Abstract
Human papillomaviruses (HPV) have been associated with the development of non-melanoma skin cancer (NMSC) but the molecular mechanisms of the role of the virus in NMSC development are not clearly understood. Abnormal epithelial differentiation seen in malignant transformation of keratinocytes is associated with changes in keratin expression. The purpose of this study was to investigate the phenotype of primary human adult keratinocytes expressing early genes of HPV8 with specific reference to their differentiation and cell cycle profile to determine whether early genes of HPV8 lead to changes that are consistent with transformation. The expression of HPV8 early genes either individually or simultaneously caused distinct changes in the keratinocyte morphology and induced an abnormal keratin expression pattern, that included simple epithelial (K8, K18, K19), hyperproliferation-specific (K6, K16), basal-specific (K14, K15) and differentiation-specific (K1, K10) keratins. Our results indicate that expression of HPV8 early genes disrupts the normal keratin expression pattern in vitro. Expression of HPV8-E7 alone caused polyploidy that was associated with decreased expression of p21 and pRb. Expression of individual genes or in combination differentially influenced cell morphology and cell cycle distribution which might be important in HPV8-induced keratinocyte transformation.
Keywords: cell cycle, cytokeratin, HPV8
Introduction
Human papillomaviruses (HPV) are small double-stranded DNA viruses, which infect keratinocytes in the basal layer of the epithelium at a variety of anatomical sites. The HPV replicative cycle is intimately linked with the differentiation of the infected keratinocyte. Infection with papillomaviruses is most frequently recognized as resulting in benign epithelial changes. However, malignancies may develop on the basis of persisting infection and particularly depending on the HPV type.
Mucosal HPV types are the best characterized, and include the high-risk types HPV16 and HPV18, which cause lesions that can progress to cervical carcinoma (1). In contrast to the genital HPV, much less is known about the biology of cutaneous HPV types, including HPV5 and HPV8, which have been linked with the development of non-melanoma skin cancer (NMSC) (2).
An important contribution towards NMSC may be the inhibition of apoptosis by E6 proteins of cutaneous HPV. The E6 proteins of HPV type 5, 10 and 77 have been shown to target Bak for proteolytic degradation and to inhibit effectively UVB-induced apoptosis (3). Additionally, E6 proteins of the cutaneous HPV77 also inhibit p53-dependent transcription of pro-apoptotic genes following UVB irradiation (4). The expression of HPV8-E7 in primary human adult keratinocytes (PHAK) caused an enhancement of terminal differentiation and invasion of keratinocytes in an in vitro skin-equivalent model (5). The carcinogenic properties of HPV8 have also been demonstrated in a transgenic mouse model, in which the complete early coding region of HPV8 is controlled by the human keratin-14 promoter. HPV8-positive mice developed single or multifocal benign tumors with varying degrees of epidermal dysplasia including the development of squamous cell carcinoma (SCC). These results support the hypothesis that HPV8 may have a direct role in promoting skin cancer (6).
Keratins are characteristic structural elements of epithelial cells, which are the target cells for HPVs. They make up the largest subgroup of intermediate filament proteins and represent the most abundant proteins in epithelial cells. The primary function of keratins is to protect epithelial cells from mechanical and non-mechanical stresses that result in cell death. Other functions include roles in cell signalling, the stress response and apoptosis (7). The pattern and quantity of keratin expression are strictly regulated in epithelial cells. Normally, K8, K18 as well as K19 are typically found in simple epithelial tissues, whereas in the epidermis, K5, K14 and K15 are expressed in basal keratinocytes, gradually replaced by K1/K10 in the suprabasal layers during terminal differentiation. Activated keratinocytes express suprabasal keratins K6 and K16 in part in response to cytokines and immune mediators (8). Results from cell transfection experiments indicated a direct role for K10 in inhibiting cell cycle entry by sequestering Akt kinase (9). In addition, ectopic expression of K10 in basal epidermal layers of transgenic mice led to a strong decrease in proliferation and reduced tumorigenesis. During skin tumor progression K1/K10 expression is lost, suggesting a possible tumor suppressor role for these keratins (10). Abnormal epithelial differentiation seen in malignant transformation is associated with the inappropriate expression of the simple epithelial keratins 8 and 18 in SCCs (11). The simple epithelial keratins K8, K18 and K19 are heterogeneously expressed in cultured keratinocytes but are absent in skin equivalents of normal skin. K8/K18 overexpression is normally associated with cells with great proliferation potential, such as those of embryonic structures, the latter stages of cancer or carcinoma in situ (Bowen's disease) (11-13). A positive correlation is found between the expression of simple epithelium keratins and epidermal malignancy, both in vitro (14) and in vivo (11,15).
The keratin expression profile of each cell is therefore influenced by the type of epithelium, the differentiation potential and the proliferative capacity of the tissue. The abnormal epithelial differentiation seen in malignant transformation is associated with changes in keratin expression (16). The use of keratin-specific antibodies has proved to be very useful for the study of both normal and abnormal differentiation of malignant transformed keratinocytes. The purpose of this study was to establish whether the expression of one or more early genes of HPV8 leads to changes in keratinocyte phenotype consistent with cell transformation with specific reference to the differentiation and cell cycle profile of the infected cell.
Material and methods
HPV expression vectors and production of high titre retroviruses
The Moloney murine leukaemia retrovirus vector pLXSN (17) was used to generate recombinant retroviruses containing HPV8 genes. HPV8-E2 and -E6 ORF were amplified by PCR by using the primers 8-E2-1: AAGCTTGAATTCGTGATCAAGAAGACGAGGGCG and 8-E2-2: AAGCTTGGATCCTGTGTTAGTAGCAAGGCAGCG; 8-E6-1: GAAGCTTGAATTCTCTGACTTGTGCAATTTTCC and 8-E6-2: AAGCTTGGATCCACAAAATCTTGCACAGTGACC, which contain EcoRI and BamHI restriction endonuclease sites at their 5′ ends respectively. After PCR, the amplimers were digested and subsequently cloned into EcoRI/BamHI-digested pLXSN, thus obtaining pLXSN-8-E2 and pLXSN-8-E6. The construction of pLXSN-8-E7 is described elsewhere (5). PLXSN-8-CER containing the complete early coding region of HPV8 (nt. 1–5111) was cloned by inserting the HpaI/BamHI fragment of HPV8 (18) into pLXSN. The positive orientation of the insert was verified by restriction digestion and by sequencing. The HPV8 genes were cloned downstream of the Moloney murine leukaemia virus 5′ LTR sequence. Recombinant retroviruses were produced by transfecting pLXSN-derived DNA into PT67 cells with Fugene 6 (Roche, Burgess Hill, UK), following the manufacturer's instructions. Two days after transfection, cells were plated into selection media containing 500 μg/ml G418 for 3 days. Resistant cells were grown to confluence at which time retrovirus-containing cellular supernatants were collected.
Cell culture and infection of keratinocytes with recombinant retroviruses
Cultivation of PT67 cells, which were used to replicate amphotropic retroviruses, and infection of PHAK were performed as described in Akgul et al. (5). Keratinocytes used in this study were isolated from a breast reduction of a 22-year-old patient. Infected and selected cells in passage 3 were seeded onto sterilized 13 mm diameter glass coverslips (Chance Propper, Wasley, UK) and placed in 12-well tissue culture plates. Cells were left to grow for 5 days before being fixed for immunocytochemical analysis.
Detection of HPV8 transcripts by RT-PCR
Total RNA was isolated with Trizol Reagent (Life Technologies, Inc., Gaithersburg, MD, USA) according to the manufacturer's instructions. RNA was suspended in DEPC-treated water. Reverse transcriptase polymerase chain reaction (RT-PCR) was performed using the First-Strand cDNA Synthesis Kit (Amersham, Buckinghamshire, UK) and 5 μg of total RNA from pLXSN-8-E6, pLXSN-8-E7 and pLXSN-8-CER-infected keratinocytes. The oligonucleotides designed for amplification of HPV8-E6 were E6-RT-1: CATTGCAGGACTGTTCAGTACCG and E6-RT-2: ACAAGCAGTTTTGACACCTAACGTCTA, for HPV8-E7 were E7-RT-1: CTTTGTGAAGAGGAATTACCAAACGA and E7-RT-2: AAGATGTATCAACTCCCTTCGGGTA, for HPV8-E1 were E1-RT-1: TCATGAGGTGGAGGTACCGG and E1-RT-2: AGACAATATCTGCTGCTCTTGCA, for HPV8-E2 were E2-RT-1: GCAAAACATACAGGGCGACTTG and E2-RT-2: AAGATGTATCAACTCCCTTCGGGTA, for HPV8-E1∧E4 were E1E4-RT-1: GGTATCAGGACCTTTCAAGAATTGC and E1E4-RT-2: CAGTAGCACTATATCTACGTGCATC. Sequence of the E1∧E4 primer pair is based on published data for HPV5 (19).
Flow cytometric analysis
For DNA cell cycle analysis, cells were treated with Hoechst 33342 (10 μg/ml; Sigma, Dorset, UK) for 45 min at 37°C. The cells were harvested by trypsinization, pelleted by centrifugation and washed twice with phosphate-buffered saline (PBS). After the second wash cells were resuspended in 500 μl PBS containing 5 μg/ml propidium iodide (PI; Sigma). The relative cellular DNA content of stained cells was measured by using an LSR flow cytometer (Becton Dickinson, San Jose, CA, USA). Hoechst 33342 was excited by a 325-nm UV laser and fluorescence was collected using a 424/44 nm bandpass filter. A 488-nm laser was used to generate scatter signals and to excite PI with fluorescence being collected above 620 nm. Single cells were selected for analysis by excluding dead cells on the basis of PI positivity and by pulse processing of the Hoechst signal. The relative DNA content in single cells was plotted as a frequency histogram of the Hoechst fluorescence. The proportions of cells in G0/G1, S and G2/M were calculated using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
For the analysis of cell cycle profiles of cells labelled with 5-bromodeoxyuridine (BrdU), cells were incubated with 10 μm of BrdU (Sigma) for 30 min. The cells were harvested by trypsinization and pelleted by centrifugation. After fixation in 70% ethanol, the samples were pelleted, washed twice in PBS, and treated with 2 m HCl for 30 min. The samples were then washed twice in PBS and once in PBST (PBS containing 0.1% BSA and 0.2% Tween 20). Cells were then incubated with anti-BrdU monoclonal antibody (2.5 μg/ml; Becton Dickinson, Oxford, UK) for 20 min in the dark. Cells were then washed twice in PBST and stained with FITC-conjugated rabbit anti-mouse fragments (10 μg/ml; Dako Cytomation, Ely, UK) for 20 min at RT in the dark. Cells were treated with ribonuclease A (100 mg/ml; Sigma) and PI was added at a final concentration of 50 μg/ml. Samples were incubated at RT for 30 min prior to flow cytometric analysis. Samples were analysed on a FACSCalibur (Becton Dickinson, San Jose, CA, USA) with FITC fluorescence collected between 515 and 545 nm and PI fluorescence collected above 620 nm. Forward and right angle scatter were used to define the cellular population, and pulse processing of the PI signal was used to distinguish single cells from double cells.
Western blot
For Western blot analysis cells were trysinized, pelleted by centrifugation and resuspended in lysis buffer [150 mm NaCl, 50 mm Tris (pH 7.4), 1% NP-40, 1% SDS] containing protease inhibitors. The protein concentration of the cell lysate, containing cytoplasmic and nuclear proteins was determined using Bio-Rad protein assay reagent (Bio Rad, Hemel Hempstead, UK). After adding an equal volume of 2× Laemmli buffer and boiling for 5 min, equal amounts of proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The blots were probed with specific antibodies: p53 (Do-1; Cancer Research UK Laboratories, Cancer Research UK, London, UK), p21 (sc-817; Santa Cruz Biotechnology, Mile Elm, UK), pRB (sc-IF8; Santa Cruz Biotechnology) and GAPDH (Abcam Ltd, Cambridge, UK). The signal was developed using the ECL+ kit (Amersham Biosciences, Little Chalfont, UK) and quantified with the imaging analysis software Quantity One (Bio-Rad).
Immunocytochemistry
Cells were fixed on coverslips in 4% paraformaldehyde for 10 min at RT and further permeabilized with 0.2% Triton X-100 for 10 min at RT. Cells were rinsed in PBS and incubated with primary mouse antibodies for 90 min at RT: LHK1: anti-K1 (8), LHK6: anti-K6 (8), LE41: anti-K8 (20), LHP2: anti-K10 (21), LL001: anti-K14 (22), LHK15: anti-K15 (23), LL025: anti-K16 (8), LE61: anti-K8/K18 complex (24), Lp2K: anti-K19 (25). Following several rinses in PBS, coverslips were incubated for 30 min with a biotinylated secondary antibody and processed with a streptavidin-biotin-peroxidase detection system (Vectastain ABC Kit; Vector Laboratories, Orton Southgate, UK) as recommended by the manufacturer. The staining was developed using TSA Fluorescein System (Perkin Elmer Life Sciences, Inc., Waltham, MA, USA). Prior to mounting with Immunomount (ThermoShandon, Pittsburgh, PA, USA), the cells were counterstained with PI (1:1000 working concentration from 1 mg/ml in PBS) for 1 min before washing twice in PBS. Images were recorded using a Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany).
Results
Morphology of HPV8-positive keratinocytes
PHAKs were infected with the recombinant retroviruses encoding different HPV8 early region genes, pLXSN-8-E6, pLXSN-8-E7 and pLXSN-8 complete early region (CER) and the empty control vector pLXSN. The transduced cells were selected in G418, pooled in passage 1 and passaged a second time. To confirm HPV8 gene expression in these cells, total RNA was extracted and RT-PCR was performed. The primers used in this study were able to amplify PCR fragments for HPV8-E6 and -E7 in the corresponding infected cells (Fig. 1a). We could also confirm the expression of E6, E7, E1 and E2 in PHAKs infected with pLXSN-8-CER but no PCR products were generated with the E1∧E4 primers. However, the specificity of the E1∧E4 primers was demonstrated on total RNA isolated from skin tumors of HPV8-transgenic mice. The E1∧E4 protein is synthesized in the late phase of HPV life cycle (26).
Figure 1.
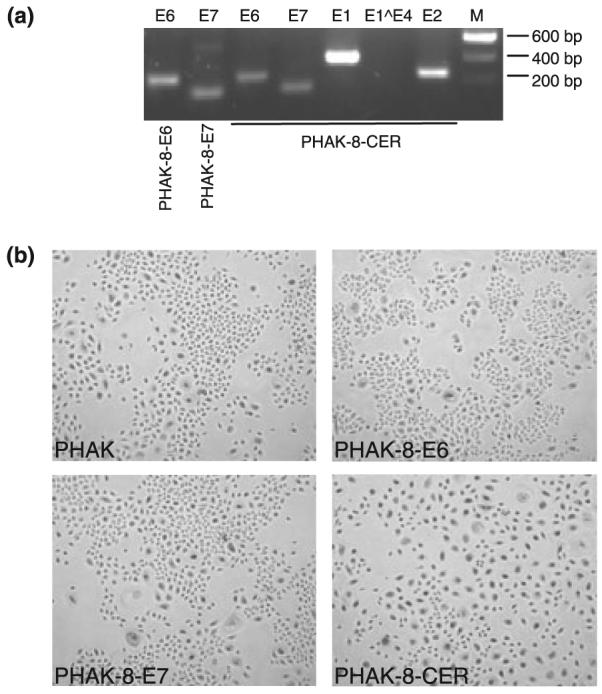
HPV transcripts expressed in human adult primary keratinocytes and morphology of cells transdused with different HPV8 genes. (a) Detection of HPV8 early gene transcripts in PHAKs infected with recombinant retroviruses. cDNA was amplified with primer for E6 and E7 to verify the presence of transcripts in PHAK-8-E6 (lane 1) and PHAK-8-E7 (lane 2) cells. These and also the primers for E1, E2 and E1∧E4 were used to show the presence of all early genes of HPV8 in PHAK-8-CER except for E1∧E4 (lanes 3–7). (b) Morphology of PHAK cells infected with recombinant retroviruses. Phase contrast photographs of PHAK, PHAK-8E6, PHAK-E7 and PHAK-8-CER at passage 2 are shown. Magnification, ×100.
Transduced keratinocytes were plated on plastic dishes at passage 2 and initially analysed for differences in cell morphology. Pooled cultures containing pLXSN-8-E6 (PHAK-8-E6) and pLXSN-8-E7 (PHAK-8-E7) displayed a morphology similar to that of control cells (PHAK), which had been infected with virus produced by the empty vector pLXSN. These cells were a homogeneous population of small proliferating cells, which appear to grow in clusters and maintain cell–cell contacts. In marked contrast, infection with retrovirus containing the CER of HPV8 (PHAK-8-CER) conferred a dramatic difference on keratinocyte morphology when compared with empty vector or E6 and E7-expressing cells. In the PHAK-8-CER culture we noted the appearance of larger cells, which were more frequent compared with PHAK or PHAK-8-E6 and PHAK-8-E7-infected cultures. In addition, these cells lost cell–cell contacts and dissociated from one another (Fig. 1b).
Keratin expression profile in HPV8-positive keratinocytes
The abnormal epithelial differentiation seen in malignant transformation is associated with a number of changes in keratin expression (13,27). To investigate whether HPV8 genes were able to alter the keratin profile in the transduced keratinocytes, changes in keratin expression were analysed at passage 3, when specific early genes were known to be expressed (Fig. 2).
Figure 2.
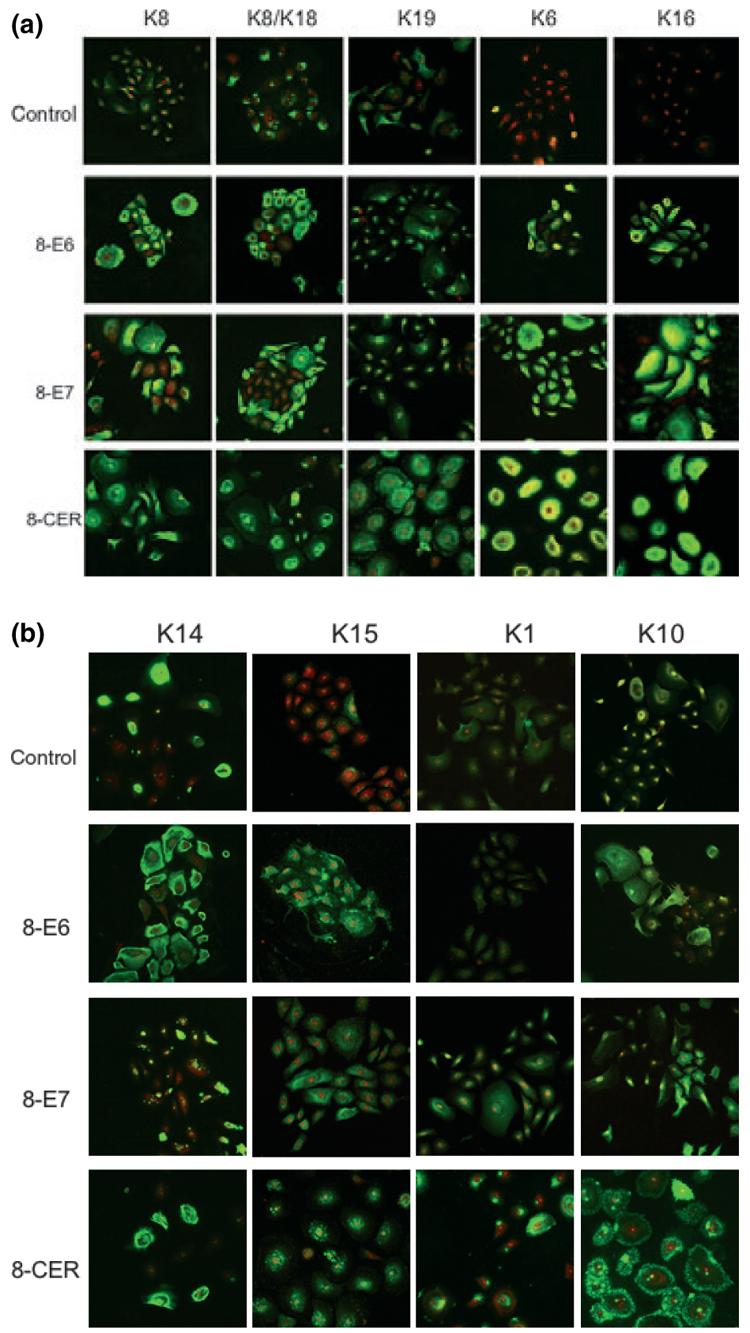
Keratin immunofluorescence of PHAK, PHAK-8-E6, PHAK-8-E7 and PHAK-8-CER cell lines. Keratin staining, which fluoresces green, was detected using the TSA Fluorescein System. Propidium iodide (PI) staining, which fluoresces red, highlights DNA stain in the nuclei. The apparent yellow colour present in the K6 of the HPV8-CER cells was the result of a very intense fluorescence that sometimes obscures the red staining of the nuclei.
Simple epithelia-associated keratins 8/18 and 19
As expression of simple epithelial keratins is associated with malignant conversion, we first investigated the expression profile of K8, K18 and K19 in the different transduced cultures. About 50% of the PHAK-8-E6 and PHAK-8-E7 cells were K8 and K8/K18 positive whereas nearly all cells were positive for K8 and K18 expression in the PHAK-8-CER cells. All HPV-transduced and the empty pLXSN vector-transduced cells showed well-formed K8 and K18 filament network. K19 was expressed at higher levels in PHAK-8-E6 and PHAK-8-E7 compared with the control. In contrast, PHAK-8-CER cells, however, showed very strong speckled immunoreactivity for K19 at the cell membrane (Fig. 2a).
Hyperproliferation-associated K6/16
The expression of keratins 6 and 16 in the epidermis is observed in hyperproliferative conditions such as wound healing or carcinomas but not in normal skin; however, some patchy expression of K6 and K16 is seen on normal keratinocytes when cultured in vitro (28). In PHAK-8-E7 and PHAK-8-CER, strong immunoreactivity for both K6 and K16 was observed, while PHAK-8-E6 cells showed weaker staining (Fig. 2a).
Basal cell-associated K14 and K15
Antibodies to the basal cell-associated keratin K14 indicated that about half of the PHAK, PHAK-8-E6 and PHAK-8-E7 cells stained positive. The expression of HPV8-E7 had a more pronounced impact on the distribution pattern of K14 as only aggregates were formed and no filament formation was observed. In PHAKs, K15 was detected only in few cells. In cultures expressing HPV8 proteins, nearly all cells were positive for K15 and showed punctate distribution pattern (Fig. 2b).
Differentiation-associated K1/10
When cultured in vitro in low calcium medium, normal keratinocytes display minimal reactivity with anti-K1 and -K10 monoclonal antibodies (28). Staining of the terminal differentiation-associated keratins K1 and K10 was detected in PHAK-8-E7 and PHAK-8-CER, whereas K1 expression was absent in PHAK-8-E6 where K10 was detectable. However, K1 and K10 distribution was significantly disturbed in PHAK-8-CER resulting in K10 aggregation that was located around the cell margins (Fig. 2b).
Cell cycle profile of HPV8-positive keratinocytes
In normal keratinocytes proliferation and differentiation are strictly coupled. The differences observed in the differentiation status of the HPV8-expressing cells suggested that they might have altered cell cycle profiles. To examine this, living cells were stained with Hoechst 33342 and analysed via flow cytometry for cell cycle distribution. The result of a representative experiment is shown in Fig. 3a. Scatter profiles showed two populations – one with low forward and side scatter and one with higher forward and side scatter characteristics. The percentage of cells falling in each of these populations showed that the PHAK-8-CER culture had a higher percentage (41%) of high scatter cells compared with control (28%), E2 (27%), E6 (22%) and E7 (21%) cells. When the DNA profiles were examined, there was no significant difference between PHAK, PHAK-8-E2 and PHAK-8-E6 cultures when looking at either the whole population or the populations with low or high scatter characteristics. However, in the PHAK-8-E7 culture, the higher scatter cells showed a significant increase in the percentage of cells in G2/M and also a significant increase in cells with a greater than 4n DNA content (P < 0.05). In addition, the PHAK-8-CER culture showed reduced cell proliferation as judged by the percentage of cells in the G2/M region.
Figure 3.
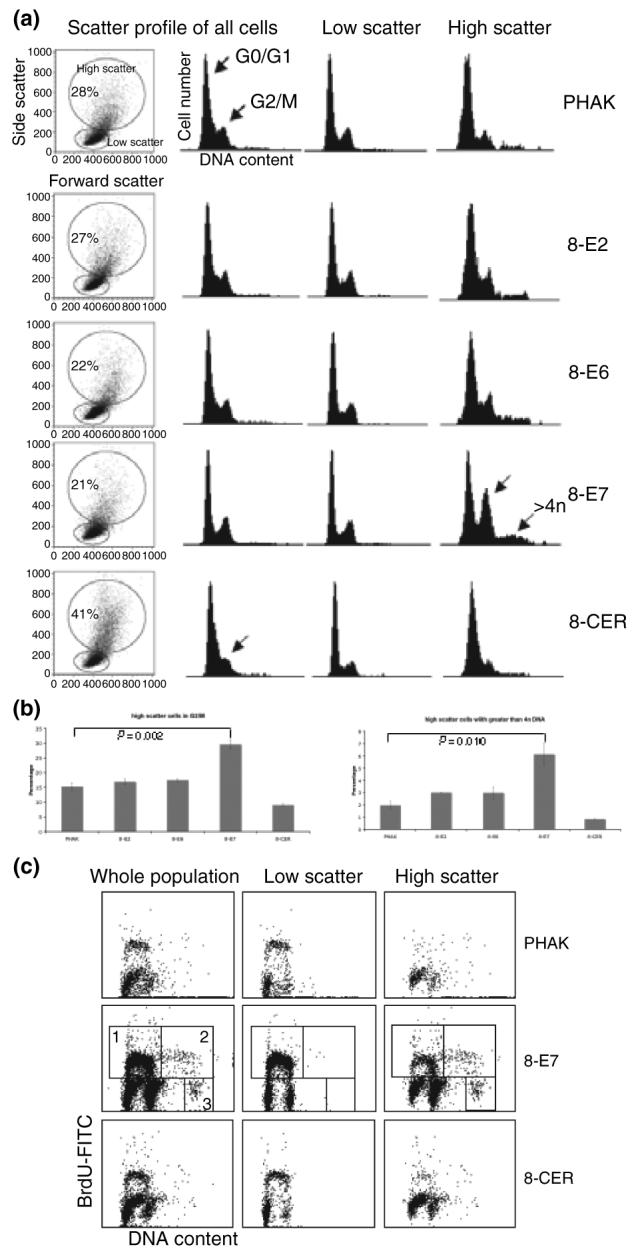
Flow cytometric analysis of PHAKs expressing HPV8 early genes. (a, b) Relative DNA content of cells was determined by staining living cells with Hoechst 33342. Results shown are representative of at least three independent experiments. (c) BrdU incorporation into PHAK expressing HPV8 early genes. Box 1: 2n to 4n cycling cells; box 2: 4n to 8n cycling cells; box 3: octaploid cell population.
To assess further the effect of HPV-E7 on cell cycle progression, BrdU incorporation was measured. Figure 3b shows that PHAK-8-E7 cells had significantly higher BrdU incorporation (29%) compared with PHAK (14%) and PHAK-8-CER (14%). In addition, the PHAK-8-E7 culture shows a significant number of octoploid/8n cells (Fig. 3c, box 3). By gating according to light scatter, these cells are found almost exclusively amongst the population with higher scatter characteristics. The fact that these cells also incorporate BrdU (Fig. 3c, box 2) shows that they actively synthesize DNA without undergoing cell division.
HPV8 proteins differentially modulate p53, pRb and p21 protein levels in keratinocytes
E6 and E7 of genital HPV types are known to associate with the cellular tumor suppressor proteins p53 and pRb and neutralize their cell cycle regulatory functions in the G1/S and G2/M checkpoints (29-31). The E7 protein of HPV8 binds to pRb with only 34% of the affinity of HPV16 E7 (32). The E6 protein of cutaneous HPVs fails to bind to p53 and promote its proteolytic degradation (33), whereas E2 is able to bind to p53 (34).
To investigate the mechanism by which E7 overcomes cell cycle checkpoints, we analysed the levels of the tumor suppressor proteins p53, pRb and the cyclin-dependent kinase inhibitor p21 protein in cell extracts of keratinocytes expressing HPV8 early genes. After normalizing to GAPDH levels, p53 was not changed in PHAK-8-E2 and PHAK-8-CER cells, but was slightly higher in E6 (1.13-fold) and E7 (1.12-fold) cells (Fig. 4). The levels of p21 were constant in PHAK-8-E2 and PHAK-8-E6 cells when compared with the control but lower in PHAK-8-E7 (1.8-fold lower) and PHAK-8-CER (fourfold lower) cells. Interestingly, pRb levels were also lower in PHAK-8-E7 (2.2-fold lower) and PHAK-8-CER (2.9-fold lower) cells compared with control cell extracts. PHAK-8-E2 and PHAK-8-E6 cell extracts show no decrease in pRb levels.
Figure 4.
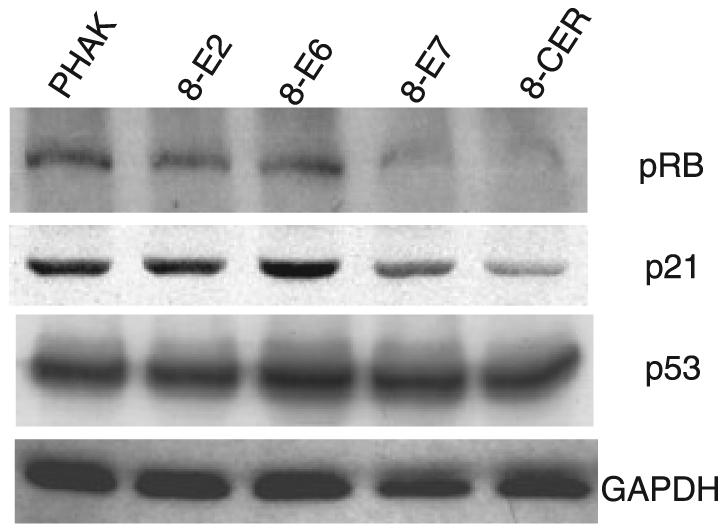
Western blot analysis for p53, pRb, p21 and GAPDH with extracts from keratinocytes expressing HPV8 early genes.
Discussion
Morphology of keratinocytes expressing HPV8 early genes
In this study we cultivated PHAK in vitro and infected them with recombinant retroviruses coding for HPV8 early proteins. Under the culture conditions employed, there were noticeable differences in the morphology of PHAK-8-CER cells compared with vector only, E6 or E7 cells. In PHAK-8-CER cultures, cells were characterized by loss of tight cell packing, irregular cell and colony shapes and larger cell size and complexity (Figs 1 and 3a).
Cultures of normal keratinocytes generate a range of different in vitro colony forms, classified as holoclones (derived from stem cells), meroclones and paraclones. These cells are capable of generating progenies that differentiate along the epidermal lineage and give raise to transient amplifying (meroclones) and terminally differentiated (paraclones) keratinocytes (35). Comparing the morphology of the PHAK-8-CER cultures to those derived from normal keratinocyte colonies, it seems that the expression of the CER of HPV8 may have enhanced the differentiation of the keratinocyte population by altering the proportion of stem and transit amplifying cells in the culture. Further work will be needed to ascertain whether the expression of HPV8 genes alters the number of holoclones in transduced keratinocytes or influences the proliferation of meroclones and paraclones.
Mechanism of control of keratin expression by HPV8 early genes
We began to explore the molecular basis for the observed cell morphology and characterized the differential expression of keratins in these cultured epidermal cells. Our results have shown the overexpression of hyperproliferative (K6, K16), simple epithelial (K8, K18, K19) and the differentiation-specific (K10) keratins by immunocytochemistry due to HPV8 gene expression. The reason how the overexpression of these keratins is regulated differently between normal and HPV8-positive cells however remains to be explored further. A potential explanation for the observed overexpression of certain keratins could be that these keratins have been post-transcriptionally modified by HPV8 early proteins, a mechanism of keratin regulation that has previously been postulated (36-39). An alternative explanation is that immediate degradation of keratins occurs in normal epithelia (40), whereas they might have been stabilized in HPV8-transformed cells.
Staining on these cells also revealed a disruption of K1, K10 and K15 filaments as they appear only as aggregates in PHAK-8-CER cells. A similar collapse of the K1 and K10 network was first described by Doorbar et al. (41), showing that the expression of HPV16 E1∧E4 protein in primary keratinocytes resulted in keratin aggregation. The failure to detect E1∧E4 message in PHAK-8-CER cells may be due to HPV gene expression being directed by the retroviral LTR rather than the homologous HPV promoter. This study shows that even without the expression of E1∧E4, HPV8 is capable of inducing K1 and K10 filament collapse. We have been unable to generate organotypic skin equivalents on de-epidermized human dermis using PHAK-8-CER cells (personal observation) as shown previously for HPV8-E7 (5), which may in part be explained by the alteration of the differentiation programme in these cells. In this context it needs to be mentioned that keratins undergo post-translational modifications, like glycosylation and phosphorylation, with phosphorylation being the most important regulatory modification in epithelial tissue (42-44). Phosphorylation can modulate the solubility of keratins regulating keratin–keratin interaction or keratin interaction with other associated proteins, thereby causing filament collapse (45). It is therefore possible that phosphorylation may be a molecular mechanism by which keratin organization is regulated in cells early during infection with HPV8, a stage where E4 is not present (19).
Control of proliferation in HPV8-expressing keratinocytes
In the course of the normal viral life cycle, to establish a persistent infection, HPV must reside in the mitotically active keratinocytes in the basal layer, including the epidermal stem cells. In addition, HPV5 has been found in cultured keratinocytes from psoriatic lesions suggesting that the virus may be involved in the development of this hyperproliferative disease (46). In normal skin, the keratinocytes in basal layer express K14 (47). Our results show that E7 expression alone is able to perturb the K14 filament network resulting in the appearance of collapsed filaments. However, when E7 was coexpressed with E6, E1 and E2, no great impact on keratin 14 network was observed suggesting that changes in gene expression at different stages of the viral life cycle could have a pronounced effect on the infected keratinocytes.
In normal epithelia, when keratinocytes begin to stratify and differentiate, they acquire the expression of differentiation-specific keratins K1 and K10. Recent studies have shown that the overexpression of K10 inhibits cell proliferation and suppresses tumor development (48) and could explain a requirement for keratin filament breaking in keratinocyte transformation.
Under certain conditions, such as tissue culture, terminally differentiating epidermal keratinocytes express K6 and K16 in preference to K1 and K10 (28,49,50). HPV8-E6 expression induced K6 and K16 expression only slightly, whereas PHAK-8-E7 and PHAK-8-CER cells are strongly positive for K6 and K16 suggesting that E7 was the major protein responsible for conferring the hyperproliferative status of the keratinocytes. This is in line with the observation that when expressed in in vitro organotypic skin cultures E7, and not E6, caused hyperproliferation of the cells (5). Transgenic mice overexpressing K16 display skin lesions characterized by hyperkeratinization, dyskeratosis, acanthosis, acantholysis and hyperkeratosis, histological features which have recently been described for the skin tumors of HPV8-transgenic mice (6). To determine whether PHAK-8-CER cells also exibited a hyperproliferative phenotype we examined their cell cyle profile. The cell cycle profiles of these cells indicated that PHAK-8-CER cells, although strongly positive for hyperproliferation-specific keratins, are less proliferative compared with control, E2, E6 and E7 cells as judged to the number of cells in the G2/M. This observation is in line with data from Kinjo et al. showing that the growth rate of combined transfected cells with HPV16 E2 and E6-E7 was reduced (51). E7, when expressed on its own, acted at the G1–S phase of the cell cycle as there were twice as many cells in the S-phase than in the other cell lines. In line with previous observations and with the K6 and K16 staining presented here, HPV8-E7 causes hyperproliferation of primary keratinocytes. In addition to a role affecting the G1/S transition, we have found evidence that HPV8-E7 also influences G2/M as we observed octaploid cells present in the E7 culture.
The major mechanisms controlling cell cycle progression are mediated by p53 and retinoblastoma proteins (52). Our results show that the p53 levels are unchanged in keratinocytes expressing HPV8 early proteins, either alone or together. However, an E7-induced reduction in pRb levels could be responsible for the manipulation of the G1/S transition control, a property which was also recently shown for HPV38-E7, another virus type associated with NMSC (53). It seems that the weak binding affinity of HPV8-E7 for pRb (32) may be sufficient to target pRb for degradation, a mechanism which has been published before for other HPV E7 genes. Whether the lower levels of pRb observed in HPV8-E7-expressing cells are due to lower rates of synthesis or increased proteolysis remains to be determined. Surprisingly, only cells expressing E7 alone are more proliferative (loss of G1/S control) and able to overcome the mitotic checkpoint and form octaploid cells (loss of G2/M control), which could be a pRb-dependent mechanism. Similar to pRb, p21 has been implicated in G1, G2 and S-phase arrest. It is a transcriptional target of p53 and plays a crucial role in mediating growth arrest. Recently, Decesse et al. (54) showed that p21 can also be transcriptionally activated by pRb only in epithelial cells in a p53-independent manner. These findings suggest that inactivation of pRb by HPV8-E7 can result in p21 transcriptional repression, thus enhancing uncontrolled cell proliferation.
Biological significance underlying simple epithelial keratin regulation
In contrast to keratin 14, the basal-specific K15 has been poorly characterized. It has been shown that K15 appears to be preferentially expressed in stable basal cells that turn over slowly (55). The expression of K15 is downregulated in hyperproliferative situations when K6 and K16 are overexpressed, perhaps to maintain the activated cell phenotype (23). A characteristic expression pattern of K15 has also been demonstrated in the interfollicular epidermis where it is expressed within patches of basal keratinocytes in the rete ridges (23,56). The reactivity of hair follicle bulge keratinocytes for K15 had led to the hypothesis for K15 to be a hair follicle stem cell marker (57,58). The expression of simple epithelial keratins 8 and 18 is a common finding in transformed cells and cultured SCCs (11,13,59). These keratins are considered as markers of malignancy in human skin tumors. When overexpressed in transgenic mice, keratin 8 alters epidermal cell differentiation, favours the neoplastic transformation of cells, and is ultimately responsible for the invasive behaviour of transformed epidermal cells leading to conversion of benign to malignant tumors (60). These malignancy-associated simple keratins are overexpressed in the more flattened and morphological larger cells in the PHAK-8-E6, PHAK-8-E7 and PHAK-8-CER cells compared with the control cells leading to the hypothesis that K8 and K18 are present in more differentiated cells. Keratin 19 is detected in tumor cells of primary skin SCC with lymph node metastasis, and K19 expression is considered to be a possible marker for the metastatic potential of SCC (61). The very strong immunoreactivity of K19 in PHAK-8-CER may suggest that these cells have a more transformed phenotype. Reports of increased simple epithelial keratin expression in invasive squamous cell carcinomas of skin and other keratinocyte-derived tumors (62) support the supposition that PHAK-8-CER cells are more transformed as evidenced by increased expression of K8, K18 and K19.
In summary, this study shows that HPV8-infected cells lose their normal keratin expression profile as a consequence of viral gene expression. This may have important implications for HPV8-induced keratinocyte transformation.
Acknowledgements
We thank Dr Ahmed Waseem and Dr Bishr Omary for critical reading of the manuscript and useful discussions. This study was supported by grants from the Dr Mildred Scheel Stiftung für Krebsforschung/Deutsche Krebshilfe, the Research Advisory Board of the St Bartholomew's and The Royal London Charitable Foundation and Cancer Research UK.
Abbreviations
- HPV
human papillomavirus
- K
cytokeratin
- NMSC
non-melanoma skin cancer
- PHAK
primary human adult keratinocytes
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Pfister H. Chapter 8: Human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003;31:52–56. doi: 10.1093/oxfordjournals.jncimonographs.a003483. [DOI] [PubMed] [Google Scholar]
- 3.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giampieri S, Garcia-Escudero R, Green J, Storey A. Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation. Oncogene. 2004;23:5864–5870. doi: 10.1038/sj.onc.1207711. [DOI] [PubMed] [Google Scholar]
- 5.Akgul B, Garcia-Escudero R, Ghali L, et al. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 2005;65:2216–2223. doi: 10.1158/0008-5472.CAN-04-1952. [DOI] [PubMed] [Google Scholar]
- 6.Schaper ID, Marcuzzi GP, Weissenborn SJ, et al. Development of skin tumors in mice transgenic for early genes of human papillomavirus type 8. Cancer Res. 2005;65:1394–1400. doi: 10.1158/0008-5472.CAN-04-3263. [DOI] [PubMed] [Google Scholar]
- 7.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 8.Machesney M, Tidman N, Waseem A, Kirby L, Leigh I. Activated keratinocytes in the epidermis of hypertrophic scars. Am J Pathol. 1998;152:1133–1141. [PMC free article] [PubMed] [Google Scholar]
- 9.Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos M, Paramio JM, Bravo A, Ramirez A, Jorcano JL. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J Biol Chem. 2002;277:19122–19130. doi: 10.1074/jbc.M201001200. [DOI] [PubMed] [Google Scholar]
- 11.Markey AC, Lane EB, Churchill LJ, MacDonald DM, Leigh IM. Expression of simple epithelial keratins 8 and 18 in epidermal neoplasia. J Invest Dermatol. 1991;97:763–770. doi: 10.1111/1523-1747.ep12486607. [DOI] [PubMed] [Google Scholar]
- 12.Hashido K, Morita T, Matsushiro A, Nozaki M. Gene expression of cytokeratin endo A and endo B during embryogenesis and in adult tissues of mouse. Exp Cell Res. 1991;192:203–212. doi: 10.1016/0014-4827(91)90177-v. [DOI] [PubMed] [Google Scholar]
- 13.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 14.Caulin C, Bauluz C, Gandarillas A, Cano A, Quintanilla M. Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp Cell Res. 1993;204:11–21. doi: 10.1006/excr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 15.Larcher F, Bauluz C, Diaz-Guerra M, et al. Aberrant expression of the simple epithelial type II keratin 8 by mouse skin carcinomas but not papillomas. Mol Carcinog. 1992;6:112–121. doi: 10.1002/mc.2940060206. [DOI] [PubMed] [Google Scholar]
- 16.Proby CM, Churchill L, Purkis PE, Glover MT, Sexton CJ, Leigh IM. Keratin 17 expression as a marker for epithelial transformation in viral warts. Am J Pathol. 1993;143:1667–1678. [PMC free article] [PubMed] [Google Scholar]
- 17.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:989–990. [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs PG, Iftner T, Weninger J, Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986;58:626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haller K, Stubenrauch F, Pfister H. Differentiation-dependent transcription of the epidermodysplasia verruciformis-associated human papillomavirus type 5 in benign lesions. Virology. 1995;214:245–255. doi: 10.1006/viro.1995.0028. [DOI] [PubMed] [Google Scholar]
- 20.Lane EB. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982;92:665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane EB, Bartek J, Purkis PE, Leigh IM. Keratin antigens in differentiating skin. Ann N Y Acad Sci. 1985;455:241–258. doi: 10.1111/j.1749-6632.1985.tb50415.x. [DOI] [PubMed] [Google Scholar]
- 22.Purkis PE, Steel JB, Mackenzie IC, Nathrath WB, Leigh IM, Lane EB. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990;97(Pt 1):39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Waseem A, Dogan B, Tidman N, et al. Keratin 15 expression in stratified epithelia: downregulation in activated keratinocytes. J Invest Dermatol. 1999;112:362–369. doi: 10.1046/j.1523-1747.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- 24.Waseem A, Lane EB, Harrison D, Waseem N. A keratin antibody recognizing a heterotypic complex: epitope mapping to complementary locations on both components of the complex. Exp Cell Res. 1996;223:203–214. doi: 10.1006/excr.1996.0074. [DOI] [PubMed] [Google Scholar]
- 25.Stasiak PC, Purkis PE, Leigh IM, Lane EB. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol. 1989;92:707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- 26.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane EB, Alexander CM. Use of keratin antibodies in tumor diagnosis. Semin Cancer Biol. 1990;1:165–179. [PubMed] [Google Scholar]
- 28.Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Leonardo A, Khan SH, Linke SP, Greco V, Seidita G, Wahl GM. DNA rereplication in the presence of mitotic spindle inhibitors in human and mouse fibroblasts lacking either p53 or pRb function. Cancer Res. 1997;57:1013–1019. [PubMed] [Google Scholar]
- 30.Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- 31.Münger K, Basile JR, Duensing S, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt A, Harry JB, Rapp B, Wettstein FO, Iftner T. Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J Virol. 1994;68:7051–7059. doi: 10.1128/jvi.68.11.7051-7059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steger G, Pfister H. In vitro expressed HPV 8 E6 protein does not bind p53. Arch Virol. 1992;125:355–360. doi: 10.1007/BF01309654. [DOI] [PubMed] [Google Scholar]
- 34.Akgul B, Karle P, Adam M, Fuchs PG, Pfister HJ. Dual role of tumor suppressor p53 in regulation of DNA replication and oncogene E6-promoter activity of epidermodysplasia verruciformis-associated human papillomavirus type 8. Virology. 2003;308:279–290. doi: 10.1016/s0042-6822(02)00133-2. [DOI] [PubMed] [Google Scholar]
- 35.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 36.Bloor BK, Su L, Shirlaw PJ, Morgan PR. Gene expression of differentiation-specific keratins (4/13 and 1/10) in normal human buccal mucosa. Lab Invest. 1998;78:787–795. [PubMed] [Google Scholar]
- 37.Su L, Morgan PR, Lane EB. Protein and mRNA expression of simple epithelial keratins in normal, dysplastic, and malignant oral epithelia. Am J Pathol. 1994;145:1349–1357. [PMC free article] [PubMed] [Google Scholar]
- 38.Su L, Morgan PR, Lane EB. Expression of cytokeratin messenger RNA versus protein in the normal mammary gland and in breast cancer. Hum Pathol. 1996;27:800–806. doi: 10.1016/s0046-8177(96)90452-9. [DOI] [PubMed] [Google Scholar]
- 39.Tyner AL, Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol. 1986;103:1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X, Lane EB. Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell. 1990;62:681–696. doi: 10.1016/0092-8674(90)90114-t. [DOI] [PubMed] [Google Scholar]
- 41.Doorbar J, Ely S, Sterling J, McLean C, Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991;352:824–827. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- 42.Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–140. [PubMed] [Google Scholar]
- 43.Steinert PM. The dynamic phosphorylation of the human intermediate filament keratin 1 chain. J Biol Chem. 1988;263:13333–13339. [PubMed] [Google Scholar]
- 44.Zhou X, Liao J, Hu L, Feng L, Omary MB. Characterization of the major physiologic phosphorylation site of human keratin 19 and its role in filament organization. J Biol Chem. 1999;274:12861–12866. doi: 10.1074/jbc.274.18.12861. [DOI] [PubMed] [Google Scholar]
- 45.Ku NO, Liao J, Chou CF, Omary MB. Implications of intermediate filament protein phosphorylation. Cancer Metastasis Rev. 1996;15:429–444. doi: 10.1007/BF00054011. [DOI] [PubMed] [Google Scholar]
- 46.Simeone P, Teson M, Latini A, Carducci M, Venuti A. Human papillomavirus type 5 in primary keratinocytes from psoriatic skin. Exp Dermatol. 2005;14:824–829. doi: 10.1111/j.1600-0625.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs E. Epidermal differentiation. Curr Opin Cell Biol. 1990;2:1028–1035. doi: 10.1016/0955-0674(90)90152-5. [DOI] [PubMed] [Google Scholar]
- 48.Paramio JM, Casanova ML, Segrelles C, Mittnacht S, Lane EB, Jorcano JL. Modulation of cell proliferation by cytokeratins K10 and K16. Mol Cell Biol. 1999;19:3086–3094. doi: 10.1128/mcb.19.4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kopan R, Fuchs E. The use of retinoic acid to probe the relation between hyperproliferation-associated keratins and cell proliferation in normal and malignant epidermal cells. J Cell Biol. 1989;109:295–307. doi: 10.1083/jcb.109.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoler A, Kopan R, Duvic M, Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988;107:427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinjo T, Kamiyama K, Chinen K, Iwamasa T, Kurihara K, Hamada T. Squamous metaplasia induced by transfection of human papillomavirus DNA into cultured adenocarcinoma cells. Mol Pathol. 2003;56:97–108. doi: 10.1136/mp.56.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picksley SM, Lane DP. p53 and Rb: their cellular roles. Curr Opin Cell Biol. 1994;6:853–858. doi: 10.1016/0955-0674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 53.Caldeira S, Zehbe I, Accardi R, et al. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol. 2003;77:2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decesse JT, Medjkane S, Datto MB, Cremisi CE. RB regulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2001;20:962–971. doi: 10.1038/sj.onc.1204169. [DOI] [PubMed] [Google Scholar]
- 55.Porter RM, Lunny DP, Ogden PH, et al. K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab Invest. 2000;80:1701–1710. doi: 10.1038/labinvest.3780180. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 57.Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in whole-mounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 58.Lyle S, Christofidou-Solomidou M, Liu Y, Elder DE, Albelda S, Cotsarelis G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- 59.Wu YJ, Parker LM, Binder NE, et al. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 60.Casanova ML, Bravo A, Martinez-Palacio J, et al. Epidermal abnormalities and increased malignancy of skin tumors in human epidermal keratin 8-expressing transgenic mice. FASEB J. 2004;18:1556–1558. doi: 10.1096/fj.04-1683fje. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe S, Ichikawa E, Takahashi H, Otsuka F. Changes of cytokeratin and involucrin expression in squamous cell carcinomas of the skin during progression to malignancy. Br J Dermatol. 1995;132:730–739. doi: 10.1111/j.1365-2133.1995.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 62.Proby CM, Purdie KJ, Sexton CJ, et al. Spontaneous keratinocyte cell lines representing early and advanced stages of malignant transformation of the epidermis. Exp Dermatol. 2000;9:104–117. doi: 10.1034/j.1600-0625.2000.009002104.x. [DOI] [PubMed] [Google Scholar]


