Abstract
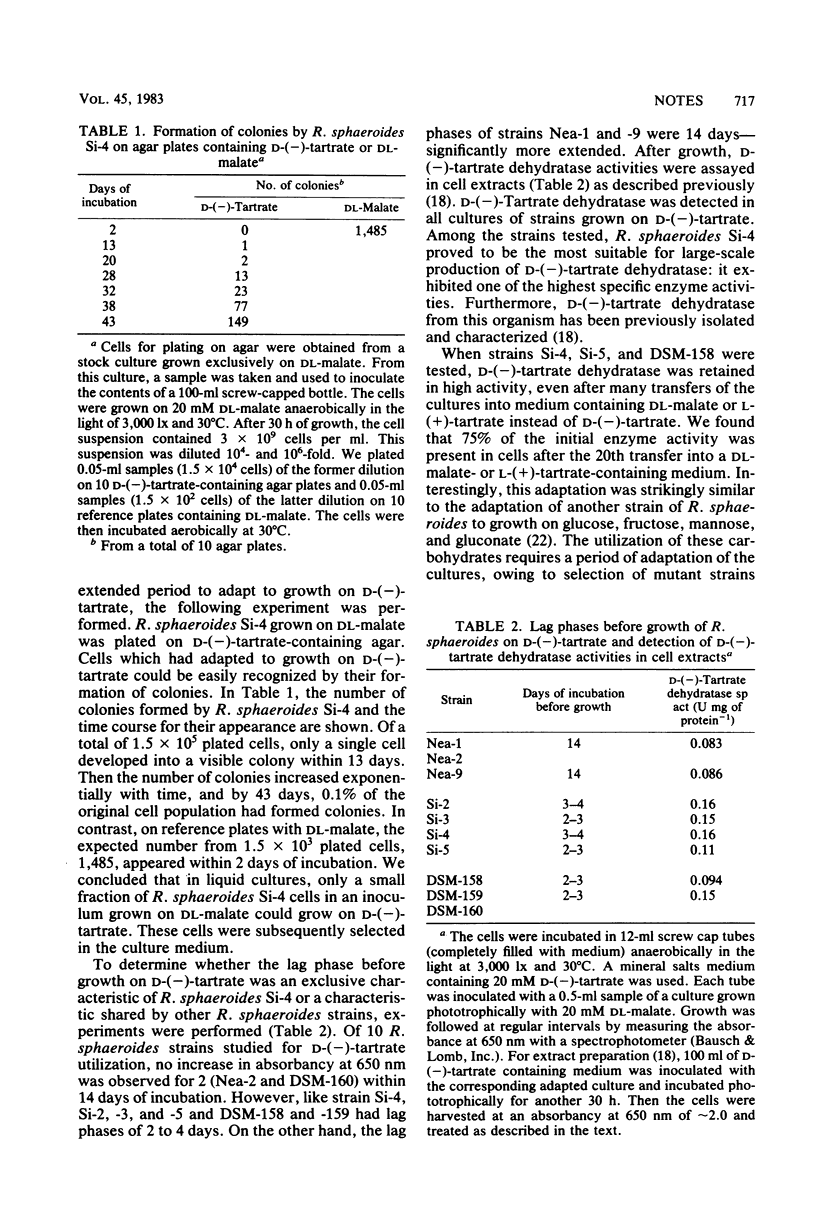
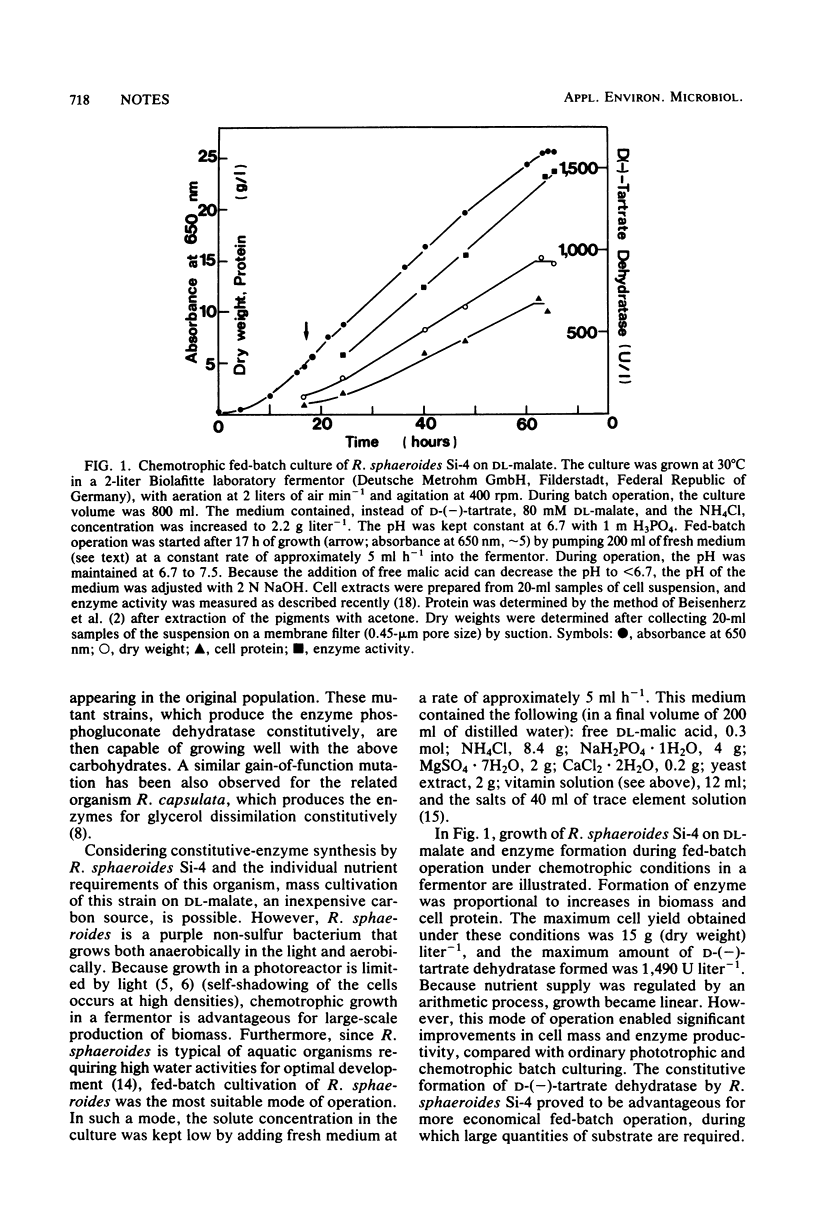
Of 10 strains of the purple non-sulfur bacterium Rhodopseudomonas sphaeroides, 8 acquired the ability to grow on d-(—)-tartrate; however, growth occurred only after extended lag phases ranging from 2 to 14 days. These lag phases occurred because only a small number of inoculum cells were able to grow by forming the enzyme d-(—)-tartrate dehydratase [d-(—)-tartrate hydro-lyase; EC number not yet available]. Once cells had grown on d-(—)-tartrate, d-(—)-tartrate dehydratase was formed constitutively. Therefore, mass cultivation of R. sphaeroides for production of large quantities of enzyme was possible on substrates much cheaper than d-(—)-tartrate. When 0.38 mol of dl-malate was used as a substrate in a chemotrophic fed-batch culture, a final biomass of 15 g (dry weight) liter−1 and 1,500 U of d-(—)-tartrate dehydratase liter of culture−1 were formed. The enzyme can be used for selective cleavage of racemic tartaric acid and for quantitative determination of d-(—)-tartrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfredsson G. A., Barker R. M., Old D. C., Duguid J. P. Use of tartaric acid isomers and citric acid in the biotyping of Salmonella typhimurium. J Hyg (Lond) 1972 Dec;70(4):651–666. doi: 10.1017/s0022172400022518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Alfredsson G. A., Barker R., Old D. C. A new biotyping scheme for Salmonella typhimurium and its phylogenetic significance. J Med Microbiol. 1975 Feb;8(1):149–166. doi: 10.1099/00222615-8-1-149. [DOI] [PubMed] [Google Scholar]
- LA RIVIERE J. W. Specificity of whole cells and cell-free extracts of Pseudomonas putida towards (+), (-), and meso-tartrate. Biochim Biophys Acta. 1956 Oct;22(1):206–207. doi: 10.1016/0006-3002(56)90250-5. [DOI] [PubMed] [Google Scholar]
- Lueking D., Tokuhisa D., Sojka G. Glycerol assimilation by a mutant of Rhodopseudomonas capsulata. J Bacteriol. 1973 Sep;115(3):897–903. doi: 10.1128/jb.115.3.897-903.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W. R., FOSTER J. W. Adaptation patterns in the utilization of the stereo-isomers of tartaric acid by a pseudomonad. J Bacteriol. 1957 May;73(5):683–684. doi: 10.1128/jb.73.5.683-684.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode H., Giffhorn F. D-(--)-tartrate dehydratase of Rhodopseudomonas sphaeroides: purification, characterization, and application to enzymatic determination of D-(--)-tartrate. J Bacteriol. 1982 Jun;150(3):1061–1068. doi: 10.1128/jb.150.3.1061-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode H., Giffhorn F. Ferrous- or cobalt ion-dependent D-(-)-tartrate dehydratase of pseudomonads: purification and properties. J Bacteriol. 1982 Sep;151(3):1602–1604. doi: 10.1128/jb.151.3.1602-1604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHILO M., STANIER R. Y. The utilization of the tartaric acids by pseudomonads. J Gen Microbiol. 1957 Apr;16(2):482–490. doi: 10.1099/00221287-16-2-482. [DOI] [PubMed] [Google Scholar]
- SHILO M. The enzymic conversion of the tartaric acids to oxaloacetic acid. J Gen Microbiol. 1957 Apr;16(2):472–481. doi: 10.1099/00221287-16-2-472. [DOI] [PubMed] [Google Scholar]
- SZYMONA M., DOUDOROFF M. Carbohydrate metabolism in Rhodopseudomonas sphreoides. J Gen Microbiol. 1960 Feb;22:167–183. doi: 10.1099/00221287-22-1-167. [DOI] [PubMed] [Google Scholar]
- Vaughn R. H., Marsh G. L., Stadtman T. C., Cantino B. C. Decomposition of Tartrates by the Coliform Bacteria. J Bacteriol. 1946 Sep;52(3):311–325. [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]


