Abstract
Genetic modification of crop plants to introduce desirable traits such as nutritional enhancement, disease and pest resistance, and enhanced crop productivity is increasingly seen as a promising technology for sustainable agriculture and boosting food production in the world. Independently, cultural practices that utilize alternative agriculture strategies including organic cultivation subscribe to sustainable agriculture by limiting chemical usage and reduced tillage. How the two together affect fruit metabolism or plant growth in the field or whether they are compatible has not yet been tested. Fruit-specific yeast S-adenosylmethionine decarboxylase (ySAMdc) line 579HO, and a control line 556AZ were grown in leguminous hairy vetch (Vicia villosa Roth) (HV) mulch and conventional black polyethylene (BP) mulch, and their fruit analysed. Significant genotype×mulch-dependent interactions on fruit phenotype were exemplified by differential profiles of 20 fruit metabolites such as amino acids, sugars, and organic acids. Expression patterns of the ySAMdc transgene, and tomato SAMdc, E8, PEPC, and ICDHc genes were compared between the two lines as a function of growth on either BP or HV mulch. HV mulch significantly stimulated the accumulation of asparagine, glutamate, glutamine, choline, and citrate concomitant with a decrease in glucose in the 556AZ fruits during ripening as compared to BP. It enables a metabolic system in tomato somewhat akin to the one in higher polyamine-accumulating transgenic fruit that have higher phytonutrient content. Finally, synergism was found between HV mulch and transgenic tomato in up-regulating N:C indicator genes PEPC and ICDHc in the fruit.
Keywords: Alternative agriculture, black polyethylene, hairy vetch, metabolite profiles, NMR, tomato fruit ripening, transgene expression
Introduction
Genetic modification of crop plants to introduce desirable traits such as nutritional enhancement, disease and pest resistance, and enhanced crop productivity has revolutionized agriculture and is increasingly sought as a promising technology to boost food production in the world (Chrispeels et al., 2002). In 2007, 23 countries planted biotech crops and the biotech crop area increased by 12%, reaching a total of 114.3 million hectares (James, 2007). The increasing global percentages of maize (14%), canola (18%), cotton (28%), and soybean (60%) that are genetically modified (GM) indicate that genetic engineering of crop plants has rapidly taken hold (James, 2005). Environmental impact data obtained from 42 field experiments with Bt cotton and maize have revealed that genetically modified crops can contribute to sustainable agriculture (Marvier et al., 2007). The need for agricultural sustainability is gaining more relevance now than before. Intensive conventional agriculture based on non-renewable resources has led to the depletion of natural resources and the loss of fertile agricultural lands, by soil erosion and contamination of two major natural resources, water and air, thereby raising concerns for human and animal health (National Research Council, 1989; Smil, 1997). It is now perceived that cultural practices that utilize alternative agriculture strategies, including organic cultivation practices, can subscribe to sustainable agriculture by limiting chemical usage and reduced tillage (National Research Council, 1989; Trewavas, 2004).
The most promising among the alternative farming practices for growing solanaceous crops is a reduced-tillage, cover crop based sustainable system (Abdul-Baki and Teasdale, 1993; Lu et al., 2000). The use of cover crop mulches such as the leguminous hairy vetch (Vicia villosa; HV) and non-leguminous rye (Secale cereale) increases crop productivity in traditionally bred crop plants including tomatoes (Abdul-Baki et al., 1996). In addition, the use of HV mulch is economically beneficial because it limits disease incidence and delays leaf senescence (Teasdale and Abdul-Baki, 1997; Mills et al., 2002). Despite these advantages, there is a lack of information on the integration of genetically engineered crops using such agricultural practices. To date, genetically engineered crops have not been tested in sustainable, alternative agriculture. Nor do we know the nature of changes brought about by cover crops in GM crops. This is of particular interest since the longer foliage life and tolerance to disease of tomato plants grown on HV mulch were correlated to activation of select signalling pathways and cross-talk among plant organs (Kumar et al., 2004, 2005; Mattoo and Abdul-Baki, 2006).
Previously, homozygous transgenic tomato lines were developed following transformation with yeast S-adenosylmethionine decarboxylase (ySAMdc) gene, Spe2, fused to a ripening-specific E8 promoter (Mehta et al., 2002). The fruit of these transgenic tomatoes accumulate higher polyamines, spermidine (Spd), and spermine (Spm), are longer-lasting on the vine, and richer in juice and nutritional quality. In addition, the high Spd-Spm tomatoes have higher fructose/glucose and sugar/acid ratios (Mattoo et al., 2006). The transgenic fruit have proven to be a good resource to study the role of Spd-Spm in regulating gene expression (Srivastava et al., 2007; Mattoo et al., 2007) and metabolite profiles (Mattoo et al., 2006) in fruit during ripening. These studies have been reviewed (Mattoo and Handa, 2008). The effect on plant phenotype is reported here, as exemplified by profiles of 20 fruit metabolites and expression patterns of the ySAMdc transgene, and tomato genes SAMdc, E8, PEPC, and cytosolic ICDH (ICDHc), by growing the homozygous transgenic tomato line along with a control azygous line in the field on beds made up of either black polyethylene (BP) or HV. Significant genotype×mulch-dependent interactions were revealed, including the finding that HV mulch enables a metabolic system in untransformed tomato somewhat akin to the one in polyamine-accumulating transgenic fruit.
Materials and methods
Plant materials, growth conditions, and fruit yield
The parental tomato cultivar used was Ohio 8245. A homozygous transgenic tomato line with ySAMdc gene (579HO) was generated as previously described by Mehta et al. (2002). The azygous control 556AZ line was randomly selected from 10 independent azygous lines generated in the laboratory. None of these azygous lines had exhibited any significant differences in agronomic characteristics during field trials in previous years. Plants were grown in the North Farm of Beltsville Agricultural Research Center, Maryland on leguminous hairy vetch (Vicia villosa; HV) beds next to black polyethylene (BP) beds. They were arranged in a randomized complete block design with the mulch being the main block (plot) and the tomato genotypes (lines) as the sub-plots. The tomato lines were then randomized within the main plots. Each treatment was replicated six times using 15 or more plants per replicate. Two plants at each end were used as border plants. Preparation of the field and seeding the HV cover crop with a no-till seeder were done during late summer (September) as previously described by Abdul-Baki et al. (1996). Hairy vetch was seeded at the rate of 45 kg ha−1 on raised beds of 15 cm high. When HV reached the flowering stage in May of the following year, it was mowed mechanically with a flair mower to 5 cm above the bed surface without disrupting the soil. The residue formed a uniform layer of mulch, several centimetres thick. BP beds were set up with trickle irrigation lines laid at 5 cm deep in the soil and 5 cm away from the plants. Drip irrigation lines were also set up over the surface of HV residue for both irrigation and fertilizer applications (Mills et al., 2002). Five-week-old tomato seedlings grown in the greenhouse were planted using a no-till mechanical transplanter, leaving 45 cm space between plants within a row and 185 cm between each row. Water was delivered through the drip lines as needed. Ammonium nitrate as fertilizer was applied at the rate of 82 kg ha−1 for HV (50% less than BP) and 164 kg ha−1 for BP beds, respectively, through the drip lines at 3-week intervals and spread over six applications per season, starting a week after transplantation of seedlings. Soil in the field plots was optimum in phosphorus and potassium, therefore no additional fertilizer was applied. Fruit yield, weight, and number were measured five times at weekly intervals and the cumulative data obtained. Yield data for the lines grown on BP and HV mulch were compiled from 4–8 replications each consisting of 10–20 plants. Average fruit weights for BP and HV-grown 556AZ and 579HO lines were calculated from an average of 175–225 fruits from each replication. Fruits were harvested at green (GR), breaker (BR), pink (PK), and red (RD) stages (Mehta et al., 2002) for biochemical and molecular analyses.
NMR spectroscopy-based metabolite profiling
Mature green fruit from the lines field grown on BP and HV mulches were harvested and transported to a temperature-controlled laboratory (at 25 °C), and sampled at different ripening stages. Skin-free pericarp was freeze-dried at –80 °C and the dry powder analysed for metabolite profiles by NMR spectrometry as previously described (Sobolev et al., 2003; Mattoo et al., 2006).
RNA extraction and RNA-DNA hybridizations
Fruit at GR, BR, PK, and RD stages were harvested from field-grown plants and total RNA was isolated (Mehta et al., 2002). Twenty μg of total RNA were electrophoresed in 1% formaldehyde agarose gels and blotted onto Nytran SuperCharge Nylon membranes (Schleicher and Schuell) with 20× SSC. DNA probes were prepared from plasmid clones after digestion with appropriate restriction enzymes followed by gel purification. Radiolabelled probes for hybridization were made with 32P using the High Prime Random Labelling kit (Roche). Yeast SAMdc, LeSAMdc, and E8 exon 1 probes were prepared from respective restriction enzyme-digested plasmid vectors as described by Mehta et al. (2002). Northern blots were hybridized in 50% formamide, 6× SSC, 5× Denhardt's reagent, 0.5% SDS, and 100 μg ml−1 heterologous DNA at 42 °C. Hybridizations were carried out overnight with probe concentrations of >1×106 cpm ml−1. Membranes were washed twice in 2× SSC and 0.1% SDS at 55 °C and final washes (two to four) were carried out in 0.2× SSC and 0.1% SDS at 55 °C. Blots were exposed to X-ray films at –70 °C with intensifying screens. Hybridization probes were stripped from RNA blots in a solution of 0.1× SSC and 0.1% SDS and blots rehybridized with 18S ribosomal tomato RNA probe.
Phosphoenolpyruvate carboxylase (PEPC) and cytosolic NADP-dependent isocitrate dehydrogenase (ICDHc) transcript analysis by real-time polymerase chain reaction (PCR)
Analysis of PEPC2 and ICDHc gene transcripts was conducted by real-time PCR as previously described by Mattoo et al. (2006). PCR primers were designed for tomato PEPC2 gene (GenBank accession no. AJ313434) (Guillet et al., 2002) and NADP+-ICDH gene (TIGR tomato gene index no. TC164449). The amplicon for PEPC2 gene included 144 bp of the 3′ UTR region and a 39 bp coding sequence; the forward and reverse primers were: 5′-ATGAAAGGTATTGCTGCTGGA-3′ (LePEPC2RTF) and 5′-TCGAGAAGCTACTAAACAAAGAGG-3′ (LePEPC2RTR), respectively. The ICDH amplicon included 98 bp of the 3′ UTR region and a 63 bp coding sequence; the forward and reverse primers were: 5′-GGAGAGTTCATCGATGCTGA-3′ (LeICDHRTF) and 5′-TCTGACACCTTAATCCCAAACA-3′ (LeICDHRTR), respectively. Tomato 18S primers were designed from rRNA gene sequence (accession number X51576) and a product of 160 bp in size was amplified. The forward and reverse 18S primers were: 5′-GCCCGGGTAATCTTTGAAAT-3′ (Le18SRTF) and 5′-CGGATCATTCAATCGGTAGG-3′ (Le18SRTR), respectively. Details on real-time PCR conditions were the same as before (Mattoo et al., 2006) and levels of PEPC and ICDHc transcripts were quantified in control and transgenic fruit during ripening by the comparative CT method using the 2–ΔΔCT formula as described by Livak and Schmittgen (2001). The amount of the target was normalized to the 18S rRNA reference. Relative efficiency of LePEPC2 or LeICDHc in relation to Le18S amplification with respective primer sets was found to be approximately equal after plotting the log concentrations of serial dilutions of cDNA input amounts against the delta CT values. The slope of the plot was less than 0.1 for the LePEPC2, LeICDH, and 18S primer sets.
Methods for polyamine analysis and statistics
Putrescine (Put), spermidine (Spd), and spermine (Spm) levels in GR, BR, PK, and RD fruit pericarp were determined as previously described (Minocha et al., 1990). Statistical methods were the same as previously described (Mattoo et al., 2006).
Results
Mulch×genotype interactions coexist in transgenic and non-transgenic tomato genotypes
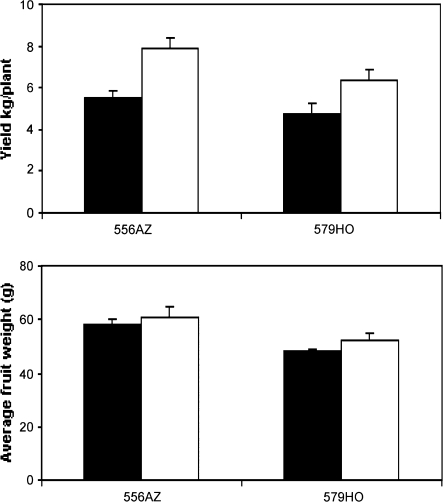
Transgenic tomato genotype (579HO), along with its azygous non-transgenic control line (556AZ), was evaluated for agronomic performance (fruit yield and fruit weight) when grown in black polyethylene (BP) and reduced-till, alternative hairy vetch (HV) cover crop (Fig. 1). The 556AZ (control) line produced a significantly higher fruit yield (P ≤0.05) when grown in HV mulch as compared to BP (Fig. 1, upper panel), with only a slight but positive effect on average fruit weight (Fig. 1, lower panel). These data are consistent with earlier observations on a fresh market tomato variety (Abdul-Baki et al., 1996). Fruit yield and fruit weight for the 579HO line were also higher when grown on HV compared with BP (Fig. 1). However, the HV-mediated increase in fruit yield for 579HO line was less pronounced than the non-transgenic 556AZ line. Fruits per plant and fruit yield were positively linked in HV-grown 556AZ (control) and 579HO lines. These data show ‘environment (cover crop mulches)×genotype (tomato)’-interactions in influencing the agronomic characteristics of both transgenic and non-transgenic tomato plants.
Fig. 1.
Field performance, measured as fruit weight and yield, of transgenic 579HO tomato and the non-transgenic azygous (556AZ) control line grown in BP and HV mulches. Data are from independent samples shown as mean ±SE (n=4–8). Closed and open bars represent BP-grown and HV-grown plants, respectively.
Differential accumulation of polyamines in HV- and BP-grown 556AZ and 579HO fruits during ripening
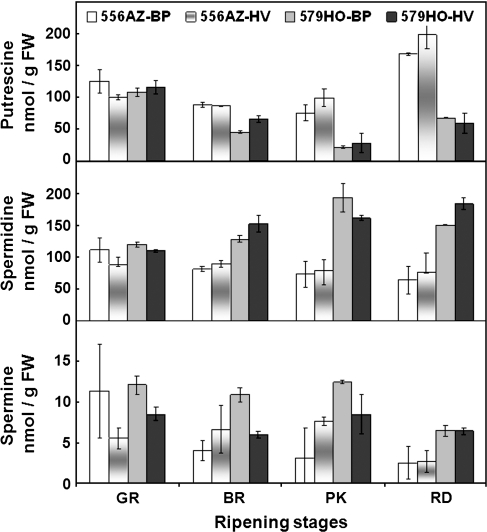
Fruits of transgenic 579HO line accumulate spermidine (Spd) and spermine (Spm) at the cost of putrescine (Put) during ripening compared with the 556AZ control line (Mehta et al., 2002; Fig. 2). The effect of the production system on the concentrations of Put, Spd, and Spm in the fruits from 556AZ control and 579HO transgenic lines as related to the stage of ripening is shown in Fig. 2. The focus here is particularly on changes occurring at the later stages of ripening since the transgene is induced in a ripening-specific manner (Mehta et al., 2002). BP and HV mulch did not affect the Put content in fruits from 556AZ line, except at the red stage in HV fruit that showed a slightly higher concentration (Fig. 2). In the 579HO transgenic fruit, Put content declined upon fruit ripening and was impervious to the growth condition except at the breaker stage when it was higher in HV fruit compared with BP fruit. Spd content in 556AZ fruit at the different stages of ripeness was similar, irrespective of the growth conditions. In the transgenic 579HO fruit, Spd content increased as ripening progressed, and was stimulated in breaker and red fruit from HV plants with a slight decrease in pink fruit compared with BP fruit (Fig. 2). Spm content decreased during ripening of 556AZ control fruits from plants grown in BP but significantly increased in HV-grown breaker and pink fruit by 62% and 47%, respectively, versus the corresponding BP-grown fruits (Fig. 2, 556AZ). At the red stage of 556AZ fruit, Spm content in BP- and HV-grown plants was similar. Interestingly, the Spm accumulation pattern during ripening of the transgenic fruit from BP-grown plants paralleled that seen with HV-grown 556AZ control fruit (Fig. 2, 579HO). In both 556AZ and 579HO fruits, the Spm accumulation pattern was similar when grown in HV mulch except that the decrease seen in the 556AZ red fruit did not occur in the corresponding HV- or BP-grown 579HO transgenic red fruit. In other words, the HV-mulch-mediated pattern of Spm accumulation in 556AZ fruit during ripening is similar to the transgene-mediated pattern in BP-grown 579HO fruit. In non-transgenic tomatoes, HV could therefore signal pathways that are similar to those regulated by higher polyamines in the transgenic line.
Fig. 2.
Pattern of polyamines in ripening fruit of transgenic (579H0) and non-transgenic (556AZ) plants grown in BP and HV mulches. Data shown are for putrescine, spermidine, and spermine at different stages of ripening from independent samples, as mean ±SE.
Differential accumulation of select metabolites in non-transgenic and transgenic fruit when grown on HV compared to BP mulch
The unexpected finding of differential effects of the growth conditions on polyamine accumulation in 556AZ and 579HO fruits prompted us to obtain profiles of select metabolites to determine which of them are sensitive to G×E interactions. Twenty metabolites, including three unverified compounds A, B, and C, at defined stages of ripening of 556AZ and 579HO fruits were quantified using NMR spectroscopy as described previously (Sobolev et al., 2003; Mattoo et al., 2006). Compounds labelled ‘A’, ‘B’, and ‘C’ could not be assigned because they remained unidentified; however, ‘A’ gave a spectral pattern identical to that of citrate and may, therefore, be a complex of citrate with a small molecule, ‘B’ is an unidentified multiplet, and ‘C’ a singlet, possibly a choline derivative.
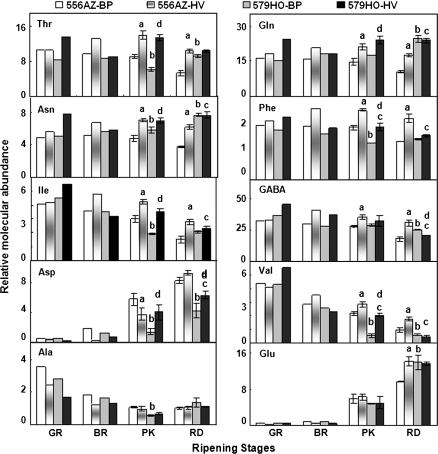
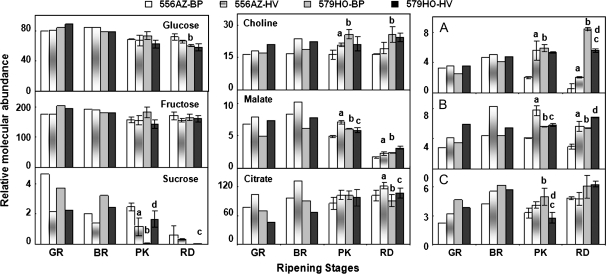
Interactions of HV mulch with the 556AZ line resulted in its fruit having significantly higher concentrations of Thr, Gln, Asn, Phe, Ile, GABA, and Val at the later stages of ripening (Fig. 3). The concentration of Glu was significantly different in 556AZ HV fruit only at the red stage compared with the fruit from the same line grown in BP; Ala levels remained more or less similar at each ripening stage (Fig. 3). In the red fruit from control plants grown in either BP or HV mulch, concentrations of Asp, Ala, and compound C were similar (Figs 3, 4, 556AZ). Similarly, the concentrations of the organic acids citrate and malate were higher in the fruit from the HV-grown 556AZ line than those grown on BP, whereas the micronutrient choline was only slightly higher in HV fruit compared with BP-grown fruit from 556AZ line (Fig. 4). Concentrations of compounds A and B were significantly higher in pink and red 556AZ fruits from HV-grown plants compared with those grown on BP. In contrast, sucrose concentration was higher in the BP-grown control fruit throughout ripening compared with HV fruit (Fig. 4). The red 556AZ fruit from BP-grown plant had an apparent trend towards slightly higher levels of Glc and Fru compared with the fruit from the HV-grown 556AZ plant.
Fig. 3.
NMR spectroscopic analysis of amino acids in ripening fruit of transgenic (579HO) and non-transgenic (556AZ) plants grown in BP and HV mulch systems. Letters a, b, c, and d marked above the bars indicate significant differences, P <0.05, respectively, between 556AZ-BP and 556AZ-HV, 556AZ-BP and 579HO-BP, 556AZ-HV and 579HO-HV, and 579HO-BP and 579HO-HV. Data shown are means ±SE (n=3–5). Only significant differences during late ripening stages are highlighted. Other details are as in Fig. 2.
Fig. 4.
NMR spectroscopic analysis of sugars, organic acids, choline, and three unidentified compounds (A, B, C) in ripening fruit of transgenic (579HO) and non-transgenic (556AZ) plants grown in BP and HV mulches. Only significant differences during late ripening stages are highlighted. Other details are the same as in the legends to Figs 2 and 3.
The interactions between the production system and the transgenic 579HO line were different from that seen with the 556AZ control in regard to the metabolite profiles (Figs 3, 4, 579HO). In the HV-grown transgenic (579HO) pink (PK) fruit, the concentrations of Thr, Gln, Asn, Phe, Ile, Asp, Val, and sucrose were higher while those of compound C were lower compared to the same stage BP-grown fruit (Figs 3, 4, 579HO). These differences become less pronounced as the transgenic fruit from the BP-mulch-grown plants turned red. The concentrations of GABA and compound A were lower in the red transgenic fruit grown in HV compared with BP (Figs 3, 4, 579HO, RD). Although HV-grown 556AZ and 579HO fruit responded to a large extent similarly, the magnitude of response varied and the pattern of changes in the class of metabolites identified were distinct. For instance, in the BP-grown azygous (control) fruit, Asn and Gln levels remained constant until the pink stage and declined thereafter, while choline levels remained constant. In the red transgenic fruit, Ile, Val, Ala, Asn, Glu, Gln, choline, Glc, Fru, and compound C levels were similar, irrespective of the growth conditions (BP or HV) of the plant. Together, these data suggest that a part of the HV-mulch-mediated differential pattern of metabolites in 556AZ fruit during ripening may be linked to the higher accumulation of polyamines, as shown here by spermine, at the penultimate stages of ripening.
HV mulch influences accumulation of transgene and endogenous gene transcripts during fruit ripening
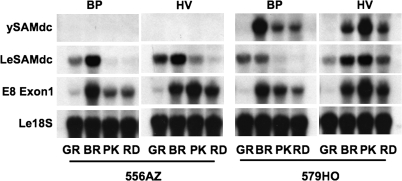
The effects seen with the type of mulch on differential accumulation of metabolites in 556AZ and 579HO fruits provided a good indication that gene expression may also be differentially influenced. To test this, transcript levels of the yeast SAMdc (ySAMdc), endogenous tomato SAMdc and E8 genes were analysed by northern blots. As expected, no ySAMdc (transgene) transcripts were apparent in azygous fruit from plants grown in either mulch (Fig. 5, 556AZ). In the 579HO fruit from BP-grown plants, ySAMdc transcripts accumulated early in the breaker fruit, declined precipitously at the pink stage and remained at that level in the red fruit (Fig. 5, 579HO, BP). By contrast, the accumulation of ySAMdc transcripts in 579HO fruit from HV-grown plants occurred at the breaker stage, as in the BP-grown transgenic line, but peaked at the pink stage of ripening (579HO, HV).
Fig. 5.
RNA gel blot analysis of ySAMdc, endogenous SAMdc (LeSAMdc), and E8 (E8 Exon1) gene transcripts at different ripening stages of fruit from non-transgenic (556AZ) and transgenic (579HO) plants grown in BP and HV mulches. Le18S RNA signals indicate loading levels of total RNA in each lane.
HV mulch promoted the accumulation of the endogenous SAMdc gene both in the 556AZ and the transgenic 579HO fruit for a longer duration than did the BP mulch, but the effect was longer lasting in the 579HO fruit (Fig. 5, LeSAMdc). In BP-grown 556AZ and 579HO fruit, LeSAMdc gene expression was evident during the early stages of fruit ripening (mature green and breaker), but decreased as ripening progressed (Fig. 5, see 556AZ PK, and RD).
The E8 transcript accumulation pattern during ripening of the transgenic fruits mirrored that of the ySAMdc expression profile whether the plants were grown on BP or HV (Fig. 5, compare E8 Exon1 with ySAMdc). HV mulch notably enhanced the expression of the E8 gene in both the non-transgenic control and the transgenic 579HO fruits as compared to the fruits from BP-grown plants. Even though the expression of the E8 gene was enhanced in HV-grown tomatoes, the patterns of appearance and accumulation were specific to each growth condition, BP or HV, independent of the genotype tested (compare 556AZ and 579HO in BP versus HV).
Synergism between HV mulch and transgenic tomato in up-regulating N:C indicator genes PEPC and ICDHc in the fruit
Nitrogen and carbon interactions heralded by the HV system (Kumar et al., 2004) are, to some extent, reflected in the metabolic changes observed in the polyamine (Spd and Spm), accumulating 579HO transgenic fruit (Mattoo et al., 2006). Carbon metabolism linkage with N sensing is known in leaves and roots to be largely achieved through the up-regulation of phosphoenolpyruvate carboxylase (PEPC) and cytosolic NADP-dependent isocitrate dehydrogenase (ICDHc) (Scheible et al., 2000; Foyer and Noctor, 2002; Rademacher et al., 2002). Therefore, the effects of BP and HV on the transcripts of these two N:C indicator genes in the fruit from 556AZ and 579HO lines was determined by real-time RT-PCR. Relative transcript levels were calculated using the 2–ΔΔCT method, using the transcript levels at the green (GR) stage of azygous non-transgenic fruit from the BP mulch as a reference.
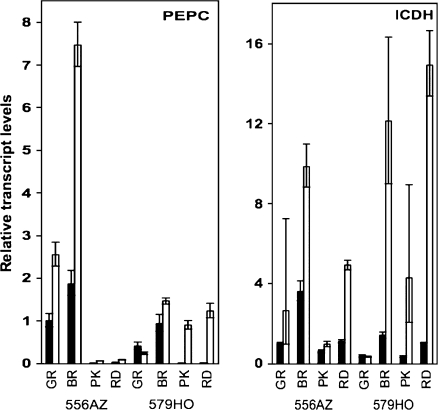
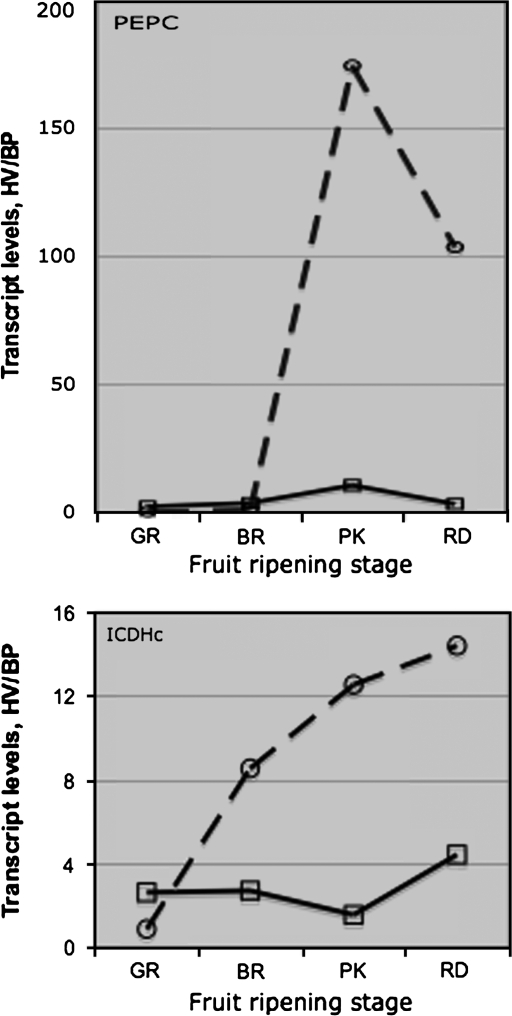
The PEPC and ICDHc transcript levels peaked at the breaker (BR) stage in the fruit from both non-transgenic (556AZ) and transgenic (579HO) lines grown in BP mulch, but decreased thereafter (Fig. 6); however, at each ripening stage, the levels in 579HO fruit were relatively lower compared with the 556AZ control fruit. In contrast to fruit from BP-grown plants, fruit from HV-grown plants contained much higher levels of PEPC and ICDHc transcript levels at each stage of fruit ripening. Early during ripening, in GR and BR stage fruit, PEPC transcript levels were higher in the 556AZ control than the 579HO transgenic line in both the mulch systems, but markedly higher PEPC transcripts were apparent in HV-grown transgenic fruit during the later stages, PK and RD, of ripening (Fig. 6, left panel). In fact, PEPC transcripts in the HV-grown 579HO transgenic fruit at the PK and RD stages of ripening were, respectively, 174-fold and 103-fold higher than the corresponding fruit from transgenic plants grown on BP (Fig. 7). It is noted here that PEPC transcript levels in the non-transgenic 556AZ fruit grown on HV were only 9.9 at pink and 2.6 at the red (RD) stages compared with BP (Fig. 7).
Fig. 6.
Real-time PCR analysis of LePEPC2 (A) and LeICDH (B) transcripts at different ripening stages of fruit from control 556AZ line and the transgenic 579HO line grown in BP (filled bars) and HV (open bars) mulches. The levels of PEPC2 and ICDHc transcripts were determined relative to the calibrator azygous (556AZ) at green (GR) stage from BP-grown plants. The range in variation is shown as error bars, which was determined by evaluating the expression 2–ΔΔCT with ΔΔCT+s and ΔΔCT–s, where s is the standard deviation of the ΔΔCT value (n=3). Closed and open bars represent BP-grown and HV-grown fruits, respectively.
Fig. 7.
Ratio of PEPC and ICDHc transcript levels, respectively, in HV mulch to that in BP in 556AZ (open rectangles) and 579HO (open circles with dotted line) fruit at different stages (GR, BR, PK, RD) of ripening. Data from Fig. 6 were used to generate this figure. Solid and broken lines represent fruits from 556AZ and 579HO genotypes, respectively.
The patterns for ICDHc transcripts were different from the PEPC transcripts in HV-grown 556AZ control and transgenic 579HO lines (Fig. 6, right panel). At the BR, PK, and RD stages of fruit ripening, ICDHc transcripts showed a relatively higher increase in HV-grown transgenic (579HO) fruit than the 556AZ control line. Relatively, increases in ICDHc transcripts at the PK and RD stages were several-fold higher in HV-grown transgenic fruit compared with BP-grown fruit, a much larger effect than seen in HV-grown non-transgenic fruit compared with BP grown fruits (Fig. 7).
Discussion
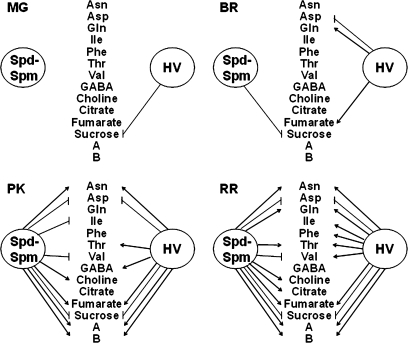
It has been demonstrated here that growth environments created by using cover crop mulch (HV) or black polyethylene (BP) interact with the genetic make-up of a plant (tomato genotypes) in a unique manner. The levels and profile of metabolite accumulation at different stages of fruit ripening were distinct among transgenic and non-transgenic tomatoes, metabolite signatures distinguishing the transgenic from the control fruit whether grown on BP or HV. Notably, while HV-grown 556AZ and 579HO fruit responded to a large extent similarly, albeit to varying magnitudes, their patterns of change in the class of metabolites identified were more revealing. A summary showing the sequence of changes in the content of particular metabolites affected by the transgene introduction, thereby attributed to the accumulation of Spd-Spm in the transgenic fruit from plants grown on BP, and those in fruit from 556AZ control line grown on HV mulch is illustrated in Fig. 8. Following the sequence of changes in the metabolites with the progression of ripening, it is evident that transgene-mediated accumulation of Spd-Spm produced metabolite profiles in the fruit (grown on BP) similar to the one seen in control fruits grown on HV mulch. Interestingly, the influence and effect of the HV system on fruit metabolism are manifested as ripening progressed, as is enabled in the transgenic fruit by the ripening-regulated promoter E8 (Fig. 8). These data unearth a linkage between polyamines and metabolite content in tomato fruit, revealing a surprising finding that HV activates the same metabolic pathways and genes as are activated by the introduction of the ySAMdc transgene. HV also modulates transgene expression and seemingly affects the pattern and quantity of the indicated metabolites during fruit ripening.
Fig. 8.
Illustration of ripening stage-specific modulation of metabolites in the Spd-Spm accumulating transgenic fruit from BP-grown plants and in the azygous, control fruit from HV mulch-grown plants. Metabolites whose levels increased (arrow head) or decreased (vertical bar) at least by 50% in 556AZ-HV or 579HO-BP compared with 556AZ-BP at the indicated stages of ripening were recorded are shown. Profiles of Ala, Glu, compound C, glucose, fructose, and malic acid which were more or less similar in the two genotypes under both growth conditions are not shown. MG, BR, PK, and RR represent mature green, breaker, pink, and red-ripe fruits, respectively.
Sensing of polyamines as organic-N could be the underlying cause for increases in the other N forms such as Glu, Gln, and Asn in the fruit, a response similar to N availability seen in plant roots and leaves (Foyer et al., 2003) as well as trees (Rennenberg et al., 1998; Bauer et al., 2004). Polyamine accumulation correlates with the expression of ySAMdc transgene in transgenic fruit or the expression pattern of the endogenous SAMdc in the non-transgenic fruit, both genes showing HV-mediated enhancement. Similarly, the E8 gene expression pattern was identical within a mulch system both in non-transgenic and transgenic fruit and enhanced in the fruit from HV-grown plants.
The observed molecular changes translated into effects on the agronomic characteristics of azygous and transgenic 579HO lines grown on BP and HV. Fruit number and yield per plant were positively linked in HV-grown 556AZ (control) as well as transgenic line 579HO. The increase in yield of non-transgenic tomatoes on HV is a reflection of increased weight of these fruit and increased number of fruit on each plant, an observation previously attributed to increased fruit set, photosynthetic activity, and physiological fitness of HV-grown plants compared with those grown on BP (Teasdale and Abdul-Baki, 1997). However, transgenic line 579HO, that accumulates higher polyamines, showed a slight reduction in the average weight of the fruits compared to non-transgenic tomatoes, which was independent of the BP or HV mulch. The reduction in fruit weight is probably due to high levels of metabolism and decreased sugar content in the transgenic fruit (Mattoo et al., 2006, 2007). Potatoes engineered to over-express the SAMdc gene produced smaller tubers (Pedros et al., 1999) while over-expression of hexokinase in tomato fruit, which altered carbon metabolism, also resulted in reduced fruit and seed size (Menu et al., 2003).
An interesting outcome of these studies relates to the nutritionally superior 579HO red fruit (Mehta et al., 2002) over the non-transgenic in terms of the relative content of Phe. Phe accumulates in the HV-grown 556AZ control red fruit, but is significantly lower in the corresponding HV-grown 579HO fruit. Higher dietary levels of Phe are not desirable for patients suffering from phenylketonuria, a genetic disorder caused by the deficiency of Phe-metabolizing enzyme, Phe hydroxylase (Levy, 1999). Thus, the transgenic 579HO tomato fruit in which Phe is down-regulated when grown in HV provides a resource to investigate the mechanism of this down-regulation and thereby find clues to fine-tune this process.
Our investigation into the transcript accumulation of two key genes in carbon metabolism and nitrogen assimilation, previously found to be critical in maintaining a balance of carbon and nitrogen in leaves and roots (Scheible et al., 2000; Foyer and Noctor, 2002; Rademacher et al., 2002; Stitt et al., 2002), PEPC and ICDH, showed that HV mulch promotes their gene activity in both transgenic and non-transgenic fruit during ripening. Notably, the HV system is synergistic with transgenic, SAMdc expressing fruit in up-regulating PEPC and ICDHc genes in tomato fruit. Over-expression of PEPC in transgenic potato plants was shown to increase plant productivity through the direction of starch and sugars to organic acids, and amino acids such as malate and glutamine (Rademacher et al., 2002). Timing of N availability and cytokinins may activate C:N metabolism in a fruit as shown for maize (Sugiharto et al., 1992) and tomato leaves (Kumar et al., 2004, 2005). HV system preferentially affects gene activity related to N-sensing and cytokinin signalling (Kumar et al., 2004; Mattoo and Abdul-Baki, 2006). Here it is shown that HV-mediated increase in the expression of SAMdc in fruit parallels increased expression of PEPC and ICDHc transcripts. It was also found that HV increases cytokinin receptor kinase (Kumar et al., 2004) transcripts in the fruit (data not shown). These correlative data suggest that SAMdc, in concert with N and cytokinins, orchestrates the C:N metabolism in tomato fruit. Thus, specific pathways and genes are activated in tomato plants in response to the HV mulch (Mattoo and Abdul-Baki, 2006). A co-ordination exists between the environmental (mulch type) cues and differential gene expression and metabolism, revealing environment-dependent and transgene-dependent changes in fruit metabolism, but without any apparent qualitative deviation from normal fruit metabolites. This observation bodes well for the incorporation of genetically engineered (transgenic) crops into alternative agriculture practices.
Acknowledgments
We thank Mary Camp for statistical analysis of field data, and Drs M Edelman, J Teasdale, and N Uphoff, and the anonymous reviewers for constructive comments on the original version of the manuscript. This study was supported in part by Israel–US Binational Agricultural Research Development grant IS-3441-03 (to AKM and AKH), Società Italiana di Spettrochimica e Spettrofisica (to APS and ALS) and a DBT Fellowship, Government of India (to RKG). Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Glossary
Abbreviations
- BP
black polyethylene
- HV
hairy vetch
- ICDHc
cytosolic NADP-dependent isocitrate dehydrogenase
- NMR
nuclear magnetic resonance
- PEPC
phosphoenolpyruvate carboxylase
- SAMdc
S-adenosylmethionine decarboxylase
- ySAMdc
yeast S-adenosylmethionine decarboxylase
References
- Abdul-Baki AA, Teasdale J. A no-tillage tomato production system using hairy vetch and subterranean clover mulches. HortScience. 1993;28:85–95. [Google Scholar]
- Abdul-Baki AA, Teasdale JR, Korcak RF, Chitwood DJ, Huettel RN. Fresh-market tomato production in a low-input alternative system using cover-crop mulch. Journal of the American Society of Horticultural Sciences. 1996;31:65–69. [Google Scholar]
- Bauer GA, Bazzaz FA, Minocha R, Long S, Magill A, Aber J, Berntson GM. Effects of chronic N additions on tissue chemistry, photosynthetic capacity, and carbon sequestration potential of a red pine (Pinus resinosa Ait.) stand in NE United States. Forest Ecology Management. 2004;196:173–186. [Google Scholar]
- Chrispeels MJ, Sadaya DE, Martinj C. Plants, genes and crop biotechnology. Boston: Jones and Bartlett Publishers; 2002. [Google Scholar]
- Guillet C, Just D, Benard N, Destrac-Irvine A, Baldet P, Hernould M, Causse M, Raymond P, Rothan C. A fruit-specific phosphoenolpyruvate carboxylase is related to rapid growth of tomato fruit. Planta. 2002;214:717–726. doi: 10.1007/s00425-001-0687-z. [DOI] [PubMed] [Google Scholar]
- James C. Global status of commercialized biotech/GM crops. 2005. ISAAA Briefs 34–2005. [Google Scholar]
- James C. Global status of commercialized biotech/GM crops. 2007. ISAAA Briefs 37–2007. [Google Scholar]
- Foyer CH, Noctor G, editors. Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. Boston: Kluwer Acadademic Publishers; 2002. [Google Scholar]
- Foyer CH, Parry M, Noctor G. Markers and signals associated with nitrogen assimilation in higher plants. Journal of Experimental Botany. 2003;54:585–593. doi: 10.1093/jxb/erg053. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abdul-Baki A, Anderson JD, Mattoo AK. Cover crop residues enhance growth, improve yield and delay leaf senescence in greenhouse-grown tomatoes. HortScience. 2005;40:1307–1311. [Google Scholar]
- Kumar V, Mills DJ, Anderson JD, Mattoo AK. An alternative agriculture system is defined by a distinct expression profile of select gene transcripts and proteins. Proceedings of the National Academy of Sciences, USA. 2004;101:10535–10540. doi: 10.1073/pnas.0403496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy HL. Phenylketonuria: old disease, new approach to treatment. Proceedings of the National Academy of Sciences, USA. 1999;96:1811–1813. doi: 10.1073/pnas.96.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu YC, Watkins KB, Teasdale JR, Abdul-Baki AA. Cover crops in sustainable food production. Food Reviews International. 2000;16:121–157. [Google Scholar]
- Marvier M, McCreedy C, Regetz J, Kareiva P. A meta-analysis of effects of Bt cotton and maize on non-target invertebrates. Science. 2007;316:1475–1477. doi: 10.1126/science.1139208. [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Abdul-Baki AA. Crop genetic responses to management: evidence of root–shoot communication. In: Uphoff N, Ball AS, Fernandes E, Herren H, Husson O, Laing M, Palm C, Thies J, editors. Biological approaches to sustainable soil systems. Bocan Raton, FL: CRC Taylor & Francis; 2006. pp. 221–230. [Google Scholar]
- Mattoo AK, Chung SH, Goyal RK, Fatima T, Solomos T, Srivastava A, Handa AK. Overaccumulation of higher polyamines in ripening transgenic tomato fruit revives metabolic memory, up-regulates anabolism-related genes, and positively impacts nutritional quality. Journal of AOAC International. 2007;90:1456–1464. [PubMed] [Google Scholar]
- Mattoo Al, Handa AK. Higher polyamines restore and enhance metabolic memory in ripening fruit. Plant Science. 2008;174:386–393. [Google Scholar]
- Mattoo AK, Sobolev AP, Neelam A, Goyal RK, Handa AK, Segre AL. Nuclear magnetic resonance spectroscopy-based metabolite profiling of transgenic tomato fruit engineered to accumulate spermidine and spermine reveals enhanced anabolic and nitrogen-carbon interactions. Plant Physiology. 2006;142:1759–1770. doi: 10.1104/pp.106.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RA, Cassol T, Li N, Ali N, Handa AK, Mattoo AK. Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality and vine life. Nature Biotechnology. 2002;20:613–618. doi: 10.1038/nbt0602-613. [DOI] [PubMed] [Google Scholar]
- Menu T, Saglio P, Granot D, Dai N, Raymond P, Ricard B. High hexokinase activity in tomato fruit perturbs carbon and energy metabolism and reduces fruit and seed size. Plant, Cell and Environment. 2003;27:89–98. [Google Scholar]
- Mills DJ, Coffman CB, Teasdale JR, Everts KL, Anderson JD. Factors associated with foliar disease of staked fresh market tomatoes grown under differing bed strategies. Plant Disease. 2002;86:356–361. doi: 10.1094/PDIS.2002.86.4.356. [DOI] [PubMed] [Google Scholar]
- Minocha SC, Minocha R, Robie CA. High-performance liquid chromatographic method for the determination of dansyl-polyamines. Journal of Chromatography. 1990;511:177–183. [Google Scholar]
- National Research Council. Alternative agriculture. Washington, DC: National Academy Press; 1989. [Google Scholar]
- Pedros RA, Macleod MR, Ross HA, McRae D, Tiburcio AF, Davies HV, Taylor MA. Manipulation of S-adenosylmethionine decarboxylase activity in potato tubers: an increase in activity leads to an increase in tuber number and a change in tuber size distribution. Planta. 1999;209:153–160. doi: 10.1007/s004250050617. [DOI] [PubMed] [Google Scholar]
- Rademacher T, Hausler RE, Hirsch H-J, Zhang L, Lipka V, Weier D, Kreuzaler F, Peterhansel C. An engineered phosphoenolpyruvate carboxylse redirects carbon and nitrogen flow in transgenic potato plants. The Plant Journal. 2002;32:25–39. doi: 10.1046/j.1365-313x.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Kreutzer K, Papen H, Weber P. Consequences of high loads of nitrogen for spruce (Picea abies) and beech (Fagus sylvatica) forests. New Phytologist. 1998;139:71–86. doi: 10.1046/j.1469-8137.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Krapp A, Stitt M. Reciprocal diurnal changes of phosphoenolpyruvate carboxylase expression and cytosolic pyruvate kinase, citrate synthase and NADP-isocitrate dehydrogenase expression regulate organic acid metabolism during nitrate assimilation in tobacco leaves. Plant, Cell and Environment. 2000;22:1155–1167. [Google Scholar]
- Smil V. Global population and the nitrogen cycle. Scientific American. 1997;277:76–81. [Google Scholar]
- Sobolev AL, Segre AL, Lamanna R. Proton high-field NMR study of tomato juice. Magnetic Resonance Chemistry. 2003;41:237–245. [Google Scholar]
- Srivastava A, Chung SH, Fatima T, Datsenka T, Handa AK, Mattoo AK. Polyamines as anabolic growth regulators revealed by transcriptome analysis and metabolite profiles of tomato fruits engineered to accumulate spermidine and spermine. Plant Biotechnology. 2007;24:57–70. [Google Scholar]
- Stitt M, Muller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible W-R, Krapp A. Steps towards an integrated view of nitrogen metabolism. Journal of Experimental Botany. 2002;53:959–970. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- Sugiharto B, Suzuki I, Burnell JN, Sugiyama T. Glutamine induces the N-dependent accumulation of mRNAs encoding phosphoenolpyruvate carboxylase and carbonic anhydrase in detached maize leaf tissue. Plant Physiology. 1992;100:2066–2070. doi: 10.1104/pp.100.4.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JR, Abdul-Baki AA. Growth analysis of tomatoes in black polyethylene and hairy vetch production systems. HortScience. 1997;32:659–663. [Google Scholar]
- Trewavas A. A critical assessment of organic farming and food assertions with particular respect to the UK and the potential environmental benefits of no-till agriculture. Crop Protection. 2004;23:757–781. [Google Scholar]