Abstract
The effects of chloroplast number and size on the capacity for blue light-dependent chloroplast movement, the ability to increase light absorption under low light, and the susceptibility to photoinhibition were investigated in Arabidopsis thaliana. Leaves of wild-type and chloroplast number mutants with mean chloroplast numbers ranging from 120 to two per mesophyll cell were analysed. Chloroplast movement was monitored as changes in light transmission through the leaves. Light transmission was used as an indicator of the ability of leaves to optimize light absorption. The ability of leaves to deal with 3 h of high light stress at 10 °C and their capacity to recover in low light was determined by measuring photochemical efficiencies of PSII using chlorophyll a fluorescence. Chloroplast movement was comparable in leaves ranging in chloroplast numbers from 120 to 30 per mesophyll cell: the final light transmission levels after exposure to 0.1 (accumulation response) and 100 μmol photons m−2 s−1 (avoidance response) were indistinguishable, the chloroplasts responded quickly to small increases in light intensity and the kinetics of movement were similar. However, when chloroplast numbers per mesophyll cell decreased to 18 or below, the accumulation response was significantly reduced. The avoidance response was only impaired in mutants with nine or fewer chloroplasts, both in terms of final transmission levels and the speed of movement. Only mutants lacking both blue light receptors (phot1/phot2) or those with drastically reduced chloroplast numbers and severely impacted avoidance responses showed a reduced ability to recover from high light stress.
Keywords: Chloroplast movement, chloroplast number mutants, chloroplast size, optimizing light absorption, photoinhibition
Introduction
Photosynthetic organisms have evolved a variety of strategies to optimize light absorption and the photochemical and biochemical machinery necessary to process the absorbed photons. One of these adaptations is the capacity for the intracellular positioning of chloroplasts in leaf mesophyll cells (for a recent review, see Wada et al., 2003). The location of the chloroplasts within a given mesophyll cell is to a great extent determined by the species, ambient light environment, and the growth history of the organism (Senn, 1908; Trojan and Gabrys, 1996; Williams et al., 2003). In general, under low light chloroplasts minimize self-shading and move to the intracellular surfaces parallel to the leaf surface. This is referred to as the accumulation reaction or face position and is believed to facilitate photosynthetic light absorption and thus carbon assimilation. When leaves are exposed to higher light intensities, chloroplasts move to the edges of the mesophyll cells, perpendicular to the leaf surface to maximize self-shading and to minimize the excess absorption of photons that could lead to photoinhibition or photo-oxidation. This is referred to as the avoidance reaction or profile position (Kasahara et al., 2002; Wada et al., 2003). For chloroplast movement, the nature of the ambient light environment is monitored by the mesophyll cell's blue light receptors phototropin1 (phot1) and phototropin 2 (phot2). The two photoreceptors, which contain two LOV domains and have serine/threonine kinase activity, have partially redundant functions mediating the accumulation response of chloroplasts, whereas phot2 is solely responsible for the avoidance response (for recent reviews, see Kagawa, 2003; Celaya and Liscum, 2005; Christie, 2007). Chloroplasts are thought to move along actin cables via a myosin motor (Takagi, 2003; Wada et al., 2003). It has been shown that chloroplasts are surrounded by a basket of actin filaments that is linked directly or indirectly to larger actin cables (Kandasamy and Meagher, 1999; Takagi, 2003; Kumatani et al., 2006). Actin depolymerization agents such as Latrunculin B impede chloroplast movement and disrupt the actin filament basket surrounding the chloroplasts (Kandasamy and Meagher, 1999), providing further evidence for the involvement of actin filaments in movement. Various studies have shown that several myosin inhibitors such as 2,3-butanedione monoxime also adversely affect chloroplast movement in plants (Paves and Truve, 2007), and myosins of the subclass XI have been co-localized with plastids in species such as maize (Wang and Pesacreta, 2004), but not yet in Arabidopsis thaliana (Reisen and Hanson, 2007). How the phototropins communicate information on light intensity to regulate the vectorial activity and speed of the motor is not well understood (Kagawa and Wada, 2004). It is only clear that the excitation of the photoreceptors leads to their autophosphorylation (Sakai et al., 2001) and a subsequent change in cytoplasmic calcium levels (Tlalka and Fricker, 1999; Harada et al., 2003). Surprisingly, a recent study by Krzeszowiec et al. (2007) showed no blue-light induced changes in the actin cytoskeleton arrangement in Arabidopsis mesophyll cells, while red light led to drastic changes. This suggests that either the chloroplasts are moving on pre-existing cables or the blue light induced changes were too subtle to be picked up with current imaging methods. There is also evidence that red light modulates the blue light response by increasing cytoplasmic motility and alters the light intensity at which chloroplasts switch from an accumulation to an avoidance response (Kagawa and Wada, 2000; DeBlasio et al., 2003). A cytoplasmic protein, named CHUP1, with a putative actin-binding domain, has been shown to be crucial for proper chloroplast movement. This protein localizes to the outer chloroplast membrane and is thought to be involved in mediating the interactions of chloroplasts with their cytoskeletal tracks (Oikawa et al., 2003). Mutant screens have also identified several other proteins essential for proper chloroplast movement such as pmi1 (plastid movement impaired1), pmi2, pmi5, and JAC1 (auxilin-like-J-domain protein), but their exact roles are yet to be determined (DeBlasio et al., 2005; Suetsugu et al., 2005; Luesse et al., 2006).
Recent evidence suggested that chloroplast size and number might also influence the ability of leaves to position their chloroplasts effectively (Jeong et al., 2002). Chloroplast division is a complicated and highly regulated process that involves the growth of chloroplasts, which are then separated into two equally sized chloroplasts with the help of plastid division (PD) rings (for recent reviews, see Aldridge et al., 2005; Maple and Møller, 2007a, b). Many of the components involved in chloroplast division have been elucidated using bacterial cell division as a paradigm and by studying chloroplast division mutants such as the arc (accumulation and replication of chloroplasts; Pyke and Leech, 1991) mutants. Initially, a Z-ring forms, the positioning of which is mediated by the interplay of MinD (ARC11) and MinE in higher plants (Colletti et al., 2000; Itoh et al., 2001; Fujiwara et al., 2004). The Z-ring is made up of FtsZ1 and FtsZ2 and forms on the stromal side of the chloroplast (Maple and Møller, 2007a, b). Recent evidence suggests that a protein encoded by the ARC3 gene is involved in regulating the formation of this stromal Z-ring and directly interacts with FtsZ1. Arc3, which shows similarities to prokaryotic FtsZ and a kinase, also interacts with the MinD and MinE proteins in Arabidopsis suggesting its involvement in proper positioning of the Z-ring (Shimada et al., 2004; Maple et al., 2007). A DnaJ-like protein, encoded by the ARC6 gene, spans the inner chloroplast envelope membrane with the N-terminus extending into the chloroplast stroma (Vitha et al., 2003) and specifically interacts with FtsZ2. It has been suggested that arc6 connects FtsZ2 to the inner envelope and that FtsZ1 is recruited to the Z-ring through interactions with FtsZ2. The Z-ring allows the recruitment of new proteins to form an inner PD ring, which is followed by the formation of an outer PD ring (El-Kafafi et al., 2005; Maple et al., 2005). The composition of these PD rings is still unknown. The stromal PD division ring contracts without a change in thickness, meaning subunits are lost during constriction while the outer PD ring thickens as it contracts (Aldridge et al., 2005). A cytosolic dynamin-like protein with putative GTPase activity, encoded by the ARC5 gene, is localized to the outer PD ring and is involved in facilitating the separation of the daughter plastids (Gao et al., 2003). Recently, two interacting partners of arc5, namely pdv1 and pdv2, were identified and shown to be important in recruiting arc5 to the plastid division site (Miyagishima et al., 2006). The loss-of-function or change in ratio of any of these chloroplast division proteins results in abnormal numbers, sizes, and shapes of the chloroplasts.
The goal of the investigation was to attempt to confirm and extend the study done by Jeong et al. (2002) who investigated a tobacco mutant with one to three large chloroplasts per mesophyll cell (NtFtsZ1-2) and showed compromised chloroplast movements accompanied by a greater susceptibility to photoinhibitory damage. The aim was to determine the effects of chloroplast size and number on movement and stress tolerance using 13 chloroplast division mutants in Arabidopsis thaliana ranging in chloroplast numbers from two to 120. Also the ability of leaves to move their chloroplasts was tested under a wide range of blue light intensities, rather than simply two extreme intensities, in an effort to gain insights into the ability of the leaves to fine-tune their chloroplast positions. It is reported here that, with increasing chloroplast numbers, the leaves were able to reach more extreme accumulation and avoidance positions and could fine-tune their positions in response to small changes in light intensities. While a reduction to two chloroplasts per mesophyll cell had the most severe effects, a reduction to 18 already adversely affected the ability of chloroplasts to exhibit accumulation responses. When cells contain nine or fewer chloroplasts, the speed of chloroplast movement was seriously impacted in addition to an impairment of the degree of the avoidance reaction. The inability to move out of the light quickly and to achieve low light transmission levels seems to contribute to a higher susceptibility of these leaves to high light stress.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana plants were grown under controlled chamber conditions for 3–4 weeks under a cycle of 8 h light (170 μmol photons m−2 s−1) and 16 h dark. The day/night temperatures were 23/20 °C. The plants were fertilized weekly with all-purpose fertilizer (Peters 20–20–20). The wild type (WT) and arc mutants (accumulation and replication of chloroplasts) of the three ecotypes [Columbia (Col), Landsberg erecta (Ler), and Wassilewskija (Ws)] were obtained from the Arabidopsis Biological Resource Center (www.arabidopsis.org). The phototropin mutants were a gift from Winslow Briggs (Carnegie Institute, Stanford University, Pasadena, CA, USA). The arc6-4 (2) mutant was detected in a chloroplast movement screen of EMS-induced M2 Columbia populations of Arabidopsis thaliana (Lehle Seeds, Tucson, AZ, USA).
PCR amplification and sequencing
DNA isolation and PCR amplification was done using the REDExtract-N-Amp Plant PCR Kit (Sigma-Aldrich, St Louis, MO, USA). The following primers were used to amplify the upper and lower strands of the arc-6 gene: forward primer, 5′-GTGGCGACTGTAACATCATA-3′, reverse primer, 5′-AGAACCCTGAACTACCTCCA-3’; forward primer, 5′-GGGCTTAGACAGTGAGGATT-3′, reverse primer, 5′-GTTCTGCTTGTACCTGATTG-3′. The PCR products were purified using a MinElute PCR Purification Kit (Qiagen, Valencia, CA, USA) and then sequenced using the BigDye v3.1 terminator protocol (Applied Biosystems, Foster City, CA, USA) in an ABI3100 capillary sequencer. The primers used for sequencing the upper DNA strand were: 5′-TAGAAGAGAGTACAATGAAGGT-3′, 5′-GTGAGAAGTTTATGAATGAGG-3′, 5′-AAGGAGGCAAGTGTGAAGAT-3′, and 5′-CCTTGTTTGATTCTGTTATTT-3′. The primers used for sequencing the lower DNA strand were: 5′-ATCAACCTACCACACAACAT-3′, 5′-CTCAGTCTCACCACCTTCTT-3′, 5′-AAATAACAGAATCAAACAAGG-3′, and 5′-GAACAGTGAAATCAGTCCAA-3′. Sequences were compiled, edited, and aligned in Sequencher v. 4.5 (GeneCodes Corporation, Ann Arbor, MI, USA).
Chloroplast movement
Chloroplast movement was analysed by monitoring light transmission through Arabidopsis thaliana leaves (Berg et al., 2006). Blue light-dependent changes in the percentage leaf transmittance in overnight dark-adapted leaves were recorded during a 19 h run. Dark-adapted leaves were placed in a leaf clip and for the first 4 h the transmission was measured in the dark. The leaves were then exposed to 0.1 μmol photons m−2 s−1 for 3 h. After that the blue intensity was increased every hour (7–8 h, 0.2 μmol photons m−2 s−1; 8–9 h, 0.4 μmol photons m−2 s−1; 9–10 h, 0.8 μmol photons m−2 s−1; 10–11 h, 1.6 μmol photons m−2 s−1; 11–12 h, 5 μmol photons m−2 s−1; 12–13 h, 10 μmol photons m−2 s−1; 13–14 h, 30 μmol photons m−2 s−1; 14–15 h, 40 μmol photons m−2 s−1; 15–16 h, 50 μmol photons m−2 s−1; 16–17 h, 60 μmol photons m−2 s−1; 17–18 h, 90 μmol photons m−2 s−1; 18–19 h, 100 μmol photons m−2 s−1).
For statistical analysis, the leaf percentage transmission data were normalized by calculating the percentage changes in light transmission compared with the dark steady-state level of light transmission. This was done because the percentage transmission after dark adaptation varied due to the different optical properties of each leaf. The percentage change in transmission was negative when the percentage transmission was less than the steady-state dark-adapted value and conversely positive when the transmittance of the leaf was greater than the dark-adapted value. To test whether there were statistically significant differences in the behaviour of the mutants or WT plants one-way ANOVA tests followed by Tukey–Kramer HSD tests were performed (JMP; SAS, Cary, NC, USA).
Light stress
Arabidopsis thaliana leaves were exposed to different light intensities in a treatment chamber that was maintained at 10 °C and flushed with humidified air (Königer et al., 1998). The high light stress test consisted of 30 min under low light (0.5 μmol photons m−2 s−1), 3 h high light (1500 μmol photons m−2 s−1), followed by 1 h of low light (0.5 μmol photons m−2 s−1). Yield (photochemical efficiency of photosystem II) was measured as chlorophyll a fluorescence with a modulated fluorometer (PAM-2000; Heinz Walz GmbH, Effeltrich, Germany) every 10 min during low light and every hour in high light. The yields of each plant variety at the end of the recovery period were analysed with a one-way ANOVA followed by a Tukey–Kramer HSD test (JMP; SAS).
Results
Chloroplast division mutants investigated include a novel arc6 mutant
The capacity for blue light-dependent chloroplast movement was investigated in the leaf tissue of a series of Arabidopsis chloroplast number mutants of three ecotypes [Columbia (Col), Landsberg erecta (Ler), and Wassilewskija (Ws)], and in mutants that lack one (phot1 or phot2) or both (phot1/phot2) of the phototropin receptors. Table 1 summarizes some of the pertinent information on the ecotypes and mutants utilized in the work reported here. These mutants have been well characterized in terms of the number and sizes of their chloroplasts with the mean chloroplast number per mesophyll cell ranging from 2 to 120. Using confocal microscopy of individual cells isolated from leaves of these mutants the numbers and sizes of chloroplasts per cell (data not shown) were confirmed. In the text, average chloroplast number will be noted in brackets following the term wild type (WT) or mutant acronym, i.e. WT (100). Mutants with decreased chloroplast numbers in general compensate by increasing the size of their individual chloroplasts (Pyke and Leech, 1994).
Table 1.
Background on wild-type (WT) and mutant Arabidopsis thaliana plants
| Plant | Ecotype | Chloroplast number/cell | Chloroplast size (μm2) | Gene locus | Role of gene product | References |
| WT | Col | 100 | 50 | |||
| phot 1 | Col | nd | nd | At3g45780 | Blue-light receptor that mediates chloroplast movement (accumulation) | Christie, 2007 |
| phot2 | Col | nd | nd | At5g58140 | Blue-light receptor that mediates chloroplast movement (accumulation and avoidance) | Christie, 2007 |
| phot1/phot2 | Col | nd | nd | See information above | Christie, 2007 | |
| arc6-4 | Col | 2 | 1000 | At5g42480 | DnaJ-like protein localized to the plastid division site; contributes to assembly, stabilization of the Z-ring | Isolated by authors |
| WT | Ler | 120 | 50 | |||
| arc1-1 | Ler | 108 | 25 | nd | Accelerates proplastid division; acts independent of arc 3, 5, 6, and 11 | Marrison et al., 1999 |
| arc11-1 = MinD | Ler | 30 | 110 | At5g24020 | Stromal, Ca2+-dependent ATPase required for correct positioning of chloroplast division apparatus | Fujiwara et al., 2004 |
| arc3-1 | Ler | 18 | 200–300 | At1g75010 | Stromal protein with FtsZ-like part and kinase; involved in division site placement | Maple et al., 2007 |
| arc5-1 | Ler | 13 | 300–900 | At3g19720 | Cytosolic dynamin-related protein; facilitates separation of daughter chloroplasts | Gao et al., 2003 |
| arc5-1/11-1 | Ler | 12 | 160 | See above | Marrison et al., 1999 | |
| arc6-1/1-1 | Ler | 9 | 530 | See above | Marrison et al., 1999 | |
| arc6-1 | Ler | 2 | 1000 | See above | Vitha et al., 2003 | |
| WT | Ws | 85 | 50 | |||
| arc7-1 | Ws | 80 | 40 | nd | Involved in chloroplast development rather than division itself | Rutherford, 1996 |
| arc8-1 | Ws | 45 | 110 | nd | nd | Rutherford, 1996 |
| arc10-1 | Ws | 38 | 170 (mix) | nd | nd | Rutherford, 1996 |
| arc9-1 | Ws | 34 | 140 | nd | nd | Rutherford, 1996 |
| arc6-1 | Ws | 2 | 1000 | See above | Vitha et al., 2003 |
Three ecotypes [Columbia (Col), Landsberg erecta (Ler), and Wassilewskija (Ws)] were used in this study. The information on chloroplast numbers, sizes, and the affected genes was obtained from Aldridge et al. (2005), TAIR (www.arabidopsis.org), and the references cited. nd = not determined.
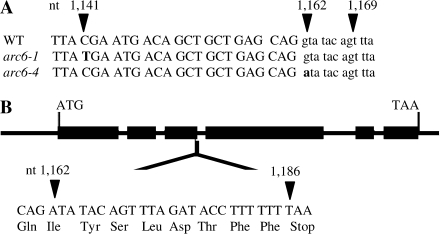
One of the mutants studied was discovered in a screen of EMS mutants and sequencing revealed that it was an allelic mutant of arc6 (from here on designated arc6-4; Fig. 1). arc6-1, which was also used in this study, shows a C to T transition at nucleotide 1141 in exon 3, which introduces a premature stop codon, leading to a truncated protein with 324 rather than 801 amino acids (Vitha et al., 2003). The mutant, arc6-4, shows a point mutation (G to A) at the first base of the donor site of intron 3, which means the intron will be expressed. There is, however, a premature stop codon at nucleotide 1186 within intron 3, resulting in a truncated protein. If a possible alternate splice site at nucleotides 1169–1170 (GT; Alexandrov et al., 2006) were used in pre-mRNA processing, then the resulting frame shift would lead to a premature stop in exon 4 at nucleotides 1247–1249 and thus a truncated protein.
Fig. 1.
(A) Comparison of the genomic sequences of wild-type Arabidopsis thaliana arc6 (WT; gene At5g42480), arc6-1, and arc6-4. A single point mutation at nucleotide (nt) 1141 (C to T) is present in arc6-1, while arc6-4 has a single point mutation at nt 1162 (G to A) which is the first base in the donor site for the third intron. A putative splice site at nt 1169–1170 (GT) may lead to alternative splicing. The use of upper- and lower-case lettering for the nucleotides denotes exon and intron regions, respectively. (B) Gene structure. Exons are depicted as black rectangles; ATG and TAA are the translation initiation and termination codons, respectively. The point mutation in arc6-4 probably prevents excision of the intron and allows for its translation, which if the alternative splice site is not recognized, will lead to a truncated protein product due to a stop codon (TAA) at nt 1186–1188.
Chloroplast number and its effects on chloroplast movement and ability to adjust to different light intensities
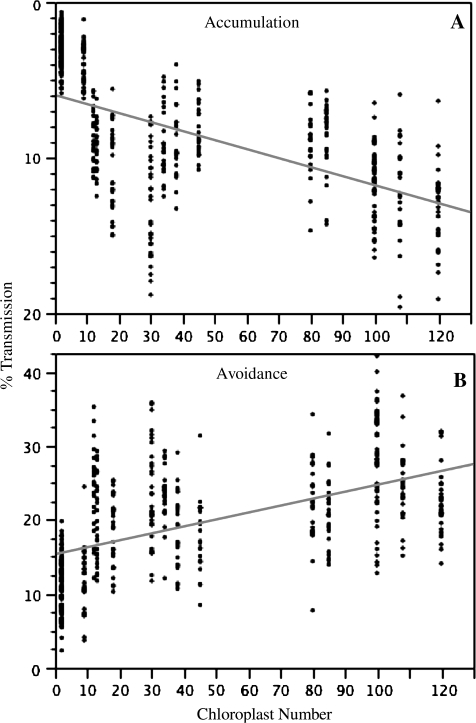
Chloroplast movement can be studied using microscopy or indirectly by measuring changes in light transmission which have been shown to reflect changes in chloroplast positioning (for example, Jeong et al., 2002; Berg et al., 2006). The latter method was chosen because it allowed a quantitative investigation of a large number of mutants at 13 different light intensities each, a task that would have been impossible using light microscopy. Changes in leaf transmission to red light were monitored after exposure to blue light intensities ranging from 0.1 to 100 μmol photons m−2 s−1 (Berg et al., 2006). When leaves were exposed to low light, the chloroplasts accumulated at the perpendicular surface leading to a decrease in the percentage transmission relative to the dark values, while high light led to an increase in percentage transmission as chloroplasts clustered at the cell edges. Pooling all data for the three ecotypes and chloroplast number mutants revealed that there was a significant negative relationship between chloroplast number and the percentage transmission at low light relative to the dark, meaning the larger the number of chloroplasts per mesophyll cell the more efficient the leaves were at absorbing the incoming low light (0.1 μmol photons m−2 s−1, Fig. 2A). There was a significant positive relationship between chloroplast number and percentage transmission at high light (100 μmol photons m−2 s−1, Fig. 2B), indicating that with increasing chloroplast number the leaves were better at avoiding high light. In fact, at any of the blue light intensities up to 10 μmol photons m−2 s−1 there was a significant negative relationship between chloroplast number and percentage transmission, while at light intensities above 30 μmol photons m−2 s−1 there was a significant positive relationship (data not shown).
Fig. 2.
The percentage transmission at the end of a given light exposure (A, accumulation at 0.1 μmol photons m−2 s−1; B, avoidance at 100 μmol photons m−2 s−1) relative to the value at the end of the 4 h dark treatment (y) was plotted against chloroplast number per mesophyll cell (x). All data for wild-type and mutant plants of ecotypes Columbia, Landsberg erecta, and Wassilewskija (see Table 1) were included except those for phot1, phot2, and the phot1/phot2 double mutant. Regression lines were fitted through the data (accumulation: y=–5.98–0.58x; avoidance: y=15.36 + 0.093x). All regressions were highly significant (P < 0.0001).
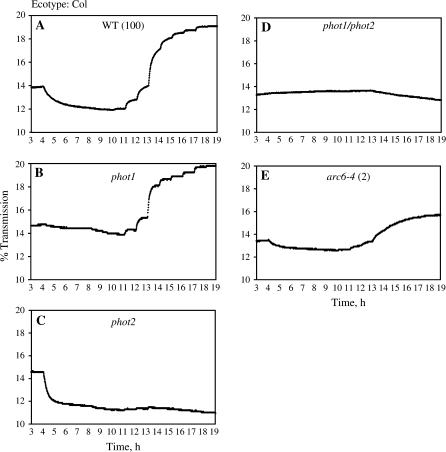
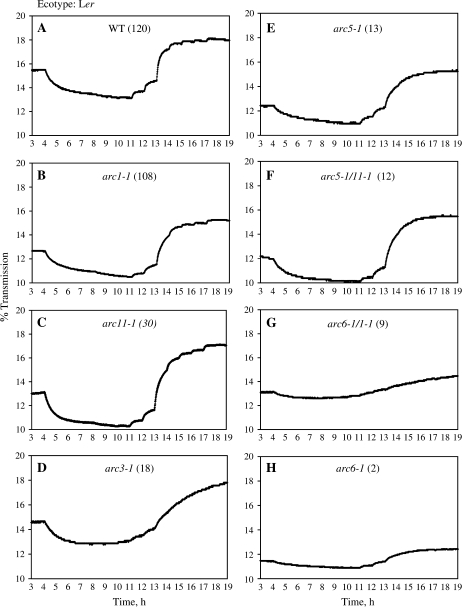
When examining the chloroplast movement behaviour of WT A. thaliana Col (100), Ws (85), and Ler (120) leaves, it became obvious that they exhibited a similar pattern of leaf transmission changes in response to blue light (Figs 3A, 4A, 5A). The accumulation and avoidance reactions were kinetically similar and the transition from accumulation to avoidance occurred at approximately the same light intensity (between 10 and 30 μmol photons m−2 s−1) in all ecotypes. The light intensities required for saturation were also quite similar (90–100 μmol photons m−2 s−1). However, WT Ler (120) showed a significantly stronger accumulation response than WT Col (100) and WT Ws (85), while WT Col showed a stronger avoidance response than the two other WT ecotypes (Tables 2, 3), based on the percentage transmission relative to dark reached after exposure to 0.1 or 100 μmol photons m−2 s−1. It was, therefore, decided to analyse WT and mutant behaviour separately for the different ecotypes.
Fig. 3.
The percentage transmission of Arabidopsis thaliana leaves (ecotype Columbia). Each graph is a representative trace for the respective plant types. Plants were dark-adapted overnight. Mature leaves were then harvested and placed in a device measuring the light transmission of the leaves at increasingly high blue light intensities for a total of 19 h (1–4 h, dark; 4–7 h, 0.1 μmol photons m−2 s−1; 7–8 h, 0.2 μmol photons m−2 s−1; 8–9 h, 0.4 μmol photons m−2 s−1; 9–10 h, 0.8 μmol photons m−2 s−1; 10–11 h, 1.6 μmol photons m−2 s−1; 11–12 h, 5 μmol photons m−2 s−1; 12–13 h, 10 μmol photons m−2 s−1; 13–14 h, 30 μmol photons m−2 s−1; 14–15 h, 40 μmol photons m−2 s−1; 15–16 h, 50 μmol photons m−2 s−1; 16–17 h, 60 μmol photons m−2 s−1; 17–18 h, 90 μmol photons m−2 s−1; 18–19 h, 100 μmol photons m−2 s−1).
Fig. 4.
The percentage transmission of Arabidopsis thaliana leaves (ecotype Landsberg erecta). Each graph is a representative trace for the respective plant types. Plants were dark-adapted overnight. Mature leaves were then harvested and placed in a device measuring the light transmission of the leaves at increasingly high blue light intensities for a total of 19 h (1–4 h, dark; 4–7 h, 0.1 μmol photons m−2 s−1; 7–8 h, 0.2 μmol photons m−2 s−1; 8–9 h, 0.4 μmol photons m−2 s−1; 9–10 h, 0.8 μmol photons m−2 s−1; 10–11 h, 1.6 μmol photons m−2 s−1; 11–12 h, 5 μmol photons m−2 s−1; 12–13 h, 10 μmol photons m−2 s−1; 13–14 h, 30 μmol photons m−2 s−1; 14–15 h, 40 μmol photons m−2 s−1; 15–16 h, 50 μmol photons m−2 s−1; 16–17 h, 60 μmol photons m−2 s−1; 17–18 h, 90 μmol photons m−2 s−1; 18–19 h, 100 μmol photons m−2 s−1).
Fig. 5.
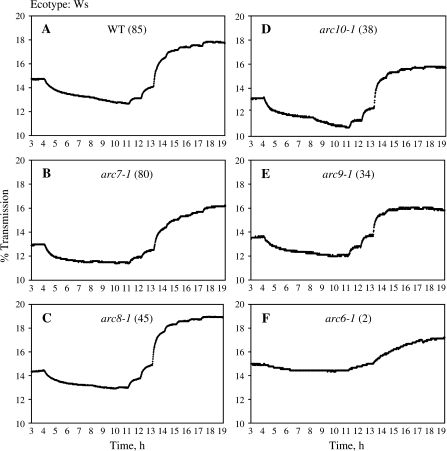
The percentage transmission of Arabidopsis thaliana leaves (ecotype Wassilewskija). Each graph is a representative trace for the respective plant types. Plants were dark-adapted overnight. Mature leaves were then harvested and placed in a device measuring the light transmission of the leaves at increasingly high blue light intensities for a total of 19 h (1–4 h, dark; 4–7 h, 0.1 μmol photons m−2 s−1; 7–8 h, 0.2 μmol photons m−2 s−1; 8–9 h, 0.4 μmol photons m−2 s−1; 9–10 h, 0.8 μmol photons m−2 s−1; 10–11 h, 1.6 μmol photons m−2 s−1; 11–12 h, 5 μmol photons m−2 s−1; 12–13 h, 10 μmol photons m−2 s−1; 13–14 h, 30 μmol photons m−2 s−1; 14–15 h, 40 μmol photons m−2 s−1; 15–16 h, 50 μmol photons m−2 s−1; 16–17 h, 60 μmol photons m−2 s−1; 17–18 h, 90 μmol photons m−2 s−1; 18–19 h, 100 μmol photons m−2 s−1).
Table 2.
Chloroplast accumulation response as measured by leaf light transmission
| Tukey level | % Transmission (means±SD) | |
| Wild-type ecotypes | ||
| Ws (85) | A | –8.5±2.2 |
| Col (100) | B | –11.7±2.3 |
| Ler (120) | C | –13.2±2.8 |
| Ecotype Col | ||
| phot1/phot2 | A | 1.0±0.6 |
| arc6-4 (2) | B | –3.8±1.3 |
| phot1 | B | –4.4±1.5 |
| Wild type (100) | C | –11.7±2.3 |
| phot2 | D | –16.2±2.4 |
| Ecotype Ler | ||
| arc6-1 (2) | A | –2.1±0.9 |
| arc6-1/1–1 (9) | A | –4.0±1.4 |
| arc5-1/11–1 (12) | B | –8.5±1.8 |
| arc5-1 (13) | BC | –9.3±1.5 |
| arc3-1 (18) | CD | –10.9±2.7 |
| arc1-1 (108) | DE | –12.2±3.5 |
| arc11-1 (30) | DE | –12.7±3.4 |
| Wild type (120) | E | –13.2±2.8 |
| Ecotype Ws | ||
| arc6-1 (2) | A | –3.3±0.7 |
| arc8-1 (45) | B | –8.1±1.7 |
| Wild type (85) | B | –8.5±2.2 |
| arc10-1 (38) | B | –8.9±2.4 |
| arc9-1 (34) | B | –8.9±2.3 |
| arc7-1 (80) | B | –9.0±2.2 |
Arabidopsis thaliana [ecotypes Columbia (Col), Landsberg erecta (Ler), Wassilewskija (Ws)] wild-type and mutant plants were dark-adapted overnight. Mature leaves were then harvested and placed in a device measuring the light transmission of the leaves at increasingly high blue light intensities for a total of 19 h. The values below represent the percentage changes in transmission at the end of the 3 h exposure to 0.1 μmol photons m−2 s−1 relative to the values at the end of the dark period (accumulation response). The numbers in brackets after the plant types represent the average chloroplast numbers per mesophyll cell. One-way ANOVA for Col: F=350.9, df = 4,128, P < 0.0001; for Ler F=72.6; df = 7,191, P < 0.0001; for Ws: F=29.3, df = 5,132, P < 0.0001. Groups not connected by the same letter are significantly different (Tukey–Kramer HSD).
Table 3.
Chloroplast avoidance response as measured by leaf light transmission
| Tukey level | % Transmission (means±SD) | |
| Wild-type ecotypes | ||
| Col (100) | A | 27.4±7.6 |
| Ler (120) | B | 22.6±4.8 |
| Ws (85) | B | 21.0±4.4 |
| Ecotype Col | ||
| Wild type (100) | A | 27.4±7.6 |
| phot1 | A | 24.5±4.9 |
| arc6-4 (2) | B | 11.9±4.1 |
| phot1/phot2 | C | –5.0±1.6 |
| phot2 | D | –18.2±2.7 |
| Ecotype Ler | ||
| arc1-1 (108) | A | 24.5±5.3 |
| arc5-1/11–1 (12) | A | 24.3±5.9 |
| arc11-1 (30) | A | 23.5±6.8 |
| Wild type (120) | AB | 22.6±4.8 |
| arc5-1 (13) | AB | 20.1±5.7 |
| arc3-1 (18) | B | 18.2±4.8 |
| arc6-1/1–1 (9) | C | 12.0±4.4 |
| arc6-1 (2) | C | 11.1±3.5 |
| Ecotype Ws | ||
| arc9-1 (34) | A | 23.2±3.9 |
| arc7-1 (80) | AB | 22.3±5.8 |
| Wild type (85) | ABC | 21.0±7.6 |
| arc10-1 (38) | BC | 18.5±5.1 |
| arc8-1 (45) | C | 17.8±4.9 |
| arc6-1 (2) | D | 10.8±3.9 |
Arabidopsis thaliana [ecotypes Columbia (Col), Landsberg erecta (Ler), Wassilewskija (Ws)] wild-type and mutant plants were dark adapted overnight. Mature leaves were then harvested and placed in a device measuring the light transmission of the leaves at increasingly high blue light intensities for a total of 19 h. The values below represent the percentage changes in transmission at the end of the 1 h exposure to 100 μmol photon m−2 s−1 relative to the values at the end of the dark period (avoidance response). The numbers in brackets after the plant types represent the average chloroplast numbers per mesophyll cell. One-way ANOVA for Col: F=372.9, df = 4128, P < 0.0001; for Ler: F=28.7; df = 7,191, P < 0.0001; for Ws: F=24.1, df = 5,132, P < 0.0001. Groups not connected by the same letter are significantly different (Tukey–Kramer HSD).
For the ecotype Col, the phot1/phot2 or phot1 mutant showed significantly reduced accumulation response when compared with WT, while phot2 exhibited a significantly stronger accumulation response (Fig. 3A–D; Table 2). WT (100) showed an avoidance response similar to phot1, while phot1/phot2 and phot2 exhibited seriously impaired avoidance responses (Table 3). The arc6-4 (2) mutant showed significant and severe reductions in both accumulation and avoidance reactions (Fig. 3E; Tables 2, 3); however, the leaves with these two large chloroplasts were capable of significantly stronger chloroplast movements than the phot1/phot2 double mutant. The arc6-4 (2) mutant could not reach very distinctly different light transmission levels in response to increases in high blue light and the movements occurred at slow speed. Interestingly, all three arc6 mutants tested behaved similarly despite coming from different ecotype backgrounds.
Experiments with Landsberg erecta (Ler) chloroplast number mutants revealed that all mutants with 18 or fewer chloroplasts [arc3-1 (18), arc5-1 (13), arc5-1/1–1 (12), arc6-1/1–1 (9), arc6-1 (2)] showed a significantly reduced ability to accumulate their chloroplasts in response to low light when compared with WT (Fig. 4A, D–H; Table 2). In general, the lower the number of chloroplasts the lower the accumulation response. The other mutants with higher chloroplast numbers [arc1-1 (108), arc11-1 (30)] were indistinguishable in their accumulation response (Fig. 4B–C; Table 2) from WT (120). The avoidance response of WT (120) was indistinguishable from that of mutants ranging in chloroplast number from 18 to 108, but significantly higher than in those mutants with nine or fewer chloroplasts (arc6-1/1-1 (9), arc6-1 (2); Fig. 4G, H; Table 3). In terms of the kinetics it was clear that especially arc6-1/1-1 (9) and arc6-1 (2) showed slower kinetics (Fig. 4G–H), meaning it took longer for the leaves to reach a steady-state level of transmission and again not many distinct levels of transmission were achieved.
Among the Wassilewskija ecotype (Ws) mutants (Fig. 5), only arc6-1 (2) showed significant impairment in accumulation and avoidance when compared with the WT (85). The other chloroplast number mutants that ranged in chloroplast number from 34 to 80 were indistinguishable from WT (Tables 2, 3) with regard to their leaf transmission values.
Chloroplast number and high light stress
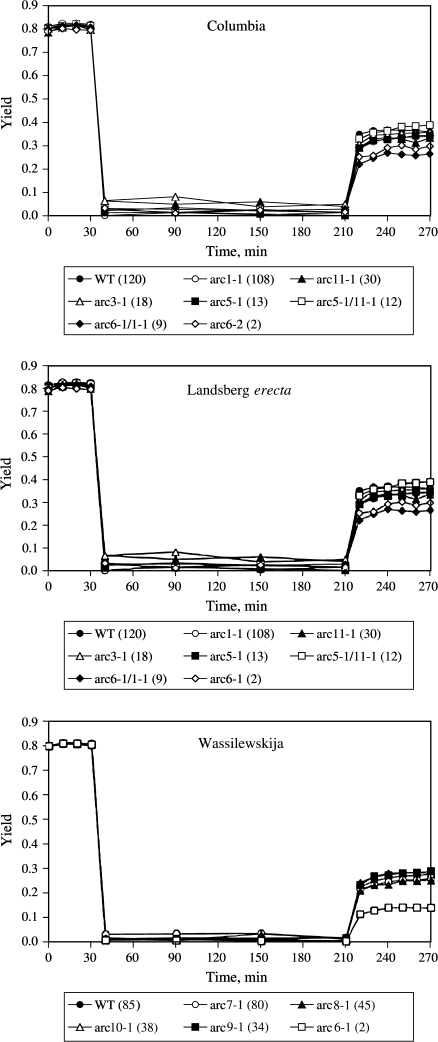
In addition to the chloroplast movement studies, the chloroplast number mutants of the three A. thaliana ecotypes were also used to investigate the effects of chloroplast number/movement on high light stress tolerance (Fig. 6; Table 4). Leaves were exposed to low light (5 μmol photons m−2 s−1) for 30 min, followed by 3 h of high light (1500 μmol photons m−2 s−1), followed by a 1-h-long recovery period at low light (5 μmol photons m−2 s−1). In general, all plants responded similarly to the treatments. The yields (PSII photochemical efficiency) were around 0.8 for all leaves at the beginning of the treatment and then rapidly dropped to very low values (<0.1) that were sustained throughout the high light-treatment period. When returned to low light, a two-phased recovery in yield was observed, a rapid increase followed by a continued slow recovery in some cases. Again, the data for the three ecotypes were kept separately. The final level of recovery from the high light treatment was the same for WT Col (100) and phot1 and phot2 mutants, while phot1/phot2 and arc6-4 (2) were significantly less capable of recovering their photochemical efficiency. For ecotypes Ler and Ws all chloroplast number mutants recovered to the same degree as their respective WT plants with the exceptions of the arc6 (2) and arc6-1/1–1 (9) mutants.
Fig. 6.
Arabidopsis thaliana leaves from mature wild-type or mutant plants were exposed for 30 min to low light (5 μmol photons m−2 s−1), followed by 180 min of high light (1500 μmol photons m−2 s−1), followed by 60 min of recovery at low light (5 μmol photons m−2 s−1). The temperature was kept at 10 °C throughout the experiment. The yield (photochemical efficiency of photosystem II) was measured at 10 min intervals throughout the low light periods, and at 30 min intervals throughout the high light period (n=12–16).
Table 4.
Recovery from high light stress as determined by chlorophyll a fluorescence
| Tukey level | % Recovery (means±SD) | |
| Ecotype Col | ||
| Wild type (100) | A | 37.0±4.2 |
| phot2 | A | 35.4±13.2 |
| phot1 | AB | 34.7±9.3 |
| phot1/phot2 | BC | 25.5±6.7 |
| arc6-4 (2) | C | 20.6±5.8 |
| Ecotype Ler | ||
| arc5-1/11–1 (12) | A | 47.7±4.6 |
| arc3-1 (18) | A | 45.0±9.9 |
| wild type (120) | A | 44.1±6.4 |
| arc5-1 (13) | A | 42.5±5.5 |
| arc1-1 (108) | AB | 41.4±3.5 |
| arc11-1 (30) | AB | 40.9±8.0 |
| arc6-1 (2) | AB | 37.4±7.7 |
| arc6-1/1–1 (9) | B | 32.8±12.9 |
| Ecotype Ws | ||
| arc9-1 (34) | A | 36.2±3.8 |
| Wild type (85) | A | 34.6±4.4 |
| arc10-1 (38) | A | 34.5±6.2 |
| arc7-1 (80) | A | 32.2±7.8 |
| arc8-1 (45) | A | 31.3±7.8 |
| arc6-1 (2) | B | 17.5±4.7 |
Arabidopsis thaliana leaves from wild-type and mutant plants (ecotypes Columbia, Landsberg erecta, and Wassilewskija) were exposed for 30 min to low light (5 μmol photons m−2 s−1), followed by 180 min of high light (1500 μmol photons m−2 s−1), followed by 60 min of recovery at low light (5 μmol photons m−2 s−1). The temperature was kept at 10 °C throughout the entire experiment. The yield (photochemical efficiency of photosystem II) was measured at 10 min intervals throughout the low light periods, and at 30 min intervals throughout the high light period (n=12–16). The values for percentage recovery were calculated using the yield values just before the start of the 180 min high light stress treatment and those at the end of the 60 min recovery at low light. The numbers in brackets after the plant types represent the average chloroplast number per mesophyll cell. One-way ANOVA for Col: F=8.3, df = 4,63, P < 0.0001; for Ler: F=4.6; df = 7,92, P=0.0002; for Ws: F=15.8, df = 5,66, P < 0.0001. Groups not connected by the same letter are significantly different (Tukey–Kramer HSD).
Discussion
Effect of chloroplast number and size on chloroplast movement and ability to fine-tune light absorption
In 1998, McCain hypothesized that chloroplast movement could be impeded by the dense packing or ‘crowding’ of the chloroplasts in the cytoplasm based on a NMR study on Acer leaves that were water-stressed to different degrees (McCain, 1998). However, when a wide variety of Arabidopsis mutants ranging in chloroplast number per mesophyll cell from two to 120 was investigated, no evidence that large numbers of chloroplasts impeded movement was seen, as judged either by the total transmission changes achieved or by the rates of change. Quite the opposite, with increasing chloroplast numbers, leaves displayed both stronger accumulation and avoidance responses, and showed faster changes in percentage transmission. Maybe if the chloroplast numbers were increased past WT levels a reversal of this trend would be observed, since an increase in chloroplast number in the arc mutants is accompanied by a decrease in chloroplast size, leaving the chloroplast volume at comparable levels (Pyke and Leech, 1994).
Looking at the levels of accumulation and avoidance in more detail, it became clear that the final levels of transmission that were achieved after at least 1 h of exposure to low or high light were indistinguishable from WT for plants with chloroplast numbers ranging from 120 to 30 for the accumulation response, and 120 to 12 for the avoidance response. It is remarkable that cells can compensate for a decrease in chloroplast numbers and an increase in chloroplast size to such a remarkable degree and still achieve ‘normal’ chloroplast movement. How this is possible is not clear. However, this is not surprising given that knowledge of ‘normal’ chloroplast movement is scant. For example, it is still not known if chloroplasts move on pre-existing or newly formed actin cables, and if myosins are really the motor molecules that provide the force for the movement (Wada et al., 2003). Only after these fundamental questions have been answered for WT plants will it become possible to address how larger chloroplasts are moved. For example, is there simply a proportional increase in the number of motor molecules per chloroplast with increasing organelle size that allows for comparable movements? Why is it not possible to move chloroplasts effectively after a certain size is reached?
When chloroplast numbers per mesophyll cell were reduced to or below 18 and 9, respectively, accumulation and avoidance reactions were severely inhibited in terms of the final steady-state levels of leaf transmission. Interestingly, very small differences in chloroplast numbers had substantial effects. For example, there were significant differences in accumulation responses in plants with 9 versus 12 versus 18 chloroplasts. This indicates that plants can compensate for reductions in chloroplast numbers relatively well up to some point, but then every small decrease in chloroplast number and thus increase in chloroplast size significantly alters how well these plastids can move. The present data support previous research in transgenic tobacco leaves that had shown that tobacco leaves with one to three large chloroplasts per mesophyll (NtFtsZ1-2) exhibited an impaired ability to move their chloroplasts (Jeong et al., 2002). However, the present results also clearly show that, in Arabidopsis leaves, even less extreme reductions in chloroplast numbers can have drastic effects on chloroplast movement. Interestingly, the avoidance response was impacted at lower chloroplast numbers (nine or less) than the accumulation response (18 or less). The reasons for these different sensitivities are not as yet clear but many investigations have shown that accumulation and avoidance responses show distinct characteristics. For example, avoidance movements are faster than accumulation responses (e.g. Fig. 3), and signals inducing accumulation responses can be sensed at greater distances and have longer lasting effects than those inducing avoidance reactions (Wada et al., 2003). On a biochemical level, it has been shown that the accumulation and avoidance reactions show differential sensitivities to the phosphoinositide-3-kinase inhibitor wortmannin. Phosphorylated phosphoinositides are important regulators of actin polymerization (Grabalska and Malec, 2004). This indicates that the pathways have some essential components that are not shared. Mutant analyses have also shown that while certain proteins such as CHUP and pmi1 are essential for both accumulation and avoidance responses (Oikawa et al., 2003; DeBlasio et al., 2005), pmi2 and pmi5 are only involved in the avoidance response (Luesse et al., 2006) and JAC1 only in the accumulation response (Suetsugo et al., 2005). It will be interesting to see which roles these proteins play and how they may act differently in plants with different chloroplast numbers and sizes.
It was also interesting to see in the present study that having more, smaller chloroplasts allowed leaves to optimize and fine-tune their light absorption according to need. This seems particularly important given that field measurements have shown that chloroplasts are in motion most of the time due to the ever-changing light intensities in most environments (Williams et al., 2003). When the ability to move chloroplasts quickly is impaired in mutant Arabidopsis plants with reduced chloroplast numbers, the consequences can be severe. Under the ideal growth conditions, the plants showed fairly normal growth because they compensated for their reduced chloroplast numbers with an increase in chloroplast size (Pyke and Leech, 1994). However, the mutants with severely reduced chloroplast numbers exhibited reduced leaf areas, impaired photosynthetic capacities, and an inability to adapt to higher growth light conditions (Austin and Webber, 2005). Impaired chloroplast movement accompanied by an inability to adjust and fine-tune leaf transmission levels as documented in the present study may in part explain these findings.
Effect of chloroplast number on tolerance to high light stress
The few studies that have investigated the relationship between the capacity for chloroplast movement and the sensitivity to high light stress (photoinhibition) have shown chloroplast movement to be an important mechanism of photoprotection (Park et al., 1996). For example, Kasahara et al. (2002) tested the effects of continuous high light (1400 μmol photons m−2 s−1) on phot2 plants, which lacked the ability to exhibit a high light-avoidance response. The phot2 plants were severely photobleached and necrotic within 22 h, while WT Col tolerated the stress treatment for 31 h. Tobacco plants (NtFtsZ1-2) with compromised chloroplast movement due to severely reduced chloroplasts numbers (one to three) also exhibited an increased sensitivity to photoinhibition (Jeong et al., 2002). This is not surprising given that photoinhibition is caused by an imbalance between the amount of light absorbed by chloroplasts and their ability to utilize the light in photosynthesis or dissipate it safely in the form of heat (for reviews, see Niyogi, 1999; Demmig-Adams and Adams, 2006). In general, a positive, linear relationship between the ability of chloroplasts to move out of the high light and the ability to recover from the stress treatment (data not shown) was seen. In particular, those plants with seriously impaired chloroplast movement [phot1/phot2, arc6 (2), arc6-1/1-1 (9)] exhibited the most extreme photoinhibition. This is probably in part due to increased absorption of light. However, previous work had also shown that Arabidopsis plants with severely reduced chloroplast numbers (arc3, arc5, arc6) have reduced photosynthetic rates at high light intensities and highly reduced plastoquinone pools (Austin and Webber, 2005). This would also contribute to their enhanced sensitivity to light stress.
Under the present treatment conditions of chilling temperatures combined with ∼9-fold higher light intensities than those experienced during growth, there was no difference in stress tolerance between WT and phot2 despite the fact that the mutant showed significantly impaired chloroplast movement. This is in contrast to previous work that had shown phot2 to be more prone to photoinhibition than WT plants (Kasahara et al., 2002). The difference in response could be due to a variety of reasons. For example, the present stress treatments were conducted at 10 °C and it has been shown that the velocity of chloroplast movement is reduced at lower temperatures. However, the same levels of light transmission are eventually achieved at light saturation and the maximum accumulation response is reached at lower light intensities, suggesting that the system is more sensitive to blue light at lower temperatures (Weisenseel, 1968a, b; Gabrys and Konopacka, 1980). It is therefore possible that under the conditions of the present stress treatments differences between WT and phot2 were masked. Another possibility is that the mutants up-regulated other photoprotective mechanisms to compensate for their impaired chloroplast movement abilities. Plants from stressful environments (e.g. those from high light environments) have been shown to increase their ability to dissipate excess light as heat via the pigments of the xanthophyll cycle (Königer et al., 1995; Demmig-Adams and Adams, 1996). As far as is known, no information exists about the pigment composition of any of the phot mutants. However, phot2 plants grown at 100 μmol photons m−2 s−1 exhibited similar degrees of non-photochemical quenching (an indicator of heat dissipation via the xanthophyll-cycle pigments) as WT plants (Kasahara et al., 2002). The plants in the present study were grown at higher light intensities and thus it is possible that they up-regulated the amount of xanthophyll-cycle pigments. It has been shown that phot2 has comparable oxygen radical scavenging systems as WT when grown at lower light intensities (Kasahara et al., 2002). These antioxidants and enzymes are important for scavenging radicals that result from PSII overexcitation and potentially cause photo-oxidation of PSII proteins such as D1 (Niyogi, 1999). Again, these scavenging systems may have been up-regulated in the plants that were grown at higher light intensities.
Acknowledgments
We would like to thank Dr Sequeira for help with sequencing. Funding was provided by the following sources: Jerome Shiff Fellowship (JAD), Faculty Award (MK), and Howard Hughes Medical Institute Fellowship (EDM).
References
- Aldridge C, Maple J, Møller SG. The molecular biology of plastid division in higher plants. Journal of Experimental Botany. 2005;56:1061–1077. doi: 10.1093/jxb/eri118. [DOI] [PubMed] [Google Scholar]
- Alexandrov NN, Troukhan ME, Brover VV, Tatarinova T, Flavell RB, Feldmann KA. Features of Arabidopsis genes and genome discovered using full-length cDNAs. Plant Molecular Biology. 2006;60:69–85. doi: 10.1007/s11103-005-2564-9. [DOI] [PubMed] [Google Scholar]
- Austin J, II, Webber AN. Photosynthesis in Arabidopsis thaliana mutants with reduced chloroplast number. Photosynthesis Research. 2005;85:373–384. doi: 10.1007/s11120-005-7708-x. [DOI] [PubMed] [Google Scholar]
- Berg R, Königer M, Schjeide B-M, Dikmak G, Kohler S, Harris GC. A simple low-cost microcontroller-based photometric instrument for monitoring chloroplast movement. Photosynthesis Research. 2006;87:303–311. doi: 10.1007/s11120-005-9012-1. [DOI] [PubMed] [Google Scholar]
- Celaya RB, Liscum E. Phototropins and associated signaling: providing the power of movement in higher plants. Photochemistry and Photobiology. 2005;81:73–80. doi: 10.1562/2004-08-22-IR-282. [DOI] [PubMed] [Google Scholar]
- Christie JM. Phototropin blue-light receptors. Annual Review of Plant Biology. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- Colletti KS, Tattersall EA, Pyke KA, Froehlich JE, Stokes KD, Osteryoung KW. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Current Biology. 2000;10:507–516. doi: 10.1016/s0960-9822(00)00466-8. [DOI] [PubMed] [Google Scholar]
- DeBlasio SL, Luesse DL, Hangarter RP. A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiology. 2005;139:101–114. doi: 10.1104/pp.105.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio SL, Mullen JL, Luesse DL, Hangarter RP. Phytochrome modulation of blue-light-induced chloroplast movement in Arabidopsis. Plant Physiology. 2003;133:1471–1479. doi: 10.1104/pp.103.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophylls cycle carotenoids in the protection of photosynthesis. Trends in Plant Science. 1996;1:21–26. [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytologist. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- El-Kafafi E-S, Mukherjee S, El-Shami M, Putaux J-L, Block MA, Pignot-Paintrand I, Lerbs-Mache S, Falconet D. The plastid division proteins, FtsZ1 and FtsZ2, differ in their biochemical properties and sub-plastidial localization. The Biochemical Journal. 2005;387:669–676. doi: 10.1042/BJ20041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara MT, Nakamura A, Itoh R, Shimada Y, Yoshida S, Møller SG. Chloroplast division site placement requires dimerization of the ARC11/AtMinD1 protein in Arabidopsis. Journal of Cell Science. 2004;117:2399–2410. doi: 10.1242/jcs.01092. [DOI] [PubMed] [Google Scholar]
- Gabrys H, Konopacka M. The effect of temperature on chloroplast phototranslocation in Tradescantia albiflora leaves. Acta Physiologiae Plantarum. 1980;4:291–297. [Google Scholar]
- Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proceedings of the National Academy of Sciences, USA. 2003;100:4328–4333. doi: 10.1073/pnas.0530206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabalska M, Malec P. Blue light-induced chloroplast reorientations in Lemna trisulca L. (duckweed) are controlled by two separable cellular mechanisms as suggested by different sensitivity to wortmannin. Photochemistry and Photobiology. 2004;79:343–348. doi: 10.1562/le-03-16.1. [DOI] [PubMed] [Google Scholar]
- Harada A, Sakai T, Okada K. phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proceedings of the National Academy of Sciences, USA. 2003;100:8583–8588. doi: 10.1073/pnas.1336802100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R, Fujiwara M, Nagata N, Yoshida S. A chloroplast protein homologous to the eubacterial topological specificity factor MinE plays a role in chloroplast division. Plant Physiology. 2001;127:1644–1655. [PMC free article] [PubMed] [Google Scholar]
- Jeong WJ, Park Y-I, Suh KH, Raven JA, Yoo OJ, Liu YR. A large population of small chloroplasts in tobacco leaf cells allows more effective chloroplast movement than a few enlarged chloroplasts. Plant Physiology. 2002;129:112–121. doi: 10.1104/pp.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T. The phototropin family as photoreceptors for blue light-induced chloroplast relocation. Journal of Plant Research. 2003;116:77–82. doi: 10.1007/s10265-002-0072-4. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M. Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant and Cell Physiology. 2000;41:84–93. doi: 10.1093/pcp/41.1.84. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M. Velocity of chloroplast avoidance movement is fluence rate dependent. Photochemistry and Photobiology. 2004;3:592–595. doi: 10.1039/b316285k. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Meagher RB. Actin-organelle interaction: association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motility and the Cytoskeleton. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- Königer M, Harris GC, Pearcy RW. Interaction between photon flux density and elevated temperatures on photoinhibition in Alocasia macrorrhiza. Planta. 1998;205:214–222. [Google Scholar]
- Königer M, Harris GC, Virgo A, Winter K. Xanthophyll-cycle pigments and photosynthetic capacity in tropical forest species: a comparative field study on canopy, gap and understory plants. Oecologia. 1995;104:280–290. doi: 10.1007/BF00328362. [DOI] [PubMed] [Google Scholar]
- Kumatani T, Sakurai-Ozato N, Miyawaki N, Yokota E, Shimmen T, Terashima I, Takagi S. Possible association of actin filaments with chloroplasts of spinach mesophyll cells in vivo and in vitro. Protoplasma. 2006;229:45–52. doi: 10.1007/s00709-006-0189-8. [DOI] [PubMed] [Google Scholar]
- Krzeszowiec W, Rajwa B, Dobrucki J, Gabrys H. Actin cytoskeleton in Arabidopsis thaliana under blue and red light. Biology of the Cell. 2007;99:251–260. doi: 10.1042/BC20060077. [DOI] [PubMed] [Google Scholar]
- Luesse DR, DeBlasio SL, Hangarter RP. Plastid movement impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in Arabidopsis. Plant Physiology. 2006;141:1328–1337. doi: 10.1104/pp.106.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple J, Aldridge C, Møller SG. Plastid division is mediated by combinatorial assembly of plastid division proteins. The Plant Journal. 2005;43:811–823. doi: 10.1111/j.1365-313X.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- Maple J, Møller SG. Plastid division: evolution, mechanism and complexity. Annals of Botany. 2007a;99:565–579. doi: 10.1093/aob/mcl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple J, Møller SG. Plastid division coordination across a double-membraned structure. FEBS Letters. 2007b;581:2162–2167. doi: 10.1016/j.febslet.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Maple J, Vojita L, Soll J, Møller SG. ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Reports. 2007;8:293–299. doi: 10.1038/sj.embor.7400902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrison JL, Rutherford SM, Robertson EJ, Lister C, Dean C, Leech RM. The distinctive roles of five different ARC genes in the chloroplast division process in Arabidopsis. The Plant Journal. 1999;18:651–662. doi: 10.1046/j.1365-313x.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- McCain DC. Chloroplast movement can be impeded by crowding. Plant Science. 1998;135:219–225. [Google Scholar]
- Miyagishima S-Y, Froehlich JE, Osteryoung KW. PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. The Plant Cell. 2006;18:2517–2530. doi: 10.1105/tpc.106.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanagae T, Niwa Y, Kadota A, Wada M. Chloroplast unusual positioning 1 is essential for proper chloroplast positioning. The Plant Cell. 2003;15:2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y-I, Chow WS, Anderson JM. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiology. 1996;111:867–875. doi: 10.1104/pp.111.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paves H, Truve E. Myosin inhibitors block accumulation movement of chloroplasts in Arabidopsis thaliana leaf cells. Protoplasma. 2007;230:165–169. doi: 10.1007/s00709-006-0230-y. [DOI] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. Rapid image analysis screening procedure for identifying chloroplast number mutants in mesophyll cells of Arabidopsis thaliana (L.) Heynh. Plant Physiology. 1991;96:1193–1195. doi: 10.1104/pp.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Leech RM. A genetic analysis of chloroplast division and expansion in Arabidopsis thaliana. Plant Physiology. 1994;104:201–207. doi: 10.1104/pp.104.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen D, Hanson MR. Association of six YFP-myosin XI-tail fusions with mobile plant cell organelles. BMC Plant Biology. 2007;7:6. doi: 10.1186/1471-2229-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SM. 1996. The genetic and physical analysis of mutants of chloroplast number and size in Arabidopsis thaliana. PhD thesis, University of York. [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proceedings of the National Academy of Sciences, USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn G. Die Gestalts- und Lageveränderung der Pflanzenchromatophoren. Leipzig: W. Engelmann; 1908. [Google Scholar]
- Shimada H, Koizumi M, Kuroki K, Mochizuki M, Fujimoto H, Ohta H, Masuda T, Takamiya K-I. ARC3, a chloroplast division factor, is a chimera of prokaryotic FtsZ and part of eukaryotic phosphatidylinositol-4-phosphate 5-kinase. Plant and Cell Physiology. 2004;45:960–967. doi: 10.1093/pcp/pch130. [DOI] [PubMed] [Google Scholar]
- Suetsugu N, Kagawa T, Wada M. An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiology. 2005;139:151–162. doi: 10.1104/pp.105.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S. Actin-based photo-orientation of chloroplast in plant cells. Journal of Experimental Biology. 2003;206:1963–1969. doi: 10.1242/jeb.00215. [DOI] [PubMed] [Google Scholar]
- Tlalka M, Fricker M. The role of calcium in blue-light-dependent chloroplast movement in Lemna trisulca L. The Plant Journal. 1999;20:461–473. doi: 10.1046/j.1365-313x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiology. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Froehlich JE, Koksharova O, Pyke KA, van Erp H, Osteryoung KW. ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. The Plant Cell. 2003;15:1918–1933. doi: 10.1105/tpc.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Kagawa T, Sato Y. Chloroplast movement. Annual Review of Plant Biology. 2003;54:455–468. doi: 10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pesacreta TC. A subclass of myosin XI is associated with mitochondria, plastids and the molecular chaperone subunit TCP-1a in maize. Cell Motility and the Cytoskeleton. 2004;57:218–232. doi: 10.1002/cm.10168. [DOI] [PubMed] [Google Scholar]
- Weisenseel M. Vergleichende Untersuchungen zum Einfluss der Temperatur auf lichtinduzierte Chloroplastenverlagerungen. I. Die Wirkung verschiedener Lichtintensitäten auf die Chloroplastenanordnung und ihre Abhängigkeit von der Temperatur. Zeitschrift für Pflanzenphysiologie. 1968a;59:56–69. [Google Scholar]
- Weisenseel M. Vergleichende Untersuchungen zum Einfluss der Temperatur auf lichtinduzierte Chloroplastenverlagerungen. II. Die statistische Bewegungsgeschwindigkeit der Chloroplasten und ihre Abhängigkeit von der Temperatur. Zeitschrift für Pflanzenphysiologie. 1968b;59:153–171. [Google Scholar]
- Williams WE, Gorton HL, Witiak SM. Chloroplast movements in the field. Plant, Cell and Environment. 2003;26:2005–2014. [Google Scholar]