Abstract
Regulation of the rat glutathione S-transferase A2 (GSTA2) gene by glucocorticoids is biphasic in its concentration dependence to glucocorticoids, with concentrations of 10−100 nM repressing gene activity (GR-dependent), and concentrations above 1 μM increasing transactivation (PXR-dependent) in adult rat hepatocytes or transient transfection assays. Over-expression of either C/EBPα or β negatively regulates basal and inducible expression of a 1.65 Kb GSTA2 luciferase reporter, and synergizes the response to glucocorticoids (GC). C/EBP responsive elements have been identified in the GSTA2 5’-flanking sequence, associated with the palindrominic Glucocorticoid Responsive Element (GRE), the Ah receptor response elements, and the antioxidant response element. In reporters lacking the palindromic GRE, negative regulation by GC is observed only when C/EBPα is co-expressed. Co-transfection of C/EBPα/β induced gene expression of the GSTA2 XRE reporter, but negatively regulated the GSTA2 ARE-reporter. In contrast, the ARE from the rat NAD(P)H quinone oxidoreductase gene was induced by co-transfection of C/EBPs, but was still negatively regulated by GC. PXR-induction of the GSTA2 reporter was partially ablated by co-transfection of C/EBPα and enhanced by co-transfection of C/EBPβ. We conclude that C/EBPα and β are involved in GC-dependent repression of GSTA2 gene expression and ARE sequences that bind C/EBPs appears to be critical for these responses.
Keywords: NF-E2-related factor-2, Pregnane X receptor, Glucocorticoid receptor, C/EBPα, C/EBPβ, Glutathione S-transferase A2, NAD(P)H:quinone oxidoreductase.
INTRODUCTION
Hypothesis
Our previous studies indicated that glutathione S-transferase A2 (GSTA2) gene expression was regulated developmentally by glucocorticoids (Linder and Prough, 1993; Sherratt et al., 1990). In neonatal rat liver and primary fetal hepatocytes, GSTA2 gene expression was induced by glucocorticoid treatment, while in adult rat liver and primary hepatocytes, gene expression was repressed at glucocorticoid concentrations that activate the glucocorticoid receptor, but do not activate PXR. In primary cultures of adult rat hepatocytes, GSTA2 was regulated by glucocorticoids in a biphasic manner (Prough et al., 1996), with repression of gene expression being observed at lower glucocorticoid concentrations (10−100 nM), while higher concentrations of glucocorticoids (>1 μM) caused gene expression to be significantly increased. These effects could be reproduced in transient transfection experiments (Falkner et al., 1998, 2001) using HepG2 cells and 1.65 kb full-length GSTA2 promoter-based luciferase reporter construct in the presence of both GR (low concentration negative regulation) and PXR (high concentration induction). The effect mediated by low concentrations of glucocorticoid (Falkner et al., 2001) was mediated in part through the palindromic GRE (−1602 bp) and several GRE half sites (−1524, −1360, and −1063) found between −1.65 kb and −1.0 kb in the 5’-flanking region of the GSTA2 and was blocked by addition of 1 μM RU 38486. By itself, this same region acted as a positively-regulated GRE in transient luciferase reporter assays, indicating that GR must act with another transcription factor that is developmentally regulated to mediate this negative regulation of the GSTA2 gene.
Subsequent studies by others (Ki et al., 2005) identified C/EBPβ as a transcription factor that can mediate similar negative regulatory responses in GSTA2. This study confirmed our earlier published work that the response of the GSTA2 gene to glucocorticoids is biphasic and that the palindromic GRE and half sites located between base pairs −1.65 and −1.0 kb are involved in negative regulation of this gene through action of the glucocorticoid receptor (Fig. 1). Multiple C/EBP binding sites have been identified in the GSTA2 gene including a C/EBP site located at the ARE at −695 bp (Chen and Ramos, 2000), the XRE at −899 bp (Pimental et al., 1993) and the palindromic GRE at −1602 bp (Falkner et al., 1998; Voss et al., 1998) of the 5’-flanking region. Our previous work (Falkner et al., 2001) indicated that the PXR-mediated induction of the GSTA2 was mediated through sequences at or near the ARE, suggesting involvement of this cis-acting element in nuclear receptor-dependent regulation of the GSTA2 gene. Finally, expression of the GSTA2 gene is developmentally regulated. High expression is observed in pre-neoplastic nodules (Pickett et al., 1984), an early stage of hepatocarcinogenesis in which glucocorticoid receptor function and C/EBPα have been implicated. In addition, it is highly expressed during early adult life (for review see Schrem et al., 2004). These observations led us to develop the hypothesis that glucocorticoids regulate the GSTA2 gene negatively via GR-dependent mechanisms involving other developmentally-regulated transcription factors, either C/EBPα or C/EBPβ, acting through the nuclear-receptor sensitive C/EBP binding site located at or within the ARE.
Figure 1.
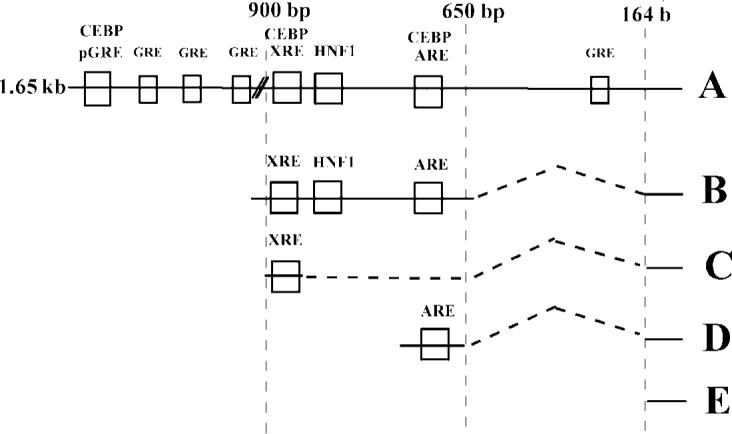
Schematic Map of the responsive elements on the 5’-flanking region of the rat glutathione S-transferase gene and reporter constructs prepared.
Regulation of the GSTA2 Gene
The transcriptional regulation of rat glutathione S-transferase A2 by xenobiotic compounds has been well characterized by Pickett and coworkers (Rushmore et al., 1990; Rushmore and Pickett, 1990; Rushmore et al., 1991). This gene's promoter has two independent xenobiotic response elements (Fig. 1). The first characterized (Rushmore et al., 1990) is an XRE (−899 in the 5’-flanking region), which binds the Ah receptor whose ligands include polycylic and polyhalogenated aromatic compounds. In addition to binding the Ah receptor, this element is thought to bind C/EBPα (Pimental et al., 1993). Electrophoretic mobility shift experiments indicated the GSTA2 XRE sequences formed DNA-C/EBP protein complexes that were disrupted by pre-incubation with antiserum against C/EBPα, but not by C/EBPβ or δ antiserum. It was proposed that C/EBPα facilitates recruitment of the Ah-receptor and therefore, positively regulates GSTA2 gene transcription.
The other responsive element (−695 in the 5’-flanking region) known either as the antioxidant (ARE) or electrophile response element (Rushmore and Pickett, 1990; Rushmore et al., 1991), binds the transcription factor NF-E2-related factor-2 (Nrf-2) and its hetero-dimerization partner, a member of the small maf family (Nguyen et al., 2003; Itoh et al., 1997; Itoh et al., 1999). The ARE is partially responsible for the basal transcription rate of the GSTA2 gene, in addition to the xenobiotic inducibility observed with a broad range of electrophilic compounds. Interestingly, C/EBP family members have also been implicated in binding to this site. Chen and Ramos (2000) demonstrated that over-expression of either C/EBPα or C/EBPβ negatively regulated expression of GSTA2 reporters in transient transfection experiments in murine vascular smooth muscle cells.
In addition to the response elements activated by xenobiotic compounds, several other response elements have been defined in the basal expression of GSTA2 (Paulson et al., 1990). These include a HNF1 site (−859 bp) and an HNF4 site (−760 bp). The HNF1 site is of particular importance in understanding the modes of negative regulation of the GSTA2 gene by glucocorticoids, since it has been implicated in negative regulation of the GSTA2 gene by glucocorticoids in cells treated with interleukin-6 (Voss et al., 1998).
C/EBP Proteins
The C/EBP proteins are a family of transcription factors comprised of at least 6 different genes whose expression varies depending on tissue and developmental stage (Birkenmeier et al., 1989). C/EBP transcription factors are members of the leucine zipper superfamily, that form homodimers or heterodimers with other C/EBP family members or with other leucine zipper transcriptions factors, such as AP-1 family members or NF-κB. The C/EBP proteins α and β prefer to bind to the consensus sequence RTTGCGYAAY (Osada et al., 1996). In addition, the various C/EBP genes generate different protein transcripts by alternate initiation through ribosomal scanning mechanisms. C/EBP α and β are both expressed in liver, adipose, lung and intestine.
C/EBPα is an important regulator of hepatocyte differentiation and function (for review see Schrem et al., 2004). Studies in mice whose C/EBPα gene is ablated display hepatic architecture resembling proliferative or pseudo-glandular hepatocarcinoma, and have severe disruptions in lipid and carbohydrate metabolism. C/EBPα has been implicated with glucocorticoid receptor signaling, in the action of cell cycle regulators, like p21 (Timchenko et al., 1996; Cram et al., 1996) and is poorly expressed in rat liver following partial hepatectomy (Zhao et al., 2002) or in hepatocarcinomas (Osada et al., 1995). In immortalized hepatocarcinoma cell lines, C/EBP expression is very low and stable over-expression is difficult to achieve due to C/EBP-mediated cell cycle arrest.
C/EBPβ was originally identified as a transcription factor induced by interleukin-6 and is considered an important part of the acute phase response (Akira et al., 1990; Poli et al., 1990). C/EBPβ has high sequence homology with C/EBPα and can compensate for some of the hepatic functions of C/EBPα, if expressed from the C/EBPα promoter (Chen et al., 2000). However, either ablation of the C/EBPα gene or disruption of the C/EBPα gene in adult animals (Lee et al., 1997) results in severe changes in gene regulation suggesting that C/EBPβ expression in normal animals is insufficient to fully compensate for the loss of C/EBPα expression and indicates that C/EBPα is the predominant C/EBP controlling many hepatic functions. C/EBPβ has been shown to interact with GR tethering to C/EBPβ to form a hormone responsive element complex (Rudiger et al., 2002).
This review written in honor of Ronald W. Estabrook will describe two methods by which GR regulates the rat GSTA2 gene. One involves a palindromic GRE associated with several GRE half-sites, possibly through a putative C/EBP binding sites to recruit C/EBPs to the 5’-flanking region. The other region is the ARE of GSTA2 that appears to be part of a novel Glucocorticoid Regulatory Unit (GRU), like those GRUs seen in other genes, such as phosphoenol pyruvate carboxykinase characterized by Hanson's group (Hatzoglou et al., 1991.) The studies shown in this review will link the action of GR and C/EBP proteins in regulating the rat GSTA2 gene.
MATERIALS AND METHODS
Materials
Restriction endonucleases, T4 ligase and pGL3-basic were obtained from Promega (Madison, WI) or New England Biolabs (Beverly, MA). pCMV-β was obtained from Stratagene (La Jolla, CA). The expression vector for the human glucocorticoid receptor pRSVGR and the glucocorticoid responsive reporter plasmid, p2XDEX-Luc was a gift from Michael Mathis (LSU Medical Center, Shreveport LA). The reporter plasmid for the GSTA2 gene, p1.65Ya-CAT was provided by T.H. Rushmore and Cecil Pickett. Expression plasmids for the C/EBP proteins, pMEX, pMEX C/EBPα and pMEX C/EBPβ were generously provided by Peter Johnson, National Cancer Institute, NIH, Frederick, MD. The expression vector pPXR was provided by Steven Kliewer (The U. Texas Southwestern Medical School, Dallas, TX).
Media for culturing E. coli, were purchased from Difco Laboratories (Detroit, MI). Antimycotic/antibiotic solution, non-essential amino acids and Dulbecco's modified essential medium (high modified) was obtained from Mediatech (Hernon, VA). Fetal bovine serum was purchased from Harlan Bioproducts for Science (Indianapolis, IN). DNA purification kits were obtained from Qiagen (Chatworth, CA) and were used to produce transfection quality DNA. Oligonucleotides were purchased from Operon Technologies (Alameda, CA). PCR reagents were purchased from Fisher Scientific (Pittsburgh, PA). BA and DEX were obtained from Sigma Chemical Co (St. Louis, MO). t-Bu-DEX was purchased from Research Plus, Inc (Bayonne, NJ). RU 38486 (17β-hydroxy-11β(4-dimethylamino-phenyl)-17α(propyl-1-ynyl)-estra-4,9-dien-3-one) was obtained from Roussel Uclaf (Romainville, Cedex, France). Chlorophenol red β-D-galactopyranoside was purchased from Boehringer-Mannheim (Piscataway, NJ). All other reagents used were purchased from commercial sources and were either American Chemical Society or Molecular Biology grade.
Cells and Culture Conditions
E. coli DH5α cells were purchased from Invitogen (Carlsbad, CA) and were routinely transformed with plasmids of interest. HepG2 cells (ATCC HB8065), a human hepatoblastoma derived cell line, were obtained from the American Type Culture Collection (Rockville, MD). Cells were maintained in Dulbecco's modified Eagles Medium (high modified) supplemented with 10% fetal bovine serum, antimycotic/antibiotic and non-essential aminoacids. The cells were incubated at 37°C in a 5% carbon dioxide atmosphere and subcultured every 2 days.
Reporter Constructs
Luciferase deletion constructs of GSTA2 gene have been described previously (Falkner 1997, 2001). The pQARE-Luc reporter from the rat NAD(P)H:quinone oxidoreductase gene described by Favreau and Pickett (1995) was synthesized by annealing a single copy of the oligonucleotide for the ARE from this gene into 0.164YaLuc.
Transient Transfection
HepG2 cells were plated in 12-well plates and transfected at 40% confluency by calcium phosphate-based transfection techniques described previously (Falkner et al., 2001). Following transfection for 24 h, the cells were treated with various agents made up as 500 X stock solutions in DMSO; controls received DMSO alone. After an additional 24 h, the cells were harvested with 100 μl of cell lysis buffer (Promega, Madison, WI) according to the manufacturer’ instructions and subjected to a single freeze thaw event. All cells were co-transfected with 500 ng of pCMV-β as a transfection control. Routinely 125 ng of receptor expression plasmids and 1 μg of the various GSTA2 reporter plasmids were added to each well.
Assays of β-galactosidase and Luciferase Activity
Luciferase activity was determined on 5 μl of cell extract with using the Luciferase Assay system (Promega, Madison, WI) using a Wallac 1420 Victor3 Multilabel counter (Perkin Elmer Life and Analytical Sci., Waltham MA). Cell extracts (5 μl) were incubated with chlorophenol red β-galactopyranoside at 37°C for approximately 15−20 min. β-Galactosidase activity was determined spectrophotometrically at 595 nm using a Bio-Tek μQuant microplate spectrophotometer. (Winooski, VT).
Statistical Analysis
Students t-tests were used to define significance between groups. Fold-induction was analyzed by fitting to theoretical equations with the least squares regression program Kineti77 (Clark and Carrol, 1986).
RESULTS
C/EBPα and C/EBPβ Negatively Regulate GSTA2 Gene Expression
In our earlier work using tissues from neonatal and young adult rats, we noted that treatment of these animals with BA and co-treatment with either corn oil or DEX (25 mg/kg body wt.) in corn oil caused differences in expression of many hepatic genes involved in drug metabolism between these two developmental states (Linder and Prough, 1993). BA caused induction of GSTA2 mRNA and protein; however, BA-induction was increased by DEX in the neonatal rat liver and decreased in the young adult rat liver. For P4501A1, another AhR-regulated gene, DEX potentiated BA induction in both models (Linder and Prough, 1993), suggesting the involvement of a second transcription factor in the regulation of GSTA2 in the adult developmental models.
Among the known transcription factors that increase during development of the rat liver are the C/EBP transcription factors which play a role in the developmental expression of hepatic genes, like albumin, 442/Ap2 protein, stearoyl acyl-CoA desaturase 1, insulin-responsive glucose transporter, and many others (McKnight et al., 1989). To test the hypothesis that C/EBP transcription factors (C/EBPα and C/EBPβ) regulated the expression of the rat GSTA2, we prepared a rat GSTA2 promoter-dependent luciferase reporter construct by cloning double-stranded PCR products into cloning sites on 0.164YaLUC and subsequently developed a set of deletion constructs (A–E, Fig. 1). DEX negatively regulated both the basal and BA-dependent gene expression of the 1.65 kb 5’-flanking region luciferase reporter construct as shown in Fig. 2. In this experiment, the GC-dependent repression of luciferase activity was 61% and 55 % for control and BA-induced cells, respectively, consistent with our previously published results using intact liver of young adult rats (see above) and in transient transfection experiments (Falkner et al., 1998). Co-transfection of expression vectors for the C/EBP proteins dramatically reduced basal level transcription rates for both C/EBPα and C/EBPβ by 95% and 89%, respectively. Under these conditions, the expression of a chloramphenicol acetyl-transferase reporter containing the C/EBP responsive element in the 5’-flanking region of the albumin gene was positively increased by C/EBPα and C/EBPβ, 9.4 ± 0.3 and 5.6 ± 0.4-fold, respectively (data not shown). However, glucocorticoids negatively regulated both the basal and BA-dependent gene expression of luciferase reporters in cells co-transfected by C/EBPα by 46% and 70%, respectively. With cells transfected with C/EBPβ, no significant repression of basal level expression was observed in this experiment. However, the BA-induced gene expression was repressed by 65%. Thus, a synergistic negative regulation by DEX treatment and C/EBPα co-transfection was observed with the GSTA2 luciferase reporter genes, suggesting that the GR and C/EBP proteins must act through a single concerted mechanism. The results of co-transfection of C/EBP expression vectors is in agreement with those obtained in vascular smooth muscle cells (Chen and Ramos, 2000) and in H4IIE cells co-transfected with C/EBPβ (Ki et al., 2005).
Figure 2.
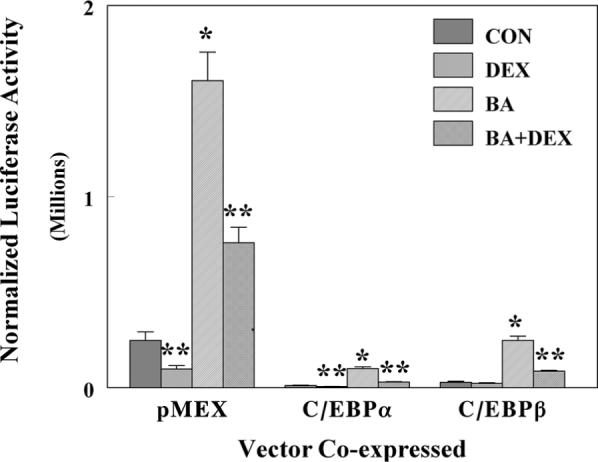
Effect of co-transfection of either C/EBPα or β on the GR-dependent repression of GSTA2 reporter construct activities in HepG2 cells transfected with either p1.65YaLuc. Luciferase and β-galactosidase assays were performed on lysed HepG2 cells that had been transfected with p1.65Ya-Luc, a control vector pCMV-β, an expression vector for the glucocorticoid receptor pRSV-GR, and pMEX expression vectors for either pC/EBPα or β, as described in materials and methods. Cells were treated with either 0.1 μM DEX, 50 μM BA, or a combination of both compounds (BA+DEX). Control cells (CON) received DMSO alone. The normalized luciferase activity is expressed as the relative light units divided by β-galactosidase activity and is the average activity ± SD for three independent samples. *P < 0.05 statistically significant difference from control cells, **P < 0.05 statistically significant different from basal or BA-induced cells.
Over-expression of C/EBPα Alters the Action of RU 38486
To further test the hypothesis that the effects of DEX are mediated by the glucocorticoid receptor (GR), we used the antagonist RU 38486 to ablate the response of DEX action through GR. As shown in Fig. 3, RU 38486 was an effective antagonist in control cells and those co-transfected with C/EBPβ, but not C/EBPα. DEX caused a 25% decrease in expression of BA-induced activity, but RU 38486 modestly increased basal expression. However, RU 38486 caused reversal of the DEX-dependent suppression of expression. When C/EBPα was co-expressed with GR, reporter expression was suppressed to 30% of control and DEX suppressed expression by an additional 48%. This suppression was partially reversed by addition of RU 38486. In contrast to control cells, RU 38486 displayed partial agonist activity in C/EBPα co-transfected cells, suppressing basal expression by 31%. In cells co-transfected with C/EBPβ, less pronounced suppression of GSTA2 reporter expression was observed. C/EBPβ co-expression repressed basal expression to 70% of control and DEX treatment further suppressed expression by 29%. In contrast to cells co-transfected with C/EBPα, RU 38486 did not display any partial agonist activity and reversed the effect of DEX.
Figure 3.
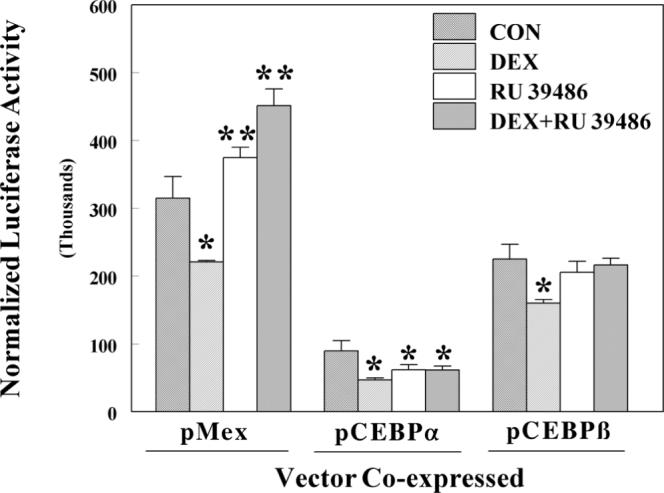
Effects of DEX and RU 38486 on luciferase activity of HepG2 cells transfected with plasmid p1.65Ya-Luc and expression vectors for C/EBPα and β. Luciferase and β-galactosidase assays were performed on lysed HepG2 cells that had been transfected with p1.65Ya-Luc, a control vector pCMV-β, an expression vector for the glucocorticoid receptor pRSV-GR, and pMEX expression vectors for either pC/EBPα or β, as described in materials and methods. Cells were treated with either 0.1 μM DEX, 1 μM RU 38486 or a combination of the two compounds. The normalized luciferase activity is expressed as the relative light units divided by β-galactosidase activity and is the mean ± SD of three wells. *P < 0.05 statistically significant decrease from control cells. **P < 0.05 statistically significant increase from control cells.
Deletion Mapping of the C/EBP:GR Loci of Regulation
In order to define the cis-acting element involved in the C/EBP and GR regulated regions of the 5’-flanking region, we prepared a series of deletion constructs starting with a 1.65 kb 5’-flanking region luciferase construct. The longest construct (Fig. 1, Construct A) was composed of 1.65 kb of 5’-flanking region contains an upstream region of a palindromic GRE (−1602) and 3 GRE half-sites (−1524, −1360, −1063, and −546), a XRE at −899 bp that binds the Ah receptor, an HNF1 site at −859 bp, an HNF4 site at −760 bp, and the antioxidant responsive element (ARE) at −695 bp. All these reporter constructs contain the 164 bp minimal promoter of GSTA2 defined by Rushmore and Pickett (1990).
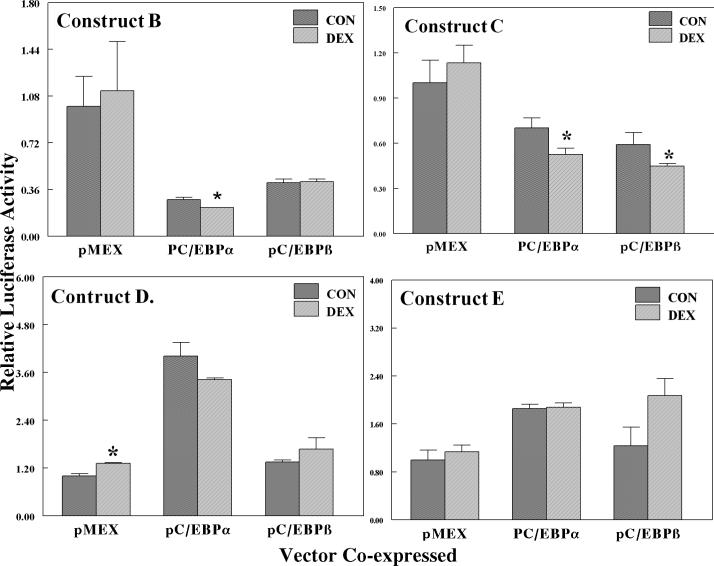
As seen in Fig. 2, the basal expression of the full-length construct was negatively regulated by DEX. Co-expression of C/EBPα strikingly repressed basal expression and DEX further decreased expression, while C/EBPβ was less effective in its negative regulation. The central construct (914 bp to −639 bp) that contains the XRE, HNF1, and ARE cis-acting elements, but does not contain any sequences with homology to a concensus GRE, was potently repressed by C/EBPα and C/EBPβ. However, there was much less effect of DEX on the construct (Fig. 4, Construct B), clearly indicating that the palindromic GRE (−1602 bp) is an important element in the glucocorticoid-dependent regulation of this gene. Treatment with DEX did not negatively regulate reporter gene expression in cells co-transfected with the empty expression vector pMEX, in agreement with previous studies (Falkner et al., 1998; Ki et al., 2005). In contrast to control cells, DEX-treatment suppressed basal expression by 20% in cells co-transfected with C/EBPα. Observation of DEX-dependent suppression of this reporter gene requires co-expression of C/EBPα. In contrast to C/EBPα, C/EBPβ co-transfection did not support DEX-dependent suppression of gene expression.
Figure 4.
Effects of DEX on the expression of luciferase activity in HepG2 cells transfected with deletion constructs of GSTA2 genes co-transfected with either C/EBPα or β. Deletions constructs are shown in Figure 1. Luciferase and β-galactosidase assays were performed on lysed HepG2 cells that had been transfected with deletion constructs, 0.914−0.638Ya-Luc (Construct B), XRE-Luc (Construct C), 0.721−0.681Ya-Luc (Construct D), or 0.164YaLuc (Construct E). All cells were co-transfected with a control vector pCMV-β, an expression vector for the glucocorticoid receptor pRSV-GR, and pMEX expression vectors for either pC/EBPα or β, as described in materials and methods. Cells were treated with either DMSO alone or 0.1 μM DEX. The normalized luciferase activity is expressed as the relative light units divided by β-galactosidase activity and is the average normalized luciferase ± SD of three wells. *P < 0.05 statistically significant difference from control cells.
The central reporter construct B (−914 to −639 bp) has two C/EBP response elements. The first described was a C/EBPα response element located within the XRE sequence (Pimental et al., 1993), while the second element is located at the ARE (Chen and Ramos). We tested the ability of DEX to negatively regulate either a XRE or ARE reporter constructs co-transfected with either C/EBPα or β (Fig. 4, Constructs C & D). The XRE construct C (−913 to −881 bp) was positively regulated by C/EBPα, increasing basal activity by 4-fold, consistent with the study of Pimental et al. (1993). Likewise, co-transfection of C/EBPβ also increased basal gene expression (1.35-fold), albeit to a lesser extent than C/EBPα (Fig. 4, Construct C). The effect of DEX on the XRE luciferase construct co-transfected with either C/EBPα or β was not statistically significant. Therefore, it appears unlikely that the C/EBP element located at the XRE mediates GR-dependent negative regulation of this gene.
Basal expression of the construct containing the ARE responsive element (−721 bp to −681 bp) construct was decreased by co-transfection of C/EBP proteins, to 70% and 40% for C/EBPα and β, respectively (Fig. 4, Construct D). Unlike the XRE element, this reporter was negatively affected by DEX by C/EBPα, but DEX in the presence of the empty expression vector pMex did not affect expression. However, DEX in the presence of C/EBPα suppressed reporter expression 70% of that seen in control cells. C/EBPβ also suppressed basal level expression by 40%, but there was no affect of DEX on expression.
Expression of the minimal −164 bp promoter construct was only slightly increased basal expression by either C/EBPα or β proteins (Fig. 4, Construct E) and DEX did not have any significant effect in the presence of either C/EBP. From these results, GR negatively regulates this gene by mechanisms involving the palindromic GRE at −1602 bp and another transcription factor, namely C/EBPα and C/EBPβ. When the palindromic GRE is studied in a luciferase construct, it was positively regulated by GR plus DEX (Falkner et al., 1998). C/EBPs induce expression of the XRE reporter and is not DEX sensitive, while DEX negatively regulates the ARE reporter in the presence of C/EBPs. Thus crosstalk between the transcription factors and cooperativity between the response elements must be a crucial factor in determining the response of the gene to GC. The GR may assist in recruitment of C/EBPs to the ARE site and possibly the XRE site that bind the C/EBP proteins through a looping mechanism.
Effect of C/EBP Proteins on Other ARE Constructs
Since the possible interactions of the transcription factors that bind to ARE may have importance in regulation of other ARE-containing genes upon treatment with GC, we prepared a luciferase reporter construct, pQARELuc, containing the ARE of the rat NAD(P)H:quinone oxidoreductase 5’-flanking region described by Favreau and Pickett (1995). We had previously shown this rat gene is negatively regulated by glucocorticoids, yet there was no defined GRE that could be located in the 5’-flanking region (data not shown). While the effect of C/EBP proteins was to increase its basal expression by 2- and 3.5-fold in the presence of C/EBPα or β, respectively (Fig. 5), the ARE reporter from the QOR gene was also negatively regulated by DEX, similar to the DEX-response of the GSTA2 ARE reporter. In cells co-transfected with the control pMEX expression vector, DEX caused a 30% suppression of gene activation, but in the presence of C/EBPα and β the effect of DEX was greater with a 57% and 46% suppression of gene expression. This clearly demonstrates a synergistic effect of C/EBP co-transfection on the ability of GR to negatively regulate this construct. These results also suggest that AREs may be loci of C/EBP action, allowing the definition of a C/EBP-ARE composite element, allowing GR to negatively regulate gene expression.
Figure 5.
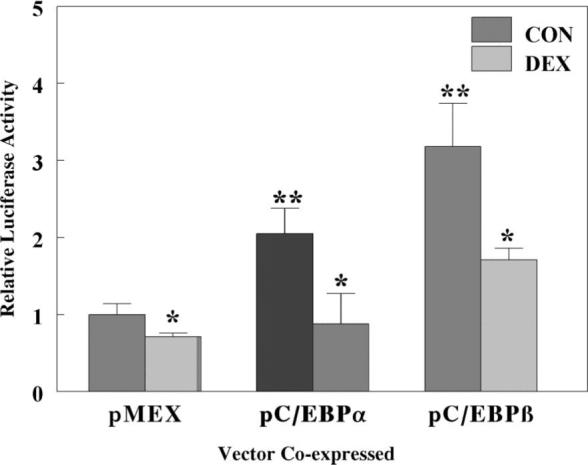
Effect of co-transfection of either C/EBPα or β on the GR-dependent repression of a rat NAD(P)H:quinone oxidoreductase ARE luciferase reporter construct activities in HepG2 cells. Luciferase and β-galactosidase assays were performed on lysed HepG2 cells that had been transfected with pQARE-Luc, a control vector pCMV-β, an expression vector for the glucocorticoid receptor pRSV-GR, and pMEX expression vectors for either pC/EBPα or β, as described in Materials and Methods. Cells were treated with either DMS0 or 0.1 μM DEX. The normalized luciferase activity is expressed as the relative light units divided by β-galactosidase activity and is the average activity ± SD for three independent samples. *P < 0.05 statistically significant decrease by DEX from control cells, **P < 0.05statistically significant induction by C/EBP.
Effects of C/EBP Proteins on the PXR-dependent Induction of GSTA2
Previously, we demonstrated that the GSTA2 is regulated through action of the Pregnane X Receptor (Falkner et al., 2001). The expression of this gene in primary rat hepatocytes displayed a biphasic concentration dependence. The induction of GSTA2 by high concentrations of glucocorticoids or pregnane derivatives was similar to the induction of P4503A23 regulated by PXR ligands, but surprisingly the locus of PXR interaction was defined as the ARE (Falkner et al., 2001). Thus, the ARE and its associated C/EBP response element appears to be involved in both PXR- and GR-dependent regulation of gene expression. We co-transfected C/EBPs and PXR prior to measuring responsiveness of the GSTA2 ARE reporter to t-Bu-DEX, a ligand-activator of PXR. Co-transfection with PXR alone increased basal level expression by 4-fold (Falkner et al., 2001) and 10 nM t-Bu-DEX caused a further 1.5-fold increase in luciferase activity (Fig. 6). C/EBPs suppressed basal expression 20% and 70% for C/EBPα and β, respectively. Interestingly, t-Bu-DEX did not induce gene activation in cells co-transfected with C/EBPα, but did increase expression by t-Bu-DEX by 1.9-fold in the presence of C/EBPβ. These and our previous results suggest that C/EBPs affect the PXR-dependent induction of gene expression, as well as the GR-dependent gene repression, through the ARE indicating there are interactions or crosstalk between the transcription factors that apparently bind to these ARE; namely, C/EBPα/β and Nrf2 interacting with GR and PXR.
Figure 6.
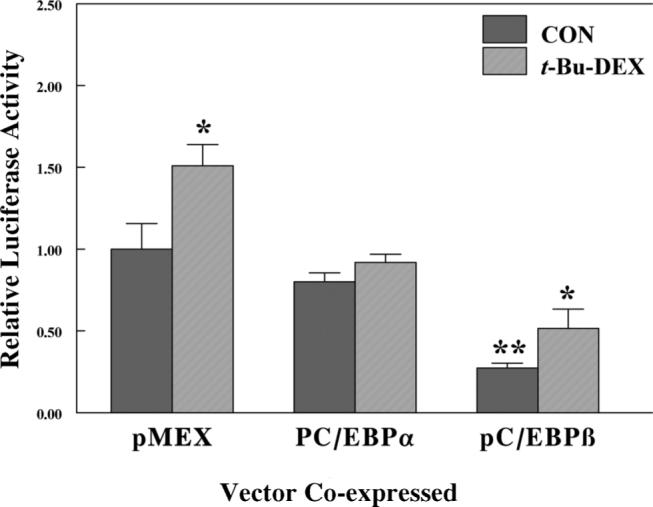
Effect of co-transfection of either C/EBPα or β on the GR-dependent repression of a rat GSTA2 ARE luciferase reporter construct activities in HepG2 cells. Luciferase and β-galactosidase assays were performed on lysed HepG2 cells that had been transfected with pARE-Luc, a control vector pCMV-β, an expression vector for the glucocorticoid receptor pRSV-GR, and pMEX expression vectors for either pC/EBPα or β as described in Materials and Methods. Cells were treated with either DMS0 or 50 μM t-butyl-DEX. The normalized luciferase activity is expressed as the relative light units divided by β-galactosidase activity and is the average activity ±SD for three independent samples. *P < 0.05 statistically significant induction from control cells.
DISCUSSION
The rat glutathione S-transferase A2 gene encodes a protein that can either homodimerize or heterodimerize with subfamily members to produce an enzyme which catalyzes conjugation of glutathione with the electrophilic centers of a wide range of xenobiotic and endobiotic compounds. These reactions inactivate electrophiles, preventing protein or nucleic acid alkylation, and target molecules for export from the cell by members of the multidrug resistance-associated proteins, most notably MRP1 (for review see Cole and Deeley, 2006). In addition, GSTA2 may also serve as an intracellular binding protein for non-substrate ligands, such as the PXR ligands, the pregnane steroids (Homma et al., 1986) and bile acids such as lithocholic acid (Takikawa and Kaplowitz, 1988). Regulation of the rat glutathione S-transferase A2 (GSTA2) gene by glucocorticoids is complex and two different mechanisms involving either GR or PXR have been proposed to address this complexity (Falkner et al., 2001).
REGULATION OF THE GSTA2 GENE IN TISSUES AND ISOLATED HEPATOCYTES
Our studies (Linder and Prough, 1993) suggest that there are important developmental differences in the ability to regulate this gene. For example, in liver from two developmental models (neonatal or adult rats), there is a difference in the action of glucocorticoid agonists. In neonates, DEX caused induction of GSTA2 message and protein, while in adult animals this GR-dependent response was suppressed. Similar results were obtained in isolated hepatocyte cultures (Xiao et al., 1995, Voss et al., 1996, Prough et al., 1996). In addition, the adult rat hepatic glutathione S-transferase A2 gene was regulated by glucocorticoids in a biphasic manner, with lower concentrations of GC repressing gene transcription, while high concentrations of GC induced gene transcription.
Similar results were obtained in studies by Boyer and coworkers (Voss et al., 1996), who demonstrated the same biphasic regulation of the GSTA2 gene by glucocorticoids in cultured rat hepatocytes; however, this occurred only in the presence of added recombinant interleukin-6. In these studies neither recombinant interleukin-6 nor corticosteroid treatment had any significant effect on GSTA2 transcript levels. This observation was important, since it linked the glucocorticoid-dependent regulation of GSTA2 with rIL6 signaling. In our studies, we utilized a Matrigel based cell culture system, while the studies by Voss et al employed collagen coated plates. (Xiao et al., 1995, Voss et al., 1996, Prough et al., 1996). The significance of the cell culture methods and the degree of de-differentiation hepatocytes undergo in culture may account for the differences in GC sensitivity.
Negative Regulation of the Rat GSTA2 Gene Involving the Glucocorticoid Receptor and C/EBP Ramily Members
Our initial studies performed in isolated rat hepatocytes indicated a biphasic response to glucocorticoids in isolated rat hepatocytes. We reproduced those effects in transient transfection assays in HepG2 cells (Falkner et al., 1998). Using mutation and deletion constructs we documented the importance of a palindromic glucocorticoid response element and three glucocorticoid receptor half sites located between −1.65 and −1.0 kb in the 5’-flanking region of the GSTA2 gene. If these response elements were either mutated or deleted, the glucocorticoid response was significantly reduced. In contrast, this region has no effect on the IL-6 glucocorticoid mediated mechanism of gene suppression. However, this region did not negatively regulate expression by itself, since when a construct containing the sequences between −1,710 to −1,000 bp of the 5’-flanking region of the GSTA2 gene was studied in isolation, it was induced by DEX, strongly suggesting that another transcription factor must mediate the negative regulatory effect of the entire gene.
An important advance in our understanding of this mechanism of regulation was the demonstration that C/EBPβ and Nrf-2 are both involved in this mechanism and act to recruit SMRT (silencing mediator for retinoid and thyroid receptors, Ki et al., 2005). Although this study assumed that C/EBPβ was an inducer of GSTA2 gene expression presumably acting through the C/EBP element located at the XRE, the results of over-expression studies presented in the current article and by others (Chen and Ramos, 2000) suggest that C/EBPs are negative regulators of GSTA2 gene expression. The study of Ki et al. (2005) demonstrates through chromatin immunoprecipitation assays that the co-repressor SMRT binds to C/EBPβ and Nrf-2 in a GC-dependent manner to form a negative regulatory complex located between base pairs −989 and −643 in the 5’-flanking region. We propose that the C/EBP site responsible for mediating negative regulation in this region is located at the ARE, rather than the XRE, and both C/EBPα and C/EBPβ can mediate this effect.
The significance of C/EBPα as a silencer is consistent with the observation that GSTA2 gene expression is elevated in chemical hepatocarinogenesis models (Pickett et al., 1984). In rats during chemically induced hepatocarcinogenesis, C/EBPα levels are reduced, while C/EBPβ levels are unchanged (Osada et al., 1995). This function of C/EBPβ may contribute to the increased expression of glutathione S-transferase P1 (Osada et al., 1995), a marker of chemically induced hepatocarcinogenesis in the rat.
Alternative Mechanisms for Glucocorticoid Regulation of GSTA2
Subsequent studies by Boyer and coworkers (Voss et al., 1996; Voss et al., 2002; Whalen et al., 2004; Whalen et al., 2006) have also demonstrated that the negative regulation of the GSTA2 gene requires interleukin-6 treatment for synthesis of a novel transcriptional repressor, a short form of ubiquitin-specific protease 3. Initially, transient transfection assays were used to determine that the response was mediated by elements residing between base pairs −914 and −663 in the 5’-flanking region of the gene; this area contains the XRE, ARE, HNF1 and HNF4 response elements. Using EMSA analysis, a specific DNA-protein complex was identified in cells treated with both rIL-6 and DEX (Voss et al., 2002), which was subsequently identified as a short form of ubiquitin-specific protease 3 (Whalen et al., 2006). Nuclear extract from these cells induced an electro-phoretic mobility shift of a double-stranded oligonucleotide corresponding to the sequences −881 to −852 in 5’-flanking region, a core sequence of TGATT that is part of the HNF1 response element. In these studies, reduction in GSTA2 gene expression was correlated initially with a loss of HNF1 binding and at later time points to an increase in binding of ubiquitin-specific protease 3.
Cross Talk Between GR, Nrf2 and C/EBPs
The studies we have reported provide evidence that the palindromic and half sites between −1,710 and −1,000 bp in the 5’-flanking region of GSTA2 impart the negative regulatory properties of GR upon binding to the palindromic and half site GREs. The deletion studies reported suggest that the ARE may be a locus where C/EBPα and β interact with GR, Nrf2, or PXR in regulating this gene. The ARE of NAD(P)H:quinone oxidoreductase also displays negative regulation in the presence of GR and C/EBP, further suggesting a common mechanism of these transcription factors acting at AREs. Additional experiments are required to establish whether the ARE sequences of other genes may be similarly regulated by GR and C/EBP proteins. These interactions may be important for developmental differences in gene expression, as was seen for GSTA2 and NAD(P)H:quinone oxidoreductase.
CONCLUSIONS
As defined by the careful analysis of the ARE of the rat GSTA2 gene (Rushmore et al., 1989; Rushmore and Pickett, 1993), this responsive element is required for both basal and inducible transcriptional activity of this gene, and probably by other genes regulated by Nrf2. The ARE sequence has similarity to both the TRE (Fos/Jun) and C/EBP responsive elements. The detailed studies of Rushmore et al. (1990, 1991) were critical to defining the differences between AP1 TREs and the ARE. For example, the GSTA2 ARE is not an AP1 site, although the ARE from the rat NAD(P)H:quinine oxidoreductase gene is an AP1 responsive element. Therefore, the sequence specificity required for determining the sequence specificity of which ARE elements are C/EBP responsive should be determined for human genes.
| C/EBP sequence | RTTGCGYAAY |
| ARE | GTGACAAAGC- |
| AP1 TRE | TGACTCA |
In addition, the physical interactions of the transcription factors that bind to the GSTA2 ARE, namely Nrf2, GR, PXR, need to be elucidated to understand tissue- and development-specific regulation of genes containing the ARE enhancer element. In the case of C/EBPs that are differentially expressed during development, expression of genes can be either attenuated or enhanced depending on the relative expression of these transcription factors. This knowledge may help explain the complex pattern of expression of GSTA2 seen during life of the rat described by Listowsky's group, demonstrating high levels of expression at birth, low levels during neonatal life and high levels during adolescent and adult life (Abrahamovitz et al., 1988). Further, our results suggest that since the ARE in at least 2 genes (rat GSTA2 and NAD(P)H:quinone oxidoreductase) displays complex regulation by C/EBPs, other genes with AREs may also be sensitive to cross-talk with basic leucine zipper transcription factors. GSTA2 plays an important role in binding lipids, sterols, and bile acids, and in the disposition of exogenous and endogenous compounds, protective mechanisms important for hepatic homeostasis. GSTA2 is an abundant protein and over-expression of this protein could require a significant energy burden on the cells. Thus, the complex expression pattern of GSTA2 may reflect a trade-off between the advantage of high expression protecting DNA in rapidly proliferating tissues and the energy conservation of reduced expression in post-mitotic cells in which regulation is controlled by a combination of xenobiotic and endobiotic compounds and hormonal action.
TRIBUTE TO RONALD W. ESTABROOK
Those of us who have worked with Ron in the laboratory or class room have been significantly influenced by his personality, intellect, and advice. Ron pushed hardest those he felt would be successful and provided constructive guidance in how to handle difficult personal situations, perform experiments, and compete with other scientists working in similar research areas. His active attendance and frequent participation in meetings provided information about the latest experiments being performed by others and stimulated either new key experiments or frustration in moving away from other well-planned experiments. No matter what the issue, life in Dallas with Ron was exciting and stimulating, due to the many invited seminar speakers, research workers, and famous scientists passing through the Department of Biochemistry. My career has benefited from my exposure to Ron and provided me with the skills to weather difficult experiences as a Biochemistry department chair and the good experiences of having skilled young people and expert visitors in Louisville after I left Dallas. I remember fondly the many interactions between Ron and those of us recruited to Dallas, such as Bettie Sue Masters, Julian “Bill” Peterson, Mike Waterman, Evan Simpson, Ian Mason, Jorge Capdevila, and Jurgen Werringloer, as well as, their many students. These colleagues are the scientific family of Ron who have continued in the field, many of whom have contributed to this volume, and who spread his legacy as a chair, a scientist and a mentor.
ACKNOWLEDGMENT
Supported by National Institutes of Health Grant DK54774 (RAP) and Projects 2 (RAP) and 5 (KCF) of NASA grant NAG5-12874. The authors wish to thank Mary Pendleton for her technical assistance. The authors are indebted to Immaculate Amunom, Thomas Geoghegan, and Stephanie Webb for their positive critical comments about this manuscript.
ABBREVIATIONS
- AhR
aryl hydrocarbon receptor
- ARE
antioxidant response element, also known as electrophilic (EpRE) responsive element
- BA
1,2-benzanthracene
- C/EBP
CCAAT-enhancer binding protein
- DEX
dexamethasone
- DMSO
dimethyl sulfoxide
- GC
glucocorticoids
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- GST
glutathione S-transferase
- Nrf2
NF-E2-related factor-2
- PXR
pregnane X receptor
- QOR
NAD(P)H:quinone oxidoreductase
- RU 38 486
17β-hydroxy-11β-(4-dimethylamino-phenyl)-17α-(prop-1-ynyl)-estra-4,9-dien-3-one
- t-Bu-DEX
t-butyl-dexamethasone acetate
- t-bHQ
t-butylhydroquinone
- XRE
xenobiotic responsive element
REFERENCES
- Abramovitz M, Listowsky I. Developmental regulation of glutathione S-transferases. Xenobiotica. 1988;18:1249–1254. doi: 10.3109/00498258809042248. [DOI] [PubMed] [Google Scholar]
- Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier EH, Gwynn B, Howard S, Jerry J, Gordon JI, Landschulz WH, McKnight SL. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989;3:1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Chen SS, Chen JF, Johnson PF, Muppala V, Lee YH. C/EBPbeta, when expressed from the C/EBPalpha gene locus, can functionally replace C/EBPalpha in liver but not in adipose tissue. Mol. Cell Biol. 2000;20:7292–7299. doi: 10.1128/mcb.20.19.7292-7299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Ramos KS. A CCAAT/enhancer-binding protein site within the antioxidant/electrophile response element along with CREB-binding protein participate in the negative regulation of rat GST-Ya gene in vascular smooth muscle cells. J. Biol. Chem. 2000;275:27366–27376. doi: 10.1074/jbc.M000405200. [DOI] [PubMed] [Google Scholar]
- Clark AG, Carrol N. Suppression of high affinity ligand binding to the major glutathione S-transferase for galleria mellonella by physiological concentrations of glutathione. Biochem. J. 1986;233:325–331. doi: 10.1042/bj2330325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006;27:438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Cram EJ, Ramos RA, Wang EC, Cha HH, Nishio Y, Firestone GL. Role of the CCAAT/enhancer binding protein-alpha transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J. Biol. Chem. 1998;273:2008–2014. doi: 10.1074/jbc.273.4.2008. [DOI] [PubMed] [Google Scholar]
- Falkner KC, Rushmore TH, Linder MW, Prough RA. Negative regulation of the rat glutathione S-transferase A2 gene by glucocorticoids involves a canonical glucocorticoid consensus sequence. Mol. Pharmacol. 1998;53:1016–1026. [PubMed] [Google Scholar]
- Falkner KC, Pinaire JA, Xiao G-H, Geoghegan TE, Prough RA. Regulation of the rat glutathione S-transferase A2 gene by glucocorticoids: Involvement of both the glucocorticoid and pregnane X receptors. Mol. Pharmacol. 2001;60:611–619. [PubMed] [Google Scholar]
- Favreau LV, Pickett CB. The rat quinone reductase antioxidant response element. Identification of the nucleotide sequence required for basal and inducible activity and detection of antioxidant response element-binding proteins in hepatoma and non-hepatoma cell lines. J. Biol. Chem. 1995;270:24468–24474. doi: 10.1074/jbc.270.41.24468. [DOI] [PubMed] [Google Scholar]
- Hatzoglou M, Bosch F, Park EA, Hanson RW. Hormonal control of interacting promoters introduced into cells by retroviruses. J. Biol. Chem. 1991;266:8416–8425. [PubMed] [Google Scholar]
- Homma H, Maruyama H, Niitsu Y, Listowsky I. A subclass of glutathione S-transferases as intracellular high-capacity and high-affinity steroid-binding proteins. Biochem. J. 1986;235:763–768. doi: 10.1042/bj2350763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh T, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/Small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap-1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Develop. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Cho IJ, Choi DW, Kim SG. Glucocorticoid receptor (GR)-Associated SMRT binding to C/EBP beta TAD and Nrf2 Neh4/5: Role of SMRT recruited to GR in GSTA2 gene repression. Mol. Cell Biol. 2005;25:4150–4165. doi: 10.1128/MCB.25.10.4150-4165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Sauer B, Johnson PF, Gonzalez FJ. Disruption of the C/EBP alpha gene in adult mouse liver. Mol. Cell Biol. 1997;17:6014–6022. doi: 10.1128/mcb.17.10.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder MW, Prough RA. Developmental aspects of glucocorticoid regulation of polycyclic aromatic hydrocarbon-inducible enzymes in rat liver. Arch. Biochem. Biophys. 1993;302:92–102. doi: 10.1006/abbi.1993.1185. [DOI] [PubMed] [Google Scholar]
- McKnight SL, Lane MD, Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989;3:2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Ann. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- Osada S, Takano K, Nishihara T, Suzuki T, Muramatsu M, Imagawa M. CCAAT/enhancer-binding proteins alpha and beta interact with the silencer element in the promoter of glutathione S-transferase P gene during hepatocarcinogenesis. J. Biol. Chem. 1995;270:31288–31293. doi: 10.1074/jbc.270.52.31288. [DOI] [PubMed] [Google Scholar]
- Osada S, Yamamoto H, Nishihara T, Imagawa M. DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J. Biol. Chem. 1996;271:3891–3896. doi: 10.1074/jbc.271.7.3891. [DOI] [PubMed] [Google Scholar]
- Paulson KE, Darnell JE, Jr, Rushmore T, Pickett CB. Analysis of the upstream elements of the xenobiotic compound-inducible and positionally regulated glutathione S-transferase Ya gene. Mol. Cell Biol. 1990;10:1841–1852. doi: 10.1128/mcb.10.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CB, Williams JB, Lu AYH, Cameron RG. Regulation of glutathione transferase and DT diaphorase mRNAs in persistent hepatocyte nodules during chemical hepatocarcinogenesis. Proc. Natl. Acad. Sci. USA. 1984;83:5091–5095. doi: 10.1073/pnas.81.16.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimental RA, Liang B, Yee GK, Wilhelmsson A, Poellinger L, Paulson KE. Dioxin receptor and C/EBP regulate the function of the glutathione S-transferase Ya gene xenobiotic response element. Mol. Cell Biol. 1993;13:4365–4373. doi: 10.1128/mcb.13.7.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Prough RA, Xiao G-H, Pinaire JA, Falkner KC. Hormonal regulation of xenobiotic drug metabolizing enzymes. FASEB J. 1996;10:1369–1377. doi: 10.1096/fasebj.10.12.8903507. [DOI] [PubMed] [Google Scholar]
- Rudiger JJ, Roth M, Bihl MP, Cornelius BC, Johnson M, Ziesche R, Block LH. Interaction of C/EBPalpha and the glucocorticoid receptor in vivo and in non-transformed human cells. FASEB J. 2002;16:177–184. doi: 10.1096/fj.01-0226com. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: Identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc. Natl. Acad. Sci. USA. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element: activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the glutathione S-transferase Ya subunit gene: Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990;265:4648–14653. [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Glutathione S-transferases, structure, regulation, and therapeutic implications. J. Biol. Chem. 1993;268:11475–11478. [PubMed] [Google Scholar]
- Sherratt AJ, Banet DE, Prough RA. Glucocorticoid regulation of polycyclic aromatic hydrocarbon induction of cytochrome P450IA1, glutathione S-transferases, and NAD(P)H:quinone oxidoreductase in cultured fetal rat hepatocytes. Mol. Pharmacol. 1990;37:198–205. [PubMed] [Google Scholar]
- Schrem H, Klempnauer J, Borlak J. Liver-enriched transcription factors in liver function and development. Part II: the C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol. Rev. 2004;56:291–330. doi: 10.1124/pr.56.2.5. [DOI] [PubMed] [Google Scholar]
- Takikawa H, Kaplowitz N. Comparison of the binding sites of GSH S-transferases of the Ya- and Yb-subunit classes: effect of glutathione on the binding of bile acids. J. Lipid Res. 1988;29:279–286. [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- Voss SH, Park Y, Kwon SO, Whalen R, Boyer TD. Role of interleukin 6 and corticosteroids in the regulation of expression of glutathione S-transferases in primary cultures of rat hepatocytes. Biochem. J. 1996;317(Pt 2):627–632. doi: 10.1042/bj3170627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss SH, Whalen R, Boyer TD. Mechanism of negative regulation of rat glutathione S-transferase A2 by the cytokine interleukin 6. Biochem. J. 2002;365:229–237. doi: 10.1042/BJ20011514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R, Voss SH, Boyer TD. Decreased expression levels of rat liver glutathione S-transferase A2 and albumin during the acute phase response are mediated by HNF1 (hepatic nuclear factor 1) and IL6DEX-NP. Biochem. J. 2004;377:763–768. doi: 10.1042/BJ20031256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R, Liu X, Boyer TD. Identification of a short form of ubiquitin-specific protease 3 that is a repressor of rat glutathione S-transferase gene expression. Biochem. J. 2006;394:519–526. doi: 10.1042/BJ20051392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Pinaire JA, Rodrigues AD, Prough RA. Regulation of the Ah gene battery via Ah receptor-dependent and independent processes in cultured adult rat hepatocytes. Drug Metab. Dispos. 1995;23:642–650. [PubMed] [Google Scholar]
- Zhao G, Nakano K, Chijiiwa K, Ueda J, Tanaka M. Inhibited activities in CCAAT/enhancer-binding protein, activating protein-1 and cyclins after hepatectomy in rats with thioacetamide-induced liver cirrhosis. Biochem. Biophys. Res. Commun. 2002;292:474–481. doi: 10.1006/bbrc.2002.6630. [DOI] [PubMed] [Google Scholar]