yet perfectly applicable without change or case-to-case modifications for wide range of organisms, including plants. Lately, some considerably successful achievements have been made to develop powerful, more precise, and unbiased population-based association-mapping methodology for plants. Here, we provide a brief overview for a basic concept and ideology of widely used pioneer methodologies for association mapping, and then highlight the latest developments in the methodology and experimental design of association mapping in plant population with the examples of association mapping of useful traits in crop species.
The classical methodology and design of association mapping is “case and control” (also referred to as “case-control”) approach that identifies the causative gene tags in the comparison of allele frequencies in a sample of unrelated affected (referred to as “cases”) individuals and a sample of uninfected or healthy individuals (referred to as “controls”) [93, 94]. This design requires an equal numbers of unrelated and unstructured “case-control” samples for accurate mapping. The Pearson chi-square test, Fisher's exact test, or Yates continuity correction can be used for a comparison of allele frequencies and detection of an association between a disease phenotype and marker. Although favored, the random sampling individuals from a population do not provide the equal representation of case and controls in the mapping population since cases in the population are usually low, thus requires special efforts to collect the cases. Case and control approach is seriously affected by the existence of population structure and stratification that caught the attention of scientist [93]. Falk and Rubinstein [95] developed a haplotype relative risk (HRR) approach that minimizes, but not eliminates population stratification issues in association mapping [96]. In that, first, a “pseudocontrol” group (containing combination of two alleles that are not transmitted to affected offspring) is created; then, the marker allele frequencies in case and “pseudocontrol” groups are correlated [93].
To efficiently eliminate the confounding effects coming from population structure and stratification, Spielman et al. [97] developed transmission disequilibrium test (TDT) method that compares transmission versus nontransmission of marker alleles to affected offspring by using chi-square test [93], assuming a linkage between marker and trait. The TDT design requires genotyping of markers from three individuals: one heterozygous parent, one homozygous parent, and one affected offspring. Although HRR performs better with unstructured sample than TDT because of its power to completely eliminate spurious association with good experimental design, later is widely used as a tool for unbiased fine mapping of traits in the presence of linkage with a biallelic, one marker model that can accommodate pedigree structure [30, 93].
Nonetheless, initial TDT approach had issues with the use of multiallelic markers, multiple markers, missing parental information, extended (larger) pedigrees, and complex quantitative traits [93]. To address these issues, a variety of extensions of TDT approach were developed and applied for multiallelic markers (i.e., GTDT, ETDT, MC-Tm) [98–102], multiple markers [103–105], missing parental information (Curtis-test, S-TDT, SDT, 1-TDT, C-TDT or RC-TDT) [96, 106–110], which were reviewed by Schulze and McMahon [93] in detail. Shortly after publication of various extensions of TDT to multiallelic and multiple markers, the extensions for X-linked genes, such as XS-TDT or XRC-TDT were developed and applied [111]. TDT approach was also extended to pedigrees of any size as a PDT approach [112, 113] that was demonstrated more powerful than TDT, and S-TDT or SDT under the assumption of high disease prevalence [93, 114].
Further, there were many studies to extend the TDT approaches to QTL and covariates [93]. One of the comprehensive approaches, QTDT was developed with its three different extensions for quantitative traits for any pedigree structure [115, 116]. These family-based association-mapping approaches have their other improvements using more powerful statistical and robust algorithmic procedures, such as likelihood-base statistics and EM algorithm (TDTLIKE, LRT, EM-LRT) [117–119]. The unified family-based association test package (FBAT) incorporating some of TDT is also developed [120–122] to deal with wide types of experimental designs. The next generation of association mapping approaches in both “case and control” and family-based designs, referred to as identity by decent (IBD) mapping [123], haplotypes-sharing analysis (HSA) [124], and decay of haplotypes sharing (DHS) [125], involves the analysis of haplotypes by testing the length of haplotypes in the data sample, assuming affected individuals will have a longer haplotypes than controls [93].
Although family-based association mapping methodology is effective to control confounding effects of a sample and remove spurious associations, it is less powerful design [126] and have its disadvantageous sides compared to case-control [93] that led to develop the methodologies with better controlling of population structure and stratification. Such an improved methodology for a case and control design or random samples from a population involves the use of additional markers that have neutral effect (null loci) to the trait of interest in the analysis. This approach is referred to as the genomic control (GC) that finds confounding effects of a population and corrects it, thus enabling to remove spurious associations [127, 128]. Although GC is powerful then TDT [128], it will not remove spurious associations in highly structured populations. Zhao et al. [129] put it as
“Methods like “genomic control,” which simply rescale p-values without changing the ranking of loci are not likely to be useful in genome-wide scans where the existence of true positives is not in doubt.”
To better deal with highly structured populations, Pritchard et al. [47, 62] developed approach of structured association (SA). SA first searches a population for closely related clusters/subdivisions using Bayesian approach, and then uses the clustering matrices (Q) in association mapping (by a logistic regression) to correct the false associations. Population structure and shared coancestry coefficients between individuals of subdivisions of a population can be effectively estimated with STRUCTURE program using several models for linked and unlinked markers [130]. Similar type of methodology measuring and using the population subdivisions (K) in association mapping referred to as “mixture model” was proposed by several other studies [131, 132]. However, SA incorporating only population structure information in the analysis is not good enough itself when highly structured population with some degree of related individuals used in the association mapping.
Hence, recently, Yu et al. [133] developed new methodology, a mixed linear model (MLM) that combines both population structure information (Q-matrix) and level of pairwise relatedness coefficients—“kinship” (K-matrix) in the analysis. To perform MLM: (1) Q-matrix is generated using STRUCTURE, (2) the pairwise relatedness coefficients between individuals of a mapping population (K-matrix) [134] measured using SpaGedi software [135], and (3) then both Q- and K-matrices are used in association mapping to control spurious associations. Although computationally intensive, MLM approach found to be effective in removing the confounding effects of the population in association mapping [133].
Later Zhao et al. [129] extensively tested the MLM approach of Yu et al. [133] in their global set of 95 highly structured Arabidopsis population and came to overall agreement with better performance of Q + K MLM model than any of the other tests that used K- or Q-matrix alone. However, they also noted that (1) K matrix would alone be good enough if a kinship estimated as a proportion of shared haplotypes for each pair of individuals (as denoted K*); (2) the replacement of Q-matrix (from the computational intensive structure analysis) [130] with P-matrix (from more robust principal component analysis) [136] performed similarly to MLM of Yu et al. [133], thus suggesting a potential for future replacements; (3) removing of the confounding effects will also subject to remove true associations with biological effect, which is strongly correlated with population structure that requires a caution; and (4) in a small and highly structured population, the causations with major effect should be expected to be found and, perhaps, larger samples and adequate marker densities are needed for genome-wide dissection of the most traits of interest segregating in an association mapping population [129].
There are other types of mixed models for association mapping that have its own advantages to control population confounding effects and tag a genetic causative of a trait of interest. One of such mixed models utilizes a sample with pedigree information to measure a pedigree-based relatedness and incorporates it directly in QTL-mapping and association mapping [59, 137, 138]. This type of mixed model for known pedigree population combines haplotype effects with pedigree-based structure of variance-covariance relatedness matrix and random polygenic effect that control the population structure [59, 139]. The efficiency of pedigree population for association mapping depends on the population size of pedigree founders (i.e., pedigree population obtained from just two parents will not provide significant level of LD) and the level of relatedness of the founders. Latter is very important and may still lead to spurious association due to initial population structure (mostly unknown) coming from founders that needs to be analyzed also by using STRUCTURE [140].
However, as stated by Malosetti et al. [59] and others [140] the pedigree-based mixed model is highly appropriate in association mapping in crops due to (1) plant breeding programs have already generated many useful pedigree populations that contain LD useful for association studies but cannot be used as an independent LD-mapping population, and (2) many historical trait data sets in plant breeding are unbalanced that have been collected over multiple-years, and multienvironmental trials. At the same time, issues with obtaining the fine-grained pedigree information and difficulty of finding population structure of narrow-based elite cultivars are the concern in pedigree-based mixed model. There is another mixed model that combines the Bayesian variable selection for mapping multiple QTLs and LD mapping method, incorporating estimates of population structure, but not relatedness. This approach was used for association mapping in highly selfing rice germplasm [58]. Authors stated that incorporation of multiple QTL effects and population structure efficiently reduces spurious association and useful for future whole genome associations, with the development of more complex models dealing with differences of LD and effect of QTL alleles between populations.
The other mixed model approach combines QTL and LD analyses of distinct studies. In that, QTLs or candidate genes with already annotated biological function(s) are used as a priori information in association mapping [140, 141]. This is one of the effective alternative strategies in association mapping that allow reducing the total amount of marker genotyping (because of preselecting of markers restricted to QTL region) in less number of individuals. This increases the power and precision of the trait-marker correlations [142].
5.2. Power of association mapping
The power of association mapping is the probability of detecting the true associations within the mapping population size that really depends on (1) the extent and evolution of the LD in a population, (2) the complexity and mode of gene action of the trait of interest, (3) sample size and experimental design. The power can be increased utilizing the better data (knowledgeable experimental design and accurate measurements) and increasing the sample size. In QTL mapping studies, there are specific statistical approaches to estimate the false-positive level of the obtained strong (p-value) associations (control for Type I error) such as a permutation test [143] or false discovery rate (FDR) [144].
A statistical approach within the Bayesian framework is used test the reliability of obtained significance (p-values) in association mapping because of possibility of getting unreliable values due to (1) overestimation of effects (selection bias), (2) association coming from neglecting confounding effects of a sample, (3) poor experimental design, and (4) instability of genetic effects across different environments [142]. Ball [142] developed a methodology, combining the Bayesian and non-Bayesian approaches, that determines the Bayes factors guiding to properly design the experiments with given power to detect reliable effects. To detect the reliable effects in association mapping, experiments should be designed at least with the Bayes factor of 20 that may require much larger sample sizes. Bayes factor provides stronger evidence than conventional p-values [142]. If given Bayes factor value (say B = 20) reached with larger sample than the original experimental design, then, the original results indicate a very weak evidence to provide the real effects [142]. At this point, requirement for larger sample size might make association mapping disadvantageous over a traditional QTL-mapping. However, the sample size for association mapping can be decreased keeping the high power with (1) preselecting a priori known QTL regions or candidate genes (from QTL-mapping and expression analyses), (2) using the large populations with samples longer LD block that require a less number of markers to find useful associations, (3) an alternative experimental design (i.e., TDT), and (4) choosing the single marker from the haplotypes of interest that would cut also marker number and so genotyping cost [142]. Bayes factor can be calculated using R function of ld.design from ldDesign package [140]
5.3. Examples from reports
The pioneer association studies in plants were performed by Beer et al. [166] in oat, and by Virk et al. [167] in rice. Beer et al. [166] associated 13 QTL with RFLP loci using 64 oat varieties and landraces, yet without considering the population structure that resulted in more increased associations than what were obtained in separate analysis of subpopulations [11]. Virk et al. [167] predicted 6 trait values using RAPD markers in rice germplasm. Later, association mapping was extended to sea beet, barley, maize, wheat, potato, more examples in rice, and Arabidopsis that have utilized population level of LD considering a population structure. Hansen et al. [19] reported association of ALFP markers with bolting gene in sea beat. In barley, various traits such as yield, yield stability, heading date, flowering time, plant height, rachilla length, resistance to mildew and leaf rust were associated with many different types of molecular markers [17, 18, 157, 158]. In maize, flowering time and plant height [43, 69] were associated using SNP and SSRs. Following these pioneer studies of association mapping in maize, several other traits such as phenotypic variation in flowering time, endosperm color, starch production, maysin and chlorogenic acid accumulation, cell wall digestibility, and forage quality were associated using SNP markers of candidate genes [71, 87, 88, 149–153].
In wheat, Breseghello and Sorrells [52] reported first association mapping of kernel size and milling quality in a collection of USA winter wheat using SSRs. Following this work, association mapping of a high molecular-weight glutenin [159] and blotch resistance [56] were reported that utilized SNPs, SSRs, and STS markers. In rice, association mapping has not widely been applied yet due to highly structured population of rice (due to high selfing) [58, 133]. However, Zhang et al. [156] successfully used association mapping for multiple agronomic traits using discriminant analysis (DA) with SSR and AFLP markers. Recently, Iwata et al. [58] associated RFLP markers with width and length of milled rice grains in a set of 332 rice germplasm using their multiple QTL model considering the population structure. Association mapping approach was also successfully applied in tetraploid potato where resistance to wilt disease [138], bacterial blight [60], Phytophtora [59] that utilized a pedigree-based mixed model.
To date association mapping has also been extended to long lifespan plant species, forest tree populations [163], where associations of polymorphisms in cinnamoil CoA reductase (CCR) with earlywood microfibril angle trait [141], and polymorphisms a putative stress response gene with wood density and wood growth rate [163] were reported. There are also the examples of association mapping successes for cold tolerance, flowering time, water-soluble carbohydrate content, and forage quality in forges species that have recently been reviewed by Dobrowolski and Forster (Table 1) [87, 88, 161].
Table 1.
Linkage disequilibrium and association mapping studies in plants.
| Species | Mating system | LD extent | Mapped traits | *Approach used |
|---|---|---|---|---|
| Arabidopsis | Selfing | 10–250 kb and 50–100 cM [20, 21, 64, 66, 67] | Flowering time, growth response, pathogen resistance, and branching architecture [66, 129, 145–148] | One way ANOVA, simple regression, SA, MLM |
| Maize | Outcrossing | 200–2000 bp [43, 68], 3–500 kb [43, 69–71], 4–41 cM [9, 22] | Plant height, flowering time, endosperm color, starch production, maysin and chlorogenic acid accumulation, cell wall digestibility, forage quality, and oleic acid level [43, 69, 71, 87, 88, 149–154] | GLM, SA, MLM, WGA |
| Rice (indica, japonica and rufipogon) | Selfing | 5–500 kb [73, 75, 76] 50–225 cM [74], 20–30 cM [155] | Multiple agronomic traits such as plant height, heading date, flag leaf length and width, tiller number, steam diameter, panicle length, grain length and width, grain length/width ratio, grain thickness, 1000-grain weight, width and length of milled rice grains [58, 155, 156] | DA, MLM, mixed model with multiple QTL effect |
| Barley | Selfing | 10–50 cM [16, 77], 98–500 kb [51], 300 bp [78] | Yield, yield stability, heading date, flowering time, plant height, rachilla length, resistance to mildew, and leaf rust were associated with many different types of molecular markers [17, 18, 157, 158] | Pearson correlation; regression, ANOVA |
| Tetraaploid wheat | Selfing | 10 and 20 cM [50] | N/A | N/A |
| Hexaploid wheat | Selfing | <1–10 cM [52, 56, 72] | Kernel size and milling, a high molecular weight glutenin and blotch resistance [52, 56, 159] | GLM-Q, LMM |
| Potato | Selfing | 0.3–1 cM [25, 60], 3 cM [160] | Resistance to wilt disease, bacterial blight, Phytophtora, and potato quality (tuber shape, flesh color, under water weight, maturity, and etc.)[59, 60, 138, 160] | Nonparametric Mann-Whitney U test, standard two sample t-test, GMM |
| Soybean | Selfing | 10–50 cM [79, 80], | Seed protein content [80] | WGA |
| Sorghum | Outcrossing | 50 cM [44] | N/A | N/A |
| Grape | Vegetative propagation | 5–10 cM [53] | N/A | N/A |
| Sugarcane | Outcrossing/Vegetative propagation | 10 cM [10] | N/A | N/A |
| Sugar beet | Outcrossing | 3 cM [81] | N/A | N/A |
| Forage grasses (silage maize and ryegrass) | Outcrossing | 200–2000 bp [87–91] | Cold tolerance, flowering time and forage quality, water-soluble carbohydrate content [87, 88, 161, 162] | Multiple linear regression; ANOVA |
| Forest trees (Norway spruce, Loblolly pine, poplar, European aspen, Douglas-fir) | Outcrossing | 100–200 bp [86], ~500–2000 bp [83–85] | Early-wood microfibril angle trait, wood density and wood growth rate [141, 163] | ANOVA; combination of LD and QTL mapping |
* MLM: mixed linear model [133]; GLM: general linear model without population structure [71]; GLM-Q: general linear model using population structure matrix (Q) or the least square solution to the fixed effects GLM [56]; DA: discriminant analysis [156]; SA-structured association [47]; LMM: linear mixed model [52]; WGA: whole genome association [154, 164, 165]; GMM: general mixed model [59]; ANOVA: analysis of variance test; N/A—not available (search of known major online library database as of December 2007).
Association mapping of traits in Arabidopsis also has been reported and overall suitability of the approach well documented. Associations of CRY2 with flowering time were reported [145, 146]. Balasubramanian et al. [147] reported the association of PHYC with flowering and growth response in Arabidopsis. Later Zhao et al. [129] revisited to these association results with their mixed model approach and reproved some of previously reported associations (with PHYC), but challenged the power of these associations detected by using “standard linear methods without correcting population structure.” They put it as “Clearly, none of these polymorphisms would have been picked up in a genome-wide scan” while noting the use of different sample and trait measurements in the original studies. They also reported one of the significant flowering time associated polymorphisms in CLF gene in their genome-wide analysis using MLM [129]. Flowering time (in FRI gene) and pathogen resistance (in Rpm1, Rps5, and Rps2 genes) associated polymorphisms were also reported [148]. Recently, Ehrenreich et al. [66] reported polymorphisms of candidate genes (SPS1, MAX2, and MAX3) associated with branching architecture in a survey of 36 genes involved in branch development that were genotyped in a panel of 96 Arabidopsis accessions from Central Europe.
5.4. Choice of the appropriate approach
Table 1 summarizes the LD and association mapping efforts in plants including some of very recent whole genome association mapping studies. As one can see, within the frame of above highlighted association studies in plants, various association mapping methodologies (Table 1), molecular markers (both dominant and co-dominant markers), and plant germplasm resources (including landrace stocks, elite germplasm, and experimental populations—e.g., RILs) have been utilized. Identifying of the most appropriate approach and marker systems, therefore, is challenging and might be irrelevant case-to-case basis.
Choosing the appropriate association mapping depends on (1) the extent and evolution of the linkage disequilibrium in a population, (2) the level of population structure and stratification, (3) availability of pedigree information, (4) complexity of the trait of interest under study, and (5) availability of the genomic information and resources. Based on reported studies, GC is favored approach when population structure is suspected, but failed to be detected [59]; however, MLM considering both relatedness and population structure [133] and pedigree-based mixed model [59] or multiple QTL model [58] performs well in most cases with highly structured and stratified population although one still might argue based on his own experience, knowledge, and type of gemplasm used. According to Stich et al. [23], SA and MLM models do not “explicitly correct” for LD caused by selection and genetic drift, the major factors causing LD in plant germplasm and breeding materials. Hence Stich et al. [23] suggested use of family based association approach [168] with breeding materials. However, again the choice of methodology greatly depends on the germplasm used for mapping. The germplasm materials used for association mapping were comprehensively discussed by Breseghello and Sorrells [169].
6. CONCLUSIONS
Thus the association mapping methodology, initially developed by the human geneticists, has found its successive application in plant germplasm resources, in particular after recent improvements in minimization of spurious associations. The examples of association mapping studies performed in various plant germplasm resources including model plant Arabidopsis and extended to various crop germplasm largely demonstrate the flourish of crop genomics era with the utilization of powerful LD-based association mapping tool. This is also a good indicative of the potential utilization of this technology with the other crops and plant species in the future. Currently, a number of such studies are, perhaps, in progress in many laboratories worldwide. The near-future completion of genome sequencing projects of crop species, powered with more cost-effective sequencing technologies, will certainly create a basis for application of whole genome-association studies [164], accounting for rare and common copy number variants (CNV) (for review see, e.g., [165]) and epigenomics details of the trait of interest in plants, which is widely being applied in human genetics with great success. This will provide with more powerful association mapping tool(s) for crop breeding and genomics programs in tagging true functional associations conditioning genetic diversities, and consequently, its effective utilization.
ACKNOWLEDGMENTS
The authors are grateful to the Academy of Sciences of Uzbekistan and ARS-FSU Scientific Cooperation Program, Office of International Research Programs, USDA-ARS for financial support of their research in Uzbekistan. The authors would like to thank anonymous reviewer(s) of the manuscript for valuable suggestions.
References
- 1.Ross-Ibarra J, Morrell PL, Gaut BS. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(supplement 1):8641–8648. doi: 10.1073/pnas.0700643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo JEH, Leon J, editors. Neglected Crops 1492 from a Different Perspective. Botanical Garden of Córdoba, Andalusia, Spain: Food and Agriculture Organization (FAO) Corporate Document Repository; 1992. http://www.fao.org/docrep/T0646E/T0646E01.htm. [Google Scholar]
- 3. Food and Health Organization's 1999 Report on the State of Food Insecurity in the World, http://www.fao.org/News/1999/img/SOFI99-E.PDF.
- 4.Van Esbroeck GA, Bowman DT, May OL, Calhoun DS. Genetic similarity indices for ancestral cotton cultivars and their impact on genetic diversity estimates of modern cultivars. Crop Science. 1999;39(2):323–328. [Google Scholar]
- 5.Meilleur BA, Hodgkin T. In situ conservation of crop wild relatives: status and trends. Biodiversity and Conservation. 2004;13(4):663–684. [Google Scholar]
- 6.Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica. 2005;142(1-2):169–196. [Google Scholar]
- 7.Liu B-H. Statistical Genomics: Linkage, Mapping, and QTL Analysis. New York, NY, USA: CRC Press; 1998. [Google Scholar]
- 8.Wu RL, Ma C-X, Casella G. Statistical Genetics of Quantitative Traits: Linkage, Maps, and QTL. New York, NY, USA: Springer; 2007. [Google Scholar]
- 9.Stich B, Maurer HP, Melchinger AE, et al. Comparison of linkage disequilibrium in elite European maize inbred lines using AFLP and SSR markers. Molecular Breeding. 2006;17(3):217–226. [Google Scholar]
- 10.Flint-Garcia SA, Thornsberry JM, Buckler ES., IV Structure of linkage disequilibrium in plants. Annual Review of Plant Biology. 2003;54:357–374. doi: 10.1146/annurev.arplant.54.031902.134907. [DOI] [PubMed] [Google Scholar]
- 11.Jannink JL, Walsh B. Association mapping in plant populations. In: Kang MS, editor. Quantitative Genetics, Genomics and Plant Breedinging. Oxford, UK: CAB International; 2002. pp. 59–68. [Google Scholar]
- 12.Goldstein DB, Weale ME. Population genomics: linkage disequilibrium holds the key. Current Biology. 2001;11(14):R576–R579. doi: 10.1016/s0960-9822(01)00348-7. [DOI] [PubMed] [Google Scholar]
- 13.Weiss KM, Clark AG. Linkage disequilibrium and mapping of human traits. Trends in Genetics. 2002;18(1):19–24. doi: 10.1016/s0168-9525(01)02550-1. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H, Lowe CE, Cooper JD, et al. Discovery, linkage disequilibrium and association analyses of polymorphisms of the immune complement inhibitor, decay-accelerating factor gene (DAF/CD55) in type 1 diabetes. BMC Genetics. 2006;7, article 22 doi: 10.1186/1471-2156-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 16.Chapman JM, Cooper JD, Todd JA, Clayton DG. Detecting disease associations due to linkage disequilibrium using haplotype tags: a class of tests and the determinants of statistical power. Human Heredity. 2003;56(1–3):18–31. doi: 10.1159/000073729. [DOI] [PubMed] [Google Scholar]
- 17.Kraakman ATW, Niks RE, Van den Berg PMMM, Stam P, Van Eeuwijk FA. Linkage disequilibrium mapping of yield and yield stability in modern spring barley cultivars. Genetics. 2004;168(1):435–446. doi: 10.1534/genetics.104.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraakman ATW, Martínez F, Mussiraliev B, van Eeuwijk FA, Niks RE. Linkage disequilibrium mapping of morphological, resistance, and other agronomically relevant traits in modern spring barley cultivars. Molecular Breeding. 2006;17(1):41–58. [Google Scholar]
- 19.Hansen M, Kraft T, Ganestam S, Säll T, Nilsson N-O. Linkage disequilibrium mapping of the bolting gene in sea beet using AFLP markers. Genetical Research. 2001;77(1):61–66. doi: 10.1017/s0016672300004857. [DOI] [PubMed] [Google Scholar]
- 20.Nordborg M, Borevitz JO, Bergelson J, et al. The extent of linkage disequilibrium in Arabidopsis thaliana . Nature Genetics. 2002;30(2):190–193. doi: 10.1038/ng813. [DOI] [PubMed] [Google Scholar]
- 21.Nordborg M, Hu TT, Ishino Y, et al. The pattern of polymorphism in Arabidopsis thaliana . PLoS Biology. 2005;3(7):p. e196. doi: 10.1371/journal.pbio.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stich B, Melchinger AE, Frisch M, Maurer HP, Heckenberger M, Reif JC. Linkage disequilibrium in European elite maize germplasm investigated with SSRs. Theoretical and Applied Genetics. 2005;111(4):723–730. doi: 10.1007/s00122-005-2057-x. [DOI] [PubMed] [Google Scholar]
- 23.Stich B, Melchinger AE, Piepho H-P, et al. Potential causes of linkage disequilibrium in a European maize breeding program investigated with computer simulations. Theoretical and Applied Genetics. 2007;115(4):529–536. doi: 10.1007/s00122-007-0586-1. [DOI] [PubMed] [Google Scholar]
- 24.Huttley GA, Smith MW, Carrington M, O’Brien SJ. A scan for linkage disequilibrium across the human genome. Genetics. 1999;152(4):1711–1722. doi: 10.1093/genetics/152.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta PK, Rustgi S, Kulwal PL. Linkage disequilibrium and association studies in higher plants: present status and future prospects. Plant Molecular Biology. 2005;57(4):461–485. doi: 10.1007/s11103-005-0257-z. [DOI] [PubMed] [Google Scholar]
- 26.Oraguzie NC, Wilcox PL, Rikkerink EHA, de Silva HN. Linkage disequilibrium. In: Oraguzie NC, Rikkerink EHA, Gardiner SE, de Silva HN, editors. Association Mapping in Plants. New York, NY, USA: Springer; 2007. pp. 11–39. [Google Scholar]
- 27.Hedrick PW. Gametic disequilibrium measures: proceed with caution. Genetics. 1987;117(2):331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29(2):311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 29.Jorde LB. Linkage disequilibrium as a gene-mapping tool. American Journal of Human Genetics. 1995;56(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- 30.Jorde JB. Linkage disequilibrium and the search for complex disease gene. Genome Research. 2000;10(10):1435–1444. doi: 10.1101/gr.144500. [DOI] [PubMed] [Google Scholar]
- 31.Gaut BS, Long AD. The lowdown on linkage disequilibrium. Plant Cell. 2003;15(7):1502–1506. doi: 10.1105/tpc.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdallah JM, Goffinet B, Cierco-Ayrolles C, Pérez-Enciso M. Linkage disequilibrium fine mapping of quantitative trait loci: a simulation study. Genetics Selection Evolution. 2003;35(5):513–532. doi: 10.1186/1297-9686-35-6-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitt SR, Buckler ES., IV . Using natural allelic diversity to evaluate gene function. In: Grotewald E, editor. Plant Functional Genomics: Methods and Protocols. Clifton, NJ, USA: Humana Press; 2003. pp. 123–139. [DOI] [PubMed] [Google Scholar]
- 34.Weir BS. Genetic Data Analysis II. Sunderland, Mass, USA: Sinauer Associates; 1996. [Google Scholar]
- 35.Yang R-C. Zygotic associations and multilocus statistics a nonequilibrium diploid population. Genetics. 2000;155(3):1449–1458. doi: 10.1093/genetics/155.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang R-C. Analysis of mutilocus zygotic associations. Genetics. 2002;161(1):435–445. doi: 10.1093/genetics/161.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, Todhunter RJ, Lu Q, et al. Modeling extent and distribution of zygotic disequilibrium: implications for a multigenerational canine pedigree. Genetics. 2006;174(1):439–453. doi: 10.1534/genetics.106.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Molecular Biology and Evolution. 1995;12(5):921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- 39.Abecasis GR, Cookson WOC. GOLD—graphical overview of linkage disequilibrium. Bioinformatics. 2000;16(2):182–183. doi: 10.1093/bioinformatics/16.2.182. [DOI] [PubMed] [Google Scholar]
- 40. Trait Analysis by aSSociation, Evolution and Linkage (TASSEL), http://www.maizegenetics.net/tassel/.
- 41.Liu K, Muse SV. PowerMaker: an integrated analysis environment for genetic maker analysis. Bioinformatics. 2005;21(9):2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, Deng M, Chen T, Waterman MS, Sun F. A dynamic programming algorithm for haplotype block partitioning. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(11):7335–7339. doi: 10.1073/pnas.102186799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remington DL, Thornsberry JM, Matsuoka Y, et al. Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11479–11484. doi: 10.1073/pnas.201394398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamblin MT, Mitchell SE, White GM, et al. Comparative population genetics of the panicoid grasses: sequence polymorphism, linkage disequilibrium and selection in a diverse sample of Sorghum bicolor. Genetics. 2004;167(1):471–483. doi: 10.1534/genetics.167.1.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Thornton K, Berry A, Long M. Nucleotide variation along the Drosophila melanogaster fourth chromosome. Science. 2002;295(5552):134–137. doi: 10.1126/science.1064521. [DOI] [PubMed] [Google Scholar]
- 46.Cannon GB. The effects of natural selection on linkage disequilibrium and relative fitness in experimental populations of Drosophila melanogaster . Genetics. 1963;48(9):1201–1216. doi: 10.1093/genetics/48.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritchard JK, Stephens M, Rosenberg NA, Donnelly P. Association mapping in structured populations. American Journal of Human Genetics. 2000;67(1):170–181. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohlke KL, Lange EM, Valle TT, et al. Linkage disequilibrium between microsatellite markers extends beyond 1 cM on chromosome 20 in Finns. Genome Research. 2001;11(7):1221–1226. doi: 10.1101/gr.173201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McRae AF, McEwan JC, Dodds KG, Wilson T, Crawford AM, Slate J. Linkage disequilibrium in domestic sheep. Genetics. 2002;160(3):1113–1122. doi: 10.1093/genetics/160.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maccaferri M, Sanguineti MC, Noli E, Tuberosa R. Population structure and long-range linkage disequilibrium in a drum wheat elite collection. Molecular Breeding. 2005;15(3):271–289. [Google Scholar]
- 51.Caldwell KS, Russell J, Langridge P, Powell W. Extreme population-dependent linkage disequilibrium detected in an inbreeding plant species, Hordeum vulgare . Genetics. 2006;172(1):557–567. doi: 10.1534/genetics.104.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breseghello F, Sorrells ME. Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics. 2006;172(2):1165–1177. doi: 10.1534/genetics.105.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnaud A, Lacombe T, Doligez A. Linkage disequilibrium in cultivated grapevine, Vitis vinifera L. Theoretical and Applied Genetics. 2006;112(4):708–716. doi: 10.1007/s00122-005-0174-1. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Li Y, Wu S, et al. Estimation of multilocus linkage disequilibria in diploid populations with dominant markers. Genetics. 2007;176(3):1811–1821. doi: 10.1534/genetics.106.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sand PG. A lesson not learned: allele misassignment. Behavioral and Brain Functions. 2007;3, article 65 doi: 10.1186/1744-9081-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tommasini L, Schnurbusch T, Fossati D, Mascher F, Keller B. Association mapping of Stagonospora nodorum blotch resistance in modern European winter wheat varieties. Theoretical and Applied Genetics. 2007;115(5):697–708. doi: 10.1007/s00122-007-0601-6. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Wang Y, Huang H. High interpopulation genetic differentiation and unidirectional linear migration patterns in Myricaria laxiflora (Tamaricaceae), an endemic riparian plant in the three gorges valley of the Yangtze River. American Journal of Botany. 2006;93(2):206–215. doi: 10.3732/ajb.93.2.206. [DOI] [PubMed] [Google Scholar]
- 58.Iwata H, Uga Y, Yoshioka Y, Ebana K, Hayashi T. Bayesian association mapping of multiple quantitative trait loci and its application to the analysis of genetic variation among Oryza sativa L. germplasms. Theoretical and Applied Genetics. 2007;114(8):1437–1449. doi: 10.1007/s00122-007-0529-x. [DOI] [PubMed] [Google Scholar]
- 59.Malosetti M, van der Linden CG, Vosman B, van Eeuwijk FA. A mixed-model approach to association mapping using pedigree information with an illustration of resistance to Phytophthora infestans in potato. Genetics. 2007;175(2):879–889. doi: 10.1534/genetics.105.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gebhardt C, Ballvora A, Walkemeier B, Oberhagemann P, Schüler K. Assessing genetic potential in germplasm collections of crop plants by marker-trait association: a case study for potatoes with quantitative variation of resistance to late blight and maturity type. Molecular Breeding. 2004;13(1):93–102. [Google Scholar]
- 61.Hollingsworth PM, Ennos RA. Neighbor joining trees, dominant markers and population genetic structure. Heredity. 2004;92(6):490–498. doi: 10.1038/sj.hdy.6800445. [DOI] [PubMed] [Google Scholar]
- 62.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardy OJ. Estimation of pairwise relatedness between individuals and characterization of isolation-by-distance processes using dominant genetic markers. Molecular Ecology. 2003;12(6):1577–1588. doi: 10.1046/j.1365-294x.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 64.Tian D, Araki H, Stahl E, Bergelson J, Kreitman M. Signature of balancing selection in Arabidopsis . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11525–11530. doi: 10.1073/pnas.172203599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shepard KA, Purugganan MD. Molecular population genetics of the Arabidopsis CLAVATA2 region: the genomic scale of variation and selection in a selfing species. Genetics. 2003;163(3):1083–1095. doi: 10.1093/genetics/163.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehrenreich IM, Stafford PA, Purugganan MD. The genetic architecture of shoot branching in Arabidopsis thaliana: a comparative assessment of candidate gene associations vs. quantitative trait locus mapping. Genetics. 2007;176(2):1223–1236. doi: 10.1534/genetics.107.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plagnol V, Padhukasahasram B, Wall JD, Marjoram P, Nordborg M. Relative influences of crossing over and gene conversion on the pattern of linkage disequilibrium in Arabidopsis thaliana . Genetics. 2006;172(4):2441–2448. doi: 10.1534/genetics.104.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tenaillon MI, Sawkins MC, Long AD, Gaut RL, Doebley JF, Gaut BS. Patterns of DNA sequence polymorphism along chromosome 1 of maize (Zea mays ssp. mays L.) Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9161–9166. doi: 10.1073/pnas.151244298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thornsberry JM, Goodman MM, Doebley J, Kresovich S, Nielsen D, Buckler ES. Dwarf8 polymorphisms associate with variation in flowering time. Nature Genetics. 2001;28(3):286–289. doi: 10.1038/90135. [DOI] [PubMed] [Google Scholar]
- 70.Jung M, Ching A, Bhattramakki D, et al. Linkage disequilibrium and sequence diversity in a 500-kbp region around the adh1 locus in elite maize germplasm. Theoretical and Applied Genetics. 2004;109(4):681–689. doi: 10.1007/s00122-004-1695-8. [DOI] [PubMed] [Google Scholar]
- 71.Andersen JR, Zein I, Wenzel G, et al. High levels of linkage disequilibrium and associations with forage quality at a Phenylalanine Ammonia-Lyase locus in European maize (Zea mays L.) inbreds. Theoretical and Applied Genetics. 2007;114(2):307–319. doi: 10.1007/s00122-006-0434-8. [DOI] [PubMed] [Google Scholar]
- 72.Chao S, Zhang W, Dubcovsky J, Sorrells M. Evaluation of genetic diversity and genome-wide linkage disequilibrium among U.S. wheat (Triticum aestivum L.) germplasm representing different market classes. Crop Science. 2007;47(3):1018–1030. [Google Scholar]
- 73.Garris AJ, McCouch SR, Kresovich S. Population structure and its effect on haplotype diversity and linkage disequilibrium surrounding the xa5 locus of rice (Oryza sativa L.) Genetics. 2003;165(2):759–769. doi: 10.1093/genetics/165.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agrama HA, Eizenga GC. Molecular diversity and genome-wide linkage disequilibrium patterns in a worldwide collection of Oryza sativa and its wild relatives. Euphytica. 2008;160(3):339–355. [Google Scholar]
- 75.Rakshit S, Rakshit A, Matsumura H, et al. Large-scale DNA polymorphism study of Oryza sativa and O. rufipogon reveals the origin and divergence of Asian rice. Theoretical and Applied Genetics. 2007;114(4):731–743. doi: 10.1007/s00122-006-0473-1. [DOI] [PubMed] [Google Scholar]
- 76.Mather KA, Caicedo AL, Polato NR, Olsen KM, McCouch S, Purugganan MD. The extent of linkage disequilibrium in rice (Oryza sativa L.) Genetics. 2007;177(4):2223–2232. doi: 10.1534/genetics.107.079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malysheva-Otto LV, Ganal MW, Röder MS. Analysis of molecular diversity, population structure and linkage disequilibrium in a worldwide survey of cultivated barley germplasm (Hordeum vulgare L.) BMC Genetics. 2006;7, article 6 doi: 10.1186/1471-2156-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrell PL, Toleno DM, Lundy KE, Clegg MT. Low levels of linkage disequilibrium in wild barley (Hordeum vulgare ssp. spontaneum) despite high rates of self-fertilization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2442–2447. doi: 10.1073/pnas.0409804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu YL, Song QJ, Hyten DL, et al. Single-nucleotide polymorphisms in soybean. Genetics. 2003;163(3):1123–1134. doi: 10.1093/genetics/163.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jun T-H, Van K, Kim MY, Lee HS, Walker DR. Association analysis using SSR markers to find QTL for seed protein content in soybean. Euphytica. [Google Scholar]
- 81.Kraft T, Hansen M, Nilsson N-O. Linkage disequilibrium and fingerprinting in sugar beet. Theoretical and Applied Genetics. 2000;101(3):323–326. [Google Scholar]
- 82.Yin T-M, DiFazio SP, Gunter LE, Jawdy SS, Boerjan W, Tuskan GA. Genetic and physical mapping of Melampsora rust resistance genes in Populus and characterization of linkage disequilibrium and flanking genomic sequence. New Phytologist. 2004;164(1):95–105. doi: 10.1111/j.1469-8137.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- 83.Ingvarsson PK. Nucleotide polymorphism and linkage disequilibrium within and among natural populations of European aspen (Populus tremula L., salicaceae) Genetics. 2005;169(2):945–953. doi: 10.1534/genetics.104.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown GR, Gill GP, Kuntz RJ, Langley CH, Neale DB. Nucleotide diversity and linkage disequilibrium in loblolly pine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(42):15255–15260. doi: 10.1073/pnas.0404231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krutovsky KV, Neale DB. Nucleotide diversity and linkage disequilibrium in cold-hardiness- and wood quality-related candidate genes in douglas fir. Genetics. 2005;171(4):2029–2041. doi: 10.1534/genetics.105.044420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rafalski A, Morgante M. Corn and humans: recombination and linkage disequilibrium in two genomes of similar size. Trends in Genetics. 2004;20(2):103–111. doi: 10.1016/j.tig.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Guillet-Claude C, Birolleau-Touchard C, Manicacci D, et al. Genetic diversity associated with variation in silage corn digestibility for three O-methyltransferase genes involved in lignin biosynthesis. Theoretical and Applied Genetics. 2004;110(1):126–135. doi: 10.1007/s00122-004-1808-4. [DOI] [PubMed] [Google Scholar]
- 88.Guillet-Claude C, Birolleau-Touchard C, Manicacci D, et al. Nucleotide diversity of the ZmPox3 maize peroxidase gene: relationship between a MITE insertion in exon 2 and variation in forage maize digestibility. BMC Genetics. 2004;5, article 19:1–11. doi: 10.1186/1471-2156-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xing Y, Frei U, Schejbel B, Asp T, Lübberstedt T. Nucleotide diversity and linkage disequilibrium in 11 expressed resistance candidate genes in Lolium perenne . BMC Plant Biology. 2007;7, article 43:1–12. doi: 10.1186/1471-2229-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponting RC, Drayton MC, Cogan NOI, et al. SNP discovery, validation, haplotype structure and linkage disequilibrium in full-length herbage nutritive quality genes of perennial ryegrass (Lolium perenne L.) Molecular Genetics and Genomics. 2007;278(5):585–597. doi: 10.1007/s00438-007-0275-4. [DOI] [PubMed] [Google Scholar]
- 91.Skøt L, Humphreys MO, Armstead I, et al. An association mapping approach to identify flowering time genes in natural populations of Lolium perenne (L.) Molecular Breeding. 2005;15(3):233–245. [Google Scholar]
- 92.Abdurakhomonov IY, Kohel RJ, Saha S, et al. Genome-wide linkage disequilibrium revealed by microsatellite markers and association study of fiber quality traits in cotton. In: Proceedings of the 15th Plant and Animal Genome Conference; January 2007; San Diego, Calif, USA. W199. [Google Scholar]
- 93.Schulze TG, McMahon FJ. Genetic association mapping at the crossroads: which test and why? Overview and practical guidelines. American Journal of Medical Genetics B. 2002;114(1):1–11. doi: 10.1002/ajmg.10042. [DOI] [PubMed] [Google Scholar]
- 94.Ohashi J, Yamamoto S, Tsuchiya N, et al. Comparison of statistical power between 2 × 2 allele frequency and allele positivity tables in case-control studies of complex disease genes. Annals of Human Genetics. 2001;65(2):197–206. doi: 10.1017/s000348000100851x. [DOI] [PubMed] [Google Scholar]
- 95.Falk CT, Rubinstein P. Haplotype relative risks: an easy reliable way to construct a proper control sample for risk calculations. Annals of Human Genetics. 1987;51(3):227–233. doi: 10.1111/j.1469-1809.1987.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 96.Spielman RS, Ewens WJ. The TDT and other family-based tests for linkage disequilibrium and association. American Journal of Human Genetics. 1996;59(5):983–989. [PMC free article] [PubMed] [Google Scholar]
- 97.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) American Journal of Human Genetics. 1993;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- 98.Bickeböller H, Clerget-Darpoux F. Statistical properties of the allelic and genotypic transmission/disequilibrium test for multiallelic markers. Genetic Epidemiology. 1995;12(6):865–870. doi: 10.1002/gepi.1370120656. [DOI] [PubMed] [Google Scholar]
- 99.Rice JP, Neuman RJ, Hoshaw SL, Daw EW, Gu C. TDT with covariates and genomic screens with mod scores: their behavior on simulated data. Genetic Epidemiology. 1995;12(6):659–664. doi: 10.1002/gepi.1370120623. [DOI] [PubMed] [Google Scholar]
- 100.Sham PC, Curtis D. An extended transmission/disequilibrium test (TDT) for multi-allele marker loci. Annals of Human Genetics. 1995;59(3):323–336. doi: 10.1111/j.1469-1809.1995.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 101.Kaplan NL, Martin ER, Weir BS. Power studies for the transmission/disequilibrium tests with multiple alleles. American Journal of Human Genetics. 1997;60(3):691–702. [PMC free article] [PubMed] [Google Scholar]
- 102.Cleves MA, Olson JM, Jacobs KB. Exact transmission-disequilibrium tests with multiallelic markers. Genetic Epidemiology. 1997;14(4):337–347. doi: 10.1002/(SICI)1098-2272(1997)14:4<337::AID-GEPI1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 103.Lazzeroni LC, Lange K. A conditional inference framework for extending the transmission/disequilibrium test. Human Heredity. 1998;48(2):67–81. doi: 10.1159/000022784. [DOI] [PubMed] [Google Scholar]
- 104.Wilson SR. On extending the transmission/disequilibrium test (TDT) Annals of Human Genetics. 1997;61(2):151–161. doi: 10.1046/j.1469-1809.1997.6120151.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhao H, Zhang S, Merikangas KR, et al. Transmission/disequilibrium tests using multiple tightly linked markers. American Journal of Human Genetics. 2000;67(4):936–946. doi: 10.1086/303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curtis D. Use of siblings as controls in case-control association studies. Annals of Human Genetics. 1997;61(4):319–333. doi: 10.1046/j.1469-1809.1998.6210089.x. [DOI] [PubMed] [Google Scholar]
- 107.Horvath S, Laird NM. A discordant-sibship test for disequilibrium and linkage: no need for parental data. American Journal of Human Genetics. 1998;63(6):1886–1897. doi: 10.1086/302137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun F, Flanders WD, Yang Q, Khoury MJ. Transmission disequilibrium test (TDT) when only one parent is available: the 1-TDT. American Journal of Epidemiology. 1999;150(1):97–104. doi: 10.1093/oxfordjournals.aje.a009923. [DOI] [PubMed] [Google Scholar]
- 109.Knapp M. A note on power approximations for the transmission/disequilibrium test. American Journal of Human Genetics. 1999;64(4):1177–1185. doi: 10.1086/302334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Knapp M. Reconstructing parental genotypes when testing for linkage in the presence of association. Theoretical Population Biology. 2001;60(3):141–148. doi: 10.1006/tpbi.2001.1540. [DOI] [PubMed] [Google Scholar]
- 111.Horvath S, Laird NM, Knapp M. The transmission/disequilibrium test and parental-genotype reconstruction for X-chromosomal markers. American Journal of Human Genetics. 2000;66(3):1161–1167. doi: 10.1086/302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. American Journal of Human Genetics. 2000;67(1):146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin ER, Bass MP, Kaplan NL. Correcting for a potential bias in the pedigree disequilibrium test. American Journal of Human Genetics. 2001;68(4):1065–1067. doi: 10.1086/319525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hugot J-P, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 115.Abecasis GR, Cardon LR, Cookson WOC. A general test of association for quantitative traits in nuclear families. American Journal of Human Genetics. 2000;66(1):279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Monks SA, Kaplan NL. Removing the sampling restrictions from family-based tests of association for a quantitative-trait locus. American Journal of Human Genetics. 2000;66(2):576–592. doi: 10.1086/302745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Terwilliger JD. A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. American Journal of Human Genetics. 1995;56(3):777–787. [PMC free article] [PubMed] [Google Scholar]
- 118.Weinberg CR, Wilcox AJ, Lie RT. A log-linear approach to case-parent-triad data: assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parental imprinting. American Journal of Human Genetics. 1998;62(4):969–978. doi: 10.1086/301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weinberg CR. Allowing for missing parents in genetic studies of case-parent triads. American Journal of Human Genetics. 1999;64(4):1186–1193. doi: 10.1086/302337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genetic Epidemiology. 2000;19(supplement 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 121.Rabinowitz D, Laird NM. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitrary missing marker information. Human Heredity. 2000;50(4):211–223. doi: 10.1159/000022918. [DOI] [PubMed] [Google Scholar]
- 122.Lake SL, Blacker D, Laird NM. Family-based tests of association in the presence of linkage. American Journal of Human Genetics. 2000;67(6):1515–1525. doi: 10.1086/316895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.te Meerman GJ, Van der Meulen MA, Sandkuijl LA. Perspectives of identity by descent (IBD) mapping in founder populations. Clinical & Experimental Allergy. 1995;25(supplement 2):97–102. doi: 10.1111/j.1365-2222.1995.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 124.Levinson DF, Kirby A, Slepner S, Nolte I, Spijker GT, te Meerman G. Simulation studies of detection of a complex disease in a partially isolated population. American Journal of Medical Genetics B. 2001;105(1):65–70. [PubMed] [Google Scholar]
- 125.McPeek MS, Strahs A. Assessment of linkage disequilibrium by the decay of haplotype sharing, with application to fine-scale genetic mapping. American Journal of Human Genetics. 1999;65(3):858–875. doi: 10.1086/302537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Risch N, Teng J. The relative power of family-based and case-control designs for linkage disequilibrium studies of complex human diseases. I. DNA pooling. Genome Research. 1998;8(12):1273–1288. doi: 10.1101/gr.8.12.1273. [DOI] [PubMed] [Google Scholar]
- 127.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 128.Bacanu S-A, Devlin B, Roeder K. The power of genomic control. American Journal of Human Genetics. 2000;66(6):1933–1944. doi: 10.1086/302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao K, Aranzana MJ, Kim S, et al. An Arabidopsis example of association mapping in structured samples. PLoS Genetics. 2007;3(1):p. e4. doi: 10.1371/journal.pgen.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pritchard KJ, Wen W. Documentation for Structure Software. Chicago, Ill, USA: The University of Chicago Press; 2004. [Google Scholar]
- 131.Satten GA, Flanders WD, Yang Q. Accounting for unmeasured population substructure in case-control studies of genetic association using a novel latent-class model. American Journal of Human Genetics. 2001;68(2):466–477. doi: 10.1086/318195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu X, Zhang S, Zhao H, Cooper RS. Association mapping, using a mixture model for complex traits. Genetic Epidemiology. 2002;23(2):181–196. doi: 10.1002/gepi.210. [DOI] [PubMed] [Google Scholar]
- 133.Yu J, Pressoir G, Briggs WH, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nature Genetics. 2006;38(2):203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 134.Ritland K. Estimators for pairwise relatedness and inbreeding coefficients. Genetical Research. 1996;67(2):175–186. [Google Scholar]
- 135.Hardy OJ, Vekemans X. SPAGeDi: a versatile computer program to analyze spatial genetic structure at the individual or population levels. Molecular Ecology Notes. 2002;2(4):618–620. [Google Scholar]
- 136.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 137.Parisseaux B, Bernardo R. In silico mapping of quantitative trait loci in maize. Theoretical and Applied Genetics. 2004;109(3):508–514. doi: 10.1007/s00122-004-1666-0. [DOI] [PubMed] [Google Scholar]
- 138.Simko I, Costanzo S, Haynes KG, Christ BJ, Jones RW. Linkage disequilibrium mapping of a Verticillium dahliae resistance quantitative trait locus in tetraploid potato (Solanum tuberosum) through a candidate gene approach. Theoretical and Applied Genetics. 2004;108(2):217–224. doi: 10.1007/s00122-003-1431-9. [DOI] [PubMed] [Google Scholar]
- 139.Sillanpää MJ, Bhattacharjee M. Bayesian association-based fine mapping in small chromosomal segments. Genetics. 2005;169(1):427–439. doi: 10.1534/genetics.104.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ball RD. Statistical analysis and experimental design. In: Oraguzie NC, Rikkerink EHA, Gardiner SE, de Silva HN, editors. Association Mapping in Plants. New York, NY, USA: Springer; 2007. pp. 133–196. [Google Scholar]
- 141.Thumma BR, Nolan MF, Evans R, Moran GF. Polymorphisms in cinnamoyl CoA reductase (CCR) are associated with variation in microfibril angle in Eucalyptus spp. Genetics. 2005;171(3):1257–1265. doi: 10.1534/genetics.105.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ball RD. Experimental designs for reliable detection of linkage disequilibrium in unstructured random population association studies. Genetics. 2005;170(2):859–873. doi: 10.1534/genetics.103.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138(3):963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57(1):289–300. [Google Scholar]
- 145.Olsen KM, Halldorsdottir SS, Stinchcombe JR, Weinig C, Schmittt J, Purugganan MD. Linkage disequilibrium mapping of Arabidopsis CRY2 flowering time alleles. Genetics. 2004;167(3):1361–1369. doi: 10.1534/genetics.103.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caicedo AL, Stinchcombe JR, Olsen KM, Schmitt J, Purugganan MD. Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(44):15670–15675. doi: 10.1073/pnas.0406232101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Balasubramanian S, Sureshkumar S, Agrawal M, et al. The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana . Nature Genetics. 2006;38(6):711–715. doi: 10.1038/ng1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aranzana MJ, Kim S, Zhao K, et al. Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes. PLoS Genetics. 2005;1(5):p. e60. doi: 10.1371/journal.pgen.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palaisa KA, Morgante M, Williams M, Rafalski A. Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. The Plant Cell. 2003;15(8):1795–1806. doi: 10.1105/tpc.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wilson LM, Whitt SR, Ibáñez AM, Rocheford TR, Goodman MM, Buckler ES., IV Dissection of maize kernel composition and starch production by candidate gene association. The Plant Cell. 2004;16(10):2719–2733. doi: 10.1105/tpc.104.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andersen JR, Schrag T, Melchinger AE, Zein I, Lübberstedt T. Validation of Dwarf8 polymorphisms associated with flowering time in elite European inbred lines of maize (Zea mays L.) Theoretical and Applied Genetics. 2005;111(2):206–217. doi: 10.1007/s00122-005-1996-6. [DOI] [PubMed] [Google Scholar]
- 152.Szalma SJ, Buckler ES, IV, Snook ME, McMullen MD. Association analysis of candidate genes for maysin and chlorogenic acid accumulation in maize silks. Theoretical and Applied Genetics. 2005;110(7):1324–1333. doi: 10.1007/s00122-005-1973-0. [DOI] [PubMed] [Google Scholar]
- 153.Lübberstedt T, Zein I, Andersen JR, et al. Development and application of functional markers in maize. Euphytica. 2005;146(1-2):101–108. [Google Scholar]
- 154.Beló A, Zheng P, Luck S, et al. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Molecular Genetics and Genomics. 2008;279(1):1–10. doi: 10.1007/s00438-007-0289-y. [DOI] [PubMed] [Google Scholar]
- 155.Agrama HA, Eizenga GC, Yan W. Association mapping of yield and its components in rice cultivars. Molecular Breeding. 2007;19(4):341–356. [Google Scholar]
- 156.Zhang N, Xu Y, Akash M, McCouch S, Oard JH. Identification of candidate markers associated with agronomic traits in rice using discriminant analysis. Theoretical and Applied Genetics. 2005;110(4):721–729. doi: 10.1007/s00122-004-1898-z. [DOI] [PubMed] [Google Scholar]
- 157.Igartua E, Casas AM, Ciudad F, Montoya JL, Romagosa I. RFLP markers associated with major genes controlling heading date evaluated in a barley germ plasm pool. Heredity. 1999;83(5):551–559. doi: 10.1038/sj.hdy.6885890. [DOI] [PubMed] [Google Scholar]
- 158.Ivandic V, Thomas WTB, Nevo E, Zhang Z, Forster BP. Associations of simple sequence repeats with quantitative trait variation including biotic and abiotic stress tolerance in Hordeum spontaneum . Plant Breeding. 2003;122(4):300–304. [Google Scholar]
- 159.Ravel C, Praud S, Murigneux A, et al. Identification of Glu-B1-1 as a candidate gene for the quantity of high-molecular-weight glutenin in bread wheat (Triticum aestivum L.) by means of an association study. Theoretical and Applied Genetics. 2006;112(4):738–743. doi: 10.1007/s00122-005-0178-x. [DOI] [PubMed] [Google Scholar]
- 160.D’hoop BB, Paulo MJ, Mank RA, van Eck HJ, van Eeuwijk FA. Association mapping of quality traits in potato (Solanum tuberosum L.) Euphytica. 2008;161(1-2):47–60. [Google Scholar]
- 161.Dobrowolski MP, Forster JW. Linkage disequilibrium-based association mapping in forage species. In: Oraguzie NC, Rikkerink EHA, Gardiner SE, de Silva HN, editors. Association Mapping in Plants. New York, NY, USA: Springer; 2007. pp. 197–210. [Google Scholar]
- 162.Skøt L, Humphreys J, Humphreys MO, et al. Association of candidate genes with flowering time and water-soluble carbohydrate content in Lolium perenne (L.) Genetics. 2007;177(1):535–547. doi: 10.1534/genetics.107.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wilcox PL, Echt EC, Burdon RD. Gene-assisted selection: applications of association genetics for forest tree breeding. In: Oraguzie NC, Rikkerink EHA, Gardiner SE, de Silva HN, editors. Association Mapping in Plants. New York, NY, USA: Springer; 2007. pp. 211–247. [Google Scholar]
- 164.The Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Estivill X, Armengol L. Copy number variants and common disorders: filling the gaps and exploring complexity in genome-wide association studies. PLoS Genetics. 2007;3(10):1787–1799. doi: 10.1371/journal.pgen.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beer SC, Siripoonwiwat W, O’donoughue LS, Souza E, Matthews D, Sorrels ME. Associations between molecular markers and quantitative traits in an oat germplasm pool: can we infer linkages? Journal of Agricultural Genomics. 1997;3, paper 197 [Google Scholar]
- 167.Virk PS, Ford-Lloyd BV, Jackson MT, Pooni HS, Clemeno TP, Newbury HJ. Predicting quantitative variation within rice germplasm using molecular markers. Heredity. 1996;76(3):296–304. [Google Scholar]
- 168.Zhang S, Zhang K, Li J, Sun F, Zhao H. Test of association for quantitative traits in general pedigrees: the quantitative pedigree disequilibrium test. Genetic Epidemiology. 2001;21(supplement 1):S370–S375. doi: 10.1002/gepi.2001.21.s1.s370. [DOI] [PubMed] [Google Scholar]
- 169.Breseghello F, Sorrells ME. Association analysis as a strategy for improvement of quantitative traits in plants. Crop Science. 2006;46(3):1323–1330. [Google Scholar]