Abstract
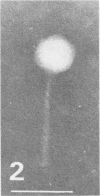
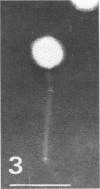
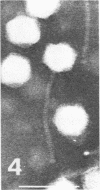
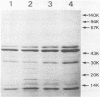
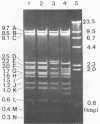
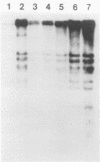
For protection from the abnormal fermentation of Lactobacillus casei S-1 caused by contamination of a virulent phage, φFSV, the origin of this phage was studied. Morphologies, viral structural proteins, and DNA structures of three independent isolates of φFSV were compared with those of φFSW, which is lysogenized in strain S-1. The results showed (i) that the morphology of φFSV phages is indistinguishable from that of φFSW and (ii) that all viral structural components found in φFSW are present in the particles of φFSV's. In addition, restriction endonuclease analyses of viral DNA showed that the HindIII-digested fragments of φFSW DNA, the sum of which covered at least 94.7% of this phage genome, were conserved in the φFSV DNA digests. Results of Southern filter hybridization of the S-1 and prophage-cured cell (C239) DNAs with φFSV DNA as a probe revealed that C239 had lost most of the φFSV DNA sequence, whereas S-1 had about one copy of the φFSV DNA sequence. These results indicate that virulent phage φFSV is derived from the lysogenized phage φFSW. Therefore, the appearance of φFSV can be eliminated by using the prophage-cured derivative of S-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels D. L., de Wet J. R., Blattner F. R. New map of bacteriophage lambda DNA. J Virol. 1980 Jan;33(1):390–400. doi: 10.1128/jvi.33.1.390-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- Flashman S. M. Mutational analysis of the operators of bacteriophage lambda. Mol Gen Genet. 1978 Oct 25;166(1):61–73. doi: 10.1007/BF00379730. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. Prophage-Cured Derivatives of Streptococcus lactis and Streptococcus cremoris. Appl Environ Microbiol. 1980 Nov;40(5):964–966. doi: 10.1128/aem.40.5.964-966.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A. R., Sandine W. E. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl Environ Microbiol. 1977 Jan;33(1):184–191. doi: 10.1128/aem.33.1.184-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L. C., Zimm B. H. Size of DNA determined by viscoelastic measurements: results on bacteriophages, Bacillus subtilis and Escherichia coli. J Mol Biol. 1972 Dec 30;72(3):779–800. doi: 10.1016/0022-2836(72)90191-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie R. J. Lysogenic strains of group N lactic streptococci. Appl Microbiol. 1974 Jan;27(1):210–217. doi: 10.1128/am.27.1.210-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T. Prophage Curing in Lactobacillus casei by Isolation of a Thermoinducible Mutant. Appl Environ Microbiol. 1982 Jun;43(6):1284–1287. doi: 10.1128/aem.43.6.1284-1287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Uesugi S. Structure and functions of the Kirsten murine sarcoma virus genome: molecular cloning of biologically active Kirsten murine sarcoma virus DNA. J Virol. 1981 May;38(2):720–727. doi: 10.1128/jvi.38.2.720-727.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]