Abstract
The shortage of strong endosperm-specific expression promoters for driving the expression of recombinant protein genes in cereal endosperm is a major limitation in obtaining the required level and pattern of expression. Six promoters of seed storage glutelin genes (GluA-1, GluA-2, GluA-3, GluB-3, GluB-5, and GluC) were isolated from rice (Oryza sativa L.) genomic DNA by PCR. Their spatial and temporal expression patterns and expression potential in stable transgenic rice plants were examined with β-glucuronidase (GUS) used as a reporter gene. All the promoters showed the expected spatial expression within the endosperm. The GluA-1, GluA-2, and GluA-3 promoters directed GUS expression mainly in the outer portion (peripheral region) of the endosperm. The GluB-5 and GluC promoters directed GUS expression in the whole endosperm, with the latter expressed almost evenly throughout the whole endosperm, a feature different from that of other rice glutelin gene promoters. The GluB-3 promoter directed GUS expression solely in aleurone and subaleurone layers. Promoter activities examined during seed maturation showed that the GluC promoter had much higher activity than the other promoters. These promoters are ideal candidates for achieving gene expression for multiple purposes in monocot endosperm but avoid promoter homology-based gene silencing. The GluC promoter did not contain the endosperm specificity-determining motifs GCN4, AACA, and the prolamin-box, which suggests the existence of additional regulatory mechanism in determining endosperm specificity.
Keywords: Endosperm-specific expression, expression pattern, genetic transformation, promoter, promoter activity
Introduction
Endosperm is the storage organ for starch and protein for cereal crops, which provide the major source of calories and protein for humans. Improvement of the endosperm composition and quality via genetic modification is attractive, and there have been great achievements (Bajaj and Mohanty, 2005). However, many agronomically important properties such as starch quality and plant secondary metabolic products are polygenic traits. Single gene introgression is thus less effective for such traits. Conventional strategies, such as repeat transformation or crossing of independent transformants, are considerably time-consuming and labour-intensive. Recently, a multigene transformation system was used for introducing several genes simultaneously through construction of one expression vector (Lin et al., 2003; Chen et al., 2006; Wakasa et al., 2006). However, these multiple genes need promoters to drive them to avoid the gene silencing caused by high homology of the promoters. A lack of suitable promoters is a critical limiting factor for such research.
Endosperm is an ideal platform for the production of recombinant proteins (Horvath et al., 2000; Takaiwa et al., 2007). Compared with other expression systems, the use of endosperm has many advantages, such as cost savings, ease of controlling the scale of production, large storage ability, a high level of safety in terms of storage of recombinant protein, and freedom from animal-derived pathogens. Moreover, its use does not involve ethical issues (Delaney, 2002; Takaiwa et al., 2007). Much progress has been made with the rice seed system used as a bioreactor for the production of recombinant protein (Katsube et al., 1999; Ye et al., 2000; Paine et al., 2005; Qu et al., 2005; Takagi et al., 2005; Yasuda et al., 2005; Yang et al., 2006, 2007). However, the shortage of strong endosperm-specific expression promoters for driving the expression of recombinant protein genes in cereal endosperm is still a major limitation in obtaining the required level and pattern of expression.
The promoter plays the most important role in determining the temporal and spatial expression pattern and transcript level of a gene, although the final amount of gene product is determined at both the transcriptional and post-transcriptional levels. To date, some strong constitutive promoters, such as the cauliflower mosaic virus 35S promoter and maize Ubiquitin-1 (Battraw and Hall, 1990; Christensen et al., 1992), are widely used in plant biotechnology research. However, the expression level of the target gene in the desired tissue is often not satisfactory (Drakakaki et al., 2000). In addition, continuous high expression of a foreign gene in all tissues may cause detrimental effects in the host plant (Cheon et al., 2004). Using a strong endosperm-specific promoter to restrict gene expression to only the endosperm may solve such problems. Moreover, foreign protein genes driven by an endosperm-specific promoter are more stable than those driven by ubiquitous promoters (Choi et al., 2003). Some endosperm-specific expression promoters have been isolated and characterized from rice (Oryza sativa L.) (Zheng et al., 1993; Qu and Takaiwa, 2004), wheat (Triticum aestivum L.) (Lamacchia et al., 2001; Wiley et al., 2007), maize (Zea mays L.) (Russell and Fromm, 1997), and barley (Hordeum vulgare L.) (Choi et al., 2003). However, few endosperm-specific expression promoters are available. In addition, the repetitive use of the same promoter is one of the reasons for transgene silencing due to promoter homology (Mol et al., 1989; Bhullar et al., 2003). To meet the demand for achieving the multiple purposes of gene expression in plants, a range of promoters that provide high and regulated expression of transgenes in monocot seeds is needed.
Rice glutelin, accounting for ∼70% of the total rice seed storage protein, is expressed exclusively in endosperm. Therefore, the glutelin gene promoters are ideal candidates for isolating and obtaining strong promoters with endosperm specificity. Recent progress in study of the rice genome revealed at least 13 glutelin gene copies (Table 1). Glutelin genes are classified into three subfamilies, GluA, GluB, and GluC, with at least three members, at least eight members, and one member, respectively (Takaiwa et al., 1987, 1991; Mitsukawa et al., 1998; Kusaba et al., 2003). Most studies of rice glutelin promoters focused on identifying cis-regulatory elements involved in endosperm specificity restricted to a few glutelin genes by observing their spatial expression patterns (Zheng et al., 1993; Croissant-Sych and Okita, 1996; Takaiwa et al., 1996; Yoshihara et al., 1996; Wu et al., 1998a; Washida et al., 1999). The potential expression activities of glutelin gene promoters were limited to Gt1 (GluA-2), GluA-3, GluB-1, GluB-2, and GluB-4 (Zheng et al., 1993; Russell and Fromm, 1997; Wu et al., 1998a; Qu and Takaiwa, 2004). The expression pattern and activity of GluA-2 and GluA-3 promoters were investigated in homologous transgenic rice (Zheng et al., 1993; Wu et al., 1998a) and heterologous transgenic maize (Russell and Fromm, 1997) via polyethylene glycol (PEG)-mediated or bolistic transformation. Since rice glutelin is encoded by more than a dozen genes, extending this investigation to other glutelin gene promoters is worthwhile.
Table 1.
Rice glutelin genes found in the genomic database
| Gene name | Gene ID | Function | Reference |
| Os01g0762500 | 4327027 | GluA-1 | Takaiwa et al. (1987) |
| Os02g0242600 | 4328851 | Glutelin | |
| Os02g0248800 | 4328876 | GluB-2 type precursor | |
| Os02g0249000 | 4328877 | GluB-2 type precursor | |
| Os02g0249600 | 4328881 | GluB-2 | Takaiwa et al. (1991) |
| Os02g0249800 | 4328883 | GluB-1 | Takaiwa et al. (1991) |
| Os02g0249900 | 4328884 | GluB-1 type precursor | |
| Os02g0268100 | 4328968 | GluB-4 | Kusaba et al.(2003) |
| Os02g0268300 | 4328969 | GluB-5 | Kusaba et al. (2003) |
| Os02g0453600 | 4329275 | GluC | Mitsukawa et al. (1998) |
| Os03g0427300 | 4333164 | GluA-3 | Takaiwa et al. (1987) |
| Os10g0400200 | 4348563 | GluA-2 | Takaiwa et al. (1987) |
| GluB-3 | Takaiwa et al. (1991) |
In this study, the expression pattern and activity of six rice seed storage glutelin gene promoters were examined by introducing them into rice via Agrobacterium-mediated transformation. These genes are GluA-1, GluA-2, GluA-3, GluB-3, GluB-5, and GluC (Takaiwa et al., 1987, 1991; Mitsukawa et al., 1998; Kusaba et al., 2003). The aim of the study was to define the spatial and temporal expression patterns of the glutelin gene promoters and evaluate their potential for driving high expression of foreign genes during rice seed maturation. The results from this study are useful for providing alternative choices of promoters for the production of high-value recombinant proteins and for multigene transformation in rice and other cereal crops.
Materials and methods
Isolation of glutelin gene promoters
A 2 kb rice seed storage glutelin gene GluA-1 promoter and 2.3 kb GluA-2, GluA-3, GluB-3, GluB-5, and GluC promoters were isolated by PCR from rice (Oryza sativa cv Taizhong 65) genomic DNA with use of ExTaq DNA polymerase (TAKARA, Japan). The primer pairs used for each promoter are as follows: GluA-1 forward, 5′-TCAAGCTTCCAAGGTTTGATC-3′ (HindIII); GluA-1 reverse, 5′-ACGCGTCGACGTTGTTGTAGTACTAATGAAC-3′ (SalI); GluA-2 forward, 5′-TCAAAGCTTATTACTATCTGAGCATT CCCC-3′ (HindIII); GluA-2 reverse, 5′-ACGCGTCGACGTTGTTGTAGGACTAATGAAC-3′ (SalI); GluA-3 forward, 5′-ACGCGTCGACGCATCTCAACGATGATGCC-3′ (HindIII); GluA-3 reverse, 5′-TTTCCCGGGGTTTTTGTGGGACTGAACTC-3′ (SmaI); GluB-3 forward, 5′-CCCAAGCTTATTTTACTTGTACTGTTTAACC-3′ (HindIII); GluB-3 reverse, 5′-AAACCCGGGAGCTTTCTGTATATG CTAATG-3′ (SmaI); GluB-5 forward, 5′-AAAGGTCGACGAGAAAAGAAGATTTGCTGAC-3′ (SalI); GluB-5 reverse, 5′-AAACCCGGGCTATTTATTGAAAGGATAATGG-3′ (SmaI); GluC forward, 5′-GGGAAGCTTGTTCAAGATTTATTTTTGG-3′ (HindIII); and GluC reverse, 5′-ACGCGTCGACAGTTATTCACTTAGTTTCCC-3′ (SalI).
The amplified 2.0 kb or 2.3 kb PCR fragments were linked with pBlue7 T-vector (Norvagen, Germany), then the inserts were sequenced.
Putative motif searching
The putative motifs contained in the promoter were analysed by use of the PLACE database (plant cis-acting regulatory DNA elements) (Higo et al., 1999; http://www.dna.affrc.go.jp/PLACE).
Construction of plant expression vector
Each promoter was introduced upstream of the uidA gene, which encodes β-glucuronidase (GUS), in the binary vector pGPTV-35S-HPT with HindIII/SalI, HindIII/SmaI, or SalI/SmaI sites as described previously (Qu and Takaiwa, 2004).
Production of transgenic plants
Transgenic rice plants (O. sativa cv. Kitaake as a host) were generated by Agrobacterium-mediated transformation. Plant expression vectors constructed as described above were transferred into A. tumefaciens strain EHA105 by heat shock. Calli, ∼5 weeks old, derived from mature rice seed or developing embryos, were infected and co-cultured with the transformed A. tumefaciens at 28 °C for 3 d. The infected calli were successively cultured for 5–6 weeks separately in N6 selection and MS regeneration medium containing 45 mg l−1 hygromycin at 25 °C. The regenerated seedlings were transplanted in a greenhouse.
Identification of transgenic plants
The regenerated seedlings were checked by PCR, with genomic DNA isolated from leaves used as templates. The sequence from GUS was used as common reverse primer paired with the forward primers mentioned above. The positive transformants were used for further analysis.
Analysis of GUS gene expression
For histochemical analysis, rootlets, leaves, leaf sheaths, and stalks cut into ∼5 mm sections and maturing seeds at 7, 12, and 17 days after flowering (DAF) sectioned longitudinally with a razor blade were stained with X-Gluc (5-brom-4-chloro-3-indolyl glucuronide) as described previously (Qu and Takaiwa, 2004). Fluorometric assays of GUS activities were conducted according to Jefferson (1987), with maturing seeds at 17 DAF used as samples. Three seeds from each independent transgenic plant were assayed.
Results
Isolation of rice glutelin gene promoters
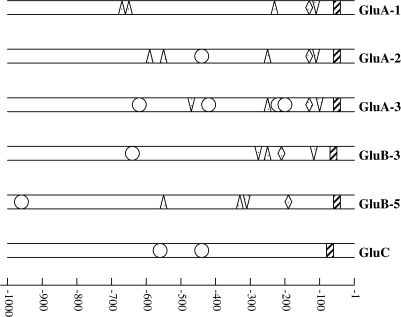
The 2 kb or 2.3 kb PCR fragment containing the promoter and the 5′-untranslated region (UTR) of the rice glutelin genes GluA-1, GluA-2, GluA-3, GluB-3, GluB-5, and GluC was amplified from rice genomic DNA. The PCR fragments were inserted into a pBlue 7 T-vector, and their nucleotide sequences were determined. The nucleotide sequences showed 42.6–50.9% identity. The putative cis-elements that could be involved in the regulation of endosperm-specific expression of the putative promoters were investigated using the PLACE database. The putative motifs which exist in a 1 kb proximal region of each promoter are summarized in Fig. 1. The GluA-1, GluA-2, GluA-3, GluB-3, and GluB-5 promoters contained the endosperm-specific essential motifs GCN4, AACA, and the prolamin box. However, the GluC promoter did not contain these cis-elements. Except for GluA-1, all promoters contained the ACGT motif.
Fig. 1.
Location of putative cis-elements in the 1 kb proximal region of glutelin promoters. Negative numbers indicate the positions of nucleotides relative to the translation start site. The triangle represents the prolamin box; the inverted triangle represents the AACA motif; the circle represents the ACGT box; the lozenge represents the GCN4 motif; and the rectangle represents the TATA box.
Construction of promoter–GUS chimeric genes and production of transgenic plants
This study aimed to characterize the expression pattern and promoter activities of glutelin genes expressed in rice seed rather than investigating the putative regulatory elements. The six putative promoters isolated were linked to the GUS reporter gene on the binary vector pGPTV-35S-HPT for transcriptional fusion. The chimeric promoter–GUS transcription fusions were introduced into the rice cultivar Kitaake via Agrobacterium-mediated transformation. The successful insertion of the chimeric gene was confirmed by PCR with genomic DNA isolated from leaves of independent transformants. From 5–22 positive individuals were obtained from each of the constructions. The promoter properties of these transformants were analysed.
Glutelin gene promoters direct GUS expression in endosperm
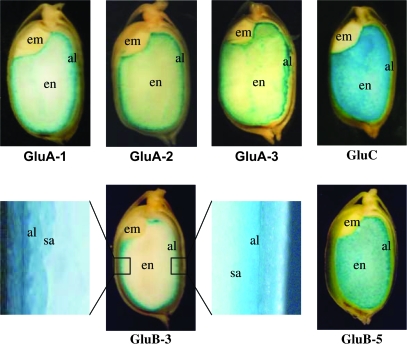
To determine the sites of GUS reporter gene expression directed by the glutelin promoters, roots, leaves, stalks, and seeds of transgenic rice were examined by RT–PCR and histochemical staining. Five seeds were obtained from individual transgenic plants 17 DAF (5–22 individuals), then longitudinally sectioned and stained with X-Gluc. A representative seed of each construct is shown in Fig. 2. The rice glutelin GluA-1, GluA-2, GluA-3, GluB-5, and GluC promoters directed GUS gene expression in the endosperm, whereas the GluB-3 promoter drove GUS expression in the aleurone and subaleurone layers (Fig. 2). No GUS expression was observed in the embryos of any transgenic plants.
Fig. 2.
Histochemical analysis of GUS expression in maturing seed of transgenic rice directed by various rice glutelin gene promoters. GUS protein was detected by incubating hand-cut longitudinal sections of transgenic seeds in X-Gluc solution. al, aleurone; em, embryo; en, endosperm; sa, subaleurone.
GUS activity differed greatly among the promoters in maturing seed. GUS activity of the GluA-1, GluA-2, and GluA-3 promoters was stronger in the outer portion but much weaker in the inner part of the endosperm. The GluB-5 and GluC promoters directed GUS expression in the whole endosperm, with the former expressed more strongly in the outer portion and the latter almost equally in the whole endosperm tissue (Fig. 2). It is of interest that GUS expression directed by the GluB-3 promoter was strictly limited to the aleurone and subaleurone layers of the endosperm.
In general, GUS expression was not observed in leaves, leaf sheaths, stalks, or roots of the transgenic rice on histochemical examination, and the uidA transcripts in these tissues were not detected by RT–PCR analysis (data not shown). These results indicated that the glutelin genes were expressed in an endosperm-specific manner.
Promoter activities during seed development
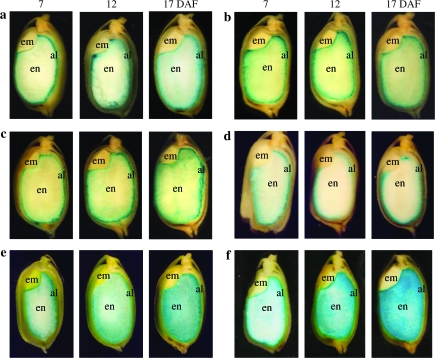
The temporal expression pattern of promoters during seed maturation was inspected in each transgenic line. The distribution of GUS expression was examined by histological staining of longitudinal sections of seed harvested at 7, 12, and 17 DAF. The site of the first detectable GUS expression was different for each construct (Fig. 3). GUS staining with GluA-1, GluA-2, GluA-3, and GluB-3 promoters was first observed in the aleurone and subaleurone layers at 7 DAF, whereas that with GluB-5 and GluC promoters was observed in the aleurone, subaleurone, and outer layers (Fig. 3). As the seed matured, GUS expression directed by the GluA-1, GluA-2, and GluA-3 promoters gradually spread to the inner portion of the endosperm (17 DAF), but the central part remained weakly stained (Fig. 3a–c). This expression pattern was remarkably different from that observed for the GluB-5 and GluC promoters: GUS staining was initiated in the outer portion of the starchy endosperm and rapidly spread throughout the whole endosperm (Fig. 3e, f). For the GluB-3 promoter, GUS staining was first observed in the peripheral region, i.e. in the aleurone and subaleurone layers, and remained unchanged during seed development (Fig. 3d).
Fig. 3.
Developmental time course of GUS activity directed by glutelin promoters during seed maturation. Histochemical X-Gluc staining of longitudinal sections of transgenic rice seeds at 7, 12, and 17 DAF. (a) GluA-1 promoter; (b) GluA-2 promoter; (c) GluA-3 promoter; (d) GluB-3 promoter; (e) GluB-5 promoter; (f) GluC promoter. al, aleurone; em, embryo; en, endosperm.
Potential strength of promoters
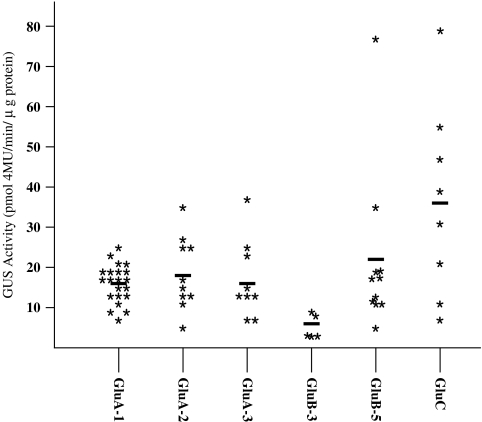
The potential strength of the various promoters was evaluated by determining the GUS expression levels in the maturing seed (17 DAF) from independent transgenic plants (5–22 individuals) for each construction. The promoter activities varied widely (Fig. 4). The seed promoters were classified into three groups on the basis of promoter strength. One promoter, GluC, showed high GUS activity; the mean GUS activity was 36.1±24.2 pmol 4MU min−1 μg−1 protein from eight individual transgenic plants. The medium GUS activity group consisted of four members: GluA-1, GluA-2, GluA-3, and GluB-5. The mean GUS activities for this group were 16.2±4.1, 19.7±9.5, 17.2±9.7, and 21.4±19.4 pmol 4MU min−1 μg−1 protein from 21, 10, 9, and 11 independent plants, respectively. The low GUS activity group had only one member, GluB-3, with GUS activity of 5.7±3.3 pmol 4MU min−1 μg−1 protein from five individuals.
Fig. 4.
GUS activity expressed using of various promoters in maturing seeds at 17 DAF. GUS activity is expressed as pmol 4MU min−1 μg−1 protein. Horizontal bars represent the average GUS activity.
Discussion
Endosperm is the storage organ for starch and protein for cereal crops. Improvement of endosperm composition and quality is one of the major tasks for breeders. Rice endosperm is an ideal platform for production of macromolecules such as recombinant protein, vitamins, and fats via biotechnology strategies (Paine et al., 2005; Takaiwa et al., 2007). Since many target traits are controlled by multiple genes, single gene transformation often has little effect on these traits. With progress in multigene transformation, suitable promoters must be developed to drive expression of these desired genes. Although some endosperm-specific expression promoters have been reported in maize, wheat, barley, and rice (Russell and Fromm, 1997; Lamacchia et al., 2001; Choi et al., 2003; Qu and Takaiwa, 2004), the number of such promoters is still too low to meet the demand for multigene stacking in plants.
The aim of the present work was to develop diverse promoters to drive multigene expression while avoiding the problem of homology-based gene silencing, and to provide ideal choices for high expression of foreign genes in transgenic rice endosperm. Transgenic plants carrying fusions of the six rice glutelin gene promoters, GluA-1, GluA-2, GluA-3, GluB-3, GluB-5, and GluC, showed strong GUS activity in maturing seeds (Fig. 2), whereas roots, leaves, sheaths, and stems showed only background GUS activity and no uidA mRNA expression (data not shown). Thus, the 5′-flanking regions of these glutelin genes were sufficient for endosperm specificity. The rice endosperm-specific promoters reported here can be added to the list of those helping to drive the expression of multiple genes targeting endosperm traits.
Although all of the six glutelin promoters drove GUS expression specifically in endosperm, their expression patterns within the endosperm varied greatly. The GluA subfamily gene promoters (GluA-1, GluA-2, and GluA-3) were specific to the outer endosperm, GluB-5 and GluC were specific to the whole endosperm, and GluB-3 was specific to the aleurone and subaleurone layers (Fig. 2). In general, the expression pattern of the GluA-3 promoter was similar to that reported previously (Wu et al., 1998a), except that the expression was slightly higher than that reported here. This difference might be caused by the different length of the GluA-3 promoter used. In addition, different transformation strategies might also play a role.
GUS activities varied significantly among the promoters. The highest GUS activity was obtained with GluC, followed by GluB-5, GluA-2, GluA-3, and GluA-1; GluB-3 showed the lowest GUS activity (Fig. 4). Except for GluB-3, the five rice glutelin promoters showed relatively high activity, as was found previously with glutelin gene promoters (Qu and Takaiwa, 2004).
The glutelin promoters reported here, together with those of seed storage protein gene promoters reported previously (Qu and Takaiwa, 2004), are good candidates for high expression of alien genes in rice endosperm without any detrimental effects on vegetative tissues. These promoters are also ideal for multigene expression in monocot endosperm while avoiding promoter homology-based gene silencing. Individual transgenic plants showed a wide range of activities for each construct, which reflect differences in insertion position and gene dosage effects (Hobbs et al., 1993). The highest individual GUS activity was 75.5 pmol 4MU min−1 μg−1 and 78.3 pmol 4MU min−1 μg−1 protein for the GluB-5 and GluC promoters, respectively, as compared with 23.1, 37.3, 38.2, and 9.6 pmol 4MU min−1 μg−1 protein for the GluA-1, GluA-2, GluA-3, and GluB-3 promoters, respectively (Fig. 4). These results suggest that the GluB-5 and GluC promoters could be used for a high level of production of recombinant protein with endosperm used as a bioreactor.
Most of the endosperm-specific expression promoters reported previously direct foreign gene expression mainly in the subaleurone layer and outer portion of the endosperm. There could be considerable loss of the target gene products expressed in cereal seeds under the control of these promoters after commercial polishing. The only inner endosperm-specific promoter is rice 26 kDa globulin Glb-1 (Qu and Takaiwa, 2004). The GluB-5 and GluC promoters directed GUS expression in the whole mature endosperm (Fig. 2). Of note, under the control of the GluC promoter, GUS expression in the inner portion of the endosperm was almost as strong as that in the peripheral region. This characteristic of the GluC promoter differentiates it from other rice glutelin promoters reported here and previously (Qu and Takaiwa, 2004). The GluB-5 and GluC promoters, together with the Glb-1 promoter, should perform well in developing health-promoting functional crops and producing recombinant protein in cereal seeds via biotechnology strategies.
It has been reported that some glutelin pseudogenes exist in rice; an example is GluB-3, which was produced by a stop codon in its open reading frame (Takaiwa et al., 1991). However, these pseudogenes cannot be easily found in the rice genomic database. Reports relating to the properties of pseudogene promoters are rare. The GluB-3 promoter showed strict tissue specificity and expression only in the aleurone and subaleurone layers (Fig. 2). This observation is particularly interesting, because the aleurone and subaleurone are layers of cells differentiated from the starchy endosperm during seed development. All of the rice glutelin promoters reported here and previously direct GUS gene expression in the aleurone, subaleurone, and outer and/or inner endosperm layers (Qu and Takaiwa, 2004). Thus, GluB-3 is a unique promoter and may be useful for investigating the metabolism of components in the aleurone and subaleurone layers.
To date, most endosperm-specific promoter studies have focused on identifying the cis-elements in promoters. The GCN4 and AACA motifs were reported to be required for endosperm-specific expression, and they are conserved in all glutelin gene promoters reported so far (Takaiwa et al., 1996; Washida et al., 1999; Qu and Takaiwa, 2004). The GCN4 motif is essential for endosperm specificity and is a key element conferring aleurone- and subaleurone-specific expression (Takaiwa et al., 1996; Wu et al., 1998b). However, Vickers et al. (2006) reported that the GCN4 motif did not affect the endosperm specificity of an oat globulin promoter (AsGlo1), but a cis-acting element [endosperm-specific palindrome (ESP), ACATGTCATCATGT] was required for endosperm specificity. Also, the prolamin box (also called the –300 element or endosperm box), which is conserved in many seed storage protein genes (Muller and Knudsen, 1993), plays a role in the endosperm-specific expression of prolamin genes (Colot et al., 1987; Ueda et al., 1994). The PLACE database (Higo et al., 1999) was searched for the motifs that could be involved in the endosperm expression of the six glutelin promoters, although the main purpose of the present study was not to investigate the putative cis-elements affecting endosperm specificity. Surprisingly, the GluC promoter did not contain the GCN4 motif or ESP element (Fig. 1), which was similar to the case of the Glb-1 promoter reported previously (Qu and Takaiwa, 2004). In addition, the GluC promoter did not contain the AACA motif and prolamin box (Fig. 1). These results indicate that multiple complex regulatory mechanisms may be involved in determining the endosperm specificity of rice glutelin promoters. The dissection of the GluC promoter will facilitate our understanding of the regulatory mechanism underlying endosperm-specific promoters.
Acknowledgments
This work was supported by the Natural Science Foundation of China (no. 30470971 and 30571035) and the Knowledge Innovation Program of the Chinese Academy of Sciences (no. KSCX2-YW-N-009).
References
- Bajaj S, Mohanty A. Recent advances in rice biotechnology—towards genetically superior transgenic rice. Plant Biotechnology Journal. 2005;3:275–307. doi: 10.1111/j.1467-7652.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- Battraw MJ, Hall TC. Histochemical analysis of CaMV 35S promoter-beta-glucuronidase gene expression in transgenic rice plants. Plant Molecular Biology. 1990;15:527–538. doi: 10.1007/BF00017828. [DOI] [PubMed] [Google Scholar]
- Bhullar S, Chakravarthy S, Advani S, Datta S, Pental D, Burma PK. Strategies for development of functionally equivalent promoters with minimum sequence homology for transgene expression in plants: cis-elements in a novel DNA context versus domain swapping. Plant Physiology. 2003;132:988–998. doi: 10.1104/pp.103.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QJ, Zhou HM, Chen J, Wang XC. A Gateway-based platform for multigene plant transformation. Plant Molecular Biology. 2006;62:927–936. doi: 10.1007/s11103-006-9065-3. [DOI] [PubMed] [Google Scholar]
- Cheon BY, Kim HJ, Oh KH, Bahn SC, Ahn JH, Chjoi JW, OK SH, Bae JM, Shin JS. Overexpression of human erythropoietin (EPO) affects plant morphologies: retarded vegetative growth in tobacco and male sterility in tobacco and Arabidopsis. Transgenic Research. 2004;13:541–549. doi: 10.1007/s11248-004-2737-3. [DOI] [PubMed] [Google Scholar]
- Choi HW, Lemaux PG, Cho MJ. Long-term stability of transgene expression driven by barley endosperm-specific hordein promoters in transgenic barley. Plant Cell Reports. 2003;21:1108–1120. doi: 10.1007/s00299-003-0630-9. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, themal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Molecular Biology. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Colot V, Robert LS, Kavanagh TA, Bevan MW, Thompson RD. Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO Journal. 1987;6:3559–3564. doi: 10.1002/j.1460-2075.1987.tb02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant-Sych Y, Okita TW. Identification of positive and negative regulatory cis-elements of the rice glutelin Gt3 promoter. Plant Science. 1996;116:27–35. [Google Scholar]
- Delaney DE. Choice of crop species and development of transgenic product lines. In: Hood EE, Howard JA, editors. Plants as factories for protein production. Dordrecht, The Netherlands: Kluwer Academic; 2002. pp. 139–158. [Google Scholar]
- Drakakaki G, Christou P, Stoger E. Constitutive expression of soybean ferritin cDNA in transgenic wheat and rice results in increased iron levels in vegetative tissues but not in seeds. Transgenic Research. 2000;9:445–452. doi: 10.1023/a:1026534009483. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SLA, Warkentin TD, DeLong CMO. Transgene copy number can be positively or negatively associated with transgene expression. Plant Molecular Biology. 1993;21:17–26. doi: 10.1007/BF00039614. [DOI] [PubMed] [Google Scholar]
- Horvath H, Huang J, Wong O, Kohl E, Okita T, Kannangara CG, von Wettstein D. The production of recombinant proteins in transgenic barley grains. Proceedings of the National Academy of Sciences, USA. 2000;97:1914–1919. doi: 10.1073/pnas.030527497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Molecular Biology Reports. 1987;5:387–405. [Google Scholar]
- Katsube T, Kurisaka N, Ogawa M, Maruyama N, Ohtsuka R, Utsumi S, Takaiwa F. Accumulation of soybean glycinin and its assembly with the glutelins in rice. Plant Physiology. 1999;120:1063–1073. doi: 10.1104/pp.120.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Miyahara K, Iida S, Fukuoka H, Takano T, Sassa H, Mishimura M, Nishio T. Low glutelin content1: a dominant mutation that suppresses the glutelin multigene family via RNA silencing in rice. The Plant Cell. 2003;15:1455–1467. doi: 10.1105/tpc.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia C, Shewry PR, Fonzo ND, Forsyth JL, Harris N, Lazzeri PA, Napier JA, Halford NG, Barcelo P. Endosperm-specific activity of a storage protein gene promoter in transgenic wheat seed. Journal of Experimental Botany. 2001;52:243–250. [PubMed] [Google Scholar]
- Lin L, Liu YG, Xu X, Li B. Efficient linking and transfer of multiple genes by a multigene assembly and transformation vector system. Proceedings of the National Academy of Sciences, USA. 2003;100:5962–5967. doi: 10.1073/pnas.0931425100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsukawa N, Hayashi H, Yamamoto K, Kidzu K, Konishi R, Masumura T, Tanaka K. Molecular cloning of a novel glutelin cDNA from rice seeds. Plant Biotechnology. 1998;15:205–211. [Google Scholar]
- Mol JN, Stuitje AR, van der Korl A. Genetic manipulation of floral pigmentation genes. Plant Molecular Biology. 1989;13:287–294. doi: 10.1007/BF00025316. [DOI] [PubMed] [Google Scholar]
- Muller M, Knudsen S. The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. The Plant Journal. 1993;4:343–355. doi: 10.1046/j.1365-313x.1993.04020343.x. [DOI] [PubMed] [Google Scholar]
- Paine JA, Shipton CA, Chaggar S, et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nature Biotechnology. 2005;23:482–487. doi: 10.1038/nbt1082. [DOI] [PubMed] [Google Scholar]
- Qu LQ, Takaiwa F. Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnology Journal. 2004;2:113–125. doi: 10.1111/j.1467-7652.2004.00055.x. [DOI] [PubMed] [Google Scholar]
- Qu LQ, Yoshihara T, Ooyama A, Goto Y, Takaiwa F. Iron accumulation does not parallel the high expression level of ferritin in transgenic rice seeds. Planta. 2005;222:225–233. doi: 10.1007/s00425-005-1530-8. [DOI] [PubMed] [Google Scholar]
- Russell DA, Fromm ME. Tissue-specific expression in transgenic maize of four endosperm promoters from maize and rice. Transgenic Research. 1997;6:157–168. doi: 10.1023/a:1018429821858. [DOI] [PubMed] [Google Scholar]
- Takagi H, Saito S, Yang LJ, Nagasaka S, Nishizawa N, Takaiwa F. Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnology Journal. 2005;3:521–533. doi: 10.1111/j.1467-7652.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Kikuchi S, Oono K. A rice glutelin gene family. A major type of glutelin mRNAs can be divided into two classes. Molecular and General Genetics. 1987;208:15–22. [Google Scholar]
- Takaiwa F, Oono K, Wing D, Kato A. Sequences of three members and expression of a new major subfamily of glutelin genes from rice. Plant Molecular Biology. 1991;17:875–885. doi: 10.1007/BF00037068. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Takagi H, Hirose S, Wakasa Y. Endosperm tissue is a good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnology Journal. 2007;5:84–92. doi: 10.1111/j.1467-7652.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Takaiwa F, Yamanouchi U, Yoshihara T, Washida H, Tanabe F, Kato A, Yamada K. Characterization of common cis-regulatory elements responsible for the endosperm-specific expression of members of the rice glutelin multigene family. Plant Molecular Biology. 1996;30:1207–1221. doi: 10.1007/BF00019553. [DOI] [PubMed] [Google Scholar]
- Ueda T, Wang Z, Pham N, Messing J. Identification of a transcriptional activator-binding element in the 27-kilodalton zein promoter, the –300 element. Molecular and Cellular Biology. 1994;14:4350–4359. doi: 10.1128/mcb.14.7.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Xue G, Gresshoff PM. A novel cis-acting element, ESP, contributes to high-level endosperm-specific expression in an oat globulin promoter. Plant Molecular Biology. 2006;62:195–214. doi: 10.1007/s11103-006-9014-1. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Yasuda H, Takaiwa F. High accumulation of bioactive peptide in transgenic rice seeds by expression of introduced multiple genes. Plant Biotechnology Journal. 2006;4:499–510. doi: 10.1111/j.1467-7652.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Washida H, Wu CY, Suzuki A, Yamanouchi U, Akihama T, Harada K, Takaiwa F. Identification of cis-regulatory elements required for endosperm expression of the rice storage protein glutelin gene GluB-1. Plant Molecular Biology. 1999;40:1–12. doi: 10.1023/a:1026459229671. [DOI] [PubMed] [Google Scholar]
- Wiley PR, Tosi P, Evrard A, Lovegrove A, Jones HD, Shewry PR. Promoter analysis and immunolocalisation show that puroindoline genes are exclusively expressed in starchy endosperm cells of wheat grain. Plant Molecular Biology. 2007;64:125–136. doi: 10.1007/s11103-007-9139-x. [DOI] [PubMed] [Google Scholar]
- Wu CY, Adachi T, Hatano T, Washida H, Suzuki A, Takaiwa F. Promoters of rice seed storage protein genes direct endosperm-specific gene expression in transgenic rice. Plant and Cell Physiology. 1998a;39:885–889. [Google Scholar]
- Wu CY, Suzuki A, Washida H, Takaiwa F. The GCN4 motif in a rice glutelin gene is essential for endosperm-specific gene expression and is activated by Opaque-2 in transgenic rice plants. The Plant Journal. 1998b;14:673–983. doi: 10.1046/j.1365-313x.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Yang LJ, Suzuki K, Hirose S, Wakasa Y, Takaiwa F. Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnology Journal. 2007;5:815–826. doi: 10.1111/j.1467-7652.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Yang LJ, Tada Y, Yamamoto MP, Zhao H, Yoshikawa M, Takaiwa F. A transgenic rice seed accumulating an anti-hypertensive peptide reduces the blood pressure of spontaneously hypertensive rats. FEBS Letters. 2006;580:3315–3320. doi: 10.1016/j.febslet.2006.04.092. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Tada Y, Hayashi Y, Jomori T, Takaiwa F. Expression of the small peptide GLP-1 in transgenic rice. Transgenic Research. 2005;14:677–684. doi: 10.1007/s11248-005-6631-4. [DOI] [PubMed] [Google Scholar]
- Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Takaiwa F. cis-regulatory elements responsible for quantitative regulation of the rice seed storage protein glutelin GluA-3 gene. Plant and Cell Physiology. 1996;37:107–111. doi: 10.1093/oxfordjournals.pcp.a028907. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kawagoe Y, Xiao S, Li Z, Okita TW, Hau TL, Lin A, Murai N. 5′ Distal and proximal cis-acting regulation elements are required for developmental control of a rice seed storage protein glutelin gene. The Plant Journal. 1993;4:357–366. doi: 10.1046/j.1365-313x.1993.04020357.x. [DOI] [PubMed] [Google Scholar]