Abstract
Background
Patients are at risk of second malignancies (SM) after treatment for Ewing sarcoma family of tumours (ESFT).
Methods
We performed a retrospective review of 237 patients with ESFT treated at our institution from September 1979 through February 2004. Cumulative incidence (CI) of SM by type of malignancy and treatment was estimated.
Results
Twelve patients with SM were identified. Secondary leukaemia (SL) developed in 8 patients (2 ALL, 6 MDS/AML) a median 2.6 years (range, 1.4-19.6 years) after diagnosis of ESFT. Four patients had secondary solid tumours a median 8.0 years (range, 7.4-9.4 years) after the ESFT diagnosis. Five- and 10-year estimates of the CI of SM were 3.0%±1.1% and 4.7%±1.5%, respectively. Patients treated on recent protocols with higher cumulative doses or an increased dose intensity of alkylators and epipodophyllotoxins and the use of G-CSF had a higher estimated CI of SL than those in earlier studies (5-year CI 6.4%±2.4% vs 0.0%±0.0%, respectively, P=0.004).
Conclusions
Patients with ESFT are at risk for SM after treatment. The cumulative incidence of SM is higher with current treatment protocols and may be related to intensification of chemotherapeutic agents.
Keywords: Ewing sarcoma family of tumours, second malignancies, dose intensification, therapy-related leukaemia
INTRODUCTION
The Ewing sarcoma family of tumours (ESFT) is a group of small, round-cell neoplasms of neuroectodermal origin arising in bone or soft tissue. Approximately 200 to 250 new cases are diagnosed in the United States each year. Current treatment with intensification of chemotherapy using alkylating agents (cyclophosphamide and ifosfamide) and topoisomerase II inhibitors (anthracyclines and epipodophyllotoxins), as well as refinements in surgical and radiation techniques, has improved the outcome for these patients. Currently, 60-70% of patients with localized ESFT survive more than 5 years.1-4 As treatments evolve, survival rates and duration of follow-up increase; thus, it is necessary to reassess the late complications of these therapies and identify those at greatest risk.
One of the most devastating complications of primary cancer therapy is the development of a second malignancy. The reported incidence of second malignancies in patients treated for ESFT varies widely, from less than 1% to more than 20%, and depends on the specific type of therapy delivered and the duration of follow-up.5-8 Despite this variability in incidence, the risk of a second malignancy in patients surviving ESFT is well established to be greater than that in the general population.9-11 However, it is less clear whether patients with ESFT have a predisposition to second malignancies.5, 7, 12, 13
The most commonly described second malignancies in patients with ESFT are radiation-induced osteosarcoma, acute myeloid leukaemia (AML), and myelodysplastic syndrome (MDS). Other second malignancies, including acute lymphoblastic leukaemia (ALL) and an array of carcinomas and soft-tissue sarcomas, have also been described.5, 10, 14, 15 The objectives of this retrospective study were to determine the histologic type of second malignancies and to estimate the incidence and investigate risk factors for the development of a second malignancy after primary treatment for ESFT at our institution.
PATIENTS AND METHODS
Patients with newly diagnosed ESFT treated at St. Jude Children’s Research Hospital, Memphis, Tennessee, between February 1979 and February 2004 were eligible for this retrospective study, which was approved by the institutional review board. Clinical characteristics (age, sex, race, sites of disease at presentation, and stage), treatment, and outcome were reviewed. Patients who had a second malignancy diagnosed after primary therapy for ESFT were identified. Second malignancy was defined as a cancer of a histologic type distinct from ESFT. Leukaemias were classified morphologically based on the French-American-British criteria. Details regarding the second malignancy, including clinical presentation, type of cancer, treatment, and outcome, were examined.
During the study period, therapy received depended on the protocol available at the time of initial diagnosis. Between 1979 and 1986, patients were treated on the ES79 protocol, which used vincristine, dactinomycin, fractionated cyclophosphamide, and doxorubicin.16 Between 1987 and 1991, patients were treated on the ES87 protocol, which was designed to explore the antitumour activity of ifosfamide and etoposide in a pretreatment window, and this combination was continued after local control, alternating with vincristine, fractionated cyclophosphamide, and doxorubicin.17 Between 1992 and 1996, patients were treated on the EWI92 protocol, which evaluated the feasibility of an aggressive early induction with cyclophosphamide, doxorubicin, ifosfamide, and etoposide, followed by prolonged maintenance therapy with intensification of alkylating agents and etoposide. Patients treated on EWI92 received granulocyte colony-stimulating factor (G-CSF) and were randomly assigned to receive either moderate (2 g/m2) or high (3 g/m2) doses of cyclophosphamide during continuation therapy.2 After completion of the EWI92 protocol, patients with localized ESFT were treated in the POG9354/CCG7942 Children’s Oncology Group (COG) study until its closure in September 1998. Patients in this study were randomized to receive cycles of vincristine, doxorubicin, and cyclophosphamide alternating with ifosfamide and etoposide over 48 weeks (Regimen A, standard arm) or 30 weeks (Regimen B, experimental arm). All patients received G-CSF.18 After September 1998, patients continued to be treated following guidelines for the standard arm of this cooperative study (St. Jude best clinical management [SJBCM]). For the first 12 weeks of therapy, patients who met the eligibility criteria may have been enrolled in a multi-institutional study evaluating the toxicity and efficacy of pegfilgrastim (Neulasta, Amgen, Thousand Oaks, CA) in pediatric patients with sarcomas. These patients received 4.8 g/m2 of cyclophosphamide and 12 mg/m2 of vincristine in the first 12 weeks of therapy, compared with 2.4 g/m2 and 3 mg/m2, respectively, on SJBCM. Between 1996 and 2000, patients with high-risk disease were also treated on the HIRISA 1 and 2 protocols, which explored the role of short-term dose-intensification regimens using cyclophosphamide, ifosfamide, vincristine, etoposide, and doxorubicin and consolidation with myeloablative chemotherapy and autologous haematopoietic stem cell rescue.19 Local control measures for all protocols included surgery and/or radiation as specified by each protocol. Table 1 summarizes the cumulative doses of cyclophosphamide, doxorubicin, etoposide, and ifosfamide as well as the radiation doses used per protocol.
Table 1.
Cumulative Doses and Dose Intensity of Chemotherapy and Radiation per Protocol
| Protocol | Doxorubicin | Cyclophosphamide | Etoposide | Ifosfamide | Radiation | G-CSF | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/m2 | mg/m2/wk | g/m2 | g/m2/wk | mg/m2 | mg/m2/wk | g/m2 | g/m2/wk | Gy | ||
| ES79 | 385 | 9.6 | 11.6 | 0.3 | – | – | – | – | 30–55 | − |
| ES87 | 315 | 5 | 9.5 | 0.15 | 3000 | 48.3 | 48 | 0.8 | 35–60 | − |
| EWI-92 | 375 | 8.1 | 12.5 | 0.24 | 4350 | 98.8 | 58 | 1.3 | 36–68.4 | + |
| EWI-92-HD | 375 | 8.1 | 16.5 | 0.36 | 4350 | 98.8 | 58 | 1.3 | 36–68.4 | + |
| HIRISA 1/2 | 330–270 | 13.8–14.2 | 21–18 | 0.88–0.95 | 2700 | 112–142 | 46–36 | 2.3–1.9 | 36–68 | + |
| POG 9354, Reg. A | 375 | 7.8 | 10.8 | 0.23 | 4000 | 83.3 | 72 | 1.5 | 45–55.8 | + |
| POG 9354, Reg. B | 375 | 12.5 | 12.0 | 0.4 | 3000 | 100 | 72 | 2.4 | 45–55.8 | + |
| SJBCM | 375 | 7.8 | 10.8–13.2 | 0.23–0.27 | 4000 | 83.3 | 72 | 1.5 | 45–55.8 | + |
Statistical Methods
Survival was defined as the interval from the date of ESFT diagnosis to the date of death from any cause or to the date of last contact. Event-free survival (EFS) was defined as the interval from the date of ESFT diagnosis to the date of the first event (relapsed or progressive disease, second malignancy, or death from any cause) or to the date of last contact for patients without events. Survival and EFS were estimated using the method of Kaplan and Meier, and associated standard errors were calculated using the method of Peto and Pike.20 The cumulative incidence of second malignancy was estimated using the methods of Kalbfleisch and Prentice.21 Competing events included relapsed or progressive disease or death prior to second malignancy. All estimates are presented as percentages ± 1 standard error. Statistical tests developed by Gray22 were used to examine differences in the cumulative incidence of second malignancy by protocol treatment and patient characteristics.
Expected numbers of cancers and leukaemias were calculated using public use data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. The number of person-years of observation was compiled for age- and gender-defined subgroups and calculated as the time from diagnosis of ESFT to diagnosis of second cancer, death or the date of last contact, whichever was first. The standardized incidence ratio (SIR) was calculated as the ratio of the observed number of cases to the expected number of cases. SIR’s are presented with 95% confidence intervals.23 Absolute excess risk was calculated by subtracting the expected number of cases from the observed number and dividing this difference by the person-years at risk. This result was multiplied by 10,000 and expressed as number of excess cases per 10,000 person-years.
RESULTS
Two hundred thirty-seven patients with newly diagnosed ESFT were treated at our institution from February 1979 through February 2004. Patient characteristics and the distribution of patients treated on each protocol are shown in Table 2. One hundred ninety-nine patients received therapy on a specific protocol, and the remaining patients received SJBCM treatment. Most patients received radiation therapy with or without surgery for local control. On earlier protocols, ES79 and ES87, radiation alone as a method of local control was more common (n=80; 65%) than in later studies (n=48; 43%). This difference was statistically significant (P=.002).
Table 2.
Patient Characteristics and Distribution by Protocol (n=237)
| Characteristic | N (%ˆ) |
|---|---|
| No. of Patients | 237 |
| Age at Diagnosis (years) | |
| Median | 13.7 |
| Range | 1.1-25.2 |
| Sex | |
| Male | 144 (60.8) |
| Female | 93 (39.2) |
| Race | |
| Caucasian | 218 (92.0) |
| African-American | 11 (4.6) |
| Hispanic | 6 (2.5) |
| Other races | 2 (0.8) |
| Stage at Diagnosis | |
| Localized | 172 (72.6) |
| Metastatic | 65 (27.4) |
| Local Control Measures | |
| Surgery alone | 49 (20.7) |
| Radiation alone | 128 (54.0) |
| Surgery and radiation | 58 (24.5) |
| No local control | 2 (0.8) |
| Treatment Protocol | |
| ES79 | 80 (33.8) |
| ES87 | 46 (19.4) |
| EWI92 | 49 (20.7) |
| HIRISA1/2 | 11 (4.6) |
| POG9354 | 13 (5.5) |
| SJBCM* | 38 (16.0) |
Percentages may not sum to 100% due to rounding
15 patients in this group received first 12 weeks of therapy on pegfilgrastim study.
Among the 237 patients, 133 (56%) were alive at the end of the study with a median follow-up of 12.2 years from diagnosis (range, 0.3-27.8 years). Follow-up for the SJBCM patients was the shortest, at a median 5 years (range, 0.3-11.5 years). Eighty-seven of the 133 survivors (65%) had been seen or contacted within 2 years. Five- and 10-year estimates of survival were 65.5±3.2% and 58.2±3.9%, respectively. Five- and 10-year estimates of EFS were 56.2±3.4% and 51.3±4.0%, respectively.
Only 12 patients had a second malignancy. Patient characteristics, type of second malignancy, and outcome for these patients are listed in Table 3. Six of the 12 were female; 9 were Caucasian, and 3 were Hispanic. The median age at the initial diagnosis of ESFT was 13.8 years (range, 2.6-22.0 years). One patient had metastatic disease in the regional lymph nodes and lungs at diagnosis. None of the patients had a known family history of predisposition to cancer or known cancer-associated syndrome. All patients had received chemotherapy, and 9 had received radiotherapy (35-68.4 Gy) as part of their primary therapy for ESFT. The second malignancy was the first event in 11 patients and second event (after relapse) in 1 patient. The median time to second malignancy was 3.3 years (range, 1.4-19.6 years). No patient had a third malignancy.
Table 3.
Characteristics, Tumor Type, and Outcome for Patients with Second Malignancies after Treatment for ESFT
| Ewing Sarcoma Family of Tumors | Second Malignancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. No. | Sex | Race | Age at Dx (yrs) | Sites of Disease | Treatment | Age at Diagnosis (yrs) | Time from Dx of ESFT (yrs) | Type | Cytogenetics | Outcome, yrs from second malignancy | |
| Chemotherapy | Radiation Dose (Site) | ||||||||||
| 1 | M | W | 14.5 | Rib | ES79 | 35 Gy (spine C7-T5) | 34.1 | 19.6 | ALL | NA | D, 1.3 |
| 2 | F | W | 12.6 | llium | ES79 | 54 Gy (pelvis) | 22.1 | 9.4 | Squamous cell carcinoma of cervix | – | A, 5.9 |
| 3 | F | H | 14.0 | Leg, LN, Lung | EWI-92-HD Paclitaxel after relapse | 66.4 Gy (inguinal and iliac LN); 16.5 Gy (WLI) | 21.4 | 7.4 | Extraosseous pelvic osteosarcoma | – | D, 4.8 |
| 4 | F | W | 7.3 | Neck | EWI-92-HD | 36 Gy (neck) | 11.3 | 4.0 | MDS (RA) | 46,XX,der(6)t(1;6)(q12;p22.2) | D, 1.3 |
| 5 | M | W | 13.7 | Radius | EWI-92 | 68.4 Gy (radius) | 22.0 | 8.3 | Osteosarcoma of radius | – | A, 4.9 |
| 6 | M | W | 14.2 | Hand | EWI-92 | 39.6 Gy (hand) | 16.9 | 2.6 | MDS (RAEB) | 46,XY,del(7)(q22) | D, 0.5 |
| 7 | F | H | 7.9 | Sacrum | POG 9354, Reg. B | 55.8 Gy (sacrum) | 15.6 | 7.7 | Thyroid papillary carcinoma | – | A, 3.5 |
| 8 | F | W | 13.8 | Foot | POG 9354, Reg. A | None | 15.6 | 1.8 | MDS (RAEB) then AML | 46,XX,del(7)(q22q34) | D, 0.4 |
| 9 | M | H | 16.2 | L3 Vertebra | POG 9354, Reg. B | 55.8 Gy (lumbar spine) | 17.6 | 1.4 | ALL | 46,XY,inv(11)(q21q23.3)der(17)t[inv(11);17](q13;q25) | D, 0.6 |
| 10 | M | W | 2.6 | Pelvis | SJBCM | None | 4.4 | 1.8 | AML (M4) | 46,XY,inv(16)(p13.1q22) | D, 0.9 |
| 11 | F | W | 22.0 | Chest wall | SJBCM | 55.6 Gy (chest wall) | 24.6 | 2.7 | MDS (RAEB) then AML | 45,XX,-7 | D, 0.2 |
| 12 | M | W | 13.7 | Hand | SJBCM | None | 16.2 | 2.5 | AML | 47, XY, inv(16)(p13.1q22), +22 | A, 1.4 |
Abbreviations: Dx, diagnosis; M, male; F, female; W, White; H, Hispanic; SJBCM, St. Jude Best Clinical Management; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; A, alive; D, dead; WLI, whole lung irradiation; NA, not available; LN, lymph node; RAEB, refractory anemia with excessive blasts; RA, refractory anemia.
Secondary Leukemia/Myelodysplastic Syndrome
In 8 patients, secondary leukaemia or MDS developed. Two patients presented with ALL, 2 with AML (both M4 subtype), and 4 with MDS. Subtypes of MDS included refractory anaemia with excessive blasts in 3 patients and refractory anaemia in 1 patient. AML subsequently developed in 2 patients with MDS. The median time to diagnosis of secondary leukaemia/MDS was 2.6 years (range, 1.4-19.6 years). Cytogenetic studies were available in 8 of 9 cases. Monosomy 7 (n=3), inversion 16 (n=2), aberration at 11q23 (n=1), and trisomy of chromosome arm 1q with partial monosomy and duplication of 6p (n=1) were detected in leukaemia cells. Details of cytogenetic abnormalities for each patient are shown in Table 3.
Seven of 8 patients received treatment for secondary leukaemia/MDS at our institution. One patient presented elsewhere with ALL, and treatment details were not available. Among the remaining 7 patients, 1 died from infectious complications of induction chemotherapy. The other 6 patients underwent allogeneic transplantation, 3 after induction chemotherapy. After transplantation, 4 patients died of multisystem organ failure and 1 from recurrent ESFT. One patient remained alive 14.1 months after haploidentical transplantation.
Secondary Solid Malignancy
Secondary solid tumours developed in 4 patients, including 2 osteosarcoma (both in the radiation field), 1 papillary thyroid carcinoma, and 1 squamous cell carcinoma of the cervix (margin of the radiation field). The median time to diagnosis of solid tumours was 8.0 years (range, 7.4-9.4 years). Three patients remained alive with no evidence of disease, 5.9, 4.9, and 3.5 years after the diagnosis of the second malignancy. The patient with cervical cancer was treated with surgery alone; the patient with papillary thyroid cancer had total thyroidectomy followed by iodine131 ablation; and 1 patient with localized osteosarcoma of the radius had above-elbow amputation after progressive disease while receiving high-dose methotrexate, cyclophosphamide, etoposide, and cisplatin. Pathologic studies showed minimal treatment effect. After the surgery, this patient received 6 courses of cyclophosphamide and topotecan. The fourth patient died of metastatic osteosarcoma 4.8 years after the diagnosis of the second malignancy. This patient had localized extra-osseous osteosarcoma near the sacrum within the irradiated field 7.4 years after salvage treatment with paclitaxel for progressive ESFT. The secondary osteosarcoma was resected initially with positive margins. Recurrent disease developed despite multiple chemotherapy regimens, including cyclophosphamide, etoposide, doxorubicin, cisplatin, and high-dose methotrexate.
Cumulative Risk and Predictors of Second Malignancy
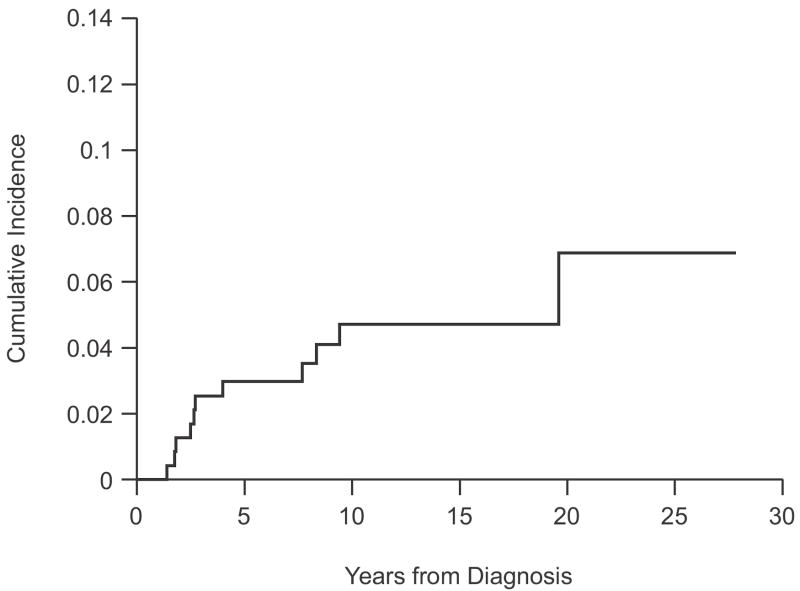
Five- and 10-year estimates of the cumulative incidence (CI) of second malignancy for all patients were 3.0%±1.1% and 4.7%±1.5%, respectively (Fig. 1). Five- and 10-year estimates of CI were 0.0%±0.0% and 1.7%±1.0%, respectively, for secondary solid malignancies, and 3.0%±1.1% and 3.0%±1.1%, respectively, for secondary leukaemias. Estimates of CI of second malignancy by treatment protocol and treatment era are shown in Table 4. Patients treated on more recent protocols (EWI92, POG9354, SJBCM, and HIRISA) had higher estimated CI than patients enrolled in earlier studies (ES79 and ES87), with a 5-year CI of 6.4%±2.4% vs. 0%±0% (P = .001), respectively. The higher estimated CI of second malignancy in the recent treatment era was solely due to secondary leukaemia.
FIGURE 1.
Cumulative Incidence of Second Malignancy for 237 ESFT Patients
Table 4.
Cumulative Incidence (Cl) of Second Malignancy
| N | Cl Estimates (SE) % 5-year | Cl Estimates (SE) % 10-year | |
|---|---|---|---|
| All Patients | 237 | 3.0 (1.1) | 4.7 (1.5) |
|
| |||
| By Protocol | |||
| ES79 | 80 | 0.0 (0.0) | 1.3 (1.3) |
| ES87 | 46 | 0.0 (0.0) | 0.0 (0.0) |
| EWI-92 | 49 | 4.1 (2.9) | 6.2 (3.5) |
| HIRISA 1/2 | 11 | 0.0 (0.0) | 0.0 (0.0) |
| POG9354/SJBCM | 51 | 10.0 (4.3) | 15.3 (6.7) |
|
| |||
| By Protocol Era | |||
| ES79/ES87 | 126 | 0 (0) | 0.8 (0.8) |
| EWI-92/HIRISA/POG9354/SJBCM | 111 | 6.4 (2.4) | 9.7 (3.2) |
Observed and expected cases of invasive cancers (and leukemias) are shown in Table 5. We observed a total of 12 second cancers in our ESFT cohort while the expected number of cases was 0.675, based on age- and gender-specific SEER rates (SIR, 17.8 (95% CI, 9.2 – 31.1). A total of 0.065 cases of leukemia were expected and we observed 8 cases in our cohort. Risks of second cancer and leukemia were significantly elevated for patients treated on more recent protocols (EWI92, POG9354, SJBCM, and HIRISA) compared to the general population (SIR for all cancers, 65.1 (95% CI, 31.2 – 119.7) There was no evidence that risks of second cancers or leukemias were significantly elevated for patients treated on earlier protocols (95% CI of SIR for all cancers, 0.4 – 13.8). The absolute excess risk of any invasive cancer was 53.6 per 10,000 person-years, or 5.4% excess risk per person per decade.
Table 5.
Observed and Expected Numbers of Cancer and Leukemia
| Type of Cancer | Observed # of cases | Expected # of cases | SIR (95% Cl) | Absolute excess risk per 10,000 person-years |
|---|---|---|---|---|
| All Cancers (invasive) | 12 | 0.675 | 17.8 (9.2–31.1) | 53.6 |
| All Cancers (invasive) by Era | ||||
| ES79/ES87 | 2 | 0.521 | 3.8 (0.4–13.8) | 10.5 |
| EWI-92/HIRISA/POG9354/SJBCM | 10 | 0.154 | 65.1 (31.2–119.7) | 138.9 |
| All Leukemia | 8 | 0.065 | 123.1 (53.0–242.5) | 37.2 |
| All Leukemia by Era | ||||
| ES79/ES87 | 1 | 0.042 | 24.0 (0.3–133.3) | 6.8 |
| EWI-92/HIRISA/POG9354/SJBCM | 7 | 0.023 | 302.8 (121.3–623.9) | 96.6 |
SRI, standardized incidence ratio; Cl, cumulative incidence
We investigated stage, tumor size, primary site, age (< vs. ≥ 14 years of age and < vs. ≥ 10 years of age), race, and sex as predictors of second malignancy (data not shown). The only factor that was significant at the .05 level was stage (P = .036). Estimates of CI of second malignancy were higher for patients with localized disease at diagnosis (5-year estimate, 4.1%±1.5%) than for patients with metastatic disease (5-year estimate, 0%±0%). However, this may be because patients with metastatic disease have an inferior outcome and thus are less likely to be at risk for second malignancy than patients with localized disease are.
DISCUSSION
Our data support the view that patients with ESFT are at risk for a second malignancy. Although the estimated CI for our entire cohort of patients was similar to those seen in most studies (Table 6), the estimated CI of secondary leukaemias exceeded what is frequently reported in the literature. Most of the secondary leukaemias in our study occurred in the recent treatment era, when patients received more intensive chemotherapy that incorporated the routine use of G-CSF and higher doses of alkylating agents and topoisomerase II inhibitors.
Table 6.
Type and Incidence of Second Cancers Reported in Literature
| Cooperative Group Study or Institution/Registry | Study Period | No. Patients | No. Second Leukemia | No. Second Solid Tumor | CI Second Cancer | Reference |
|---|---|---|---|---|---|---|
| NCI SEER & Connecticut Tumor Registry | 1935–1989 | 595 | 2 | 11 | NR | Travis et at. 199411 |
|
| ||||||
| MD Anderson Cancer Center | 1944–1976 | 173 | 0 | 4 | 35% 10-yr | Strong et al. 19796 |
|
| ||||||
| Stanford University Medical Center | 1960–1986 | 64 | 1 | 1 | 8.4% 5-yr | Smith et al. 199255 |
|
| ||||||
| NCI-PB, St. Jude, & University of Florida Medical School | 1963–1990 | 266 | 2 | 14 | 5% 10-yr
9.2% 20-yr |
Kuttesch et al. 19965 |
|
| ||||||
| Hospital La Fe, Spain | 1970–1995 | 121 | 2 | 2 | NR | Aparicio et al. 199814 |
|
| ||||||
| DFCI & Children’s Hospital in Boston | 1971–1988 | 82 | 1 | 6 | 6.7% 10-yr
42.8% 20 yr |
McLean et al 19998 |
|
| ||||||
| Istituto Ortopedico Rizzoli, Bologne, Italy | 1972–1999 | 597 | 3 | 11 | 3.0% 5-yr
6.5% 10-yr 12.7% 20-yr |
Bacci et al. 20057 |
|
| ||||||
| Istituto Nazionale Tumori, Milan, Italy | 1974–1986 | 121 | 0 | 7 | NR | Gaspirini et al. 199415 |
|
| ||||||
| CESS | 1981–1986 | 674 | 5 | 3 | 0.7% 5-yr
2.9% 10-yr 4.7% 15-yr |
Dunst et al. 199856 |
|
| ||||||
| CCG/POG INT-0091 | 1988–1992 | Bhatia et al. 20079 | ||||
| Regimen A | 262 | 3 | 2 | 0.4% 5-yr | ||
| Regimen B | 256 | 2 | 2 | 0.9% 5-yr | ||
| Regimen C | 60 | 6 | 0 | 11% 5-yr | ||
|
| ||||||
| GPOH | 1992–1999 | 690 | 4 | 2 | 0.9% 5-yr | Paulussen et al. 200110 |
|
| ||||||
| St. Jude Children’s Research Hospital | 1979–2004 | 237 | 8 | 4 | 3.0% 5-yr
4.7% 10-yr |
Navid et al. (Current Study) |
Abbreviations: NR, not reported; NCI SEER, National Cancer Institute’s Surveillance, Epidemiology, and End Results Program; CESS, Cooperative Ewing’s Sarcoma Studies; GPOH, German/Austrian/Dutch Society of Pediatric Oncology and Hematology; CCG/POG Children’s Cancer Group/Pediatric Oncology Group; DFCI, Dana-Farber Cancer Institute; NCI-PB, National Cancer Institute-Pediatric Branch; Cl, cumulative incidence.
Exposure to alkylating agents and topoisomerase II agents is a well-established risk factor for secondary leukaemias.24, 25 Dose intensification of these agents appears to result in a further increased risk of secondary leukaemia.26, 27 Most treatment regimens that use dose-intensive chemotherapy also use G-CSF. Recently, the use of G-CSF in cancer patients has been linked to an increased risk of secondary leukaemia after etoposide treatment for ALL and after cyclophosphamide and doxorubicin treatment for breast cancer.27-29 The mechanism underlying this association is not clear. The effect of G-CSF on lymphocyte DNA in stem cells of healthy volunteer donors has been evaluated. In most of these studies, reversible abnormalities in DNA structure and chromosome number have been detected, suggesting that G-CSF does not induce malignant transformation.30-32 Others postulate that G-CSF may protect myeloid stem cell or progenitor cells that have undergone lethal mutations from exposure to genotoxic chemotherapy, thereby allowing these cells to propagate and undergo malignant transformation.33, 34 In a previous study in patients with ESFT at our institution, we found that, compared with patients treated with less intensive chemotherapy and no G-CSF, patients treated with higher cumulative doses and more dose-intensive alkylators and topoisomerase II inhibitors and G-CSF had more haematologic abnormalities (lower mean platelet counts and elevated red cell mean corpuscular volume) after completion of therapy, suggestive of stem cell damage that may predispose them to secondary leukaemia/MDS.35 These observations are compelling and point out that distinguishing the contribution of intensified therapy vs. G-CSF to the development of secondary leukaemia/MDS is difficult.
The cumulative doses and the dose intensity of the chemotherapeutic agents received by the patients in the current study were not substantially different from those used in the intergroup Children’s Cancer Group/Pediatric Oncology Group (CCG/POG) ESFT study, INT-0091 Regimen B, in which the CI for secondary leukaemia at 5 years was reported to be 0.9%.9 Unlike in our patients, G-CSF was not uniformly administered to all patients in the intergroup study. This difference in practice between these two studies leads to the question of whether G-CSF plays a role in secondary leukaemia in patients with ESFT. A randomized, prospective trial would be required to resolve this point.
In our study, most secondary leukaemia occurred within 3 years of the diagnosis of ESFT. Not surprisingly, MDS and AML were the predominant histologic subtypes. Six of 7 patients with secondary leukaemia had associated cytogenetic abnormalities—11q23 (n = 1), 7q- (n = 3), and inv (16) (n = 2)—frequently seen in patients exposed to alkylating agents and topoisomerase II inhibitors.25, 36-40 One patient who presented with MDS had a complex translocation involving the long arm of chromosome 1, resulting in a dicentric derivative of the (6)t(1;6) chromosome. This patient’s unusual cytogenetic abnormality has been reported in detail elsewhere.41
The outcome for our patients with therapy-related MDS/AML was poor. Allogeneic bone marrow transplantation is currently the only approach to therapy-related MDS/AML that produces a prolonged disease-free survival, and even that is modest. This approach is associated with significant nonrelapse mortality.42-48 In our cohort of patients, 3 of 5 with MDS or AML who underwent allogeneic transplantation died of transplant-related toxicity. It has been postulated that mortality related to toxicity may be greater in patients with secondary leukaemia undergoing transplantation because of an increased risk of nonhaematologic organ toxicity and graft-versus-host disease as a result of prior chemotherapy and radiotherapy for the primary malignancy.49 Thus, refinements in conditioning regimens and medications to control graft-versus-host disease may improve the outcome for patients undergoing allogeneic transplantation.
In contrast to the commonly observed MDS/AML, ALL developed in 2 of our patients. In 1 patient, ALL did not develop until almost 20 years after his diagnosis of ESFT. This latency period was outside of what would be expected for secondary leukaemias. We suspect that this patient’s leukaemia was unrelated to his initial treatment of ESFT and represents a de novo leukaemia. The other patient had pre-B cell lineage ALL with an 11q23 abnormality, which is commonly associated with secondary ALL.50, 51 The patient had a remission after induction therapy but died of complications related to allogeneic bone marrow transplantation. Previous reports indicate that patients with secondary ALL have a dismal prognosis and that, despite an initial response to chemotherapy, the response is seldom durable.50
Secondary solid tumours were less common than secondary leukaemias in our series. It is anticipated that the incidence of secondary solid tumours will be higher with longer follow-up, because these tumours generally have a long latency period. A significant number of secondary solid tumours, but not all, arise within the field of radiation. Radiation-induced cancers are generally bone or soft tissue sarcomas, most commonly osteosarcoma.52 An increased risk of radiation-induced sarcomas has been associated with younger age, higher dose of radiation, larger treatment volume, and concomitant use of alkylating agents.5, 6, 13 In our study, the number of patients in whom secondary solid tumours developed was too small to permit assessment of risk factors; however, both patients who developed radiation-induced osteosarcoma had received > 60 Gy of radiation, a threshold dose commonly associated with an increased risk of second malignancy.5, 13
In summary, patients with ESFT treated in the current era have an increased risk of secondary leukaemia. Historical data suggest that these patients are also at increased risk for secondary solid tumours with longer follow-up. However, despite the increased risk of second malignancy, it is important to keep in mind the greater risk of death from relapse in this disease. Nonetheless, host factors, including genetic predisposition, immunodeficiency, or environmental exposures, that may predispose a patient to second malignancy clearly must be identified to prevent this devastating late complication. In addition, identifying patients with low-risk disease who would benefit from the same outcome with less intensive therapy should remain a priority. Careful attention to molecular signatures of tumour and normal tissue may help identify patients at risk for second malignancies. This approach was recently used to identify several genes in pretreatment ALL blasts whose expression distinguished patients with therapy-related leukaemia and therapy-related brain cancer from those who did not have these second malignancy.53, 54 Evaluation of whether the expression of these genes can be predictive of a second malignancy in patients with other tumours, including ESFT, may provide insight into factors that contribute to second malignancies.
Acknowledgments
We thank Alvida M. Cain for her assistance in data management and David Galloway for his editorial assistance with this manuscript. We also thank Dr. Susana Raimondi for cytogenetic analyses.
Supported in part by the American Lebanese Syrian Associated Charities (ALSAC), United States Public Health Service Cancer Center Support Grant CA21765, and Program Project Grant CA23099.
Footnotes
CONFLICT OF INTEREST STATEMENT None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 2.Marina NM, Pappo AS, Parham DM, et al. Chemotherapy dose-intensification for pediatric patients with Ewing’s family of tumors and desmoplastic small round-cell tumors: a feasibility study at St. Jude Children’s Research Hospital. J Clin Oncol. 1999;17:180–90. doi: 10.1200/JCO.1999.17.1.180. [DOI] [PubMed] [Google Scholar]
- 3.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–14. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Mercuri M, Longhi A, et al. Neoadjuvant chemotherapy for Ewing’s tumour of bone: recent experience at the Rizzoli Orthopaedic Institute. Eur J Cancer. 2002;38:2243–51. doi: 10.1016/s0959-8049(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 5.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–25. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 6.Strong LC, Herson J, Osborne BM, Sutow WW. Risk of radiation-related subsequent malignant tumors in survivors of Ewing’s sarcoma. J Natl Cancer Inst. 1979;62:1401–6. [PubMed] [Google Scholar]
- 7.Bacci G, Longhi A, Barbieri E, et al. Second malignancy in 597 patients with ewing sarcoma of bone treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. J Pediatr Hematol Oncol. 2005;27:517–20. doi: 10.1097/01.mph.0000183270.28785.33. [DOI] [PubMed] [Google Scholar]
- 8.McLean TW, Hertel C, Young ML, et al. Late events in pediatric patients with Ewing sarcoma/primitive neuroectodermal tumor of bone: the Dana-Farber Cancer Institute/Children’s Hospital experience. J Pediatr Hematol Oncol. 1999;21:486–93. [PubMed] [Google Scholar]
- 9.Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulussen M, Ahrens S, Lehnert M, et al. Second malignancies after ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol. 2001;12:1619–30. doi: 10.1023/a:1013148730966. [DOI] [PubMed] [Google Scholar]
- 11.Travis LB, Curtis RE, Hankey BF, Fraumeni JF., Jr Second cancers in patients with Ewing’s sarcoma. Med Pediatr Oncol. 1994;22:296–7. doi: 10.1002/mpo.2950220417. [DOI] [PubMed] [Google Scholar]
- 12.Menu-Branthomme A, Rubino C, Shamsaldin A, et al. Radiation dose, chemotherapy and risk of soft tissue sarcoma after solid tumours during childhood. Int J Cancer. 2004;110:87–93. doi: 10.1002/ijc.20002. [DOI] [PubMed] [Google Scholar]
- 13.Tucker MA, D’Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–93. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 14.Aparicio J, Segura A, Montalar J, Verdeguer A, Castel V, Sanchez-Heras AB. Secondary cancers after Ewing sarcoma and Ewing sarcoma as second malignant neoplasm. Med Pediatr Oncol. 1998;30:259–60. doi: 10.1002/(sici)1096-911x(199804)30:4<259::aid-mpo9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Gasparini M, Lombardi F, Ballerini E, et al. Long-term outcome of patients with monostotic Ewing’s sarcoma treated with combined modality. Med Pediatr Oncol. 1994;23:406–12. doi: 10.1002/mpo.2950230504. [DOI] [PubMed] [Google Scholar]
- 16.Hayes FA, Thompson EI, Hustu HO, Kumar M, Coburn T, Webber B. The response of Ewing’s sarcoma to sequential cyclophosphamide and adriamycin induction therapy. J Clin Oncol. 1983;1:45–51. doi: 10.1200/JCO.1983.1.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Meyer WH, Kun L, Marina N, et al. Ifosfamide plus etoposide in newly diagnosed Ewing’s sarcoma of bone. J Clin Oncol. 1992;10:1737–42. doi: 10.1200/JCO.1992.10.11.1737. [DOI] [PubMed] [Google Scholar]
- 18.Granowetter L, Womer RB, Devidas M, et al. Comparison of dose intensified and standard dose chemotherapy for the treatment of non-metastatic Ewing’s sarcoma (ES) and primitive neuroectodermal tumor (PNET) of bone and soft tisssue: A Pediatric Oncology Group-Children’s Cancer Group phase III trial. Med Pediatr Oncol. 2001;37:172. abstract. [Google Scholar]
- 19.Navid F, Santana VM, Billups CA, et al. Concomitant administration of vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide for high-risk sarcomas: the St. Jude Children’s Research Hospital experience. Cancer. 2006;106:1846–56. doi: 10.1002/cncr.21810. [DOI] [PubMed] [Google Scholar]
- 20.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: Wiley; 1980. [Google Scholar]
- 22.Gray R. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Breslow NE, Day NE. Statistical methods in cancer research: volume II - the design of analysis of cohort studies. Lyon: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 24.Felix CA. Leukemias related to treatment with DNA topoisomerase II inhibitors. Med Pediatr Oncol. 2001;36:525–35. doi: 10.1002/mpo.1125. [DOI] [PubMed] [Google Scholar]
- 25.Rowley JD, Golomb HM, Vardiman JW. Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood. 1981;58:759–67. [PubMed] [Google Scholar]
- 26.Kushner BH, Heller G, Cheung NK, et al. High risk of leukemia after short-term dose-intensive chemotherapy in young patients with solid tumors. J Clin Oncol. 1998;16:3016–20. doi: 10.1200/JCO.1998.16.9.3016. [DOI] [PubMed] [Google Scholar]
- 27.Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 28.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 29.Relling MV, Boyett JM, Blanco JG, et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood. 2003;101:3862–7. doi: 10.1182/blood-2002-08-2405. [DOI] [PubMed] [Google Scholar]
- 30.Kaplinsky C, Trakhtenbrot L, Hardan I, et al. Tetraploid myeloid cells in donors of peripheral blood stem cells treated with rhG-CSF. Bone Marrow Transplant. 2003;32:31–4. doi: 10.1038/sj.bmt.1703902. [DOI] [PubMed] [Google Scholar]
- 31.Nagler A, Korenstein-Ilan A, Amiel A, Avivi L. Granulocyte colony-stimulating factor generates epigenetic and genetic alterations in lymphocytes of normal volunteer donors of stem cells. Exp Hematol. 2004;32:122–30. doi: 10.1016/j.exphem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Shapira MY, Kaspler P, Samuel S, Shoshan S, Or R. Granulocyte colony stimulating factor does not induce long-term DNA instability in healthy peripheral blood stem cell donors. Am J Hematol. 2003;73:33–6. doi: 10.1002/ajh.10324. [DOI] [PubMed] [Google Scholar]
- 33.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–45. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 34.Kaplinsky C, Lotem J, Sachs L. Protection of human myeloid leukemic cells against doxorubicin-induced apoptosis by granulocyte-macrophage colony-stimulating factor and interleukin 3. Leukemia. 1996;10:460–5. [PubMed] [Google Scholar]
- 35.Rodriguez-Galindo C, Poquette CA, Marina NM, et al. Hematologic abnormalities and acute myeloid leukemia in children and adolescents administered intensified chemotherapy for the Ewing sarcoma family of tumors. J Pediatr Hematol Oncol. 2000;22:321–9. doi: 10.1097/00043426-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kantarjian HM, Keating MJ, Walters RS, et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4:1748–57. doi: 10.1200/JCO.1986.4.12.1748. [DOI] [PubMed] [Google Scholar]
- 37.Le Beau MM, Albain KS, Larson RA, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986;4:325–45. doi: 10.1200/JCO.1986.4.3.325. [DOI] [PubMed] [Google Scholar]
- 38.Pui CH, Behm FG, Raimondi SC, et al. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N Engl J Med. 1989;321:136–142. doi: 10.1056/NEJM198907203210302. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen-Bjergaard J, Philip P. Balanced translocations involving chromosome bands 11q23 and 21q22 are highly characteristic of myelodysplasia and leukemia following therapy with cytostatic agents targeting at DNA-topoisomerase II. Blood. 1991;78:1147–8. [PubMed] [Google Scholar]
- 40.Rowley JD, Olney HJ. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: overview report. Genes Chromosomes Cancer. 2002;33:331–45. doi: 10.1002/gcc.10040. [DOI] [PubMed] [Google Scholar]
- 41.Mathew S, Head D, Rodriguez-Galindo C, Raimondi SC. Trisomy of the long arm of chromosome 1 resulting in a dicentric derivative (6)t(1;6) chromosome in a child with myelodysplastic syndrome following treatment for a primitive neuroectodermal tumor. Leuk Lymphoma. 2000;37:213–8. doi: 10.3109/10428190009057648. [DOI] [PubMed] [Google Scholar]
- 42.Barnard DR, Lange B, Alonzo TA, et al. Acute myeloid leukemia and myelodysplastic syndrome in children treated for cancer: comparison with primary presentation. Blood. 2002;100:427–34. doi: 10.1182/blood.v100.2.427. [DOI] [PubMed] [Google Scholar]
- 43.Hale GA, Heslop HE, Bowman LC, et al. Bone marrow transplantation for therapy-induced acute myeloid leukemia in children with previous lymphoid malignancies. Bone Marrow Transplant. 1999;24:735–9. doi: 10.1038/sj.bmt.1701962. [DOI] [PubMed] [Google Scholar]
- 44.Kroger N, Brand R, van Biezen A, et al. Autologous stem cell transplantation for therapy-related acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2006;37:183–9. doi: 10.1038/sj.bmt.1705226. [DOI] [PubMed] [Google Scholar]
- 45.Leahey AM, Friedman DL, Bunin NJ. Bone marrow transplantation in pediatric patients with therapy-related myelodysplasia and leukemia. Bone Marrow Transplant. 1999;23:21–5. doi: 10.1038/sj.bmt.1701517. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell MR, Nademanee AP, Snyder DS, et al. Bone marrow transplantation for myelodysplastic and myeloproliferative syndromes. J Clin Oncol. 1987;5:1822–6. doi: 10.1200/JCO.1987.5.11.1822. [DOI] [PubMed] [Google Scholar]
- 47.Witherspoon RP, Deeg HJ, Storer B, Anasetti C, Storb R, Appelbaum FR. Hematopoietic stem-cell transplantation for treatment-related leukemia or myelodysplasia. J Clin Oncol. 2001;19:2134–41. doi: 10.1200/JCO.2001.19.8.2134. [DOI] [PubMed] [Google Scholar]
- 48.Woodard P, Barfield R, Hale G, et al. Outcome of hematopoietic stem cell transplantation for pediatric patients with therapy-related acute myeloid leukemia or myelodysplastic syndrome. Pediatr Blood Cancer. 2006;47:931–5. doi: 10.1002/pbc.20596. [DOI] [PubMed] [Google Scholar]
- 49.Bandini G, Rosti G, Calori E, Albertazzi L, Tura S. Allogeneic bone marrow transplantation for secondary leukaemia and myelodysplastic syndrome. Br J Haematol. 1990;75:442–4. doi: 10.1111/j.1365-2141.1990.tb04367.x. [DOI] [PubMed] [Google Scholar]
- 50.Pagano L, Pulsoni A, Mele L, Leone G. Clinical and epidemiological features of acute lymphoblastic leukemia following a previous malignancy. Leuk Lymphoma. 2000;39:465–75. doi: 10.3109/10428190009113377. [DOI] [PubMed] [Google Scholar]
- 51.Hunger SP, Sklar J, Link MP. Acute lymphoblastic leukemia occurring as a second malignant neoplasm in childhood: report of three cases and review of the literature. J Clin Oncol. 1992;10:156–63. doi: 10.1200/JCO.1992.10.1.156. [DOI] [PubMed] [Google Scholar]
- 52.Sheppard DG, Libshitz HI. Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol. 2001;56:22–9. doi: 10.1053/crad.2000.0599. [DOI] [PubMed] [Google Scholar]
- 53.Bogni A, Cheng C, Liu W, et al. Genome-wide approach to identify risk factors for therapy-related myeloid leukemia. Leukemia. 2006;20:239–46. doi: 10.1038/sj.leu.2404059. [DOI] [PubMed] [Google Scholar]
- 54.Edick MJ, Cheng C, Yang W, et al. Lymphoid gene expression as a predictor of risk of secondary brain tumors. Genes Chromosomes Cancer. 2005;42:107–16. doi: 10.1002/gcc.20121. [DOI] [PubMed] [Google Scholar]
- 55.Smith LM, Cox RS, Donaldson SS. Second cancers in long-term survivors of Ewing’s sarcoma. Clin Orthop Relat Res. 1992;274:275–81. [PubMed] [Google Scholar]
- 56.Dunst J, Aherns S, Paulussen M, et al. Second malignancies after treatment for Ewing’s sarcoma: a report of the CESS-studies. Int J Radiat Oncol Biol Phys. 1998;42:379–84. doi: 10.1016/s0360-3016(98)00228-4. [DOI] [PubMed] [Google Scholar]