Abstract
Enzymes that reduce the aldehyde chemical grouping (i.e. H-C=O) to its corresponding alcohol are probably crucial in maintaining plant health during stress. Succinic semialdehyde (SSA) is a mitochondrially-generated intermediate in the metabolism of γ-aminobutyrate (GABA), which accumulates in response to a variety of biotic and abiotic stresses. SSA can be reduced to γ-hydroxybutyrate (GHB) under oxygen deficiency and high light conditions. Recent evidence indicates that distinct cytosolic and plastidial glyoxylate reductase isoforms from Arabidopsis (designated hereinafter as AtGR1 and AtGR2, respectively) catalyse the in vitro conversion of SSA to GHB, as well as glyoxylate to glycolate, via NADPH-dependent reactions. In the present report, the responses of GHB and related amino acids, as well as NADP+ and NADPH, were monitored in leaves from Arabidopsis or tobacco plants subjected to various abiotic stresses (i.e. Arabidopsis during exposure to salinity, drought, submergence, cold, or heat; tobacco during exposure to, and recovery from, submergence). Time-course experiments revealed that GHB accumulated in both Arabidopsis and tobacco plants subjected to stress, and that this accumulation was generally accompanied by higher GABA and alanine levels, higher NADPH/NADP+ ratio, and lower glutamate levels. Furthermore, the analysis of gene expression in Arabidopsis revealed that the relative abundance of GR1 (salinity, drought, submergence, cold, and heat) and GR2 (cold and heat) transcripts was enhanced by the stress tested. Thus, GHB accumulation in plants is a general response to abiotic stress and appears to be regulated by both biochemical and transcriptional processes.
Keywords: Abiotic stress, aldehyde detoxification, γ-hydroxybutyrate, glyoxylate reductase, redox homeostasis, succinic semialdehyde reductase
Introduction
Under some stress conditions, aldehydes can accumulate in plants and react with DNA, oxidize membrane lipids, modify proteins, or influence the transcription of stress-related genes, thereby causing cellular and developmental problems (Weber et al., 2004; Kotchoni et al., 2006). Consequently, metabolic pathways and enzymes that reduce the aldehyde chemical grouping (i.e. H-C=O) to its corresponding alcohol are probably essential for maintaining plant health.
Succinic semialdehyde (SSA) is a mitochondrially-generated intermediate in the metabolism of γ-aminobutyrate (GABA), which accumulates in response to a variety of biotic and abiotic stresses (Bown and Shelp, 1997; Shelp et al., 1999). SSA is typically oxidized to succinate (Tuin and Shelp, 1994; Bouché et al., 2003), but evidence for the reduction of SSA to γ-hydroxybutyrate (GHB) is also available (Breitkreuz et al., 2003), at least under oxygen deficiency and high light (Allan et al., 2003a, b; Breitkreuz et al., 2003; Fait et al., 2005). Glyoxylate is a peroxisomally-generated intermediate of glycolate metabolism or photorespiration, a pathway believed to be particularly important under conditions of high light and temperature (Wingler et al., 2000). Glyoxylate is typically transaminated to Gly, but it can also undergo reduction to glycolate (Givan and Kleczkowski, 1992). Recently, recombinant expression of a cytosolic enzyme from Arabidopsis thaliana (L.) Heynh (designated hereinafter as glyoxylate reductase 1 or AtGR1; EC 1.1.1.79) revealed that it effectively catalyses the in vitro NADPH-dependent reduction of both SSA and glyoxylate (Hoover et al., 2007a). Part way through the present study, the existence of a plastidial enzyme (designated hereinafter as glyoxylate reductase 2 or AtGR2) that catalysed the same reactions in vitro was also demonstrated (Simpson et al., 2008).
Here, the responses of GHB and related amino acids (GABA, Ala, Glu), as well as NADP+ and NADPH, were monitored in mature leaves from Arabidopsis or tobacco plants subjected to various abiotic stresses (i.e. Arabidopsis during exposure to salinity, drought, submergence, cold, or heat; tobacco during exposure to, and recovery from, submergence) known to cause GABA accumulation. Other amino acids that are associated with primary N metabolism (Gln, Asp, Asn), photorespiratory N metabolism (Gly, Ser), and stress (Pro) were measured in order to gauge the broader impact of the stress. Furthermore, GR1 or GR1 and GR2 expression was monitored in Arabidopsis plants subjected to the various stresses. The results indicate that GHB accumulation is a general response to abiotic stress, and suggest that both redox balance and GR isoforms are involved in the regulation of SSA detoxification in planta.
Materials and methods
Plant material
Arabidopsis thaliana (L.) Heynh ecotype Columbia seeds were sown on Fox sandy loam (pH 6.5) in 5 cm pots, stratified at 4 °C in the dark for 48 h and grown under an 11 h photoperiod (06.00–17.00 h) in environmentally-controlled growth chambers (Controlled Environments Ltd., Winnipeg, Manitoba) set at 22/18 °C day/night temperatures, a photosynthetic photon flux density of 150 μmol m−2 s−1 at the top of the pots (supplied by a combination of cool white fluorescent and incandescent lighting; Sylvania, Mississauga, Canada), and 65% relative humidity. Arabidopsis plants were sub-irrigated with tap water as needed and fertilized once weekly with 20-20-20 fertilizer. Tobacco (Nicotiana tabacum L. cv. Samsun NN) plants were grown under a 16 h photoperiod (06.00–22.00 h) in a greenhouse with supplemental lighting and temperature as described previously (Scott-Taggart et al., 1999). Seeds were sown on Fox sandy loam soil (pH 6.5) in 5 cm diameter pots and grown for 3 weeks. Seedlings were watered with tap water as needed and fertilized once weekly with 20-20-20 fertilizer (Plant Products Co. Ltd., Brampton, Ontario, Canada). Individual seedlings were transferred to 30 cm diameter pots containing the same soil type, and grown for six more weeks with once daily watering with tap water and bi-weekly fertilization with 20–20–20.
Arabidopsis exposure to drought, salinity, submergence, cold or heat
Five different stresses (drought, salinity, submergence, cold, heat) were individually applied in separate experiments. In all experiments, pots were individually arranged in trays that were randomly located within a single growth chamber; water and nutrients were supplied via sub-irrigation, and treatments commenced when plants were 18–27 d old. For the drought experiment, sub-irrigation was withheld from one-half of the plants for up to 9 d, and plants were harvested after 0–9 d at 11.00 h. For the salinity experiment, one-half of the plants were sub-irrigated with 250 mM NaCl solution, with the other half receiving tap water, and plants were harvested at 0–24 h after first light (06.00 h). For the submergence experiment, plants were divided into two basins, one of which was filled with water at ambient temperature. The basins were covered tightly with tinfoil, and plants were harvested under dim light for up to 6 h, beginning at 11.00 h. For the cold and hot experiments, the plants were divided into two covered basins at 11.00 h and 13.00 h, respectively; one each was placed in a Kelvinator refrigerated cabinet set at 4 °C or an incubator (model 1535, VWR Scientific Products, Mississauga, Canada) set at 40 °C, and on the adjacent benchtop, and plants were harvested at 0–6 h thereafter. In all experiments, the shoots were rapidly frozen by dipping into liquid nitrogen for 10 s and then clipped at soil level into liquid nitrogen. The rosette leaves were separated from the stem and stored at –80 °C until further analysis. Each sample consisted of 3–4 replicates, containing 3–4 pooled plants. While the submergence experiment was repeated in its entirety, the results for one experiment are shown here.
Tobacco submergence and recovery
Beginning at 18.00 h, experimental plants were submerged in water equilibrated to the temperature of the greenhouse, for up to 9 h and then allowed to recover from the stress for a further 9 h. Experimental and control plants were placed in 50 l garbage pails with tight-fitting lids for the duration of the experiment. Recovering plants were transferred in the unlit greenhouse at 3, 6, and 9 h after the onset of submergence from water-filled pails to dry pails. The most fully expanded leaf was sampled from experimental and control plants (n=3 for each datum) over the 18 h time-course by dipping that leaf into liquid nitrogen for 20 s and then snipping it from the shoot directly into liquid nitrogen. All samples were subsequently stored at –80 °C until further analysis.
Extraction and analysis of GHB, amino acids, and phosphorylated pyridine nucleotides
GHB and soluble amino acids were extracted from frozen leaves (300 mg aliquot) and analysed by high performance liquid chromatography essentially as described previously (Allan et al., 2003a, b; Allan and Shelp, 2006). Total amino acids (TAA) were estimated from the amino acid profiles. Phosphorylated pyridine nucleotides (NADP+, NADPH) were extracted from frozen leaf tissue (50 mg aliquot) and assayed as described in Gibon and Larher (1997) except for the following modifications. After the neutralization of the acidic and basic extracts, 0.1 ml 16 mM phenazine ethosulphate was added and the extracts were incubated on ice for 30 min before being added to the assay solution. After the enzyme-cycling assay was completed, the reactions were stopped by adding 0.5 ml 6 M NaCl, followed by cooling on ice for 10 min. These solutions were centrifuged for 5 min (10 000 g, 4 °C) and the supernatant was carefully siphoned off. The pellet was then washed in 1 ml distilled water and solubilized in 1 ml 96% cold ethanol, and the absorbance of the formazan from 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide was determined at 570 nm.
Expression and analysis of GR1 and GR2 in Arabidopsis
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed using the Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) with a Bio-Rad iCycler (Hercules, CA). The following pairs of gene specific primers were used: GR1 gene (5′-TCGTAGAAGGTCCGGTTTCA-3′, 5′-AAACTCCTTAGTTAGGGTCG-3′); GR2 gene (5′-AAG GAT ACC GGA GCC TTG TT-3′, 5′-TCCACTGTTCGGCGATATGC-3′) and the 18S ribosomal RNA gene (5′-TCTGGCTTGCTCTGATGATT-3′, 5′-TCGAAAGTTGATAGGGCAGA-3′). For analysis of gene expression for GR1 and GR2 under stress, total RNA was isolated from the leaves of the Arabidopsis plants under each experimental condition for each time point and treated with Turbo DNase (Ambion, Austin, TX). For cDNA synthesis 300 ng of total RNA was incubated with 1 μl of random hexamer primers, brought up to 15 μl with RNase free water, incubated at 75 °C for 10 min and chilled on ice. Two microlitres of 10× reaction buffer, 40 units of RNase inhibitor (Ambion), and 10 nM dNTPs were added and then incubated at 25 °C for 10 min. One hundred units M-MLV reverse (Ambion) were added to bring the final reaction volume to 20 μl, then the reaction was incubated at 37 °C for 1 h and inactivated at 95 °C for 10 min. Each quantitative PCR reaction used 1× SYBR Green qPCR mix, 0.2 μM forward and reverse primers and 1 μl of cDNA in a 20 μl volume. All tubes were subjected to 3 min at 95 °C, followed by 40 cycles of 95 °C for 20 s, 60 °C for 20 s and 72 °C for 20 s. SYBR Green absorbance was detected at 72 °C and all reactions were conducted in triplicate. GR1 or GR2 transcript abundance in each sample was normalized to the corresponding level of 18S ribosomal RNA.
Results
Stress responsiveness of metabolites and phosphorylated pyridine nucleotides in Arabidopsis and tobacco
Six separate experiments were conducted, five with Arabidopsis (salinity, drought, submergence, cold, and heat) and another with tobacco (submergence). For those involving Arabidopsis, the pool sizes of metabolites and phosphorylated pyridine nucleotides in control plants varied somewhat because of differences in plant age at the beginning of the experiments, the duration of the experiments, and the time of harvest (see Allan and Shelp, 2006). It is significant that, where appropriate (i.e. submergence, cold, heat), control plants were placed in the dark to eliminate the complicating effects of the dark environment that was superimposed during treatment.
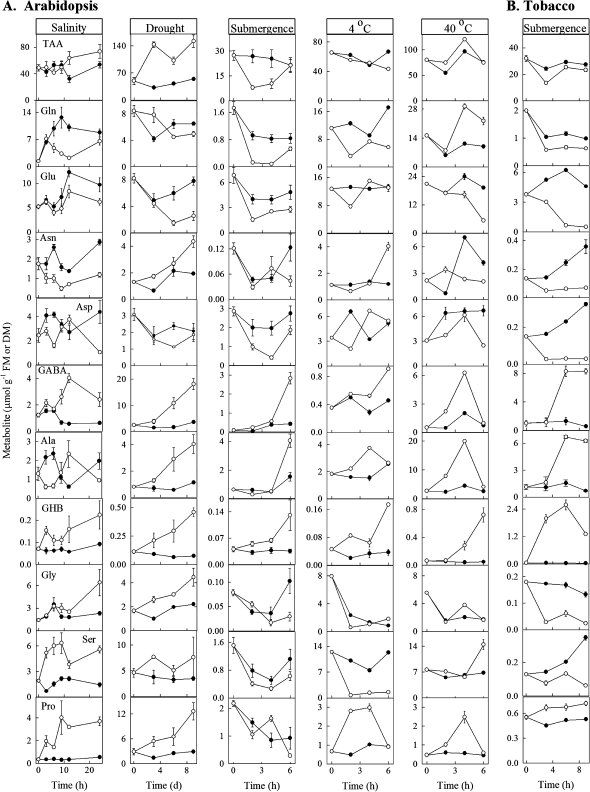
With one exception (i.e. Arabidopsis during submergence), leaf Pro accumulated in plants of both species subjected to the various treatments, indicating that these treatments initiated the stress response (Fig. 1). In all cases and regardless of the plant species, the imposition of stress increased the GHB concentration over the duration of the time-course used. With only two obvious exceptions (i.e. Ala under salinity, Glu under cold), the patterns for GHB-related metabolites were consistent, with GABA and Ala being positively related, and Glu being negatively related with GHB. The patterns for other amino acids, as well as TAA, were not consistent across the various stresses.
Fig. 1.
Response of metabolites in mature rosette leaves of Arabidopsis plants (A) and mature leaves of tobacco plants (B) subjected to salinity, drought, submergence, cold or heat. Closed and open circles represent control and experimental plants, respectively. Data represent the mean ±SE; where the bar is not shown, it is within the symbol. Note that the drought data only are expressed on a DM basis, rather than a FM basis. TAA represents total amino acids.
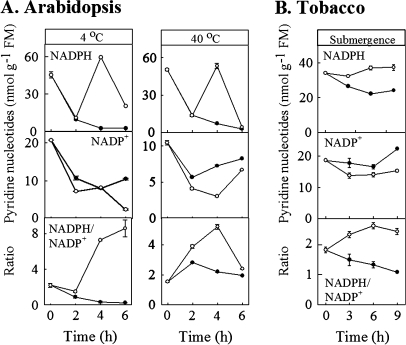
In three of the six experiments, the concentrations of phosphorylated pyridine nucleotides were also determined (Arabidopsis under cold or heat conditions, tobacco during submergence) (Fig. 2). In these cases, the plants exhibited a temporary increase in NADPH concentration together with general decline in NADP+, resulting in enhanced NADPH/NADP+ ratios over most of the time-course.
Fig. 2.
Response of phosphorylated pyridine nucleotides in mature rosette leaves of Arabidopsis plants (A) and mature leaves of tobacco plants (B) subjected to cold, heat or submergence. Closed and open circles represent control and experimental plants, respectively. Data represent the mean ±SE; where the bar is not shown, it is within the symbol.
Recovery of GHB and related metabolites, and phosphorylated pyridine nucleotides in tobacco after removal from submergence
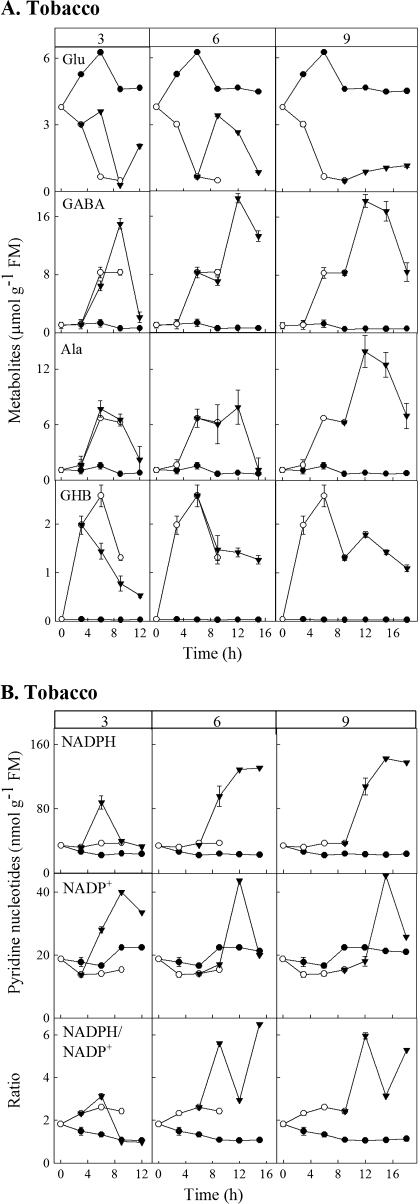
In the submergence experiment with tobacco, plants were removed from the stress at various times to test their ability to recover within a subsequent 9 h period. When plants were removed 3 h after submergence, the GHB concentration decreased immediately in a linear fashion, although it was still higher than that in control plants at the end of the time-course (Fig. 3A). By contrast, the concentrations of GABA and Ala continued to increase as in submerged plants before declining to control values, whereas the concentration of Glu exhibited a temporary increase and then approached control values. Also, there was a temporary increase in NADPH and NADPH/NADP+, which returned to control values; curiously NADP+ exhibited a longer term increase, followed by a decline (Fig. 3B). Increasing the time of submergence before transfer to air generally decreased the capacity of plants to recover from the stress; particularly evident were the unwavering GHB and NADPH concentrations and fluctuating NADPH/NADP+ ratios at the end of the time-course.
Fig. 3.
Recovery of GHB and related metabolites (A), as well as phosphorylated pyridine nucleotides (B), in mature leaves of tobacco plants after being subjected to submergence. The column headings indicate the duration of submergence (3, 6, or 9 h) before transfer to air for an additional 9 h. Data for control and submerged plants for the first 9 h are taken from Figs 1B and 2B. Closed circles, open circles, and closed triangles, respectively, represent control plants, submerged plants, and plants returned to air after a period of submergence. Data represent the mean ±SE; where the bar is not shown, it is within the symbol.
Stress responsiveness of GR transcripts in Arabidopsis
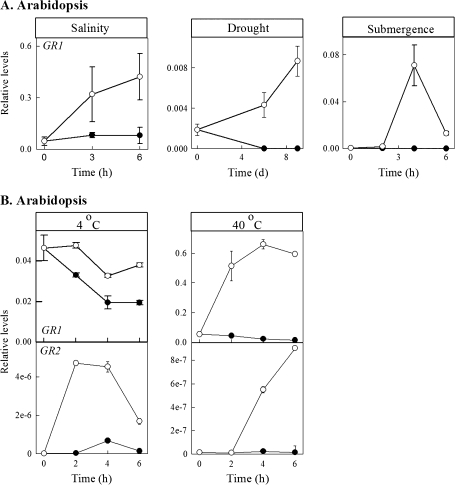
In three of the five experiments involving Arabidopsis (salinity, drought, submergence), GR1 transcript abundance was determined (Fig. 4A), whereas in the other two (cold and heat), the levels of both GR1 and GR2 transcripts were determined (Fig. 4B). In these cases, GR transcript abundance was consistently higher in treated plants than in control plants.
Fig. 4.
Response of GR transcripts in mature rosette leaves of Arabidopsis plants subjected to salinity, drought, submergence, cold, or heat. Closed and open circles represent control and experimental plants, respectively. Data represent the mean ±SE; where the bar is not shown, it is within the symbol.
Discussion
Impact of abiotic stress on GABA, Ala, and redox levels
GABA is a 4-carbon non-protein amino acid found in virtually all prokaryotic and eukaryotic organisms (Shelp et al., 1999). In plants, GABA is derived from Glu via the enzyme Glu decarboxylase (GAD). GAD is a cytosolic enzyme with an acidic pH optimum, which is stimulated by the increasing cytosolic H+ and Ca2+ levels that often accompany stress. Ca2+ in turn forms a complex with calmodulin, which binds to GAD, thereby relieving the enzyme from autoinhibition and causing the accumulation of GABA (Baum et al., 1993; Ling et al., 1994; Arazi et al., 1995; Snedden et al., 1995, 1996). A large number of studies have reported the accumulation of GABA and the concomitant loss of Glu in plant tissues and transport fluids in response to many abiotic stresses, including temperature shock, oxygen deficiency, cytosolic acidification, water stress and UV stress (Girousse et al., 1996; Valle et al., 1998; Allan et al., 2003a, b; Bartyzel et al., 2003/4; Breitkreuz et al., 2003; Fait et al., 2005; Ricoult et al., 2005; Kato-Noguchi and Ohashi, 2006; Mazzucotelli et al., 2006; Kaplan et al., 2007; see also references in Bown and Shelp, 1989, 1997; Shelp et al., 1999). Moreover, antisense suppression of GAD results in the accumulation of Glu in transgenic tomato fruit (Kisaka et al., 2006). In the present report, oxygen deficiency (submergence in water), water stress (salinity, drought) or temperature stress (4 °C, 40 °C) caused similar changes in the pool sizes of GABA and Glu in leaves of Arabidopsis and tobacco plants; the only possible exception was the relatively stable pool size of Glu at 4 °C (Fig. 1). It is noteworthy that all stresses tested, with the exception of Arabidopsis under submergence, resulted in the accumulation of Pro, findings that are consistent with the impact of abiotic stress in the literature (Kaplan et al., 2004, 2007).
SSA is produced from GABA via GABA transaminase (GABA-T), an enzyme that can apparently utilize either pyruvate or 2-oxoglutarate as an amino acceptor, resulting in the production of Ala and Glu, respectively (Shelp et al., 1995; Van Cauwenberghe and Shelp, 1999; Van Cauwenberghe et al., 2002). Evidence indicates that (i) Ala accumulation, rather than Glu accumulation, typically occurs in response to oxygen deficiency (see references in Bown and Shelp, 1989, and Miyashita et al., 2007), (ii) the primary role of an inducible Ala aminotransferase is to degrade Ala when it is in excess, for example, during recovery from oxygen deficiency (Miyashita et al., 2007), and (iii) hypoxia-induced Ala accumulation is partially inhibited in roots of gad1 and gaba-t1 knockout mutants (Miyashita and Good, 2008), indicating that pyruvate-dependent GABA-T reaction contributes to Ala formation during oxygen deficiency. In the present paper, Ala accumulation accompanied the accumulation of GABA during exposure to four of the five stresses (the exception being salinity) (Fig. 1).
Since GABA-T appears to be mitochondrial (Breitkreuz and Shelp, 1995; Van Cauwenberghe et al., 2002) GABA probably enters the mitochondrion, where it is converted to SSA, and then to succinate via an NAD-dependent SSA dehydrogenase (SSADH) (Breitkreuz and Shelp, 1995; Busch and Fromm, 1999). Biochemical characterization of plant SSADH revealed strong inhibition by NADH, as well as ADP and ATP (Busch and Fromm, 1999). Thus, under stress conditions such as oxygen deficiency, when the mitochondrion exhibits higher ratios of NADH:NAD+ and ADP:ATP, SSADH activity is probably restricted, contributing to the accumulation of SSA and feedback inhibition of GABA-T (Shelp et al., 1995; Busch and Fromm, 1999; Van Cauwenberghe and Shelp, 1999). Loss-of-function ssadh mutants are phenotypically less fit than wild-type Arabidopsis, exhibiting dwarf stature and less leaf area and chlorophyll concentration (Bouché et al., 2003). When these mutant plants are exposed to abiotic stresses, specifically high UV light and high temperature, they develop necrotic lesions and produce elevated levels of hydrogen peroxide, which is a reactive oxygen species.
Stress can cause the influx of Ca2+ and H+ into plant cells, thereby activating a number of metabolic pathways such as that involving GABA (Shelp et al., 1999; Buchanan et al., 2000). In addition, Ca2+/calmodulin activates NAD kinase, an enzyme responsible for adding phosphate to NAD+ or NADH to make NADP+ and NADPH, respectively (Harding et al., 1997; Hunt et al., 2004). In stressed plants, NADPH is required as a substrate for NADPH oxidase, a membrane protein that generates reactive oxygen species and results in activation of many stress tolerance genes and pathways in plants (Hunt et al., 2004). A balanced NAD(P)H:NAD(P)+ ratio is essential for efficient functioning of the electron transport chains on the mitochondrial and chloroplast membranes (Noctor, 2006). In the mitochondrion and chloroplast, NAD(P)+ acts as an electron acceptor for generating NAD(P)H, and, when these reducing equivalents accumulate under oxidative stress, damage could occur due to limited availability of the NAD(P)+ (Scheibe et al., 2005). Our studies confirmed that the NADPH/NADP+ ratios increase in response to oxygen deficiency and temperature stress (Fig. 2), and provide support for the involvement of redox balance in the response to stress.
GHB accumulation is a general response to abiotic stress
Other research suggests an additional coping mechanism for the detoxification of SSA, which involves its reduction to GHB. In animals, GABA functions as a signalling molecule during neurotransmission. Like plants, GABA in humans is catabolized in mitochondria to provide succinate to the Krebs cycle through an oxidative pathway (Maitre, 1997). Under oxygen deficiency, SSA is diverted to the production of GHB (Mamelak, 1989), a reaction catalysed via the enzyme SSA reductase, which is localized in the cytosol and uses NADPH as a cofactor (Schaller et al., 1999). GHB, a short chain fatty acid similar in structure to GABA, is normally present at about 1% of the GABA level (Pearl et al., 2003). This reductive pathway is proposed to minimize energy demands of the brain under stress (Mamelak, 1989). Accumulation of GHB in the brain due to a non-functional SSADH (i.e. recessive disorder called GHB aciduria) is associated with progressive mental retardation in humans. This phenotype is thought to be attributed to GHB competition with GABA for GABA receptors in the brain, thereby disrupting normal neural transmission (Pearl et al., 2003).
The first evidence for the occurrence of GHB in plants was presented in 2003 (Allan et al., 2003a, b; Breitkreuz et al., 2003). Oxygen deficiency increases GHB concentrations from about 10 to 155 nmol g−1 FM in soybean sprouts, and from 273 to 739 nmol g−1 DM in green tea leaves (Allan et al., 2003a). A cDNA [initially designated as γ-hydroxybutyrate dehydrogenase, but later renamed glyoxylate reductase 1 (GR1; Hoover et al., 2007a)], which encodes a 289 amino acid polypeptide, was identified via complementation of an SSADH-deficient yeast mutant with an Arabidopsis cDNA library (Breitkreuz et al., 2003). Overexpression of the cDNA enables growth of the mutant yeast on GABA and results in the accumulation of GHB. Furthermore, the concentrations of GHB and GABA increase in Arabidopsis plants during submergence, a condition that should increase the cellular NADH:NAD+ ratio, thereby inhibiting SSADH activity. Other work revealed that (i) ssadh mutant Arabidopsis plants, grown under high UV light, have five times the normal level of GHB and high levels of ROS (Fait et al., 2005), and (ii) the pattern of GHB in cold-acclimated Arabidopsis plants is consistent with the rise and fall of GABA (Kaplan et al., 2007). The present study provided the first evidence for GHB accumulation in response to water stress (salinity, drought) and heat stress, and confirmed that GHB accumulates under oxygen deficiency (submergence) and cold stress (Fig. 1). Together, these data indicate that GHB accumulation is a general response to abiotic stress.
Recovery of metabolite and redox levels after exposure to abiotic stress
Wallace et al. (1984) reported that changes in amino acid composition of leaflets of soybean plants exposed to 6 °C for 8 min are fully reversible within 1 h after being returned to their original growing conditions at 33 °C. Recently, Miyashita et al. (2007) showed that Ala levels in Arabidopsis plants return to normal within 24 h following a 24 h period in 5% oxygen; GABA was not assayed and Glu was unaffected by the stress. In the present study, the levels of GABA, Ala, GHB, and phosphorylated pyridine nucleotides (but not Glu) in mature tobacco leaves approached normality within 9 h following a 3 h period of submergence; 9 h was clearly inadequate for the recovery of metabolite and redox levels after 6 or 9 h of submergence (Fig. 3).
Role of GR isoforms in detoxification of SSA and glyoxylate
Recently, Hoover et al. (2007a) expressed the Arabidopsis GR1 cDNA in E. coli and purified the recombinant protein to homogeneity. Kinetic studies confirmed that the protein reduces SSA to GHB (Km=0.87 mM) in an NADPH-dependent and essentially irreversible reaction. Unexpectedly, further tests of substrate preference revealed that the protein also reduces glyoxylate (Km=4.5 μM), with a 250-fold greater preference than for SSA. The enzyme is dramatically inhibited by NADP+, but not GHB (Hoover et al., 2007b). A combination of web-based and recombinant expression tools revealed the existence of a highly homologous protein, designated as AtGR2, which also catalyses the irreversible, NADPH-dependent reduction of both SSA and glyoxylate to glycolate; GR2 has a 350-fold higher preference for glyoxylate than SSA, but the affinity for both substrates was an order of magnitude lower than that for GR1 (Simpson et al., 2008). Analysis of the transient expression of recombinant GR1 and GR2 in tobacco Bright Yellow 2 suspension cells revealed that they are localized to the cytosol and chloroplast, respectively (Simpson et al., 2008). These results are consistent with earlier biochemical evidence, which revealed the existence of distinct cytosolic (Km for glyoxylate of 70–100 μM) and chloroplastic (Km for glyoxylate of 85 μM) NADPH-dependent GRs in spinach (Kleczkowski et al., 1986; also see referencess in Givan and Kleczkowski, 1992). Also, the results lend support for the hypothesis that any glyoxylate escaping transamination to Gly in peroxisomes during photorespiration is scavenged (Givan and Kleczkowski, 1992; Hoover et al., 2007a; Simpson et al., 2008), thereby preventing the deactivation of the primary photosynthesis enzyme, ribulose bisphosphate carboxylase/oxygenase, by glyoxylate (Campbell and Ogren, 1990).
Analysis of specific gene expression using DNA micro-array technology or quantitative real-time RT-PCR, revealed that the abundance of GAD1, GABA-T1, and Ala aminotransferase1 transcripts is often elevated in plants subjected to low oxygen, water deficit or salinity (Klok et al., 2002; Cramer et al., 2007). One study, which used semi-quantitative RT-PCR, indicated that the global expression of several putative homologues of GAD and GABA-T are induced in barley during cold acclimation, but only in a frost-resistant cultivar is it maintained during subsequent freezing (Mazzucotelli et al., 2006). Another study utilized micro-array analysis to investigate gene expression in cold-acclimated Arabidopsis; increases in the transcript levels of two GAD genes precede the peak in GABA, thus demonstrating a characteristic transcript abundance-regulated response (Kaplan et al., 2007). GABA-T transcript abundance is unchanged during cold acclimation, whereas SSADH transcript abundance peaks after GABA and succinic semialdehyde reductase (presumably this identification is based on our earlier manuscript; Breitkreuz et al., 2003) is slightly down-regulated. Unfortunately, in these two studies it is unclear whether the control plants can adequately account for the conditions present during plant exposure to the cold treatment (i.e. dark). Rice GABA-T expression, as indicated by northern blot analysis, is induced by UV and abscisic acid, as well as blast fungal infection, mechanical damage, and salicylic acid, which are commonly associated with biotic stresses (Wu et al., 2006). Previous research from our laboratory suggested that the accumulation of GABA and GHB in Arabidopsis during submergence can not be attributed to the abundance of GABA-T and GR1 transcripts, as determined by relative RT-PCR (Breitkreuz et al., 2003).
In the present study, quantitative real-time RT-PCR was utilized to improve the specificity, sensitivity, and reliability of the gene expression analysis (Gachon et al., 2004), and the transcript abundance of GR1 or GR2 in Arabidopsis was consistently enhanced in response to the various abiotic stresses tested (submergence, drought, salinity, cold, heat) (Fig. 4). Since these abiotic stresses are clearly associated with the accumulation of both GABA and GHB, the results support the hypothesis that GR1 and GR2 are involved in the detoxification of SSA in planta, despite their higher affinity for glyoxylate than for SSA in vitro (Hoover et al., 2007a; Simpson et al., 2008). Currently, knockout mutants and additional environmental conditions (i.e. high light and temperature, altered CO2/O2 ratio) that influence photorespiration are being utilized in our laboratory to resolve the roles of GR1 and GR2 in the detoxification of SSA and glyoxylate in plants.
Summary
The aldehyde chemical grouping confers high reactivity on molecules (Bartels, 2001; Kotchoni et al., 2006). Thus, it is believed that: (i) aldehydes that accumulate under some stress conditions are highly toxic, reacting with DNA, oxidizing membrane lipids, and modifying proteins (Kotchoni et al., 2006), or influencing the transcription of stress-related genes (Weber et al., 2004), thereby causing cellular and overall developmental problems in the plant; and (ii) the induction and operation of reductases under stress conditions should contribute to eradication of aldehydes and redox homeostasis (Oberschall et al., 2000; Sunkar et al., 2003). Here, the imposition of abiotic stress on plants was associated with the accumulation of GHB, the product of SSA reduction, as well as altered redox levels and the induction of GR1 and GR2 genes. Thus, GHB levels appear to be regulated by a combination of biochemical and transcriptional processes. In future, GR1 and GR2 will be overexpressed in Arabidopsis in an attempt to genetically engineer improved abiotic stress tolerance.
Acknowledgments
This work was supported by funds to BJS from the Natural Sciences and Engineering Research Council of Canada. SMC was the recipient of an Ontario Graduate Scholarship.
Glossary
Abbreviations
- GABA
γ-aminobutyrate
- GABA-T
GABA transaminase
- GAD
Glu decarboxylase
- GHB
γ-hydroxybutyrate
- GR
glyoxylate reductase
- RT-PCR
reverse transcription-polymerase chain reaction
- SSA
succinic semialdehyde
- SSADH
succinic semialdehyde dehydrogenase
- TAA
total amino acids
References
- Allan WL, Peiris C, Bown AW, Shelp BJ. Gamma-hydroxybutyrate accumulates in green tea leaves and soybean sprouts in response to oxygen deficiency. Canadian Journal of Plant Science. 2003a;83:951–953. [Google Scholar]
- Allan WL, Shelp BJ. Fluctuations of gamma-aminobutyrate, gamma-hydroxybutyrate and related amino acids in Arabidopsis leaves as a function of the light-dark cycle, leaf age, and N stress. Canadian Journal of Botany. 2006;84:1339–1346. [Google Scholar]
- Allan WL, Smith R, Shelp BJ. Application Bulletin AB-0015. Agilent Technologies Inc., Mississauga, ON,: Canada; 2003b. Direct measurement of γ-hydroxybutyrate (GHB) in crude plant extracts by liquid chromatography/negative ion-ES mass spectrometry; p. 4 pp. [Google Scholar]
- Arazi T, Baum G, Snedden WA, Shelp BJ, Fromm H. Molecular and biochemical analysis of calmodulin interactions with the calmodulin-binding domain of plant glutamate decarboxylase. Plant Physiology. 1995;108:551–561. doi: 10.1104/pp.108.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D. Targeting detoxification pathways: an efficient approach to obtain plants with multiple stress tolerance. Trends in Plant Science. 2001;6:284–286. doi: 10.1016/s1360-1385(01)01983-5. [DOI] [PubMed] [Google Scholar]
- Bartyzel I, Pelczar K, Paskowski A. Functioning of the γ-aminobutyrate pathway in wheat seedlings affected by osmotic stress. Biologia Plantarum. 2003/4;47:221–225. [Google Scholar]
- Baum G, Chen Y, Arazi T, Takatsuji H, Fromm H. A plant glutamate decarboxylase containing a calmodulin binding domain. Cloning, sequence, and functional analysis. Journal of Biological Chemistry. 1993;268:19610–19617. [PubMed] [Google Scholar]
- Bouché N, Fait A, Bouchez D, Moller SG, Fromm H. Mitochondrial succinic-semialdehyde dehydrogenase of the gamma-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proceedings of the National Academy of Sciences, USA. 2003;100:6843–6848. doi: 10.1073/pnas.1037532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown AW, Shelp BJ. The metabolism and physiological roles of 4-aminobutyric acid. Biochemistry (Life Science Advances) 1989;8:21–25. [Google Scholar]
- Bown AW, Shelp BJ. The metabolism and functions of gamma-aminobutyric acid. Plant Physiology. 1997;115:1–5. doi: 10.1104/pp.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreuz KE, Shelp BJ. Subcellular compartmentation of the 4-aminobutyrate shunt in protoplasts from developing soybean cotyledons. Plant Physiology. 1995;108:99–103. doi: 10.1104/pp.108.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreuz KE, Allan WL, Van Cauwenberghe OR, Jakobs C, Talibi D, Andre B, Shelp BJ. A novel gamma-hydroxybutyrate dehydrogenase: identification and expression of an Arabidopsis cDNA and potential role under oxygen deficiency. Journal of Biological Chemistry. 2003;278:41552–41556. doi: 10.1074/jbc.M305717200. [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and molecular biology of plants. Rockville, USA: American Society of Plant Physiologists; 2000. [Google Scholar]
- Busch KB, Fromm H. Plant sucinic semialdehyde dehydrogenase. Cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiology. 1999;121:589–597. doi: 10.1104/pp.121.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL. Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation in intact, lysed, and reconstituted chloroplasts. Photosynthesis Research. 1990;23:257–268. doi: 10.1007/BF00034856. [DOI] [PubMed] [Google Scholar]
- Cramer GR, Ergül A, Grimplet J, et al. Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Functional and Integrative Genomics. 2007;7:111–134. doi: 10.1007/s10142-006-0039-y. [DOI] [PubMed] [Google Scholar]
- Fait A, Yellin A, Fromm H. GABA shunt deficiencies and accumulation of reactive oxygen intermediates: Insight from arabidopsis mutants. FEBS Letters. 2005;579:415–420. doi: 10.1016/j.febslet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Gachon C, Mingam A, Charrier B. Real-time PCR: what relevance to plant studies? Journal of Experimental Botany. 2004;55:1445–1454. doi: 10.1093/jxb/erh181. [DOI] [PubMed] [Google Scholar]
- Gibon Y, Lahrer F. Cycling assay for nicotinamide adenine dinucleotides: NaCl precipitation and ethanol solubilization of the reduced tetrazolium. Analytical Biochemistry. 1997;251:153–157. doi: 10.1006/abio.1997.2283. [DOI] [PubMed] [Google Scholar]
- Girousse C, Bournoville R, Bonnemain J-L. Water deficit-induced changes in concentrations of proline and some other amino acids in the phloem sap of alfalfa. Plant Physiology. 1996;111:109–113. doi: 10.1104/pp.111.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan CV, Kleczkowski LA. The enzymatic reduction of glyoxylate and hydroxypyruvate in leaves of higher plants. Plant Physiology. 1992;100:552–556. doi: 10.1104/pp.100.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SA, Oh SH, Roberts DM. Transgenic tobacco expressing a foreign calmodulin gene shows an enhanced production of active oxygen species. EMBO Journal. 1997;16:1137–1144. doi: 10.1093/emboj/16.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover GJ, Van Cauwenberghe OR, Breitkreuz KE, Clark SM, Merrill AR, Shelp BJ. Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta. Canadian Journal of Botany. 2007a;85:883–895. [Google Scholar]
- Hoover GJ, Prentice GA, Merrill AR, Shelp BJ. Kinetic mechanism of an Arabidopsis glyoxylate reductase: studies of initial velocity, dead-end inhibition and product inhibition. Canadian Journal of Botany. 2007b;95:896–902. [Google Scholar]
- Hunt L, Lerner F, Zeigler M. NAD: new roles in signaling and gene regulation in plants. New Phytologist. 2004;163:31–44. doi: 10.1111/j.1469-8137.2004.01087.x. [DOI] [PubMed] [Google Scholar]
- Kato-Noguchi H, Ohashi C. Effects of anoxia on amino acid levels in rice cotyledons. Plant Production Science. 2006;9:383–387. [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology. 2004;136:4159–4168. doi: 10.1104/pp.104.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Sung DY, Zhao W, Popp W, Porat R, Guy CL. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. The Plant Journal. 2007;50:967–981. doi: 10.1111/j.1365-313X.2007.03100.x. [DOI] [PubMed] [Google Scholar]
- Kisaka H, Kida T, Miwa T. Antisense suppression of glutamate decarboxylase in tomato (Lycopersicon esculentum L.) results in accumulation of glutamate in transgenic tomato fruits. Plant Biotechnology. 2006;23:267–274. [Google Scholar]
- Kleczkowski LA, Randall DD, Blevins DG. Purification and characterization of a novel glyoxylate-reductase from spinach leaves. Comparison of immunological properties of leaf glyoxylate reductase and hydroxypyruvate reductase. Biochemical Journal. 1986;239:653–959. doi: 10.1042/bj2390653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. The Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoni SO, Kuhns C, Ditzer A, Kirch HH, Bartels D. Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell and Environment. 2006;29:1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- Ling V, Snedden WA, Shelp BJ, Assmann SM. Analyses of a soluble calmodulin-binding protein from fava bean roots: identification as glutamate decarboxylase. The Plant Cell. 1994;6:1135–1143. doi: 10.1105/tpc.6.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Progress in Neurobiology. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Mamelak M. Gammahydroxybutyrate: an endogenous regulator of energy metabolism. Neuroscience and Biobehavioral Reviews. 1989;13:187–197. doi: 10.1016/s0149-7634(89)80053-3. [DOI] [PubMed] [Google Scholar]
- Mazzucotelli E, Tartari A, Cattivelli L, Forlani G. Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. Journal of Experimental Botany. 2006;57:3755–3766. doi: 10.1093/jxb/erl141. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Dolferus R, Ismond KP, Good AG. Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. The Plant Journal. 2007;49:1108–1121. doi: 10.1111/j.1365-313X.2006.03023.x. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Good AG. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant and Cell Physiology. 2008;49:92–102. doi: 10.1093/pcp/pcm171. [DOI] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Oberschall A, Deak M, Torok K, Sass L, Vass I, Kovacs I, Feher A, Dudits D, Horvath GV. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. The Plant Journal. 2000;24:437–446. doi: 10.1046/j.1365-313x.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Acosta MT, et al. Clinical spectrum of succinic semialdehyde dehydrogenase deficiency. Neurology. 2003;60:1413–1417. doi: 10.1212/01.wnl.0000059549.70717.80. [DOI] [PubMed] [Google Scholar]
- Ricoult C, Cliquet J-B, Limami AM. Stimulation of alanine amino transferase (AlaAT) gene expression and alanine accumulation in embryo axis of the model legume Medicago truncatula contribute to anoxia stress tolerance. Physiologia Plantarum. 2005;123:30–39. [Google Scholar]
- Schaller M, Schaffhauser M, Sans N, Wermuth B. Cloning and expression of succinic semialdehyde reductase from human brain: identity with aflatoxin B1 aldehyde reductase. European Journal of Biochemistry. 1999;265:1056–1060. doi: 10.1046/j.1432-1327.1999.00826.x. [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. Journal of Experimental Botany. 2005;56:1481–1489. doi: 10.1093/jxb/eri181. [DOI] [PubMed] [Google Scholar]
- Scott-Taggart CP, Van Cauwenberghe OR, McLean MD, Shelp BJ. Regulation of γ-aminobutyric acid synthesis in situ by glutamate availability. Physiologia Plantarum. 1999;106:363–369. [Google Scholar]
- Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends in Plant Science. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Walton CS, Snedden WA, Tuin LG, Oresnik IJ, Layzell DB. GABA shunt in developing soybean seeds is associated with hypoxia. Physiologia Plantarum. 1995;94:219–228. [Google Scholar]
- Simpson JP, Di Leo R, Dhanoa PK, Allan WL, Makhmoudova A, Clark SM, Hoover GJ, Mullen RT, Shelp BJ. Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification. Journal of Experimental Botany. 2008;59 doi: 10.1093/jxb/ern123. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden WA, Arazi T, Fromm H, Shelp BJ. Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiology. 1995;108:543–549. doi: 10.1104/pp.108.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden WA, Koutsia N, Baum G, Fromm H. Activation of a recombinant petunia glutamate decarboxylase by calcium/calmodulin or by a monoclonal antibody which recognizes the calmodulin binding domain. Journal of Biological Chemistry. 1996;271:4148–4153. doi: 10.1074/jbc.271.8.4148. [DOI] [PubMed] [Google Scholar]
- Sunkar R, Bartels D, Kirch HH. Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. The Plant Journal. 2003;35:452–464. doi: 10.1046/j.1365-313x.2003.01819.x. [DOI] [PubMed] [Google Scholar]
- Tuin LG, Shelp BJ. In situ [14C]glutamate metabolism by developing soybean cotyledons. Journal of Plant Physiology. 1994;143:107. [Google Scholar]
- Valle EM, Boggio SB, Heldt HW. Free amino acid composition of phloem sap and growing fruit of Lycopersicon esculentum. Plant and Cell Physiology. 1998;39:458–461. [Google Scholar]
- Van Cauwenberghe OR, Makhmoudova A, McLean MD, Clark SM, Shelp BJ. Plant pyruvate-dependent gamma-aminobutyrate transaminase: identification of an Arabidopsis cDNA and its expression in Escherichia coli. Canadian Journal of Botany. 2002;80:933–941. [Google Scholar]
- Van Cauwenberghe OR, Shelp BJ. Biochemical characterization of partially purified GABA: pyruvate transaminase from Nicotiana tabacum. Phytochemistry. 1999;52:575–581. [Google Scholar]
- Wallace W, Secor J, Schrader LE. Rapid accumulation of gamma-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiology. 1984;75:170–175. doi: 10.1104/pp.75.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Chetelat A, Reymond P, Farmer EE. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. The Plant Journal. 2004;37:877–888. doi: 10.1111/j.1365-313x.2003.02013.x. [DOI] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC. Photorespiration: metabolic pathways and their role in stress protection. Philosophical Transactions of the Royal Society of London. 2000;355:1517–1529. doi: 10.1098/rstb.2000.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Zhou S, Zhang Q, Zhao W, Peng Y. Molecular cloning and differential expression of a γ-aminobutyrate transaminase gene, OsGABA-T, in rice (Oryza sativa) leaves infected with blast fungus. Journal of Plant Research. 2006;119:663–669. doi: 10.1007/s10265-006-0018-3. [DOI] [PubMed] [Google Scholar]